Graphical abstract
Keywords: Indigofera tinctoria, Scoparia dulcis, Chronic noise stress, Immunomodulatory, Innate immunity, Adaptive immunity
Highlights
-
•
Chronic noise stress was suppressed both innate and adaptive immune response of wistar albino rats.
-
•
Noise stress also caused DNA damage in the liver and spleen tissues.
-
•
Aqueous extracts of I. tinctoria and S. dulcis prevent the immune abnormalities caused by noise stress.
Abstract
Indigofera tinctoria and Scoparia dulcis are being widely used in Indian folk medicine for the treatment of various disorders. Environmental noise pollution is thought to be an important factor for many health problems and it causes immune abnormalities. In the present study immune-regulating potential of I. tinctoria and S. dulcis aqueous extracts on innate and adaptive immune system of wistar albino rats was evaluated during normal and chronic noise induced stress conditions. The results demonstrated that both I. tinctoria and S. dulcis aqueous extracts (200 mg/kg b.w) showed immunostimulant effect on both innate and adaptive immune response of wistar albino rat compared to control group under normal condition. The noise stress (100 dB for 1 h, 20 days) induced animals showed suppressive effects on immune response by decreasing macrophage phagocytosis, antibody secretion by spleen cells, humoral immune response, proliferation of lymphocytes, cytotoxicity, TNF α expression, granzyme B and perforin expression in splenic NK cells. Similarly, noise stress also caused DNA damage in tissues. However, the suppressed effects induced by noise stress on rat immune system were significantly prevented by oral administration of both I. tinctoria and S. dulcis aqueous extracts. Considering all these results it is suggested that the selected medicinal plant’s aqueous extracts have the potential to prevent the effects of noise stress induced rat immune system and explore a strong immunostimulant potential applicable to clinical practices.
1. Introduction
Noise is one of the most important environmental problems for human beings. In the modern civilization world, rapid industrialization and usage of vehicles is unavoidable one. Therefore, nowadays the noise pollution is an ever-increasing trend in both industrial and general areas. The continuous exposure of noise by human beings is leads to oxidative stress. Oxidative stress was formed in the condition of over production of reactive oxygen species (ROS) and free radicals. The excess production of free radicals can affect the antioxidant systems and cause oxidative damage to cellular biomolecules like proteins, lipids and nucleic acids in various tissues as well as it can increase the corticosterone hormone level [1], [2], [3]. The over production of ROS directly induce posttranslational modification of ion channels leading to oxidation of specific amino acid residues and indirectly modulate channel function by disturbing the signaling pathways that control gene transcription, trafficking, and turnover [4]. The impact of oxidative stress on ion channels can lead to cardiovascular and neurodegenerative disorders [5], [6]. In previous studies it has been stated that, noise stress can lead to increase the level of oxidative stress marker enzymes such as malondialdehyde (MDA), glutathione peroxidase (GSH-Px), corticosterone and nitric oxide (NO) level [7], [8].
Moreover, continuous exposure of noise can affect the physiological and psychological process leading to many chronic diseases such as chronic inflammation, stoke, septic shock, aging, respiratory diseases, type 2 diabetes and adverse birth outcomes in humans [9], [10], [11], [12]. In recent study, Basner [13] reported that the exposure of traffic noise lead to cardiovascular disease and myocardial infarction. Many studies have explored the correlation among the various stress parameters like heat, cold water, chemicals, electric shock and immobilization are also responsible for oxidative stress and immunity [14], [15], [16]. However, only limited studies reported the auditory stressful events on the immune response of rat [7], [8], [17]. Hence, there is a need to address the impact of noise stress on vertebrate immune system and essential to find out the therapeutic compounds against the immune abnormalities caused by noise stress.
The immune system of vertebrates is a complex network and develop defense during pathogen encounter, injury, external contaminants and infectious conditions. They develop two kinds of immune responses (innate and adaptive) against infectious agents [18]. Immunomodulation is focused on accomplishment of immune system to control the infections and other unpleasant health effects with precise regulation to avoid any complications by modulating the immune system. The role of immunomodulators is to balance the immune system either by stimulation or suppression of immune response to maintain homeostasis [19]. Immunostimulators can be used during drug induced immunosuppressive conditions to reduce the side effects [20] and immunosuppressors were used under over expression of inflammatory molecules [21]. Many plants and plant based compounds have been widely used as immunostimulants during oxidative stress conditions [22], [23].
Indigofera tinctoria (I. tinctoria) is a shrub which belongs to the family Fabaceae and traditionally used in Indian and Chinese medicinal system for the treatments of various ailments including constipation, liver diseases and heart palpitation [24]. Different solvent extracts of I. tinctoria leaves showed anticancer and antioxidant activity in vitro [25], [26]. Aqueous extracts of I. tinctoria reported on neuroprotective role in noise stressed wistar rats [27]. Scoparia dulcis is a shrub belongs to the family Scrophulariaceae. Different solvent extracts of this plant showed antihyperglycemic, antioxidant and antimicrobial activity [28], [29], [30]. Scoparinol an active compound isolated from this plant showed analgesic, diuretic and anti-inflammatory activity [31]. Based on the wide range of medicinal properties of these plants, an attempt was made to study the immunoprotective role of I. tinctoria and S. dulcis aqueous extracts on both innate and adaptive immune response of wistar albino rat under noise stress conditions.
2. Materials and methods
2.1. Animals
Wistar albino rats (male, 180–200 g) were used for this study. All animals were maintained in a pathogen-free animal house (University of Madras) under normal conditions (21 ± 2 °C, 12-h light: dark cycle) and provided ad libitum access to standard rodent chow and filtered water. The experiments was approved by institutional animal ethical committee (IAEC No: 22/Feb-2013) administrated by committee for the purpose of control and supervision of experiments on animals (CPCSEA) guidelines, Government of India.
2.2. Preparation of plant extracts
tinctoria and S. dulcis plant material were collected from Tirunelveli District, Tamil Nadu; vouchers (No. PARC/2014/2250 & No. PARC/2013/2153) were deposited in a repository and authenticated by Dr. P. Jeyaraman, Director of Plant Anatomy Research Institute, Tambaram, Chennai, Tamilnadu, India. Leaves (1 kg) were washed, shade-dried and then homogenized. The aqueous extract (10%) was prepared in double distilled water with stirring at 4 °C for 24 h. The extract was filtered, lyophilized and stored at −20 °C for further analysis.
2.3. Acute toxicity study
Acute toxicity assay was carried out according to standard procedure [32] to assess the safety of the I. tinctoria aqueous extracts (ITAE) and S. dulcis aqueous extracts (SDAE) in wistar albino rats (six groups each consisting of three animals). ITAE and SDAE were given orally to 6 h fasted rats, in the dosage of 0.1, 0.25, 0.5, 1.0, 2.0 and 5.0 g/kg b.w. A control group receiving normal saline (10 mL/kg b.w) was also run parallel for comparison. The rats were observed at 1 h interval for the first 12 h and daily basis for 14 days and mortality rate was noted after 48 h. The animals were also observed for any behavioral changes and ill-health like tremors, convulsions, salivation, sweating, lacrimation, writhing reflex, somatomotor activity and behavior pattern. The level serum pro-inflammatory cytokines (IL-6 and TNF-α) were analyzed. Based on acute toxicity study, the LD50 value was calculated for both extracts and dosage was fixed as 200 mg/kg b.w for further study.
2.4. Experimental design
The rats were randomly divided into six groups (3 rats in each group). Experimental animals were administered with ITAE and SDAE orally for 48 days. At the end of the experimental period the rats were anaesthetized and euthanized by cervical dislocation for the analysis. The noise stress was given for last 20 days during the experimental period.
Group 1: Control + No stress
Group 2: No Stress + 200 mg/kg body weight ITAE treated
Group 3: No Stress + 200 mg/kg body weight SDAE treated
Group 4: Noise Stress (100 dB for 1 h for 20 days)
Group 5: Noise Stress + 200 mg/kg body weight ITAE treated
Group 6: Noise Stress + 200 mg/kg body weight SDAE treated
2.5. Noise stress induction
White noise was generated by using computer software named as NCH tone generator prepared by NCH softwares, USA (http://download.cnet. com/NCH-Tone-Generator/3000-2169_4-10019045.htmL). 1 KHz frequency of sound was generated and intensity was changed by amplifier to 100 dB, which was confirmed by digital sound level meter before the start of experiment [33].
2.6. Humoral antibody (HA) titer assay
The hemagglutination titer was determined according to Puri et al. [34]. After the experimental period blood serum samples were collected and serially diluted in 25 μL of 0.9% saline in micro titer plate. After that 25 μL of 1% sheep red blood cells (SRBC) in normal saline were added to each well and mixed. The plates were left at room temperature for 1 h and the hemagglutination was examined under a light microscope. The reciprocal of the highest dilution showing agglutination was taken as hemagglutination antibody titer.
2.7. Quantitative hemolysis of sheep red blood cells (QHS) assay
QHS assay was performed using the methods of Simpson and Gozzo [35] with slight modifications. The animals were received 0.2 mL of 5% SRBC in normal saline by intraperitoneally 6 days prior to the sacrifice. Spleen was removed from sacrificed animals and single cell suspensions (5 × 106 cells/mL) were prepared in PBS. A total of 1.0 mL of 0.2% SRBC and 1.0 mL of guinea pig serum were mixed with 1.0 mL of cell suspension and incubated for 1 h at 37 °C. The content was centrifuged at 3000 rpm for 5 min and the absorbance of the supernatant was measured at 413 nm.
2.8. Macrophage phagocytosis assay (carbon clearance)
Macrophage phagocytosis was analyzed by carbon clearance assay [36]. After the experimental period each rat was injected intravenously with the diluted carbon ink (1.6 g/mL in PBS, pH-7.4 addition with 10% gelatin) at a dose of 0.01 mL/g body weight. At 3, 6, 9, 12 and 15 min after the injection, 0.02 mL blood was obtained by retro orbital venous puncture and erythrocytes were lysed by 2 mL of 0.1% Na2CO3. The optical density (OD) of the samples was measured at 620 nm. The clearance value K was calculated by following formula:
Where, OD1 and OD2 represent the value of OD at the time t1 and t2 respectively.
2.9. Lymphocyte proliferation assay
The spleen lymphocyte proliferation was analyzed by MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-di-phenyltetrazolium bromide) assay [37]. Spleen was aseptically removed from sacrificed rats (6 groups) and single cell suspensions were prepared in PBS. The cells were seeded (5 × 106 cells/mL) in RPMI-1640 medium supplemented with 10% new born bovine serum in 96-well plate and cultured at 37 °C in 5% CO2 atmosphere for 48 h. After incubation
20 μL of MTT (5 mg/mL in PBS) solution was added to each well and cultured for further 4 h. Finally the formazan particles were dissolved by adding 100 μL of DMSO to each well and absorbance was measured in ELISA plate reader (BioTek, Winooski, USA) at 570 nm. The results were expressed as percentage of proliferation (%) compared to the control cells.
2.10. Determination of malondialdehyde (MDA)
The concentration of malondialdehyde was quantified for the assessment of lipid peroxidation level [38]. Spleen and liver samples (0.4 mL) were mixed with 1.6 mL of Tris-KCl buffer (0.15 M, pH-7.4) to which 0.5 mL of 30% trichloroacetic Acid (TCA) was added. Then 0.5 mL of 0.75% Thiobarbituric Acid (TBA) was added and incubated in a water bath for 45 min at 80 °C. After incubation a pink colored reaction mixture was cooled in ice and centrifuged at 10,000 rpm for 15 min. The absorbance of the clear pink supernatant was read at 532 nm.
Calculation:
Where, E532 is extinction coefficient of TBA at 532 nm = 1.56 × 105.
2.11. Cytotoxicity assay of splenic NK cells
NK cell cytotoxicity was assessed by according to standard method described by Kowalski [39]. The spleen single cell suspension was used as effector cells prepared as described earlier and re-suspended at a density of 5 × 105 cells/mL in RPMI-1640 medium. NK-sensitive YAC-1 cells were used as target cells and the density was adjusted to 1 × 104 cells/mL in 96-well plate. After 20 h incubation period at 37 °C in 5% CO2 condition, 100 μL of supernatant was collected from each well and mixed with 100 μL of MTT (5 mg/mL) and further incubated for 4 h. The absorbance was measured at 570 nm using ELISA plate reader. Target cells control, blank control and effector cells control were maintained. NK cell cytotoxicity (%) was calculated as following equation.
In which ODT, ODS and ODE represented the OD values of target cells control, test samples and effector control respectively.
2.12. RNA extraction and semi quantitative RT-PCR
Spleen was aseptically removed from sacrificed rat (6 groups) in PBS and total RNA was extracted using TRIzol Reagent (Invitrogen, USA) [40]. The concentration of RNA was quantified by UV spectrophotometer (Lark UV 2500, Chennai, India). First strand cDNA was synthesized from the total RNA (2 μg) using revertaid first strand cDNA synthesis kit (Thermo Scientific, USA) with random primers according to manufacturer instructions. The mRNA expression of perforin and granzyme B in spleenic NK cells was performed by reverse transcription polymerase chain reaction (Semi quantitative RT-PCR). The primer sequences of granzyme B, perforin and GAPDH are summarized in Table 1. PCR was performed for 28 cycles with initial denaturation at 98 °C for 3 min followed by 30 s denaturing at 94 °C, 1 min annealing at 60 °C and 2 min extension phase at 68 °C. PCR products were subjected to electrophoresis on 1% agarose gel containing ethidium bromide (0.5 μg/mL) and then visualized under UV illumination using a gel documentation system (GELSTAN 1312, Mediccare, India). PCR products were quantified by densitometry scanning by using Quantity One software (BIO-RAD). Perforin and granzyme B expression were normalized relative to the steady state expression of GAPDH used as internal control.
Table 1.
Forward and reverse primers used for PCR.
| Gene Name | Primer Sequence (5′-3′) |
|---|---|
| GAPDH | F: CACTCACGGCAAATTCAACGGCA |
| R: GACTCCACGACATACTCAGCAC | |
| Granzyme B | F: CCCAGGCGCAATGTCAAT |
| R: CCAGGATAAGAAACTCGA | |
| Perforin | F: AGGCAGCTGCTAATATCAAT |
| R: TGTGCTGTTTCTTCTTCTCC |
2.13. DNA damage analysis
DNA damage on rat spleen and liver tissues (6 groups) were analyzed by DNA fragmentation. DNA fragmentation was measured directly on recovered DNA by loading samples of extracted DNA onto agarose gel [41]. DNA was extracted with a mixture of phenol and chloroform, precipitated with ethanol, dried and dissolved in TE buffer. DNA samples were analyzed by electrophoresis on 1.0% agarose gel containing ethidium bromide (0.5 μg/mL) and then visualized under UV illumination and visualized using a gel documentation system.
2.14. Immunohistochemistry (IHC)
Spleen was aseptically removed from sacrificed rat and fixed in 10% buffered formalin, routinely processed, and embedded in paraffin. 5 μm thick sections were used for IHC of TNF-α. The tissue sections were incubated with hydrogen peroxide (3%) for 10 min followed by PBS wash (pH 7.4), incubated for 10 min in blocking solution (BSA-0.1%). The sections were washed and incubated with primary antibody of TNF-α (rabbit monoclonal antibody, 1:100 dilution, Santa Cruz biotechnology, USA) for 1 h at room temperature. After incubation sections were washed with PBS and incubated with secondary antibody (anti rabbit, 1:5000 dilution, Santa Cruz biotechnology, USA) for 30 min, washed three times and then incubated with horseradish peroxidase (HRP) conjugate for 30 min. After washing, the sections were incubated with the DAB substrate for 10 min, rinsed with distilled water and counterstained with mayer’s hematoxylin for 2 min. The expression of TNF-α visualized under a light microscope (Olymbus-BX43, Japan)
2.15. Histopathological analysis
Spleen and liver was collected from sacrificed rat (all groups) and fixed in buffered formalin (10%). For histopathological analysis samples were dehydrated in a different percentage of ethanol (70–100%), cleared in xylene, and finally embedded in paraffin. Thereafter, 5 μm thick sections were prepared and stained with haematoxylin and eosin (H&E) and histopathological examination were observed [42] under a light microscope (Olymbus-BX43, Japan).
2.16. Western blot analysis
Expression of Granzyme B, Perforin and TNF- α were analyzed by Western blot [43]. Total protein was extracted from the spleen of different group animal and equal amount of protein (40 μg) was separated by 12% SDS-PAGE. Protein was transferred to PVDF membrane and incubated with the primary antibodies for specific proteins (rabbit monoclonal antibody, 1:1000 dilution, Santa Cruz biotechnology, USA) followed by exposure to secondary antibody (anti rabbit, 1:5000 dilution, Santa Cruz biotechnology, USA) conjugated with horseradish peroxidise and immunodetection was performed using enhanced chemiluminescence (ECL) method. β-actin used as internal control.
2.17. Statistical analysis
The results are presented as mean ± standard deviation (mean ± SD). Differences between means were tested for statistical significance using a one- way analysis of variance (ANOVA) with the Duncan's multiple range test. All statistical analyses were carried out with Graph Pad Prism version 5.0(California, USA) with statistical significance set at P < 0.05.
3. Results
3.1. Acute toxicity assay
The results obtained in acute toxicity study revealed that the oral administration of both ITAE and SDAE showed absence of mortality after 48 h for the tested dosages. Hence, both extracts were found to be safe up to the higher dosage of 5.0 g/kg b.w in wistar rats. Moreover we noticed significance level of increase in serum cytokines at the dosage of 250 mg/kg b.w and there is no significance difference was observed above this dosage (data not shown).
3.2. Effect of plant extracts on humoral antibody (HA) titer
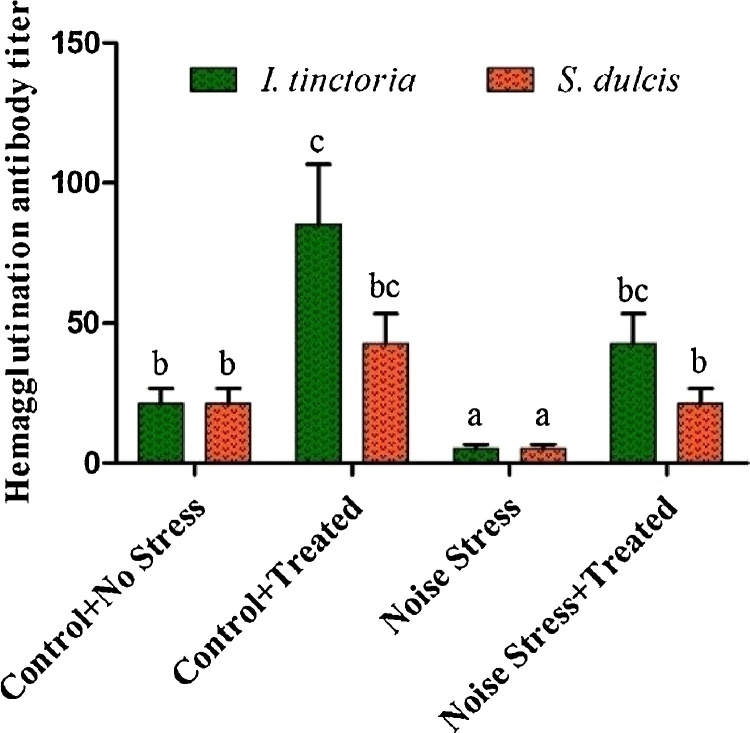
The ITAE and SDAE alone administered animals showed highest level of hemagglutinating antibody titer compared to control group. The noise stress was significantly decreased the antibody titer and the extracts administrated group animals showed increasing level of antibody titer value (Fig. 1).
Fig. 1.
Effect of ITAE and SDAE administration on humoral antibody (HA) titer of normal and noise stressed groups. Data are expressed as means ± SD (n = 9). Bars labeled with different letters represent a statistical difference at (p < 0.05).
3.3. Effect of plant extracts on humoral immune response (QHS assay)
The humoral immunity regulating potential of ITAE and SDAE was evaluated by the comparison of hemolysis capacity of spleen single cell suspension among the groups. The level of hemolysis was significantly decreased in noise stressed animals (A413- 0.542 ± 0.006) compared to control animals (0.698 ± 0.004). Whereas, both ITAE and SDAE treated groups showed significant enhancement of hemolysis activity to 0.864 ± 0.005 and 0.721 ± 0.002 respectively (Table 2).
Table 2.
Effect of ITAE and SDAE administration on macrophage phagocytosis (Carbon clearance assay) and humoral immune response (QHS assay) of different group animals under normal and noise stress condition. Each value represent mean ± SD (n = 9). Mean with different letters within a column are significantly different (p < 0.05).
| S. No | Group | Phagocytic Index (K value) | QHS Assay (A413) |
|---|---|---|---|
| 1. | Control + No stress | 0.017 ± 0.001b | 0.698 ± 0.004b |
| 2. | No stress + ITAE treated | 0.032 ± 0.004cd | 0.982 ± 0.010d |
| 3. | No stress + SDAE treated | 0.027 ± 0.002c | 0.814 ± 0.004c |
| 4. | Noise stress | 0.007 ± 0.001a | 0.542 ± 0.006a |
| 5. | Noise stress + ITAE treated | 0.024 ± 0.001c | 0.864 ± 0.005cd |
| 6. | Noise stress + SDAE treated | 0.017 ± 0.001b | 0.721 ± 0.002bc |
3.4. Effect of plant extracts on macrophage phagocytosis
Macrophage phagocytic response of ITAE and SDAE alone administered group animals showed high level of phagocytic index (K value- 0.032 ± 0.009 and 0.027 ± 0.002) compared to control group (0.017 ± 0.001). At the same time phagocytic index in noise stress induced animals was significantly decreased (0.007 ± 0.001). The ITAE and SDAE treated stress animal phagocytic index was significantly enhanced to 0.024 ± 0.001 and 0.017 ± 0.001 respectively (Table 2).
3.5. Effect of plant extracts on spleen lymphocyte proliferation
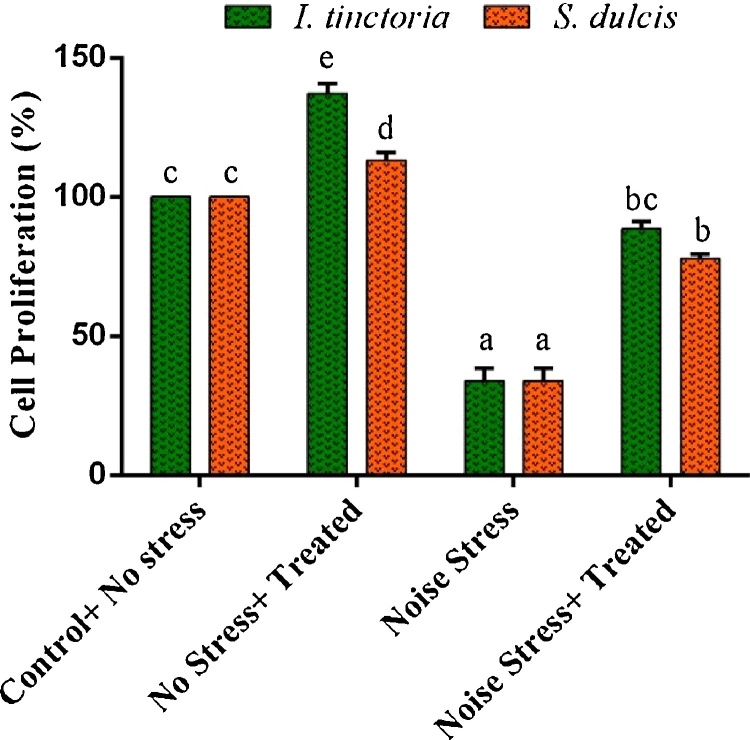
The noise stress induced animals showed significant reduction in the spleen lymphocyte proliferation (33.95 ± 4.63%) compared to control group (100.00%) and significant proliferation was observed between the control group and extracts alone treated group. Moreover the ITAE and SDAE treated stress animals showed remarkable enhancement in spleen lymphocyte proliferation to 88.54 ± 2.72% and 77.98 ± 1.54% respectively (Fig. 2).
Fig. 2.
Effect of ITAE and SDAE administration on spleen lymphocyte proliferation under normal and noise stressed groups. The results were expressed as a percentage of proliferation (%) compared to the control cells. Data are expressed as means ± SD (n = 9). Bars labeled with different letters represent a statistical difference at (p < 0.05).
3.6. Effect of plant extracts on malondialdehyde (MDA)
The effect of ITAE and SDAE on noise stress induced animal spleen and liver MDA level was shown in Table 3. The results revealed that, increased level of MDA was observed in liver (230.38 ± 2.61 nmol/mg) and spleen (120.40 ± 1.18 nmol/mg) of noise stress group animals with reference to the control group (liver- 141.18 ± 0.59 nmol/mg; spleen- 64.22 ± 1.69 nmol/mg). Moreover, the ITAE and SDAE extracts treated animals showed significant reduction in the MDA level and the results indicated that both plant extracts have ability to prevent the oxidative stress caused by noise stress.
Table 3.
Effect of ITAE and SDAE administration on MDA level of different group animals liver and spleen tissue under normal and noise stress condition. Each value represent mean ± SD (n = 9). Mean with different letters within a column are significantly different (p < 0.05).
| S. No | Group | Liver (nmol/mg) | Spleen (nmol/mg) |
|---|---|---|---|
| 1. | Control + No stress | 141.18 ± 0.59c | 64.22 ± 1.69c |
| 2. | No stress + ITAE treated | 127.35 ± 1.08cd | 48.76 ± 1.27d |
| 3. | No stress + SDAE treated | 112.73 ± 2.17d | 58.40 ± 1.86cd |
| 4. | Noise stress | 230.35 ± 2.61a | 120.40 ± 1.18a |
| 5. | Noise stress + ITAE treated | 155.27 ± 1.28bc | 68.40 ± 0.54c |
| 4. | Noise stress + SDAE treated | 171.60 ± 0.88b | 98.92 ± 2.0b |
3.7. Effect of plant extracts on cytotoxicity of splenic NK cells
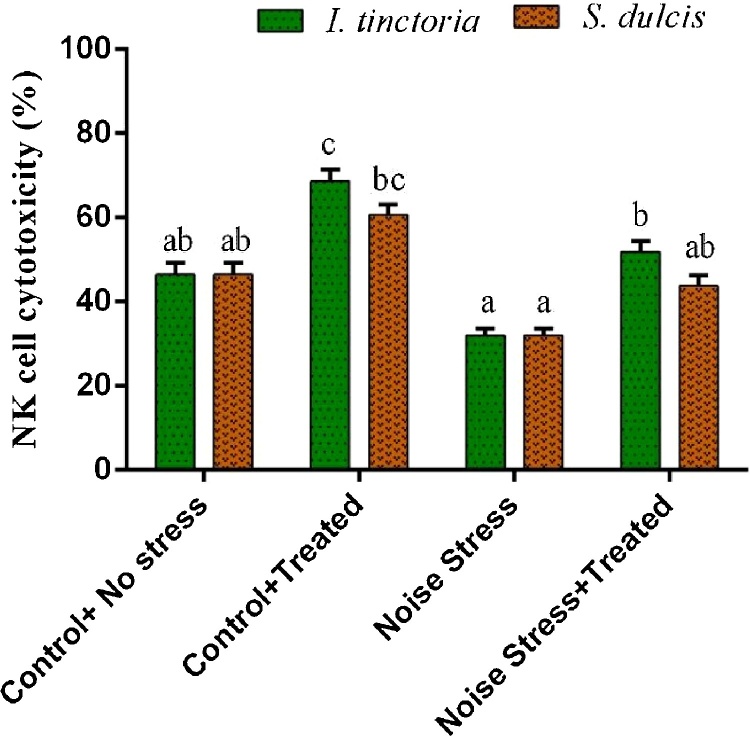
The result of in vivo cytotoxicity induction of splenic NK cells activity by extracts and noise stress shown in Fig. 3. Cytotoxicity of ITAE and SDAE treated spleen NK cells were quite similar and the activity was found decreased in noise stress subjected animals (32.02 ± 1.60%). However, noise stress induced rats treated with ITAE and SDAE showed enhanced NK cell cytotoxicity to 51.89 ± 2.60% and (3.80 ± 2.37% against YAC-1 cells.
Fig. 3.
Effect of ITAE and SDAE administration on spleen NK cell cytotoxicity against the target cells YAC-1 under normal and noise stressed groups. Data are expressed as means ± SD (n = 9). Bars labeled with different letters represent a statistical difference at (p < 0.05).
3.8. Effect of plant extracts on the expression of granzyme B and perforin in NK cells
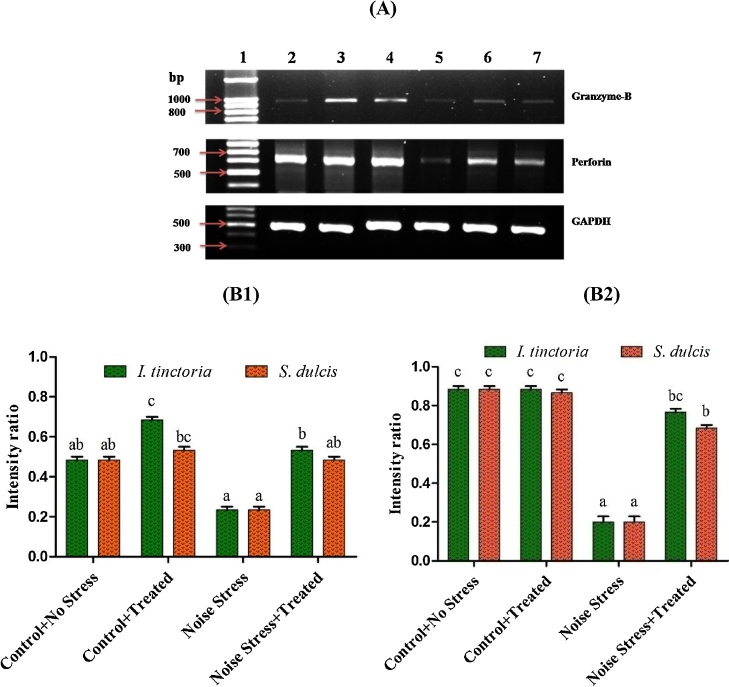
Semi quantitative RT-PCR was used to determine the effect of ITAE and SDAE on granzyme B and perforin mRNA expression by splenic NK cells on normal and noise stress animals. The ITAE and SDAE treated animals showed high level of expression on granzyme B level than the control whereas the expression of perforin level is almost similar to control. Moreover both granzyme B and perforin mRNA expression showed decreased on noise stress group, but significant enhancement on both granzyme B and perforin level was observed in noise stress group treated with ITAE and SDAE (Fig. 4A and B (B1 & B2).
Fig. 4.
Effect of both ITAE and SDAE administration on the mRNA expression of granzyme B and perforin in splenic NK cells under normal and noise stressed groups. (A) Lane 1. DNA ladder (100 bp), 2. Control + No stress, 3. No stress + ITAE treated, 4. No stress + SDAE treated 5. Noise stress, 6. Noise stress + ITAE treated, 7. Noise stress + SDAE treated. (B) PCR products were quantified by densitometric scanning and granzyme B (B1) and perforin (B2) expression was normalized relative to the steady-state expression of GAPDH used as internal control. Data are expressed as mean ± SD (n = 9). Bars labeled with different letters represent a statistical difference at (p < 0.05).
3.9. Effect of plant extracts on DNA damage of liver and spleen
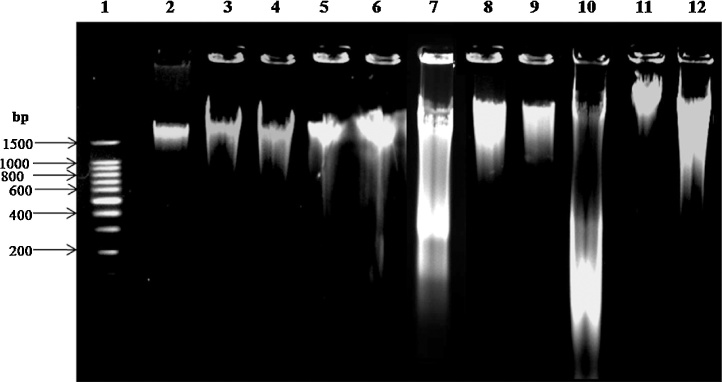
The effect of noise stress on DNA damage of liver and spleen tissues were analyzed by agarose gel electrophoresis. The results were represented in Fig. 5, which shows that the control and extracts alone administered animal liver and spleen DNA had not shown any significant changes. Besides, the noise stress animal liver and spleen tissues has showed fragmented DNA due to DNA damage and it was controlled in ITAE and SDAE extracts treated noise stress groups.
Fig. 5.
Effect of ITAE and SDAE administration on tissue DNA damage under normal and noise stress groups. Lane-1 DNA (100 bp) ladder, 2- Control + no stress liver DNA, 3- No stress + ITAE treated liver DNA, 4- No stress + SDAE treated liver DNA, 5- No stress + ITAE treated spleen DNA, 6- No stress + SDAE treated spleen DNA, 7- Noise stress liver DNA, 8- Noise stress + ITAE treated liver DNA, 9- Noise Stress + SDAE treated liver DNA, 10- Noise stress spleen DNA, 11- Noise stress + ITAE treated spleen DNA, 12- Noise stress + SDAE treated spleen DNA.
3.10. Effect of plant extracts on TNF-α expression
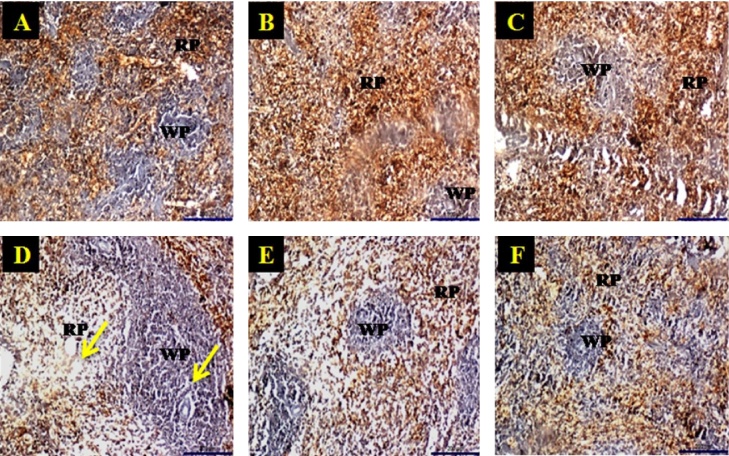
The immunohistochemistry results of control as well as extracts treated groups has showed positive expression of TNF-α in the spleen red pulp and negatively stained in white pulp whereas the noise stress group animal spleen showed very mild expression of TNF-α in red pulp area (Fig. 6). The results indicated that noise stress could potentially decrease the production of pro-inflammatory cytokine.
Fig. 6.
Immumnohistochemistry of control group animal (A) and extracts treated (B, C, E and F) group animal spleen tissue showed positive expression of TNF-α in cytoplasm within mononuclear cells in the red pulp with negatively stained white pulp and low expression of TNF-α in noise stressed group (D).
3.11. Histopathological observation of liver and spleen tissue
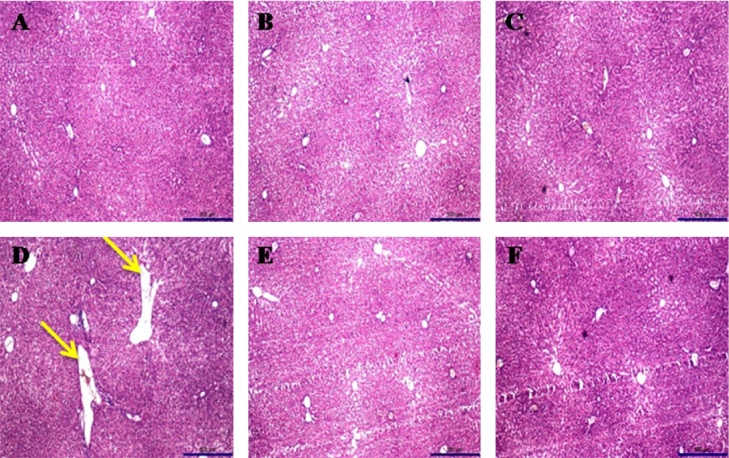
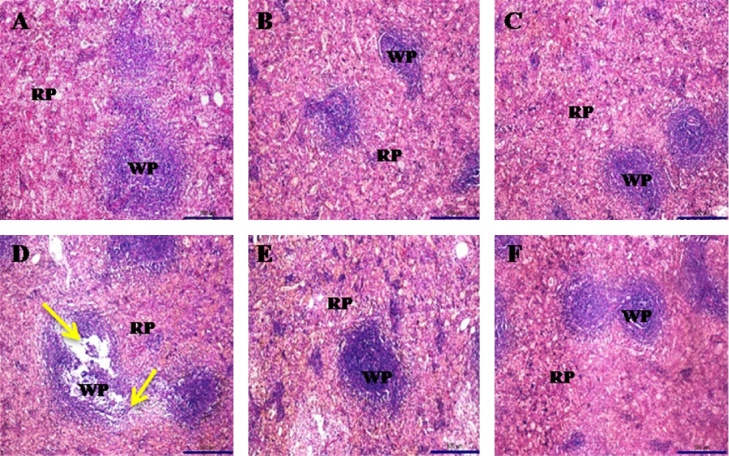
Histopathological observation of control and extract treated groups had shown normal architecture of liver cells. However, noise stress induced group showed irregular architecture and its showing a granulated cytoplasm and small uniform nuclei patterns, which refers to damage on cytoplasm (Fig. 7). On the other hand, histopathological observation of spleen showed normal architecture of red and white pulp on control and extracts treated animal whereas the stress animal showed enlargement of white pulp and irregular architecture of red pulp (Fig. 8).
Fig. 7.
Histopathology of control group animal (A) and extracts treated (B, C, E and F) group animal liver showing the normal architecture and cells with granulated cytoplasm. Noise treated (D) group animal liver showing loss of architecture as well as damage in cytoplasm (Yellow arrow).
Fig. 8.
Histopathology of control group animal (A) and extracts treated group animal (B, C, E and F) spleen showing the normal architecture of red and white pulp and noise stressed group animal (D) spleen showing loss of architecture in red pulp as well as enlargement of white pulp (Yellow arrow).
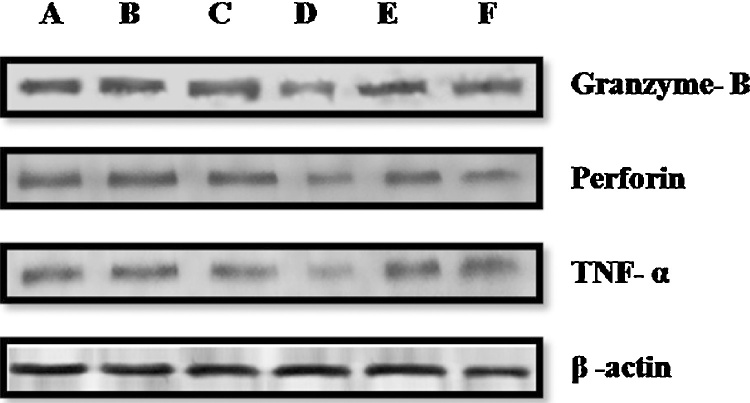
3.12. Western blot analysis of Granzyme B, Perforin and TNF-α
Expression of NK cell cytotoxic protein (Granzyme B, Perforin) and TNF-α was further examined by western blot analysis. The results indicated that the noise stress induced group animals showed significant decrease in the expression of NK cell cytotoxic protein as well as TNF- α and these results are consistent with the results of RT-PCR and IHC (Fig. 9).
Fig. 9.
Western blot analysis of granzyme B, perforin and TNF- α on spleen of different groups (A) Control (B) ITAE treated, (C) SDAE treated, (D) Noise stress, (E) Noise stress + ITAE treated and (F) Noise stress + SDAE treated group.
4. Discussion
In the present investigation both ITAE and SDAE showed a potent immunostimulant effect in both cellular and humoral immunity against noise induced immunosuppressive conditions. Both extracts were most effective at low dosage of 200 mg/kg b.w and it does not possess any toxic effects up to 5000 mg/kg b.w to the tested animals. Similarly, Olaniyan et al. [44] also reported that the aqueous and methanolic extract of Nelsonia campestris not showed any signs of toxic effect at 5000 mg/kg b.w in wistar rats after 24 h observation. Furthermore, we have already documented that the aqueous and ethanolic extracts of I. tinctoria showed immunomodulatory effect on macrophage and lymphocyte under normal condition through in vitro studies [45].
B cells and antibody secreting plasma cells play a vital role in humoral immune response and antibodies produced by B cells cause the destruction of extracellular microorganisms and prevent the spread of intracellular infections [46]. The results of the present study indicated that both extracts were significantly enhanced the humoral imnmune response through the production of antibody in noise stressed animals. Moreover, observation of low antibody titer against noise stress group animals clearly evidenced that the noise stress could suppress the humoral immune response. The enhancement of antibody titer was clearly evidenced that the augmented humoral immune responses were mediated by T and B lymphocytes [47]. Our study correlated with the result of methanolic extract of Amorphophallus commutatus (400 mg/kg b.w) significantly stimulated the humoral immune response in mice which was suppressed in cyclophosphamide induced oxidative stress conditions [48].
The immunostimulatory effects of ITAE and SDAE on humoral immune response was further assessed by hemolysis assay, which measures the ability of the primary complement pathway to augment the hemolysis of SRBC when sensitized with anti-SRBC antibody. Hemolysis of SRBC by spleen single cell suspension declared the number of immune cells producing specific antibodies against SRBC, which evidenced the enhancement of humoral immune response against particular antigen [49]. In this study both plant extracts were potentially enhanced the hemolysis and number of specific antibody production by spleen in noise stressed animals. In this contrast, polysaccharide—protein complex isolated from Lysium barbarum (100 mg/kg b.w) increased the hemolysis activity of spleen single cell suspension in mice under oxidative stress conditions [50].
Lymphocytes are the principal cells of the immune system involved in health and diseases; they participate principally in innate (monocytes and NK cells) and acquired (T and B cells) immune defenses. The previous study also reported that host defense against pathogens and tumors were directly correlated with lymphocyte proliferation [51]. The cell mediated immune response was especially mediated by T cells including cytotoxic T cells. T cells can kill tumor cells and produce various lymphocyte factors consisting of macrophage mobile factors which can enhance the macrophage phagocytosis and the efficacy of killing target cells [52]. Our study showed that the noise stress could decrease the lymphocyte proliferation, because during the chronic stress conditions the organs are failure to proliferation of lymphocytes as well as fighting against pathogens [53].
The most important non specific immune response was carried out by macrophage phagocytosis. Phagocytosis exhibits the last and most fundamental footprint of immunological defense system. It plays a crucial role in the human and animal immune defense against cancer cell development, infectious and non infectious factor [54]. In previous study, Zheng et al. [55] reported that the oxidative stress could be decreased the phagocytosis of macrophages in mice and the mice treated with polysaccharide of Trametes orientalis could enhance the phagocytosis. Similarly our study results also clearly demonstrate that both ITAE and SDAE are potentially stimulated the phagocytic response during noise induced oxidative stress condition.
Lipid peroxidation is a well known oxidative stress mechanism in animal tissues and is generated by reactive oxygen species. We have observed increased level of lipid peroxidation on liver and spleen tissue of noise stress induced animals, which was displayed by higher level of MDA. MDA is most essential biomarker of lipid peroxidation. The increasing level of lipid peroxidation clearly shows the imbalance between intracellular free radical formation and cellular defense mechanism [56]. In addition, lipid peroxidation could direct effect on the decrease of immune cells membrane fluidity, which results in decreased immune function [57]. Since, the ITAE and SDAE treated stress animals showed decreased level of MDA. It means that both extracts have ability to increase the antioxidant capacity to alleviate the effect of noise stress. Our results comparable with Abdul majeed et al. [58] reported that honey (1.5 mL/kg b.w) decrease the lipid peroxidation level and enhanced the other antioxidant enzyme level on lead induced oxidative stress in wistar rat.
NK cells involved in body’s front line defense in innate immune system particularly against virus and cancer cells. It can kill wide range of cancer cell and it is an important tool for cell therapy of cancer. In addition to that spleen NK cells have more cytotoxicity against cancer cells [59]. The cytotoxicity of NK cells were carried out by cytolytic protein perforin and granzyme-B, which was exist in NK cells. Both of them play a vital role in lytic activity and killing the target cells [60]. In connection with that, polysaccharide isolated form Trametes orientalis enhanced the cytotoxicity of spleen NK cells under cyclophosphamide induced oxidative stress conditions [55]. Moreover, in previous study we found that physical or psychological stress decreased NK cells cytotoxicity and mRNA transcription of granzymes and perforin in mice [61]. Similarly, we found significant reduction in the NK cells cytotoxicity as well as perforin and granzyme-B expression under stress conditions. Besides, both extracts could enhance the cytotoxicity of NK cells and expression of perforin and granzyme-B in noise stress induced animals.
Noise is a stressful stimulai when it exceed to more than 90 dB. The continuous exposure of noise can increase the production of free radicals and it can lead to oxidative damage of biomolecules like DNA, lipid and protein [62]. In the present study we found noise stress has caused DNA damage in liver and spleen tissues. The reason behind that DNA is a foremost early target in mammals before the lipid peroxidation and protein damage by the oxidative stress [63]. The histology results also proved the DNA damage caused by noise stress on liver and spleen tissue. In the present study we have observed histopathological changes in liver and spleen tissues during noise stress condition. This was due to the oxidative stress leading to the generation of ROS and free radicals, resulting in changes in normal architecture of tissues [64]. On the other hand, the ITAE and SDAE administered group showed protective effect against noise stress induced DNA damage and architecture changes in tissues. Similar study on cisplatin induced toxicity via attenuation of oxidative stress lead to severe DNA damage and showed abnormal architecture of tissues in wistar rats and the damage was prevented in the rat treated with chrysin (50 mg/kg b.w) a natural flavonoid [65].
TNF α is a vital pro-inflammatory molecule that regulates many feature of macrophage function and considered a mediator of NO release by macrophage via the PI 3-kinase Akt signaling pathway [66]. In our study we found reduction in the expression of TNF α level in noise stress group whereas, control and extract treated group animals showed positive expression of TNF α. Oxidative stress has been associated with the inflammatory response and lead to reduction in the pro-inflammatory cytokines production [67]. In connection with that, Meltzer et al. [68] reported that stress was significantly decreased the secretion of cytokines like TNF α and IL 1β in spleen of Sprague-Dawley rats. Other report on dietary litchi pulp polysaccharides could enhance the production of TNF α in mice, which was suppressed in oxidative stressed animals [69].
5. Conclusion
In conclusion, the present investigation clearly demonstrated that continuous exposures of noise more than 100 dB probably suppress both innate and adaptive immune response. Further, this study also confirms that both I. tinctoria and S. dulcis aqueous extracts are significantly enhanced the innate immune response by increasing the level of macrophage phagocytosis, NK cell cytotoxicity, cytolytic protein expressin (Granzyme B & Perforin), TNF α secretion and adaptive immune response through enhancing the humoral antibody (HA) titer, Quantitative hemolysis of SRBC, spleen lymphocyte proliferation and decreasing the level of malondialdehyde and tissue DNA damage during noise stress condition. Overall the results obtained from the present study clearly demonstrate that, both I. tinctoria and S. dulcis aqueous extract posses the ability to prevent the immune abnormalities caused by chronic noise stress in wistar alibino rat and explore the strong immunostimulatory potential of these plants. Further studies are underway to ascertain the bioactive compound from both plants responsible for immunostimulatory effects.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
The authors gratefully acknowledge UGC- UPE – PHASE II New Delhi (No. 2013/PFEP/C3/280) for financial support.
Contributor Information
Boothapandi Madakkannu, Email: boothapandibiotech@gmail.com.
Ramanibai Ravichandran, Email: rramani8@hotmail.com.
References
- 1.Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res. Int. 2014;2014:1–19. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noori S. An overview of oxidative stress and antioxidant defensive system. J. Clin. Cell Immunol. 2012;1:1–9. [Google Scholar]
- 3.Mendez-Cuesta L.A., Marquez-Valadez B., Perez-De la Cruz V., Maldonado P.D., Santana R.A., Escobar-Briones C., Galvan-Arzate S., Carrillo-Mora P., Santamaria A. Early changes in oxidative stress markers in a rat model of acute stress: effect of l-carnitine on the striatum. Basic Clin. Pharmacol. Toxicol. 2011;109:123–129. doi: 10.1111/j.1742-7843.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- 4.Annunziato L., Pannaccione A., Cataldi M., Secondo A., Castaldo P., Di Renzo G., Taglialatela M. Modulation of ion channels by reactive oxygen and nitrogen species: a pathophysiological role in brain aging? Neurobiol. Aging. 2002;23:819–834. doi: 10.1016/s0197-4580(02)00069-6. [DOI] [PubMed] [Google Scholar]
- 5.Fedele F., Mancone M., Chilian W.M., Severino P., Canali E., Logan S., Marchis M.L.D., Volterrani M., Palmirotta R., Guadagni F. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res. Cardiol. 2013;108:3–12. doi: 10.1007/s00395-013-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantegazza M., Curia G., Biagini G., Ragsdale D.S., Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9:413–424. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- 7.Demirel R., Mollaoglu H., Yesilyurt H., Ucok K., Aycicek A., Akkaya M., Genc A., Uygur R., Dogan M. Noise induces oxidative stress in rat. Eur. J. Gen. Med. 2009;6:20–24. [Google Scholar]
- 8.Sundareswaran L., Srinivasan S., Wankhar W., Sheeladevi R. Effect of Scoparia dulcis on noise stress induced adaptive immunity and cytokine response in immunized Wistar rats. J. Ayurveda Integr. Med. 2017;8:13–19. doi: 10.1016/j.jaim.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selander J., Nilsson M.E., Bluhm G., Rosenlund M., Lindqvist M., Nise G., Pershagen G. Long-term exposure to road traffic noise and myocardial infarction. Epidemiology. 2009;20:272–279. doi: 10.1097/EDE.0b013e31819463bd. [DOI] [PubMed] [Google Scholar]
- 10.Niemann H., Bonnefoy X., Braubach M., Hecht K., Maschke C., Rodrigues C., Robbel N. Noise-induced annoyance and morbidity results from the pan-European LARES study. Noise Health. 2006;8:63–79. doi: 10.4103/1463-1741.33537. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen M., Andersen Z.J., Nordsborg R.B., Becker T., Tjonneland A., Overvad K., Nielsen O.R. Long-term exposure to road traffic noise and incident diabetes: a cohort study. Environ. Health Perspect. 2013;121:217–222. doi: 10.1289/ehp.1205503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz J., Linares C. Traffic noise and adverse birth outcomes in Madrid: a time-series analysis. Epidemiology. 2016;27:2–3. doi: 10.1097/EDE.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 13.Basner M. Much ado about noise-health risks associated with traffic noise. Dtsch. Arztebl. Int. 2016;113:405–406. doi: 10.3238/arztebl.2016.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira M., De Abreu L.C., Valenti V.E., Meneghini A., Murad N., Ferreira C. Electric counter shock and cold stress effects on liver and adrenal gland. Clinics (Sao Paulo) 2010;65:291–296. doi: 10.1590/S1807-59322010000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C.Y., Lin M.T. Oxidative stress in rats with heatstroke-induced cerebral ischemia. Stroke. 2002;33:790–794. doi: 10.1161/hs0102.100208. [DOI] [PubMed] [Google Scholar]
- 16.Samarghandian S., Azimi-Nezhad M., Farkhondeh T., Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017;87:223–229. doi: 10.1016/j.biopha.2016.12.105. [DOI] [PubMed] [Google Scholar]
- 17.Recio A., Linares C., Banegas J.R., Diaz J. Road traffic noise effects on cardiovascular, respiratory, and metabolic health: an integrative model of biological mechanisms. Environ. Res. 2016;146:359–370. doi: 10.1016/j.envres.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Thomas A.W., Ajay C., Jeffrey W.P. Macrophages biology in development, homeostasis and diseases. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh V.K., Sharma P.K., Dubhe R., Kumar N. Immunomodulatory effects of some traditional medicinal plants. J. Chem. Pharm. Res. 2011;3:675–684. [Google Scholar]
- 20.Prendergast G.C., Jaffee E.M. Cancer immunologists and cancer biologists: why we didn’t talk then but need to now. Cancer Res. 2007;67:3500–3504. doi: 10.1158/0008-5472.CAN-06-4626. [DOI] [PubMed] [Google Scholar]
- 21.Charles A.D. Anti-inflammatory agents: present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Hai N. The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture. 2015;446:88–96. [Google Scholar]
- 23.Sethi J., Singh J. Role of medicinal plants as immunostimulants in health and disease. Ann. Med. Chem. Res. 2015;1:1009–1013. [Google Scholar]
- 24.Singh A. Oxford & IBH Publishing Co. Pvt Ltd; New Delhi: 2006. Medicinal Plants of the World; p. 68. [Google Scholar]
- 25.Kameswaran T.R., Ramanibai R. The anti-proliferative activity of flavanoidal fraction of Indigofera tinctoria is through cell cycle arrest and apoptotic pathway in A-549 cell lines. J. Biol. Sci. 2008;8:584–590. [Google Scholar]
- 26.Ramanibai R., Boothapandi M., Madhavarani A. Preliminary phytochemical screening and in vitro anti-oxidant activities of aqueous extract of Indigofera tinctoria and Indigofera astragalina. Int. J. Drug Res. Technol. 2014;4:46–54. [Google Scholar]
- 27.Sakthivel S., Wankupar W., Sheeladevi R., Ravindran R. Neuroprotective effects of Indigofera tinctoria on noise stress. J. App. Pharm. Sci. 2015;5:58–65. [Google Scholar]
- 28.Pari L., Latha M., Rao C.A. Effect of Scoparia dulcis extract on insulin receptors in streptozotocin induced diabetic rats: studies on insulin binding to erythrocytes. J. Basic Clin. Physiol. Pharmacol. 2004;15:223–240. doi: 10.1515/jbcpp.2004.15.3-4.223. [DOI] [PubMed] [Google Scholar]
- 29.Coulibaly A.Y., Kiendrebeogo M., Kehoe P.G., Sombie P.A.E.D., Lamien C.E., Millogo J.F., Nacoulma O.G. Antioxidant and anti-inflammatory effects of Scoparia dulcis L. J. Med. Food. 2011;14:1576–1582. doi: 10.1089/jmf.2010.0191. [DOI] [PubMed] [Google Scholar]
- 30.Zulfiker A.H., Siddiqua M., Nahar L., Habib R., Uddin N., Hasan N., Rana S. Invitro antibacterial, antifungal and cytotoxic activity of Scoparia dulcis L. Int. J. Pharm. Pharmaceut. Sci. 2011;3:198–203. [Google Scholar]
- 31.Ahmed M., Shikha H.A., Sadhu S.K., Rahman M.T., Datta B.K. Analgesic diuretic, and antiinflammatory principle from Scoparia dulcis. Pharmazie. 2001;56:657–660. [PubMed] [Google Scholar]
- 32.Lalitha P., Shubashini K., Jayanthi P. Acute toxicity study of extracts of Eichhornia crassipes (mart.) solms. Asian J. Pharm. Clin. Res. 2012;5:59–61. [Google Scholar]
- 33.Nawaz S.K., Hasnain S. Effects of noise exposure on catalase activity of growing lymphocytes. Bosn. J. Basic Med. Sci. 2011;11:219–222. doi: 10.17305/bjbms.2011.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puri A., Saxena R., Saxena K.C., Tandon J.S. Immunostimulant activity of Nyctanthes arbor-tristis L. J. Ethnopharmacol. 1994;42:31–37. doi: 10.1016/0378-8741(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 35.Simpson M.A., Gozzo J.J. Spectrophotometric determination of lymphocyte mediated sheep red blood cell haemolysis in vitro. J. Immunol. Methods. 1978;21:159–165. doi: 10.1016/0022-1759(78)90232-6. [DOI] [PubMed] [Google Scholar]
- 36.Salem M.L., Matsuzaki G., Madkour G.A., Nomoto K. Betaestradiol-induced decrease in ILK12 and TNF-a expression suppresses macrophage functions in the course of Listeria monocytogenes infection in mice. Int. J. Immunopharmacol. 1999;21:481–497. doi: 10.1016/s0192-0561(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 37.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application for proliferation and cytotoxicity assays. J. Imunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 38.Reilly C.A., Aust S.D. Measurement of lipid peroxidation. In: Bus J.S., editor. Curr Protoc Toxicol. Academic Press; San Diego: 1999. p 2.4. 1-2.4.13. [Google Scholar]
- 39.Kowalski J. Effect of enkephalins and endorphins on cytotoxic activity of natural killer cells and macrophages/monocytes in mice. Eur. J. Pharmacol. 1997;326:251–255. doi: 10.1016/s0014-2999(97)85420-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhu X., Lin Z. Modulation of cytokines production, granzyme B and perforin in murine CIK cells by Ganoderma lucidum polysaccharides. Carbohydr. Polym. 2006;63:188–197. [Google Scholar]
- 41.Kalinina T.S., Bannova A.V., Dygalo N.N. Quantitative evaluation of DNA fragmentation. Bull. Exp. Biol. Med. 2002;134:554–556. doi: 10.1023/a:1022957011153. [DOI] [PubMed] [Google Scholar]
- 42.Suvarna S.K., Layton C., Bancroft J.D. Techniques. 7 ed. Churchill Livingstone; Elsevier England: 2013. Bancroft's Theory and Practice of Histological. [Google Scholar]
- 43.Okur M., Chen C.P., Advis J.P., Sarkar D.K. Beta-endorphin modulation of interferon-gamma, perforin and granzyme B levels in splenic NK cells: effects of ethanol. J. Neuroimmunol. 2005;166:29–38. doi: 10.1016/j.jneuroim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Olaniyan J.M., Muhammad H.L., Makun H.A., Busari M.B., Abdullah A.S. Acute and sub-acute toxicity studies of aqueous and methanol extracts of Nelsonia campestris in rats. J. Acute Dis. 2016;5:62–70. [Google Scholar]
- 45.Boothapandi M., Ramanibai R. Immunomodulatory activity of Indigofera tinctoria leaf extract on in vitro Macrophage responses and Lymphocyte proliferation. Int. J. Pharm. Pharm. Sci. 2016;8:58–63. [Google Scholar]
- 46.Charles A.J., Paul T., Mark W., Mark J.S. fifth ed. Garland Science; New York: 2001. Immunobiology The Immune Systems in Health and Diseases. [Google Scholar]
- 47.Achkar J.M., Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe. 2013;13:250–262. doi: 10.1016/j.chom.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raj S., Gothandam K.M. Immunomodulatory activity of methanolic extract of Amorphophallus commutatus var. wayanadensis under normal and cyclophosphamide induced immunosuppressive conditions in mice models. Food Chem. Toxicol. 2015;81:151–159. doi: 10.1016/j.fct.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 49.Ding Z., Bergman A., Rutemark C., Ouchida R., Ohno H., Wang J.Y., Heyman B. Complement-activating IgM enhances the humoral but not the T cell immune response in mice. PLoS One. 2013;8:e81299. doi: 10.1371/journal.pone.0081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gan L., Hua Zhang S., Liang Yang X., Bi Xu H. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int. Immunopharmacol. 2004;4:563–569. doi: 10.1016/j.intimp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 51.Ho C.Y., Lau C.B., Kim C.F., Leung K.N., Fung K.P., Tse T.F., Chan H.H., Chow M.S. Differential effect of Coriolus versicolor (Yunzhi) extract on cytokine production by murine lymphocytes in vitro. Int. Immunopharmacol. 2004;4:1549–1557. doi: 10.1016/j.intimp.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 52.Shi Y., Liu C.H., Roberts A.I., Das J., Xu G., Ren G., Zhang Y., Zhang L., Yuan Z.R., Tan H.S., Das G., Devadas S. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 53.Engler H., Bailey M.T., Engler A., Sheridan J.F. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J. Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Yuan H., Song J., Li X., Li N., Dai J. Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides. Cancer Lett. 2006;243:228–234. doi: 10.1016/j.canlet.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y., Zong Z.M., Chen S.L., Chen A.H., Weim X.Y. Ameliorative effect of Trametes orientalis polysaccharide against immunosuppression and oxidative stress in cyclophosphamide-treated mice. Int. J. Biol. Macromol. 2017;95:1216–1222. doi: 10.1016/j.ijbiomac.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Ogur R., Coskun O., Korkmaz A., Oter S., Yaren H., Hasde M. High nitrate intake impairs liver functions and morphology in rats; protective effects of α- toicopoherol. Environ. Toxicol. Pharmacol. 2005;20:161. doi: 10.1016/j.etap.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 57.Vida C., Gonzalez E.M., De la Fuente M. Increase of oxidation and inflammation in nervous and immune systems with aging and anxiety. Curr. Pharm. Des. 2014;20:4656–4678. doi: 10.2174/1381612820666140130201734. [DOI] [PubMed] [Google Scholar]
- 58.Abdulmajeed W.I., Sulieman H.B., Zubayr M.O., Imam A., Amin A., Biliaminu S.A., Oyewole L.A., Owoyele B.V. Honey prevents neurobehavioural deficit and oxidative stress induced by lead acetate exposure in male wistar rats- a preliminary study. Metab. Brain. Dis. 2016;31:37–44. doi: 10.1007/s11011-015-9733-6. [DOI] [PubMed] [Google Scholar]
- 59.Culley F.J. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128:151–163. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trapani J.A., Sutton V.R. Granzyme B: pro-apoptotic: antiviral and antitumor functions. Curr. Opin. Immunol. 2003;15:533–543. doi: 10.1016/s0952-7915(03)00107-9. [DOI] [PubMed] [Google Scholar]
- 61.Li Q., Liang Z., Nakadai A., Kawada T. Effect of electric foot shock and psychological stress on activities of murine splenic natural killer and lymphokine-activated killer cells cytotoxic T-lymphocytes, natural killer receptors and mRNA transcripts for granzymes and perforin. Stress. 2005;8:107–116. doi: 10.1080/10253890500140972. [DOI] [PubMed] [Google Scholar]
- 62.Yun-Zhong F., Sheng Y., Guoyao W.U. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 63.Schraufstatter I.U., Hinshaw D.B., Hyslop P.A., Spragg R.G., Cochrane C.G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J. Clin. Invest. 1986;77:1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Etemadi-Aleagha A., Akhgari M., Abdollahi M. A brief review on oxidative stress and cardiac diseases. Mid. East. Pharmac. 2002;10:8–9. [Google Scholar]
- 65.Sultana S., Verma K., Khan R. Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. J. Pharm. Pharmacol. 2012;64:872–881. doi: 10.1111/j.2042-7158.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- 66.Parameswaran N., Patial S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vida C., Gonzalez E.M., De la Fuente M. Increase of oxidation and inflammation in nervous and immune systems with aging and anxiety. Curr. Pharm. Des. 2014;20:4656–4678. doi: 10.2174/1381612820666140130201734. [DOI] [PubMed] [Google Scholar]
- 68.Meltzer J.C., MacNeil B.J., Sanders V., Pylypas S., Jansen A.H., Greenberg A.H., Nance D.M. Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain. Behav. Immun. 2004;18:262–273. doi: 10.1016/j.bbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Huang F., Zhang R., Liu Y., Xiao J., Liu L., Wei Z., Yi Y., Zhang M., Liu D. Dietary litchi pulp polysaccharides could enhance immunomodulatory and antioxidant effects in mice. Int. J. Biol. Macromol. 2016;92:1067–1073. doi: 10.1016/j.ijbiomac.2016.08.021. [DOI] [PubMed] [Google Scholar]