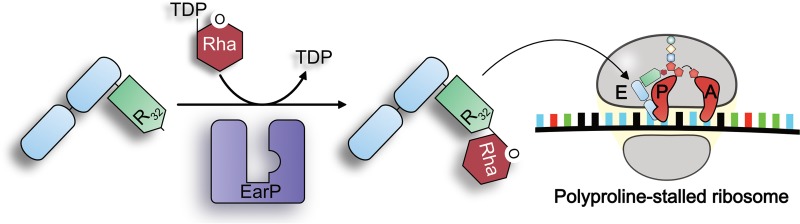
FIG 1 .
Activation and molecular function of EarP-arginine-type translation elongation factor EF-P. (Left) The bacterial translation elongation factor EF-P is composed of two OB-Fold domains (light blue) and one KOW-like N-domain (light green). In about 10% of all bacteria, EF-P is posttranslationally activated by α-glycosylation of a strictly conserved arginine (R32) (17, 26). The glycosylation reaction is catalyzed by the EF-P–arginine rhamnosyltransferase EarP (purple) using dTDP-β-l-rhamnose (TDP-Rha [red]) as a substrate. (Right) Activated EF-P is recruited to polyproline-stalled ribosomes and binds between the E and P sites. Thereby, R32EF-P and the attached rhamnose moiety presumably stabilize the CCA end of the P-site prolyl-tRNA, which in turn stimulates Pro–Pro peptide bond formation and thus alleviates the translational arrest.