Abstract
The phenomenon of a remarkable conservation of the X chromosome in eutherian mammals has been first described by Susumu Ohno in 1964. A notable exception is the cetartiodactyl X chromosome, which varies widely in morphology and G-banding pattern between species. It is hypothesized that this sex chromosome has undergone multiple rearrangements that changed the centromere position and the order of syntenic segments over the last 80 million years of Cetartiodactyla speciation. To investigate its evolution we have selected 26 evolutionarily conserved bacterial artificial chromosome (BAC) clones from the cattle CHORI-240 library evenly distributed along the cattle X chromosome. High-resolution BAC maps of the X chromosome on a representative range of cetartiodactyl species from different branches: pig (Suidae), alpaca (Camelidae), gray whale (Cetacea), hippopotamus (Hippopotamidae), Java mouse-deer (Tragulidae), pronghorn (Antilocapridae), Siberian musk deer (Moschidae), and giraffe (Giraffidae) were obtained by fluorescent in situ hybridization. To trace the X chromosome evolution during fast radiation in specious families, we performed mapping in several cervids (moose, Siberian roe deer, fallow deer, and Pere David’s deer) and bovid (muskox, goat, sheep, sable antelope, and cattle) species. We have identified three major conserved synteny blocks and rearrangements in different cetartiodactyl lineages and found that the recently described phenomenon of the evolutionary new centromere emergence has taken place in the X chromosome evolution of Cetartiodactyla at least five times. We propose the structure of the putative ancestral cetartiodactyl X chromosome by reconstructing the order of syntenic segments and centromere position for key groups.
Keywords: Pecora, Ruminantia, cattle bacterial artificial chromosome (BAC) clones, fluorescent in situ hybridization (FISH), intrachromosomal rearrangements, centromere reposition, inversion
1. Introduction
Despite the great variation in diploid number and high level of autosome reshuffling, the X chromosome of eutherian mammals is evolutionary conserved. The size and morphology of the X chromosome as defined by the position of the centromere is similar in most mammalian orders. Hypothetically, this unique conservation was guided by the establishment of a mechanism for dosage compensation in the therian ancestor [1]. The emergence of this mechanism is thought to have imposed evolutionary constraints on chromosomal rearrangements in the sex chromosome [1].
Classical cytogenetic techniques were used to describe morphology, centromere position, banding pattern, and heterochromatin distribution in a wide range of species. Comparative analysis has identified similar X chromosome morphology and G-banding patterns across species from different taxa (primates, pigs, camels, carnivores, perissodactyls) [2]. Comparative mapping of the X chromosome with gene-specific probes confirmed similarity in the gene order on the X chromosome of distantly related species (human, pig, horse, dog, cat) [3]. These studies provided strong evidence for Ohno’s rule, confirming genomic conservancy of eutherian X chromosomes. However, some notable exceptions in conservation phenomenon of X chromosome have been identified in Cetartiodactyla and Rodentia. The modified X chromosome structure in these orders is caused by inversions, changes in centromere position, heterochromatin expansion and autosome to sex chromosome translocations [4].
The order Cetartiodactyla exhibits great diversity of chromosome X morphology both within and between families. Note that in most eutherian orders only autosomal syntenic segments undergo reshuffling as shown by cross-species chromosome painting [5]. The exact mechanisms behind dynamic changes on X chromosome in Cetartiodactyla are unknown. Comparative chromosome painting with whole chromosome painting probes, including X, has been employed in several studies [6,7,8,9,10,11]. These studies showed that cetartiodactyl autosomes evolved through fission, fusion, and inversions. However, unlike autosomes, the sex chromosomes evolved through more complex chromosomal rearrangements involving reshuffling of conserved segments inside the chromosome, changes in centromere positions, heterochromatic variation, and autosomal translocations [12,13]. It is likely that centromere repositioning (shift) or so-called evolutionary new centromere phenomenon, reflecting a change of centromere position on the chromosome without a change in the gene order, also occurred in cetartiodactyl X chromosome evolution. So far it was shown only in primates, rodents and perissodactyls [14,15,16,17].
The structure of cetartiodactyl X chromosomes has been closely studied mainly in domestic species from the family Bovidae [13,18,19,20,21,22], and in a few wild species from the families Giraffidae, Cervidae, Antilocapridae and Hippopotamidae [6,23,24,25]. In previous studies, microdissection probes or arm-specific paints and several bacterial artificial chromosome (BAC) clones were used to detect intrachromosomal rearrangements. A recent investigation showed centromere repositioning and inversions in cetartiodactyl X chromosomes [25]. Interspecific X chromosome variation in the Cetartiodactyla has been a source of some controversy in the past [12]. The analysis of X chromosome rearrangements can be a potential source of phylogenetic information [12], but the X chromosome evolution in Cetartiodactyla has not yet been studied in detail.
In the present study, we report the comparative map of cetartiodactyl X chromosomes obtained by cross-species hybridization with the set of cattle BAC clones, and provide new data about X chromosome evolution in 10 cetartiodactyl families. Our analysis allows reconstruction of the ancestral X chromosome for major nodes of the cetartiodactyl tree and traces the rearrangements of X chromosome that have occurred during evolution within this order.
2. Materials and Methods
2.1. Species
The list of studied species with scientific and common names, diploid chromosome number, and source of cell lines is presented in the Table 1. All cell lines belong to the cell cultures collection of general biological purpose (No. 0310-2016-0002) of Institute of Molecular and Cellular Biology Siberian Branch of the Russian Academy of Sciences.
Table 1.
List of cetartiodactyl species included in this study and their characteristics.
| Scientific Name, Abbreviation | Code | Common Name | Family | Diploid Number | Source of Cell Line |
|---|---|---|---|---|---|
| Sus scrofa | SSC | Pig | Suidae | 38, XX | IMCB SB RAS, Novosibirsk-1* |
| Lama pacos | LPA | Alpaca | Camelidae | 74, XY | 2* |
| Eschrihtius robustus | ERO | Gray whale | Eschrichtiidae (Cetacea) | 44, XY | [11] |
| Hippopotamus amphibius | HAM | Common hippopotamus | Hippopotamidae | 36, XY | [8] |
| Tragulus javanicus | TJA | Java mouse-deer | Tragulidae | 32, XY | Frozen Zoo (San Diego Zoo’s Conservation Research, San Diego, CA, USA) |
| Antilocapra americana | AAM | Pronghorn | Antilocapridae | 58, XY | [10] |
| Giraffa camelopardalis | GCA | Giraffe | Giraffidae | 30, XY | [8] |
| Moschus moschiferus | MMO | Siberian musk deer | Moschidae | 58, XY | [8] |
| Dama dama | DDA | Fallow deer | Cervidae, Cervinae | 68, XX | Catoctin Wildlife Preserve and Zoo, Maryland, USA |
| Elaphurus davidianus | EDA | Pere David’s deer | 68, XX | 3* | |
| Alces alces | AAL | Eurasian elk | Cervidae, Capreolinae | 68, XX | IMCB SB RAS, Novosibirsk |
| Capreolus pygargus | CPY | Siberian roe deer | 70, XX | IMCB SB RAS, Novosibirsk | |
| Ovibos moschatus | OMO | Muskox | Bovidae, Antilopinae | 48, XX | IMCB SB RAS, Novosibirsk |
| Capra hircus | CHI | Goat | 60, XX | Catoctin Wildlife Preserve and Zoo, Maryland, USA | |
| Ovis aries musimon | OAR | Sheep | 54, XX | Catoctin Wildlife Preserve and Zoo, Maryland, USA | |
| Hippotragus niger | HNI | Sable antelope | 60, XX | 3* | |
| Bison bison | BBI | American bison | Bovidae, Bovinae | 60, XX | 4* |
| Bos taurus | BTA | Cattle | 60, XX | IMCB SB RAS, Novosibirsk |
1*: IMCB SB RAS - Institute of Molecular and Cellular Biology Siberian Branch of the Russian Academy of Sciences. 2*: The cell line is established by William Nash (Laboratory of Genomic Diversity, NCI, Frederick, MD, USA). The sample provided by Camelid Research Group (Oregon State University, Corvallis, OR, USA). 3*: Sample provided by Mitchell Bush (Conservation and Research Center, National Zoological Park, Front Royal, VA, USA). Cell line is established in the Laboratory of Genomic Diversity (NCI, Frederick, MD, USA). 4*: The sample is provided by Douglas Armstrong (Henry Doorly Zoo, Omaha, NE, USA). Cell line is established in the Laboratory of Genomic Diversity (NCI, Frederick, MD, USA).
2.2. Chromosome Preparation
Metaphase chromosomes were obtained from fibroblast cell lines. Briefly, cells were incubated at 37 °C and 5% CO2 in medium αMEM (Sigma Aldrich Co., St. Louis, MO, USA) supplemented with 15% fetal bovine serum, 5% AmnioMAX-II complete (GibcoTM) and antibiotics (ampicillin 100 µg/mL, penicillin 100 µg/mL, amphotericin B 2.5 µg/mL). Metaphases were obtained by adding colcemid (0.02 mg/mL) and ethidium bromide (1.5 mg/mL) to actively dividing culture for 3–4 h. Hypotonic treatment was performed with 3 mM KCl, 0.7 mM sodium citrate for 20 min at 37 °C and followed by fixation with 3:1 methanol/glacial acetic acid (Carnoy’s) fixative. Metaphase chromosome preparations were made from a suspension of fixed fibroblasts, as described previously [26]. G-banding on metaphase chromosomes prior to fluorescence in-situ hybridization (FISH) was performed using standard procedure [27].
2.3. BAC Clones
Using the cattle genome assembly version from October 2011 (Baylor Btau_4.6.1/bosTau7) in UCSC Genome Browser [28], X chromosome-located BAC clones were manually chosen from the CHORI-240 BAC library from the “BACPAC Resource Center” (BPRC, the Children’s Hospital Oakland Research Institute in Oakland, CA, USA). To download information in the Genome Browser about the localization of BACs of appropriate size (length of insertion varied from 50–300 kb), a custom track in Browser Extensible Data (BED) format was created [29]. BAC clones with appropriate insert sizes (50–300 kbp) and genetic content (unique genes, less repetitive elements) were selected. BAC sequence conservation was estimated from phyloP data [30] in the human genome (“Conservation” track in GRCh37/hg19 assembly). Genome coordinates were converted from cow to human using the Batch Coordinate Conversion (liftOver tool) in UCSC Genome Browser. Thus, 73 BAC clones evenly distributed on cattle X chromosome (2–5 Mbp gaps) were selected. For each of the manually selected 73 BACs, we defined various genomic features selected to increase the probability of a clone to hybridize with metaphase spreads of distant cetartiodactyl species. To do so, we calculated protein coding genes, cattle genes orthologous to human, GC content, and repetitive sequences in each of the selected BAC clones. By using multiple alignments, including all available cetartiodactyl genomes, we calculated the nucleotide conservation scores and conserved elements using phastCons [31]. Then, we compared the characteristics of four BACs that had previously worked on distant species with all the 73 BACs by using the classification tree from the CART algorithm [32]. A total of 51 BACs were selected to have a high probability of hybridization to distant species. These BACs contained less than 48% of repetitive sequence and more than 20% of conserved elements. A subset of 26 of these BAC clones that were evenly distributed along the cattle X chromosome with a median distance of 5 Mb were hybridized on all cetartiodactyl species studied here. Table 2 lists the CHORI-240 cattle X chromosome BAC clones used in this study. A single BAC clone (CH240-316D2) is the same as used by Fröhlich et al. [25].
Table 2.
CHORI-240 BAC’s order on cetartiodactyl X chromosomes. The color of the cells corresponds to a certain conservative syntenic segment.
| No. | BAC’s Order and Localization on Cattle X Chromosome | CHORI (CH-240) BACs Localization on Cetartiodactyl X Chromosomes | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domestic Pig, SSC | Alpaca, LPA | Gray Whale, ERO | Common Hippopota-mus, HAM | Java Mouse-Deer, TJA | Pronghorn, AAM | Giraffe, GCA | Siberian Roe Deer, CPY | Eurasian Elk, AAL | Fallow Deer, DDA | Pere David’s Deer, EDA | Muskox, OMO | Goat, CHI | Sheep, OAR | Sable Antelope, HNI | ||||
| 1 | X syntenic block 2 (XSB2) | CH240-514O22 | Start 1949353, End 2129088 |
66H2 | 66H2 | 66H2 | 66H2 | 66H2 | 108D16 | 386M8 | 386M8 | 386M8 | 93K24 | 514O22 | 66H2 | 66H2 | 66H2 | 66H2 |
| 2 | CH240-287O21 | Start 7324034, End 7488466 |
155A13 | 155A13 | 155A13 | 155A13 | 155A13 | 54D24 | 103E10 | 103E10 | 103E10 | 122N13 | 287O21 | 155A13 | 155A13 | 155A13 | 155A13 | |
| 3 | CH240-128C9 | Start 8233624, End 8391009 |
90L14 | 90L14 | 90L14 | 90L14 | 90L14 | 93K24 | 229I15 | 229I15 | 229I15 | 195J23 | 128C9 | 90L14 | 90L14 | 90L14 | 90L14 | |
| 4 | CH240-106A3 | Start 13345128, End 13540519 |
373L23 | 373L23 | 373L23 | 373L23 | 373L23 | 122N13 | 106A3 | 106A3 | 106A3 | 316D2 | 106A3 | 373L23 | 373L23 | 373L23 | 373L23 | |
| 5 | CH240-229I15 | Start 13805346, End 13950311 |
62M10 | 62M10 | 62M10 | 62M10 | 62M10 | 195J23 | 128C9 | 128C9 | 128C9 | 386M8 | 229I15 | 62M10 | 62M10 | 62M10 | 62M10 | |
| 6 | CH240-103E10 | Start 20150516, End 20286173 |
122P17 | 122P17 | 122P17 | 122P17 | 122P17 | 316D2 | 287O21 | 287O21 | 287O21 | 103E10 | 103E10 | 122P17 | 122P17 | 122P17 | 122P17 | |
| 7 | CH240-386M8 | Start 33395588, End 33587168 |
252G15 | 252G15 | 252G15 | 252G15 | 252G15 | 514O22 | 514O22 | 514O22 | 514O22 | 229I15 | 386M8 | 252G15 | 252G15 | 252G15 | 252G15 | |
| 8 | X syntenic block 3 (XSB3) | CH240-108D16 | Start 48672324, End 48917704 |
375C5 | 375C5 | 375C5 | 375C5 | 375C5 | 287O21 | 316D2 | 316D2 | 316D2 | 106A3 | 108D16 | 375C5 | 375C5 | 375C5 | 375C5 |
| 9 | CH240-54D24 | Start 53219586, End 53351583 |
130I15 | 130I15 | 130I15 | 130I15 | 130I15 | 128C9 | 195J23 | 195J23 | 195J23 | 229I15 | 54D24 | 130I15 | 130I15 | 130I15 | 130I15 | |
| 10 | CH240-93K24 | Start 57734547, End 57947720 |
118P13 | 118P13 | 118P13 | 118P13 | 118P13 | 106A3 | 122N13 | 122N13 | 122N13 | 287O21 | 93K24 | 118P13 | 118P13 | 118P13 | 118P13 | |
| 11 | CH240-122N13 | Start 62228039, End 62371946 |
25P8 | 25P8 | 25P8 | 25P8 | 25P8 | 229I15 | 93K24 | 93K24 | 93K24 | 514O22 | 122N13 | 25P8 | 25P8 | 25P8 | 25P8 | |
| 12 | CH240-195J23 | Start 62982639, End 63183460 |
14O10 | 14O10 | 14O10 | 14O10 | 14O10 | 103E10 | 54D24 | 54D24 | 54D24 | 54D24 | 195J23 | 14O10 | 14O10 | 14O10 | 14O10 | |
| 13 | CH240-316D2 | Start 68490278, End 68678635 |
214A3 | 214A3 | 214A3 | 214A3 | 214A3 | 386M8 | 108D16 | 108D16 | 108D16 | 108D16 | 316D2 | 214A3 | 214A3 | 214A3 | 214A3 | |
| 14 | X syntenic block 1 (XSB1) | CH240-214A3 | Start 84397606, End 84521707 |
108D16 | 108D16 | 108D16 | 108D16 | 316D2 | 214A2 | 214A3 | 214A3 | 214A3 | 214A3 | 214A3 | 386M8 | 386M8 | 386M8 | 386M8 |
| 15 | CH240-14O10 | Start 85224265, End 85389684 |
54D24 | 54D24? | 54D24 | 54D24 | 195J23 | 14O9 | 14O10 | 14O10 | 14O10 | 14O10 | 14O10 | 103E10 | 103E10 | 103E10 | 103E10 | |
| 16 | CH240-25P8 | Start 90681870, End 90861947 |
93K24 | 93K24 | 93K24 | 93K24 | 122N13 | 25P7 | 25P8 | 25P8 | 25P8 | 25P8 | 25P8 | 128C9 | 128C9 | 128C9 | 229I15 | |
| 17 | CH240-118P13 | Start 92264186, End 92429310 |
122N13 | 122N13 | 122N13 | 122N13 | 93K24 | 118P12 | 118P13 | 118P13 | 118P13 | 118P13 | 118P13 | 106A3 | 106A3 | 106A3 | 106A3 | |
| 18 | CH240-130I15 | Start 95938488, End 96135558 |
195J23 | 195J23 | 195J23 | 195J23 | 54D24 | 130I14 | 130I15 | 130I15 | 130I15 | 130I15 | 130I15 | 229I15 | 229I15 | 229I15 | 128C9 | |
| 19 | CH240-375C5 | Start 103959199, End 104119579 |
316D2 | 316D2 | 316D2 | 316D2 | 514O22 | 375C4 | 375C5 | 375C5 | 375C5 | 375C5 | 375C5 | 287O21 | 287O21 | 287O21 | 287O21 | |
| 20 | CH240-252G15 | Start 108195394, End 108349350 |
514O22 | 514O22 | 514O22 | 514O22 | 287O21 | 252G14 | 252G15 | 252G15 | 252G15 | 252G15 | 252G15 | 514O22 | 514O22 | 514O22 | 514O22 | |
| 21 | CH240-122P17 | Start 110284444, End 110450903 |
287O21 | 287O21 | 287O21 | 287O21 | 128C9 | 122P16 | 122P17 | 122P17 | 122P17 | 122P17 | 122P17 | 316D2 | 316D2 | 316D2 | 316D2 | |
| 22 | CH240-62M10 | Start 111125731, End 111275450 |
128C9? | 128C9 | 128C9 | 128C9? | 106A3 | 62M9 | 62M10 | 62M10 | 62M10 | 62M10 | 62M10 | 195J23 | 195J23 | 195J23 | 195J23 | |
| 23 | CH240-373L23 | Start 117191008, End 117371368 |
106A3 | 106A3 | 106A3 | 106A3 | 229I15 | 373L22 | 373L23 | 373L23 | 373L23 | 373L23 | 373L23 | 122N13 | 122N13 | 122N13 | 122N13 | |
| 24 | CH240-90L14 | Start 126821940, End 127050706 |
229I15 | 229I15 | 229I15 | 229I15 | 108D16 | 90L13 | 90L14 | 90L14 | 90L14 | 90L14 | 90L14 | 93K24 | 93K24 | 93K24 | 93K24 | |
| 25 | CH240-155A13 | Start 128339848, End 128504608 |
103E10 | 103E10 | 103E10 | 103E9 | 103E10 | 155A12 | 155A13 | 155A13 | 155A13 | 155A13 | 155A13 | 54D24 | 54D24 | 54D24 | 54D24 | |
| 26 | CH240-66H2 | Start 141101222, End 141358968 |
386M8 | 386M8 | 386M8 | 386M7 | 386M8 | 66H1 | 66H2 | 66H2 | 66H2 | 66H2 | 66H2 | 108D16 | 108D16 | 108D16 | 108D16 | |
BAC DNA was isolated using the Plasmid DNA Isolation Kit (BiosSilica, Novosibirsk, Russia) and amplified with GenomePlex Whole Genome Amplification kit (Sigma-Aldrich Co., St. Louis, MO, USA). Labeling of BAC DNA was performed using GenomePlex WGA Reamplification Kit (Sigma-Aldrich Co., St. Louis, MO, USA) by incorporating biotin-16-dUTP or digoxigenin-dUTP (Roche, Basel, Switzerland). The quality of produced BAC probes was controlled by FISH localization on cattle chromosomes.
2.4. Fluorescence In-Situ Hybridization (FISH)
Dual-color FISH experiments on G-banded metaphase chromosomes were performed as described by Yang and Graphodatsky [26]. Digoxigenin-labeled and biotin-labeled probes were detected with CyTM3 anti-digoxin (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), fluorescein avidin DCS, biotinilated anti-avidin D (Vector Laboratories, Inc., Burlingame, CA, USA), respectively. Images were captured with a Baumer Optronics CCD Camera (Baumer Ltd., Southington, CT, USA) mounted on an Olympus BX53 microscope (Olympus, Shinjuku, Japan) and processed using VideoTesT 2.0 Image Analysis System (Zenit, St. Petersburg, Russia).
2.5. Bioinformatics Analysis
An analysis in UCSC Genome Browser was performed to establish the order of CHORI-240 BAC clones on X chromosomes of one cetartiodactyl species (sheep) and four species from out-group mammalian orders (Perissodactyla, Primates, Rodentia). BAC positions in these genomes were obtained using Batch Coordinate Conversion (liftOver) in the UCSC Genome Browser that converts genome coordinates between assemblies. The cattle genome assembly (Bos_taurus_UMD3.1.1/bosTau8) was used as a reference. Sequences coordinates of all BAC clones were calculated in human (GR ch38/hg 38), mouse (GRC m38/mm10), rat (RGSC 6.0/rn6, except 386M8, which is disrupted in this genome), horse (Broad/equCab2), and sheep (ISGC Oar_v3.1/oviAri3) genomes.
2.6. Ancestral Chromosome Deduction
The morphology and conservative block orientation of the ancestral X chromosome were deduced using maximum parsimony by comparing X chromosomes across the top branches of Cetartiodactyla and assuming the most common variant to be ancestral for the order. Once the provisional ancestral chromosome was identified, we detected whether the extant X chromosome and the suggested ancestral form differ by inversions (change of BAC order) or/and by centromere repositioning (change of centromere position without change in BAC order).
3. Result
3.1. BACs Localization
We investigated the X chromosome structure across major branches of Cetartiodactyla represented by 18 species from four non-ruminant (Suidae, Camelidae, Eschrichtiidae (Cetacea), Hippopotamidae) and six ruminant (Tragulidae, Antilocapridae, Giraffidae, Moschidae, Cervidae, and Bovidae) families (Table 1). The order of 26 labeled cattle BAC clones was established on the X chromosomes of each of 18 species by a series of pairwise FISH experiments (Table 2). In total, comparative analyses of BAC orders across 18 species revealed three major chromosomal conservative segments, which were numbered and designated with colors used throughout the paper: X Syntenic Block 1 (13 BACs, XSB1, pink); X Syntenic Block 2 (seven BACs, XSB2, yellow), and; X Syntenic Block 3 (six BACs, XSB3, blue).
3.2. Intrachromosome Rearrangements
Comparative analysis of the order of BAC on the X chromosomes of 18 species identified three key scenarios that likely took place in the course of the cetartiodactyl X chromosomes’ evolution.
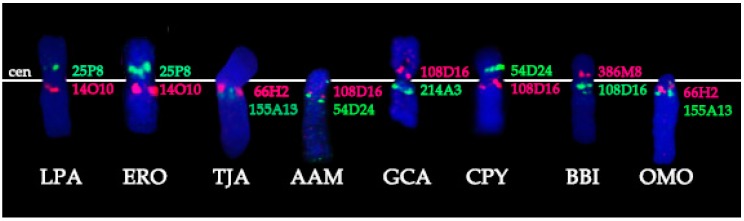
Conservation: no change in the BAC order and no change of the centromere position. We identified a group of four basal cetartiodactyl species (gray whale (ERO), common hippopotamus (HAM), alpaca (LPA), and pig (SSC)) that have an identical order of the BACs and the same relative position of the centromere (located in XSB1).
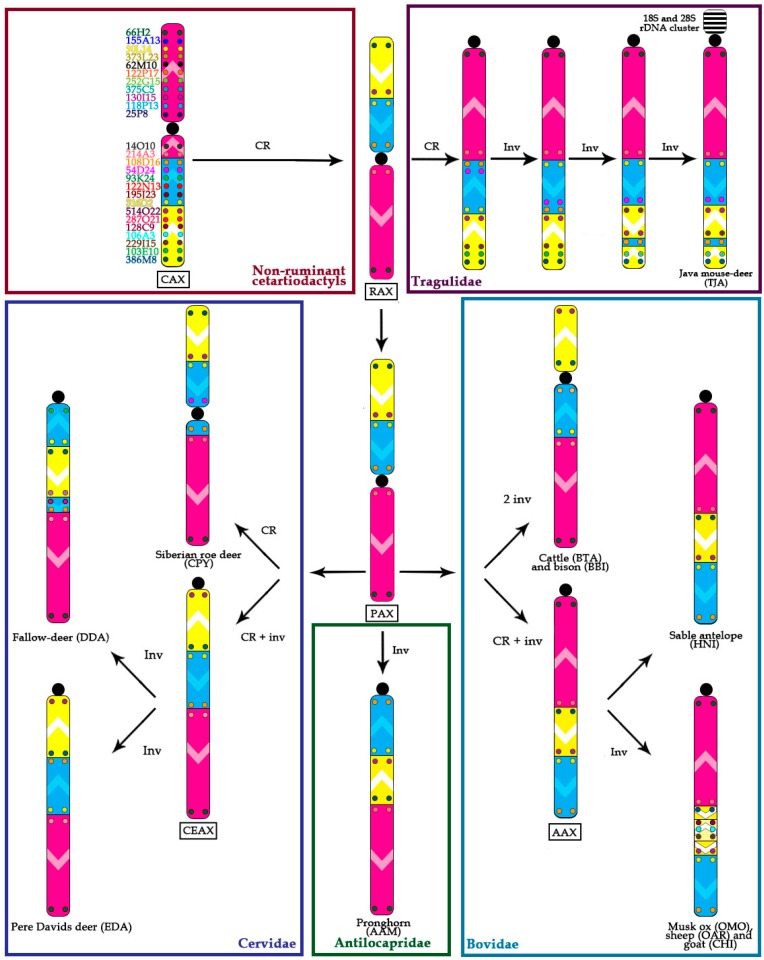
Centromere repositioning: conserved BAC order, changes in the centromere position. Centromere repositions have been shown in roe deer, and mouse-deer, resulting in metacentric (Siberian roe deer (CPY)) and acrocentric (Java mouse-deer (TJA)) X chromosomes, respectively. This event took place prior to a formation of some lineage specific ancestral chromosomes (RAX (Ruminant Ancestral X), AAX (Antilopinae Ancestral X), and CEAX (Cervinae Ancestral X)), indicating that centromere repositioning is one of the key rearrangements of the ruminant X: while maintaining a conserved order of the segments there was a displacement of the centromere (Figure 1).
Inversion: changes in the BAC order. Three kinds of inversions were identified: (A) syntenic block (SB) flip—this inversion reverses the orientation of the whole syntenic block (TJA, AAM, AAX, CEAX); (B) an inversion inside the syntenic block (goat (CHI), muskox (OMO)); (C) the exchange inversion—inversion that involves several BAC clones from two syntenic blocks (TJA, fallow deer (DDA)) (Figure 2).
Figure 1.
Centromere location (cen, white line) and positions of specific BAC clones (pink and green) on X chromosome of several cetartiodactyl species. Species three-letter codes are listed in Table 1.
Figure 2.
The scheme of evolutionary transformations of X chromosome in Cetartiodactyla. Chromosome rearrangements were identified by changes in BAC order. Three major conservative segments are designated by different colors: pink—X syntenic block 1; yellow—X syntenic block 2, and; blue—X syntenic block 3. Individual BAC clones are shown with a different color in small colored circles on corresponding conservative segment. Centromere position is indicated by a black circle. The orientation of the conservative segments is indicated by the white arrowhead. Ancestral associations are shown in black rectangle (Cetartiodactyla ancestral X (CAX), Ruminantia ancestral X (RAX), Pecora ancestral X (PAX), Antilopinae ancestral X (AAX), Cervinae ancestral X (CEAX)). CR: centromere reposition. Inv: inversion.
Taken together, we found that inversions (paracentric and pericentric) and centromere shifts were key rearrangements in the course of X chromosome evolution in Cetartiodactyla. In addition to the described rearrangements, the nucleolar organizing region (containing clusters of 18S and 28S rDNA genes) were localized on the short arm of both X and Y sex chromosomes of the Java mouse-deer (TJA) [33].
3.3. Bioinformatic Analysis of Mammalian X Chromosomes
To evaluate the unique conservation of mammalian X chromosomes [3] we calculated the coordinates of 26 BAC clone sequences in four Boreoeutherian non-cetartiodactyl genomes represented by Euarchontoglires: human (Primates); mouse, and rat (Rodentia), and; Laurasiatheria: horse (Perissodactyla). We have observed that three X chromosome syntenic blocks (XSB) found in Cetartiodactyla are conserved in Laurasiatheria and also in Euarchontoglires, indicating common Boreoeutherian structure of the X chromosome. It was previously reported that human, horse, and pig X chromosomes have similar gene order [3]. In general, this observation was confirmed by liftOver analyses (Table 3). We have identified several small inversions in XSB1 (human, horse) and in XSB2 (horse) in comparison to CAX. Interestingly, XSB1 is the most derived segment outside of Cetartiodactyla, no rearrangements in BACs order in the cetartiodactyl species were detected within this block. According to our data, XSB2 is highly conserved in non-cetartiodactyl species, while in ruminants there are inversions inside of this syntenic block (CHI, OMO, sheep (OAR)) and exchange inversions between XSB2 and XSB3 (TJA and DDA).
Table 3.
CHORI-240 (CH-240) BAC’s order on mammalian chromosomes X. Conservative syntenic segments are colored in pink, yellow and blue.
| No. | Laurasiatheria | Euarchontoglires | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAC Clones in Cattle Genome | BAC Clones in Sheep Genome | BAC Clones in Horse Genome) | BAC Clones in Human Genome | BAC Clones in Mouse Genome | BAC Clones in Rat Genome | |||||||
| 1 | 514O22 | Start 1949353 | 66H2 | Start 10045822 | 66H2 | Start 8367618 | 66H2 | Start 12497685 | 118P13 | Start 7554450 | 25P8 | Start 1711095 |
| End 2129088 | End 10306770 | End 8624882 | End 12794877 | End 7697987 | End 1907049 | |||||||
| 2 | 287O21 | Start 7324034 | 155A13 | Start 19299853 | 155A13 | Start 16543677 | 155A13 | Start 22069138 | 62M10 | Start 9209615 | 375C5 | Start 4672236 |
| End 7488466 | End 19464920 | End 16698622 | End 22228453 | End 9317028 | End 4863802 | |||||||
| 3 | 128C9 | Start 8233624 | 373L23 | Start 28630482 | 373L23 | Start 24698243 | 373L23 | Start 31328065 | 122P17 | Start 10195810 | 252G15 | Start 10936630 |
| End 8391009 | End 28810179 | End 24857300 | End 31509266 | End 10370080 | End 11107682 | |||||||
| 4 | 106A3 | Start 13345128 | 62M10 | Start 34891275 | 62M10 | Start 30220096 | 62M10 | Start 31328065 | 252G15 | Start 12644301 | 122P17 | Start 13483272 |
| End 13540519 | End 35037294 | End 30342071 | End 31509266 | End 12803364 | End 14335671 | |||||||
| 5 | 229I15 | Start 13805346 | 122P17 | Start 35738657 | 122P17 | Start 30907267 | 122P17 | Start 38298814 | 375C5 | Start 18235010 | 62M10 | Start 14415064 |
| End 13950311 | End 35910824 | End 31039631 | End 38458494 | End 18480200 | End 14541523 | |||||||
| 6 | 103E10 | Start 20150516 | 252G15 | Start 37830134 | 252G15 | Start 32879937 | 252G15 | Start 40611820 | 25P8 | Start 20507324 | 118P13 | Start 15650399 |
| End 20286173 | End 37981845 | End 33007527 | End 40767797 | End 20696050 | End 15784402 | |||||||
| 7 | 386M8 | Start 33395588 | 375C5 | Start 41973255 | 375C5 | Start 36512266 | 375C5 | Start 45036869 | 514O22 | Start 23213727 | 130I15 | Start 22235385 |
| End 33587168 | End 42128838 | End 36698919 | End 45234319 | End 23316229 | End 22435973 | |||||||
| 8 | 108D16 | Start 48672324 | 130I15 | Start 49649383 | 25P8 | Start 38190847 | 25P8 | Start 47047149 | 287O21 | Start 41535889 | 66H2 | Start 27957571 |
| End 48917704 | End 49847996 | End 38327897 | End 47226311 | End 41677049 | End 28439737 | |||||||
| 9 | 54D24 | Start 53219586 | 118P13 | Start 52564228 | 118P13 | Start 39580949 | 118P13 | Srart 49122932 | 128C9 | Start 42491010 | 155A13 | Start 40510641 |
| End 53351583 | End 52727917 | End 39734268 | End 49608099 | End 42653374 | End 40710667 | |||||||
| 10 | 93K24 | Start 57734547 | 25P8 | Start 54170178 | 130I15 | Start 44962739 | 130I15 | Start 53053920 | 106A3 | Start 47802786 | 373L23 | Start 53052665 |
| End 57947720 | End 54331345 | End 45135718 | End 53291737 | End 48012093 | End 53277814 | |||||||
| 11 | 122N13 | Start 62228039 | 14O10 | Start 59810734 | 14O10 | Start 52316685 | 14O10 | Start 70333575 | 229I15 | Start 48279488 | 14O10 | Start 70503930 |
| End 62371946 | End 59977176 | End 52471298 | End 70530493 | End 48451406 | End 70671925 | |||||||
| 12 | 195J23 | Start 62982639 | 214A3 | Start 60702841 | 214A3 | Start 53269385 | 214A3 | Start 71438703 | 103E10 | Start 57106307 | 214A3 | Start 71468323 |
| End 63183460 | End 60821565 | End 53389760 | End 71567090 | End 57244888 | End 71575467 | |||||||
| 13 | 316D2 | Start 68490278 | 386M8 | Start 80094458 | 108D16 | Start 76549898 | 108D16 | Start 97540872 | 386M8 | Start 71145260 | 108D16 | Start 100451494 |
| End 68678635 | End 80283568 | End 76701833 | End 97704621 | End 71388925 | End 100747362 | |||||||
| 14 | 214A3 | Start 84397606 | 103E10 | Start 93391997 | 93K24 | Start 81725518 | 54D24 | Start 103662230 | 373L23 | Start 84771898 | 93K24 | Start 107378470 |
| End 84521707 | End 93531761 | End 81893517 | End 103838933 | End 84970050 | End 107552526 | |||||||
| 15 | 14O10 | Start 85224265 | 287O21 | Start 101734836 | 54D24 | Start 83362409 | 93K24 | Start 105651553 | 14O10 | Start 100669857 | 54D24 | Start 109470944 |
| End 85389684 | End 101892691 | End 83480210 | End 105782858 | End 100840304 | End 109865654 | |||||||
| 16 | 25P8 | Start 90681870 | 128C9 | Start 102635193 | 122N13 | Start 86318687 | 122N13 | Start 109356460 | 214A3 | Start 101583273 | 122N13 | Start 113344277 |
| End 90861947 | End 102791210 | End 86434114 | End 109486477 | End 101676469 | End 113475228 | |||||||
| 17 | 118P13 | Start 92264186 | 106A3 | Start 107701336 | 195J23 | Start 86961327 | 195J23 | Start 110108649 | 108D16 | Start 130409135 | 195J23 | Start 114041201 |
| End 92429310 | End 107885819 | End 87148727 | End 110310972 | End 130602938 | End 114226114 | |||||||
| 18 | 130I15 | Start 95938488 | 229I15 | Start 108152302 | 316D2 | Start 89122764 | 316D2 | Start 112670755 | 93K24 | Start 136717423 | 316D2 | Start 116629155 |
| End 96135558 | End 108300381 | End 89285710 | End 112844328 | End 136874331 | End 116812891 | |||||||
| 19 | 375C5 | Start 103959199 | 514O22 | Start 111218064 | 514O22 | Start 93641183 | 514O22 | Start 117884744 | 54D24 | Start 138738349 | 514O22 | Start 121570459 |
| End 104119579 | End 111402064 | End 93800197 | End 118065524 | End 138862889 | End 121690157 | |||||||
| 20 | 252G15 | Start 108195394 | 316D2 | Start 115697759 | 287O21 | Start 97957842 | 287O21 | Start 123321223 | 122N13 | Start 142065201 | 287O21 | Start 127687918 |
| End 108349350 | End 115869258 | End 98099669 | End 123489384 | End 142200659 | End 127828202 | |||||||
| 21 | 122P17 | Start 110284444 | 195J23 | Start 118153613 | 128C9 | Start 98755966 | 128C9 | Start 124334066 | 195J23 | Start 142770604 | 128C9 | Start 128883616 |
| End 110450903 | End 118356580 | End 98902048 | End 124491573 | End 142959710 | End 129042712 | |||||||
| 22 | 62M10 | Start 111125731 | 122N13 | Start 118952818 | 106A3 | Start 102828316 | 106A3 | Start 129437403 | 316D2 | Start 145340226 | 106A3 | Start 134638127 |
| End 111275450 | End 119094106 | End 103004015 | End 129628777 | End 145507925 | End 134841751 | |||||||
| 23 | 373L23 | Start 117191008 | 93K24 | Start 122240643 | 229I15 | Start 103242991 | 229I15 | Start 129926486 | 130I15 | Start 152167108 | 229I15 | Start 135116351 |
| End 117371368 | End 122443675 | End 103384683 | End 130107731 | End 152363913 | End 135281818 | |||||||
| 24 | 155A13 | Start 128339848 | 54D24 | Start 124299513 | 103E10 | Start 108619397 | 103E10 | Start 136556505 | 155A13 | Start 157177353 | 103E10 | Start 159580103 |
| End 128504608 | End 124431515 | End 108750001 | End 136681177 | End 157384607 | End 159734497 | |||||||
| 25 | 66H2 | Start 141101222 | 108D16 | Start 129843594 | 386M8 | Start 119476270 | 386M8 | Start 150502313 | 66H2 | Start 167378561 | ||
| End 141358968 | End 130091258 | End 119683931 | End 150728735 | End 167730488 | ||||||||
We also aligned the BAC clone sequences to another cetartiodactyl genome, the domestic sheep. We observed the same BAC order as in all analyzed Caprini species except for a small inversion in XSB3. The FISH with relevant BAC clones confirmed the presence of this inversion in the sheep genome.
4. Discussion
4.1. Ancestral X Chromosome
The phenomenon of X chromosome conservation in eutherian mammals was first proposed by Susumu Ohno and was based solely on its size similarity across a wide range of species [1]. High similarity in G-banding pattern led to the hypothesis that not only size and gene content [34] but also gene order is conserved on the X chromosomes of most eutherian mammals, and this was later confirmed by fine gene mapping [3,35,36,37,38]. Remarkably, the submetacentric X chromosome morphology defined by the location of the centromere is also largely conserved across mammals. Some slight changes of otherwise conserved X chromosomes were observed in several orders, such as the difference in the distance between homologous genes between human and alpaca [39], or a shift in centromere position without a change of the gene order in Afrotheria [37]. Still, the lack or low level of rearrangements of the X chromosome in comparison to the active exchanges on autosomes during over 150 million years of eutherian evolution represents an interesting phenomenon. Comparative G-banding analysis had identified the classical chromosome X morphology and banding pattern common to most eutherian species [2]. Similar submetacentric morphology and gene order were also found in non-ruminant cetartiodactyls. A high level of X chromosome conservation was shown in Suinae [3,40], Tylopoda [41,42,43], and Cetacea [44]. Nevertheless, using G-banding analysis [4] and high-resolution mapping with BACs [25] or region specific probes, [12] intrachromosomal rearrangements were uncovered in Ruminantia species. Compared with the previous study, we have expanded the number of BACs to 26 and the species list to 18 in order to define conservative blocks and their orientation, to identify rearrangements across species, and to reconstruct the ancestral cetartiodactyl X chromosome. The analyses of BAC order across major families of Cetartiodactyla revealed three syntenic blocks on the X chromosome that in general correspond to the conserved segments reported by Fröhlich and coauthors [25].
Using available FISH and bioinformatic data on the order of cattle BACs in the genomes of different species, we were able to investigate the phenomenon of the conservation of the X chromosome in eutherian mammals represented by four superorders: Laurasiatheria; Euarchontoglires; Afrotheria, and; Xenarthra [45]. Three conserved syntenic blocks identified here can be traced in Boreoeutherians (Laurasiatheria and Euarchontoglires), and possibly in all eutherians, considering reports on Afrotheria X chromosome conserved gene order [37] (Table 2). The eutherian X chromosome ancestral condition (EUX) is represented by a submetacentric chromosome with the centromere located in XSB1. Bioinformatic analysis in outgroup species shows a common change of BAC order in XSB1 on human and horse X chromosomes. Supposedly, an inversion on EUX had occurred in the ancestor of Cetartiodactyla prior the radiation of this order. This ancestral condition was revealed in all non-ruminant cetartiodactyls and named here Cetartiodactyla Ancestral X (CAX). We confirmed the conservation of the X chromosome in basal branches of Cetartiodactyla. It occurs in Suidae (pig), Camelidae (alpaca), and Cetacea (gray whale) (Table 2 and Figure 3). Cetacea is a sister taxon to Hippopatamidae and is characterized by extremely conserved karyotypes across the whole infraorder and by uniform X chromosome morphology and banding pattern [11,46]. The Hippopotamidae X chromosome also displays the same morphology and the gene order [8,25]. However, it should be emphasized that there are some unresolved cases of the X chromosome changes in these basal groups that would require additional investigation, for example, the X chromosome of Tayassu pecari (Suinae, Taysuidae) has been changed due to a centromere reposition [40].
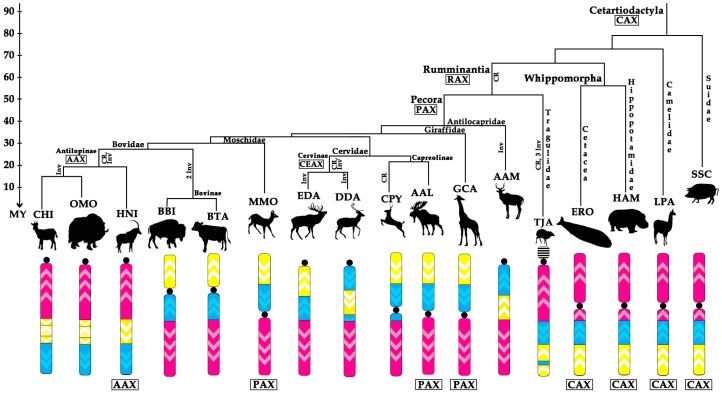
Figure 3.
The structure of the Cetartiodactyla X chromosome depicted on the phylogenetic tree of the order (the tree topology from [47]) Major conservative segments are shown by yellow, blue, and pink. Centromere positions are designated by a black circle. White arrowheads show the orientation of the conservative segments. Ancestral associations are shown under X chromosomes (Cetartiodactyla ancestral X (CAX), Ruminantia ancestral X (RAX), Pecora ancestral X (PAX), Antilopinae ancestral X (AAX). MMO X chromosome is inverted here relatively to its cytogenetic orientation for presentation purposes [8].
4.2. Ancestral Form of Ruminantia-Pecora X-Chromosome
Contrary to the conservation of the X chromosome in Suidae-Camelidae-Whippomorpha, we found that multiple rearrangements occurred during the radiation of other cetartiodactyl branches. We suggest that in the Ruminantia an ancestral centromere reposition led to changes of the X chromosome morphology from submetacentric to metacentric forming the Ruminantia Ancestral X-chromosome (RAX) (Figure 2 and Figure 3). Both ancestral forms (CAX and RAX) have same intrachromosomal structure and differ only by centromere position. The RAX form of the X chromosome is also preserved in many basal Pecora branches: Giraffidae (GCA); Moschidae (MMO); and in the Capreolini (AAL) subfamily of Cervidae. Only in the basal Pecoran family Antilocapridae, an inversion turned the ancestral metacentric X chromosome into an acrocentric element (Figure 2). Thus we expect that the Ancestral Ruminant and the Ancestral Pecoran X chromosomes have the same structure: RAX=PAX.
In the Tragulidae, the basal and the only non-Pecora ruminant group, we found a major centromere reposition resulted in the formation of an acrocentric X. Also, two kinds of inversions (SB-flip and synteny block exchange) affect syntenic block structure in the Tragulidae. These rearrangements create unique arrangement of the three syntenic blocks in the Java mouse-deer. This arrangement may occur across all tragulids, but requires confirmation in other Tragulus species.
4.3. Cervidae
There is a great variation in X chromosome morphology among cervids. Two cervid subfamilies, Capreolinae and Cervinae, exhibit a notably differing extent of sex chromosome conservation. The only detected rearrangement was a centromere shift in CPY. G-banding pattern comparison of Capreolinae X chromosomes otherwise indicates a uniform metacentric morphology [48] and suggests a similar disposition of conservative syntenic blocks.
In contrast, Cervinae is characterized by a variety of rearrangements on the X chromosome: centromere repositioning, SB flips, and many inversions disrupting the XSB2. The Cervinae Ancestral X-chromosome (CEAX) was formed by a centromere reposition and a SB flip of XSB2. Inversions change this ancestral form in EDA by SB flip of XSB3 and in DDA by the splitting of XSB2 (Figure 2). Also in the same subfamily, a translocation of an autosome to the X chromosome was reported in several Muntiacini species [7,49,50,51,52]. In total, this indicates that the level of X chromosome variation is increased in Cervinae and is caused not only by inversions and centromere repositioning but also by autosome to sex chromosome translocations.
4.4. Bovidae
The family Bovidae includes two major branches: Bovinae and Antilopinae [53]. Earlier cytogenetic studies identified two types of morphological diversity of X chromosome in Bovidae: a caprine type (acrocentric, suni type) and a bovine type (submetacentric) [12,54]. The bovine type of X chromosome was likely formed from the ancestral pecoran X (PAX) by two inversions. This form is retained in cattle (BTA) and American bison (BBI). Cytogenetic data for other studied Bovinae species demonstrated same submetacentric X chromosome morphology [48]. There are independent autosome translocations in two branches (Tragelaphini and Bosephalini) altering the bovine type X chromosome [12,23,48,55,56]. The notable exceptions are the Bubalina lineage, oryx and kudu (Tragelaphilini), whose X chromosomes have acrocentric morphology (designated as eland-type acrocentric based on eland, kudu, and nyala X chromosomes [12]).
Centromere reposition and inversion events resulted in the formation of an acrocentric Antilopinae Ancestral X-chromosome (AAX) (Figure 2) from PAX. Therefore the X of the sable antelope (HNI) could likely represent an ancestral form for all Antilopinae. Moreover, comparative analyses based on published karyotypes supports the theory that the X chromosome in antelopes is largely conserved, retaining the same morphology and banding pattern [48,57]. The exceptions are autosome to X chromosome translocations found in several Antilopini species [48,58]. In the Caprini lineage there is an additional inversion within the XSB3 (OMO, CHI, OAR). The bioinformatic and FISH analyses of X chromosome of OAR indicated that the inversion between 128C9 and 229I15 is an apomorphic phylogenetic marker for Caprini.
4.5. X Chromosome Rearrangements
All X chromosome rearrangements discovered here are in agreement with the current phylogenetic tree (Figure 3), and some of them could be used as cytogenetic markers for different Cetartiodactyla groups. Therefore, we suggest that our BAC clone set can serve as a precise instrument for a further search for cytogenetic X chromosome markers in Bovidae. The independent autosome to sex chromosome translocations that occurred in several Bovidae and Cervidae branches require special attention because they increase the previously identified rapid rate of evolution of the structure of the cetartiodactyl X chromosome [7,12,49,50,51,52,55,56].
The BAC clones that mark the borders of three conserved segments delineate regions of frequent chromosome rearrangements in cetartiodactyl X, indicating a breakpoint reuse phenomenon [59]. Several BAC clones were involved at least twice in the intrachromosomal rearrangements found here, suggesting breakpoint reuse: 108D16 and 214A3; 514O22 and 316D2; 229I15 and 103E10. We found that the regions surroundings these BACs in the cattle genome are often gene sparse. It was previously shown that chromosomal regions with evolutionary breakpoint in amniotes are enriched for structural variations (segmental duplications, copy number variants, and indels), retrotransposons, zinc finger genes, and single nucleotide polymorphisms [60]. Further investigation is required to find precise points of evolutionary chromosome breakage on the Cetatiodactyla X and to define common genomic features underlying chromosome rearrangements.
Another mammalian order characterized by the increased rate of X chromosome evolution is Rodentia. Heterochromatin expansion, amplification of tandem repeats, inversions [61], centromere reposition [62], and autosome to sex chromosome translocations [63] were shown to be involved in rearrangements of X chromosome in rodents. Comparative chromosomal analysis of X chromosomes was performed by microdissection in the Microtus genus. Rubtsov with coauthors postulated that intrachromosomal rearrangements are associated with large clusters of intrachromosomal duplications and/or repeated DNA sequences which were present in ancestral species but have subsequently disappeared during evolution [61]. We hypothesize that similar processes were involved in evolution of X chromosome in Ruminantia. Some genomic events possibly took place in the ruminant ancestor that launched multiple chromosomal rearrangements of the conservative eutherian X chromosome. Insertions of mobile repetitive elements such as long and short interspersed nuclear elements (LINE and SINE were probably involved in synteny breaks on this sex chromosome [64]. It is possible that this transforming genomic event had happend in or around the XSB2 area which demonstrates highest rate of inversions in Ruminantia.
In total, nine paracentric, two pericentric inversions, and five centromere reposition events have been revealed in Cetartyodactyla X chromosome evolution based on the analysis of 18 species. The eutherian and cetartiodactyl ancestral X differ only by one small inversion; one additional rearrangement is proposed to derive the Ruminantia ancestral X (RAX). Most other identified rearrangements happend during the remaining 55 million years of ruminant’s radiation. The cow X chromosome was formed by at least two rearrangements that distinguish it from PAX, corresponding to a rate of rearrangements of approximately 1 per 15 million years. This is comparable to 1 rearrangement per 10 million years postulated for autosomal evolution among most mammalian orders found by chromosome painting [65]. These findings are consistent with the rate of X chromosome evolution in Ruminantia being at least twice as high as in X chromosomes of average eutherian mammalian group.
5. Conclusions
High-resolution X chromosome maps of cetartiodactyl species provide unique information about evolution of intrachromosomal rearrangements. Three conserved syntenic blocks have been identified. We postulate that inversions and centromere repositioning were two key types of rearrangements in course of cetartiodactyl X chromosome evolution. The detailed analysis of the BAC order across multiple species by FISH mapping and bioinformatic analysis allowed the reconstruction of a putative cetartiodactyl ancestral X chromosome. The basal cetartiodactyl group of non-ruminants (pigs, camels, whales, and hippos) share this metacentric ancestral type of X chromosome. The submetacentric ancestral Ruminantia X chromosome was likely formed by simple centromere shift but it retained the ancestral intrachromosomal structure. Currently observed X chromosome morphological variation was formed by inversions and centromere repositioning during 55 million years of ruminant evolution. Chromosome rearrangements supporting the taxonomic status of ruminant families and subfamilies were found by mapping 26 BAC clones specific to the X chromosome. The rate of X-specific rearrangements in Ruminantia significantly exceeds that among eutherian mammals.
Acknowledgments
The work was supported by the Russian Science Foundation (RSF, 16-14-10009). Animal silhouettes were sourced from https://pixabay.com. Preliminary BAC selection and preparation was supported by the United States Department of Agriculture Federal Hatch Project (grant number 538922) and the Biotechnology and Biological Sciences Research Council grants BB/K008226/1 and BB/J010170/1 (to D.M.L). We kindly acknowledge Mary Thompson for establishing cell lines in the Laboratory of Genomic Diversity, NCI-Frederick, MD, USA and Marlys Houck, Julie Fronczek, and Suellen Charter for establishing cell cultures at the San Diego Zoo Institute for Conservation Research's Frozen Zoo. We would like to acknowledge Director of Catoctin Wildlife Preserve and Zoo Richard Hahn. We would like to deeply acknowledge D. Yudkin (IMCB SB RAS), N. Mamaev and E.r Kirillin (Institute of Biological Problems of Cryolithozone SB RAS) for providing muskox sample, A. Sharshov for providing Siberian musk deer and Siberian roe deer samples, G.G. Boeskorov for providing moose sample. We acknowledge P. Dementieva for preparing Siberian roe deer cell line. All authors read and approved the final paper.
Author Contributions
A.G. and A.K. conceived and designed the experiments; A.K., A.M., M.F., D.L. performed BAC clone selection; A.K., J.J., D.L. provide BAC clone material, A.P. performed the experiments and analyzed the data; A.P., A.M. performed bioinformatics analysis; P.P., V.B., O.R., S.O. provided cell lines, M.R., J.B. provided samples, A.P., N.L., P.P., A.K., V.B. prepared suspensions of metaphase chromosome, A.P. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ohno S., Beçak W., Beçak M.L. X-autosome ratio and the behavior pattern of individual X-chromosomes in placental mammals. Chromosoma. 1964;15:14–30. doi: 10.1007/BF00326912. [DOI] [PubMed] [Google Scholar]
- 2.Pathak S., Stock A.D. The X chromosomes of mammals: karyological homology as revealed by banding techniques. Genetics. 1974;78:703–714. doi: 10.1093/genetics/78.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy W.J., Larkin D.M., Everts-Van Der Wind A., Bourque G., Tesler G., Auvil L., Beever J.E., Chowdhary B.P., Galibert F., Gatzke L., et al. Dynamics of mammalian chromosome evolution inferred from multispecies comparative maps. Science. 2005;309:613–617. doi: 10.1126/science.1111387. [DOI] [PubMed] [Google Scholar]
- 4.Graphodatsky A.S. Conserved and variable elements of mammalian chromosomes. Cytogenet. Anim. 1989:95–124. [Google Scholar]
- 5.Ferguson-Smith M.A. History and evolution of cytogenetics. Mol. Cytogenet. 2015;8:19. doi: 10.1186/s13039-015-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C., Griffin D.K., O’Brien P.C.M., Yang F., Lin C.C., Ferguson-Smith M.A. Defining the anatomy of the Rangifer tarandus sex chromosomes. Chromosoma. 1998;107:61–69. doi: 10.1007/s004120050281. [DOI] [PubMed] [Google Scholar]
- 7.Huang L., Chi J., Nie W., Wang J., Yang F. Phylogenomics of several deer species revealed by comparative chromosome painting with Chinese muntjac paints. Genetica. 2006;127:25–33. doi: 10.1007/s10709-005-2449-5. [DOI] [PubMed] [Google Scholar]
- 8.Kulemzina A.I., Trifonov V.A., Perelman P.L., Rubtsova N.V., Volobuev V., Ferguson-Smith M.A., Stanyon R., Yang F., Graphodatsky A.S. Cross-species chromosome painting in Cetartiodactyla: Reconstructing the karyotype evolution in key phylogenetic lineages. Chromosome Res. 2009;17:419–436. doi: 10.1007/s10577-009-9032-3. [DOI] [PubMed] [Google Scholar]
- 9.Kulemzina A.I., Yang F., Trifonov V.A., Ryder O.A., Ferguson-Smith M.A., Graphodatsky A.S. Chromosome painting in Tragulidae facilitates the reconstruction of Ruminantia ancestral karyotype. Chromosome Res. 2011;19:531. doi: 10.1007/s10577-011-9201-z. [DOI] [PubMed] [Google Scholar]
- 10.Kulemzina A.I., Perelman P.L., Grafodatskaya D.A., Nguyen T.T., Thompson M., Roelke-Parker M.E., Graphodatsky A.S. Comparative chromosome painting of pronghorn (Antilocapra americana) and saola (Pseudoryx nghetinhensis) karyotypes with human and dromedary camel probes. BMC Genet. 2014;15:68. doi: 10.1186/1471-2156-15-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulemzina A.I., Proskuryakova A.A., Beklemisheva V.R., Lemskaya N.A., Perelman P.L., Graphodatsky A.S. Comparative Chromosome Map and Heterochromatin Features of the Gray Whale Karyotype (Cetacea) Cytogenet. Genome Res. 2016;148:25–34. doi: 10.1159/000445459. [DOI] [PubMed] [Google Scholar]
- 12.Robinson T.J., Harrison W.R., Ponce de Leon F.A., Davis S.K., Elder F.F.B. A molecular cytogenetic analysis of X chromosome repatterning in the Bovidae: transpositions, inversions, and phylogenetic inference. Cytogenet. Genome Res. 1998;80:179–184. doi: 10.1159/000014976. [DOI] [PubMed] [Google Scholar]
- 13.Rubes J., Musilova P., Kopecna O., Kubickova S., Cernohorska H., Kulemsina A.I. Comparative molecular cytogenetics in Cetartiodactyla. Cytogenet. Genome Res. 2012;137:194–207. doi: 10.1159/000338932. [DOI] [PubMed] [Google Scholar]
- 14.Stanyon R., Archidiacono N., Rocchi M. Comparative Primate Molecular Cytogenetics: Revealing Ancestral Genomes, Marker Order, and Evolutionary New Centromeres. In: Hirai H., Imai H., Go Y., editors. Post-Genome Biology of Primates. Springer; Tokyo, Japan: 2012. pp. 193–216. [Google Scholar]
- 15.Chiatante G., Capozzi O., Svartman M., Perelman P., Centrone L., Romanenko S.S., Ishida T., Valeri M., Roelke-Parker M.E., Stanyon R. Centromere repositioning explains fundamental number variability in the New World monkey genus Saimiri. Chromosoma. 2016:1–11. doi: 10.1007/s00412-016-0619-0. [DOI] [PubMed] [Google Scholar]
- 16.Trifonov V.A., Kosyakova N., Romanenko S.A., Stanyon R., Graphodatsky A.S., Liehr T. New insights into the karyotypic evolution in muroid rodents revealed by multicolor banding applying murine probes. Chromosome Res. 2010;18:265–275. doi: 10.1007/s10577-010-9110-6. [DOI] [PubMed] [Google Scholar]
- 17.Trifonov V.A., Musilova P., Kulemsina A.I. Chromosome evolution in Perissodactyla. Cytogenet. Genome Res. 2012;137:208–217. doi: 10.1159/000339900. [DOI] [PubMed] [Google Scholar]
- 18.Hassanane M.S., Chaudhary R., Chowdhary B.P. Microdissected bovine X chromosome segment delineates homoeologous chromosomal regions in sheep, goat and buffalo. Chromosome Res. 1998;6:213–217. doi: 10.1023/A:1009263718667. [DOI] [PubMed] [Google Scholar]
- 19.Piumi F., Schibler L., Vaiman D., Oustry A., Cribiu E.P. Comparative cytogenetic mapping reveals chromosome rearrangements between the X chromosomes of two closely related mammalian species (cattle and goats) Cytogenet. Genome Res. 1998;81:36–41. doi: 10.1159/000015004. [DOI] [PubMed] [Google Scholar]
- 20.Iannuzzi L., Di Meo G.P., Perucatti A., Incarnato D., Schibler L., Cribiu E.P. Comparative FISH mapping of bovid X chromosomes reveals homologies and divergences between the subfamilies Bovinae and Caprinae. Cytogenet. Genome Res. 2000;89:171–176. doi: 10.1159/000015607. [DOI] [PubMed] [Google Scholar]
- 21.Iannuzzi L., King W.A., Di Berardino D. Chromosome evolution in domestic bovids as revealed by chromosome banding and FISH-mapping techniques. Cytogenet. Genome Res. 2009;126:49–62. doi: 10.1159/000245906. [DOI] [PubMed] [Google Scholar]
- 22.Perucatti A., Genualdo V., Iannuzzi A., Rebl A., Di Berardino D., Goldammer T., Iannuzzi L. Advanced comparative cytogenetic analysis of X chromosomes in river buffalo, cattle, sheep, and human. Chromosome Res. 2012;20:413–425. doi: 10.1007/s10577-012-9285-0. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher D.S., Davis S.K., De Donato M., Burzlaff J.D., Womack J.E., Taylor J.F., Kumamoto A.T. A Molecular Cytogenetic Analysis of the Tribe Bovini (Artiodactyla: Bovidae: Bovinae) with an Emphasis on Sex Сhromosome Morphology and NOR Distribution. Chromosome Res. 1999;7:481–492. doi: 10.1023/A:1009254014526. [DOI] [PubMed] [Google Scholar]
- 24.Cernohorska H., Kubickova S., Kopecna O., Kulemzina A.I., Perelman P.L., Elder F.F., Robinson T.J., Graphodatsky A.S., Rubes J. Molecular cytogenetic insights to the phylogenetic affinities of the giraffe (Giraffa camelopardalis) and pronghorn (Antilocapra americana) Chromosome Res. 2013;21:447–460. doi: 10.1007/s10577-013-9361-0. [DOI] [PubMed] [Google Scholar]
- 25.Fröhlich J., Kubickova S., Musilova P., Cernohorska H., Muskova H., Rubes J. A Comparative Study of Pygmy Hippopotamus (Choeropsis liberiensis) Karyotype by Cross-Species Chromosome Painting. J. Mamm. Evol. 2016:1–10. doi: 10.1007/s10914-016-9358-5. [DOI] [Google Scholar]
- 26.Yang F., Graphodatsky A.S. Animal probes and ZOO-FISH. In: Liehr T., editor. Fluorescence in Situ Hybridization (FISH) Springer; Berlin, Germany: 2017. pp. 323–346. [Google Scholar]
- 27.Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971;2:971–972. doi: 10.1016/S0140-6736(71)90287-X. [DOI] [PubMed] [Google Scholar]
- 28.Kent W.J., Sugnet C.W., Furey T.S., Roskin K.M., Pringle T.H., Zahler A.M., Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karolchik D., Hinrichs A.S., Furey T.S., Roskin K.M., Sugnet C.W., Haussler D., Kent W.J. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004;32:D493–D496. doi: 10.1093/nar/gkh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard K.S., Hubisz M.J., Rosenbloom K.R., Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2010;20:110–121. doi: 10.1101/gr.097857.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siepel A., Bejerano G., Pedersen J.S., Hinrichs A.S., Hou M., Rosenbloom K., Clawson H., Spieth J., Hillier L.W., Richards S., et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Therneau T., Atkinson B., Ripley B. Recursive Partitioning and Regression Trees. R Package Version 4.1–10. Mayo Foundation; Rochester, MN, USA: 2015. [Google Scholar]
- 33.Proskuryakova A.A., Kulemzina A.I., Graphodatsky A.S. Localisation of NOR on sex chromosome of Tragulus javanicus. Cytogenet. Genome Res. 2018 in preparation. [Google Scholar]
- 34.Ohno S., Wolf U., Atkin N.B. Evolution from fish to mammals by gene duplication. Hereditas. 1968;59:169–187. doi: 10.1111/j.1601-5223.1968.tb02169.x. [DOI] [PubMed] [Google Scholar]
- 35.Raudsepp T., Lee E.-J., Kata S.R., Brinkmeyer C., Mickelson J.R., Skow L.C., Womack J.E., Chowdhary B.P. Exceptional conservation of horse–human gene order on X chromosome revealed by high-resolution radiation hybrid mapping. Proc. Natl. Acad. Sci. USA. 2004;101:2386–2391. doi: 10.1073/pnas.0308513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy W.J., Sun S., Chen Z.-Q., Pecon-Slattery J., O’Brien S.J. Extensive Conservation of Sex Chromosome Organization Between Cat and Human Revealed by Parallel Radiation Hybrid Mapping. Genome Res. 1999;9:1223–1230. doi: 10.1101/gr.9.12.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez Delgado C.L., Waters P.D., Gilbert C., Robinson T.J., Graves J.A.M. Physical mapping of the elephant X chromosome: conservation of gene order over 105 million years. Chromosome Res. 2009;17:917–926. doi: 10.1007/s10577-009-9079-1. [DOI] [PubMed] [Google Scholar]
- 38.Quilter C.R., Blott S.C., Mileham A.J., Affara N.A., Sargent C.A., Griffin D.K. A mapping and evolutionary study of porcine sex chromosome gene. Mamm. Genome. 2002;13:588–594. doi: 10.1007/s00335-002-3026-1. [DOI] [PubMed] [Google Scholar]
- 39.Avila F., Baily M.P., Perelman P., Das P.J., Pontius J., Chowdhary R., Owens E., Johnson W.E., Merriwether D.A., Raudsepp T. A Comprehensive Whole-Genome Integrated Cytogenetic Map for the Alpaca (Lama pacos) Cytogenet. Genome Res. 2014;144:196–207. doi: 10.1159/000370329. [DOI] [PubMed] [Google Scholar]
- 40.Adega F., Chaves R., Guedes-Pinto H. Chromosomal evolution and phylogenetic analyses in Tayassu pecari and Pecari tajacu (Tayassuidae): tales from constitutive heterochromatin. J. Genet. 2007;86:19–26. doi: 10.1007/s12041-007-0003-1. [DOI] [PubMed] [Google Scholar]
- 41.Bianchi N.O., Larramendy M.L., Bianchi M.S., Cortes L. Karyological conservatism in South American camelids. Cell. Mol. Life Sci. 1986;42:622–624. doi: 10.1007/BF01955563. [DOI] [Google Scholar]
- 42.Bunch T.D., Foote W.C., Maciulis A. Chromosome banding pattern homologies and NORs for the Bactrian camel, guanaco, and llama. J. Hered. 1985;76:115–118. doi: 10.1093/oxfordjournals.jhered.a110034. [DOI] [Google Scholar]
- 43.Balmus G., Trifonov V.A., Biltueva L.S., O’Brien P.C., Alkalaeva E.S., Fu B., Skidmore J.A., Allen T., Graphodatsky A.S., Yang F., et al. Cross-species chromosome painting among camel, cattle, pig and human: further insights into the putative Cetartiodactyla ancestral karyotype. Chromosome Res. 2007;15:499–514. doi: 10.1007/s10577-007-1154-x. [DOI] [PubMed] [Google Scholar]
- 44.Árnason Ú. Comparative chromosome studies in Cetacea. Hereditas. 1974;77:1–36. doi: 10.1111/j.1601-5223.1974.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 45.Murphy W.J., Pringle T.H., Crider T.A., Springer M.S., Miller W. Using genomic data to unravel the root of the placental mammal phylogeny. Genome Res. 2007;17:413–421. doi: 10.1101/gr.5918807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price S.A., Bininda-Emonds O.R., Gittleman J.L. A complete phylogeny of the whales, dolphins and even-toed hoofed mammals (Cetartiodactyla) Biol. Rev. 2005;80:445–473. doi: 10.1017/S1464793105006743. [DOI] [PubMed] [Google Scholar]
- 47.Hassanin A., Delsuc F., Ropiquet A., Hammer C., van Vuuren B.J., Matthee C., Ruiz-Garcia M., Catzeflis F., Areskoug V., Nguyen T.T., et al. Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. C. R. Biol. 2012;335:32–50. doi: 10.1016/j.crvi.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien S.J., Menninger J.C., Nash W.G. Atlas of Mammalian Chromosomes. John Wiley & Sons; Hoboken, NJ, USA: 2006. [Google Scholar]
- 49.Chi J., Fu B., Nie W., Wang J., Graphodatsky A.S., Yang F. New insights into the karyotypic relationships of Chinese muntjac (Muntiacus reevesi), forest musk deer (Moschus berezovskii) and gayal (Bos frontalis) Cytogenet. Genome Res. 2005;108:310–316. doi: 10.1159/000081520. [DOI] [PubMed] [Google Scholar]
- 50.Yang F., Müller S., Just R., Ferguson-Smith M.A., Wienberg J. Comparative chromosome painting in mammals: human and the Indian muntjac (Muntiacus muntjak vaginalis) Genomics. 1997;39:396–401. doi: 10.1006/geno.1996.4497. [DOI] [PubMed] [Google Scholar]
- 51.Yang F., O’Brien P.C. M., Wienberg J., Ferguson-Smith M.A. Evolution of the black muntjac (Muntiacus crinifrons) karyotype revealed by comparative chromosome painting. Cytogenet. Genome Res. 1997;76:159–163. doi: 10.1159/000134535. [DOI] [PubMed] [Google Scholar]
- 52.Yang F., O’Brien P.C. M., Wienberg J., Neitzel H., Lin C.C., Ferguson-Smith M.A. Chromosomal evolution of the Chinese muntjac (Muntiacus reevesi) Chromosoma. 1997;106:37–43. doi: 10.1007/s004120050222. [DOI] [PubMed] [Google Scholar]
- 53.Kingdon J. The Kingdon Field Guide to African Mammals. Bloomsbury Publishing; London, UK: 2015. [Google Scholar]
- 54.Buckland R.A., Evans H.J. Cytogenetic aspects of phylogeny in the Bovidae. Cytogenet. Genome Res. 1978;21:42–63. doi: 10.1159/000130877. [DOI] [PubMed] [Google Scholar]
- 55.Vozdova M., Ruiz-Herrera A., Fernandez J., Cernohorska H., Frohlich J., Sebestova H., Kubickova S., Rubes J. Meiotic behaviour of evolutionary sex-autosome translocations in Bovidae. Chromosome Res. 2016;24:325–338. doi: 10.1007/s10577-016-9524-x. [DOI] [PubMed] [Google Scholar]
- 56.Iannuzzi L. Standard karyotype of the river buffalo (Bubalus bubalis L., 2n= 50). Report of the committee for the standardization of banded karyotypes of the river buffalo. Cytogenet. Cell Genet. 1994;67:102–113. doi: 10.1159/000133808. [DOI] [PubMed] [Google Scholar]
- 57.Hsu T.C., Benirschke K. An atlas of Mammalian Chromosomes. Volume 10 Springer Science & Business Media; Berlin, Germany: 2013. [Google Scholar]
- 58.Kingswood S.C., Kumamoto A.T. Madoqua kirkii. Mamm. Species. 1997:1–10. doi: 10.2307/3504510. [DOI] [Google Scholar]
- 59.Pevzner P., Tesler G. Human and mouse genomic sequences reveal extensive breakpoint reuse in mammalian evolution. Proc. Natl. Acad. Sci. USA. 2003;100:7672–7677. doi: 10.1073/pnas.1330369100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larkin D.M., Pape G., Donthu R., Auvil L., Welge M., Lewin H.A. Breakpoint regions and homologous synteny blocks in chromosomes have different evolutionary histories. Genome Res. 2009;19:770–777. doi: 10.1101/gr.086546.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubtsov N.B., Rubtsova N.V., Anopriyenko O.V., Karamysheva T.V., Shevchenko A.I., Mazurok N.A., Nesterova T.B., Zakian S.M. Reorganization of the X chromosome in voles of the genus Microtus. Cytogenet. Genome Res. 2002;99:323–329. doi: 10.1159/000071611. [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi T., Yamada F., Hashimoto T., Abe S., Matsuda Y., Kuroiwa A. Centromere repositioning in the X chromosome of XO/XO mammals, Ryukyu spiny rat. Chromosome Res. 2008;16:587–593. doi: 10.1007/s10577-008-1199-5. [DOI] [PubMed] [Google Scholar]
- 63.Gladkikh O.L., Romanenko S.A., Lemskaya N.A., Serdyukova N.A., O’Brien P.C., Kovalskaya J.M., Smorkatcheva A.V., Golenishchev F.N., Perelman P.L., Trifonov V.A., et al. Rapid Karyotype Evolution in Lasiopodomys Involved at Least Two Autosome–Sex Chromosome Translocations. PLoS ONE. 2016;11:e0167653. doi: 10.1371/journal.pone.0167653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim H., Lee T., Sung S., Lee C., Kim H. Reanalysis of Ohno’s hypothesis on conservation of the size of the X chromosome in mammals. Anim. Cells Syst. 2012;16:438–446. doi: 10.1080/19768354.2012.724709. [DOI] [Google Scholar]
- 65.Murphy W.J., Stanyon R., O’Brien S.J. Evolution of mammalian genome organization inferred from comparative gene mapping. Genome Biol. 2001;2:reviews0005.1–reviews0005.8. doi: 10.1186/gb-2001-2-6-reviews0005. [DOI] [PMC free article] [PubMed] [Google Scholar]