Abstract
Vanillin is commonly used as an additive in food, medicine and cosmetics, but its effect has not yet been studied in gastric injury. Therefore the effect of vanillin was studied in experimental gastric ulcer. Gastric secretion and acidity were studied in pylorus ligated rats. Ulcer index, levels of gastric mucus, malondialdehyde (MDA), myeloperoxidase activity (MPO), expression of nuclear factor kappa B (NF-κB) p65, and histopathological changes were determined in ethanol induced gastric ulcer. Pre treatment with vanillin significantly reduced gastric secretion (P < 0.001) and acidity (P < 0.0001) and gastric ulcer index scores (P < 0.001). and augmented the gastric mucosal defense. Vanillin significantly restored the depleted gastric wall mucus levels (P < 0.0001) induced by ethanol and also significantly attenuated ethanol induced inflammation and oxidative stress by the suppression of gastric MPO activity (P < 0.001), reducing the expression of NF-κB p65 and the increased MDA levels (P < 0.001). Vanillin was also effective in alleviating the damage to the histological architecture and the activation of mast cells induced by ethanol.
Together the results of this study highlight the gastroprotective activity of vanillin in gastric ulcers of rats through multiple actions that include inhibition of gastric secretion and acidity, reduction of inflammation and oxidative stress, suppression of expression of NF-κB, and restoration of the histological architecture.
Keywords: Gastric ulcers, Pylorus ligation, Ethanol, Vanillin, Inflammation, Oxidative stress
1. Introduction
Gastric ulcer commonly characterized by an interruption of the mucosal integrity is a common and chronic disease in the adult population. Approximately 5–10% of people are affected by this disease during their life time [47]. A number of factors have been linked to the genesis of gastric ulcers that include malnutrition, helicobacter pylori infection, use of alcohol, anti-inflammatory drugs, stress, trauma, and burns [1], [42], [52], [56]. Another important factor involved in the formation of gastric ulcers is the disruption of the homeostasis between the defensive (mucin, prostaglandin, bicarbonate, nitric oxide and growth factors) and the offensive factors (increased gastric secretion of pepsin and acid, helicobacter pylori) [56] in the gastric mucosa.
Gastric ulcer formation may also be due to disturbances in the microcirculation [55] leading to ischemia ensuing in the temporary loss or reduction in supply of blood and oxygen to the gastric mucosa. Restoration of the blood supply through reperfusion restores the function but may also initiate adverse events such as generation of free radicals (ROS), and inflammation at the site of injury. Infiltration of activated neutrophils is also a vital component in the formation of gastric ulcers. Excessive infiltration of neutrophils is indicated by an increase in the activity of the enzyme myeloperoxidase, which is used as a marker in gastric ulcer pathogenesis [7].
Oxidative stress [49] infiltration of neutrophils, over expression of inflammatory agents and generation of pro inflammatory cytokines [7], [12] have all been shown to be involved in the pathogenesis of gastric ulcers. On the other hand the mucus layer, and endogenous antioxidants; components of the gastrointestinal defense help in the protection against ROS induced cytotoxicity [7], [49]. Consequently reducing oxidative stress, inflammation and activation of neutrophils and strengthening of the gastric mucosal defense may be helpful against gastric damage by ulcerogens.
A number of drugs are available for the treatment of gastric ulceration; the common approach for the management of gastric ulcers is based on decreasing gastric secretion through H_receptor antagonists and proton pump inhibitors [19]. However the continuous and prolong use of these drugs may result in serious adverse effects [50], thus necessitating a constant search for new anti ulcer therapies. Since gastric ulcerogenesis is a multi etiologic disease, identification of drug/s agents that possess natural antiulcer properties such as reduction of gastric secretion, and inflammation, and an ability to strengthen the endogenous defensive capacity of the gastric mucosa may be helpful in the amelioration of gastric ulcers.
Dietary consumption and use of polyphenols is helpful in protecting against different diseases [44]. Vanillin (VLN) a o-methyl catechol (4-hydroxy-3-methoxybenzaldehyde) polyphenol is a flavor compound extracted from vanilla beans, and widely used as an additive in food, medicine and perfume [18], with an estimated annual consumption of more than 2000 tons all over the world [57] (Fig. 1).
Fig. 1.
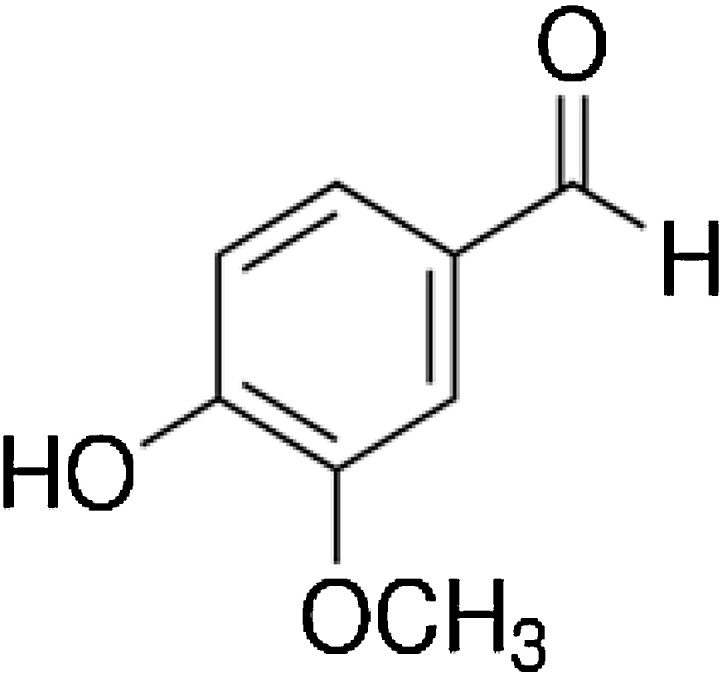
Structure of Vanillin.
Vanillin has been reported to possess anti inflammatory [24], anti colitis [59], [22], antimutagenic [17], antioxidant and antitumor properties [15], [34]. Furthermore VLN also ameliorates depression like behaviors in rats [61], and has a preventive effect in animal model of Huntington’s disease through the reduction of oxidative and nitrosative stress [15]. In addition vanillin was also reported to reduce the expression of proinflammatory cytokines interleukin 1L 1β, IL-6, interferon-γ and tumor necrosis factor in colonic tissues of mice subjected to inflammatory bowel disease [60].
Keeping in view the strong antioxidant and potent anti inflammatory properties of vanillin we hypothesized that vanillin may provide protection against chemically induced gastric ulcers in rats. At the same time a thorough review of literature showed that the effect of vanillin has not been studied against ethanol induced gastric ulcers in rats. Therefore the present study investigates the gastroprotective potential of vanillin against ethanol induced gastric ulcers in rats.
2. Methods
The study protocol was approved by the institutional research and ethics committee. The institutional guidelines for the maintenance and use of animals were strictly followed. Male Wistar rats, weighing 200–225 g maintained at 12 h light/dark cycles and provided a standard chow diet and water ad libitum were used in all the experiments. The animals were divided into groups of six animals each. The aqueous solution of vanillin in doses of 50, 100 and 200 mg/kg body weight was freshly prepared and administered orally by gavage for both gastric secretion and antiulcer studies to different groups. The concentrations of drug were prepared in such a way that each rat received 0.5 ml of drug solution/100 g body weight.
2.1. Gastric secretion study
Pylorus Ligated (Shay) Rats: Pylorus ligation was performed under anesthesia in 36 h fasted rats with ad libitum access to water as per the method of Shay [43]. All essential precautions were taken to prevent bleeding or to occlude blood vessels. Vanillin in the desired doses was administered immediately after the ligation in three groups. One group served as control and received only water in the same volume as that of vanillin treated groups. The rats were killed six hours after the ligation and the stomachs were removed, The stomachs were emptied and the volume was measured for gastric secretion and centrifuged and analyzed for titratable acidity against 0.01 N NaOH to pH 7 using a pH meter and total acid output was calculated.
2.2. Induction of gastric ulcers
Gastric Lesions Induced by Ethanol (Cytoprotection Studies): Gastric ulcers were induced in rats by the administration (i.g.) of 1 ml of absolute ethanol as per the method of Natale et al. [30]. Vanillin in different doses was given (i.g.) 30 min before the administration of ethanol. The animals in the control and drug alone groups did not receive any ethanol. One hour after the administration of ethanol the animals in the different groups were killed and examined for the lesions in stomachs.
Scoring of Lesions: The method as described by Schiantarelli [39] was used for scoring the ethanol induced patched lesions of the stomach.
2.3. Histological and biochemical studies
Separate batches of animals (with six animals in each group) were used for biochemical and histological studies. The same protocol of ethanol and vanillin administration as mentioned above for gastric lesions was followed for these studies. Each batch of animals was comprised of six groups. Group one was considered as control and did not receive any ethanol. Group two was given ethanol, group three, four and five received vanillin in different doses 30 min before the administration of ethanol. Group six was drug alone control group and was administered the highest dose of vanillin only. Stomachs were collected after killing the animals at one hour after the administration of ethanol and assays were performed for gastric wall mucus, myeloperoxidase (MPO) activity, and malondialdehyde (MDA) levels. For histological studies stomach was preserved in neutral buffered formaldehyde.
2.4. Determination of gastric wall mucus
The procedure as described by Corne et al. [11] was used for the determination of gastric wall mucus.
2.5. Determination of malondialdehyde
Malondialdehyde (MDA) concentration a determinant of lipid peroxidation was measured in the gastric mucosa by the thiobarbituric acid test as per the method of Ohkawa et al. [32].
2.6. Determination of MPO
MPO activity in the gastric mucosa was measured according to the method described by Bradley et al. [9].
2.7. Mast cell staining
For the detection of mast cells, paraffin sections of 5 μm were cut on poly-l-lysine-coated microscopic slides and stained with 0.1% toluidine blue (pH 2.3) in 1% sodium chloride solution for 5 min. The slides were then washed with distilled water and dehydrated, cleared in xylene, and mounted. The slides were evaluated under the light microscope (Olympus BX50, Japan). Toluidine blue allows the identification of mast cells metachromatically, resulting in deep purplish blue granular cytoplasmic staining.
2.8. Immuno-histochemical detection of NF-κB expression
Stomach sections of 5 μ thickness cut on poly-l-lysine coated glass slides were deparaffinized 3 times (5 min each) in xylene and dehydrated in graded ethanol and then rehydrated in running tap water. To retrieve antigen, the sections were boiled for 15–20 min 10 mM citrate buffer (pH 6.0) and incubated with protein block (abcam) for 10 min at room temperature and washed with TBST (Tris Buffered Saline). Normal goat serum (ab138478) was used as a blocker for 2 h before overnight treatment with primary antibodies (rabbit anti-NF-κB p105/p50 (phosphor S927 dilution1:150, Abcam, ab131493)), at 4 °C. Mouse and rabbit specific HRP (ABC) detection IHC kit (ab93697) from Abcam were used for further processing. The peroxides complex was visualized with 3,3-diaminobenzidine. The slides were counter stained with haematoxylin, cleaned in water, xylene and ethanol and mounted in DPX and microscopic (BX53 microscope, Olympus, Japan), analysis was performed by a blinded observer.
2.9. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Differences with a P value less than 0.05 were considered statistically significant. The statistical program SPSS (Version 14) was used to analyze the data.
3. Results
3.1. Effect of vanillin on gastric secretion and acidity in 6 h pylorus-ligated rats
Pylorus ligation for 6 h in control rats resulted in the accumulation of 6.0 ± 0.3 ml of gastric secretion (Table 1). Treatment of rats with 50, 100 and 200 mg/kg of vanillin resulted in a significant and dose dependent decrease in the volume of gastric secretion to 4.83 ± 0.47 ml, 3.66 ± 0.21 ml and 3.33 ± 0.33 ml respectively (ANOVA F = 12.97 P < 0.001). The total acid output in untreated control rats was 589.66 ± 49.81 mEq. A significant and dose dependent reduction in total acid output was observed in rats treated with vanillin at 50 mg/kg (354 ± 28.14 mEq), 100 mg/kg (314 ± 28.6 mEq) and 200 mg/kg (230 ± 31.35 mEq) (ANOVA F = 18.68 P < 0.0001) (Table 1).
Table 1.
Effect of vanillin on gastric secretion and acidity in pylorus ligated rats.
| Treatment | Volume of gastric secretion (ml) | Total acid output (mEq) |
|---|---|---|
| Control (pylorus ligation only) | 6.0 ± 0.3 | 589.66 ± 49.81 |
| P. ligation + VLN 50 mg/kg | 4.83 ± 0.47 | 354.66 ± 28.14* |
| P. ligation + VLN 100 mg/kg | 3.66 ± 0.21** | 314.83 ± 28.6** |
| P. ligation + VLN 200 mg/kg | 3.33 ± 0.30** | 230 ± 31.35** |
Values are mean ± standard error of mean.
P < 0.05 as compared with control group using ANOVA followed by Dunnett’s multiple comparison test.
P < 0.001 as compared with control group using ANOVA followed by Dunnett’s multiple comparison test.
3.2. Effect of vanillin on ethanol-induced gastric lesions
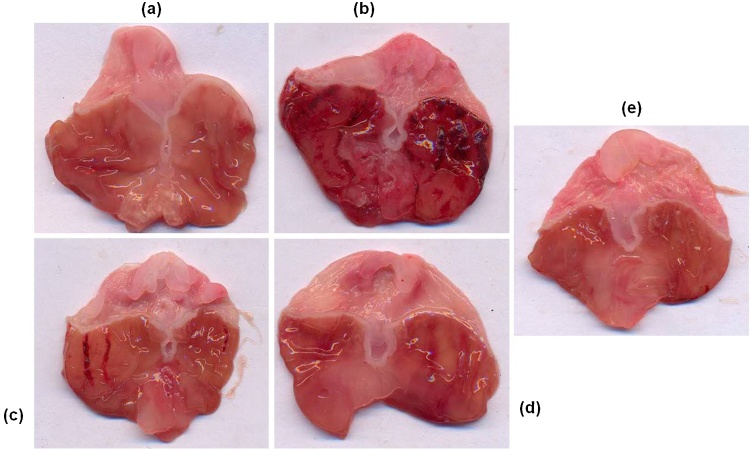
Treatment of rats with ethanol caused extensive gastric ulcerations in the gastric mucosa of the stomach in the control animals. These lesions were characterized by multiple hemorrhagic red bands (patches) of different sizes along the axis of the glandular stomach. Pretreatment with vanillin reduced ethanol-induced mucosal damage dose-dependently (Fig. 2).
Fig. 2.
Macroscopic examination of gastric mucosal changes in ethanol and vanillin pretreated rats. Representative stomachs of: (a) control (saline only); (b) Mucosal damage induced by ethanol; (c–e) vanillin pretreatment at low dose (50 mg/kg), medium dose (100 mg/kg) and high dose (200 mg/kg) and ethanol treated animals.
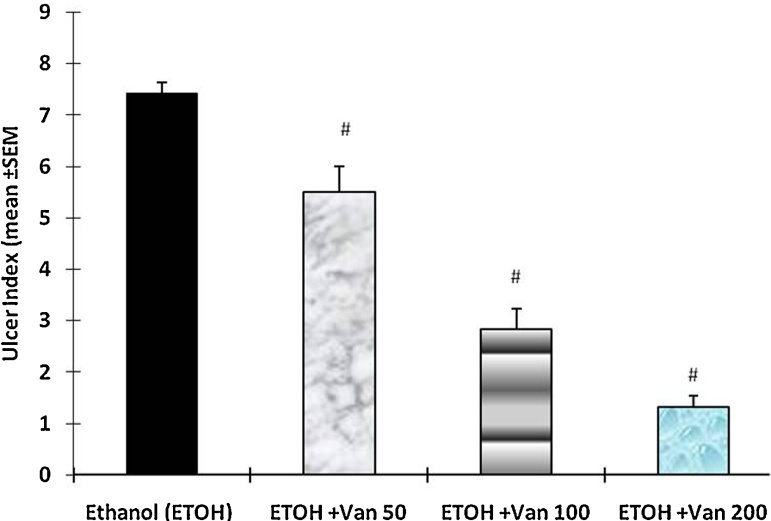
The ulcer index calculated from the size and number of gastric lesions was 7.43 ± 0.2 in ethanol treated rats. Pretreatment of rats with vanillin resulted in a significant and dose dependent reduction of ulcer index of rats. Vanillin treatment at a dose of 50 mg/kg (ulcer index = 5.5 ± 0.50), 100 mg/kg (2.83 ± 0.4) and 200 mg/kg (1.33 ± 0.21) significantly inhibited the formation of gastric lesions (ANOVA, F = 74.60 P < 0.001) (Fig. 3).
Fig. 3.
Effect of vanillin on ethanol induced gastric mucosal damage (ulcer index) in rats. Values are mean ± SEM. #P < 0.001 as compared with ethanol alone treated group using Dunnett’s multiple comparison test. Animals in control group were killed 1 h after the oral administration of ethanol. In the test group vanillin was given by gavage 30 min before the administration of ethanol. Van 50, 100 and 200 = Vanillin 50 mg, 100 mg and 200 mg/kg.
3.3. Effect of vanillin on ethanol-induced histological changes in the gastric mucosa
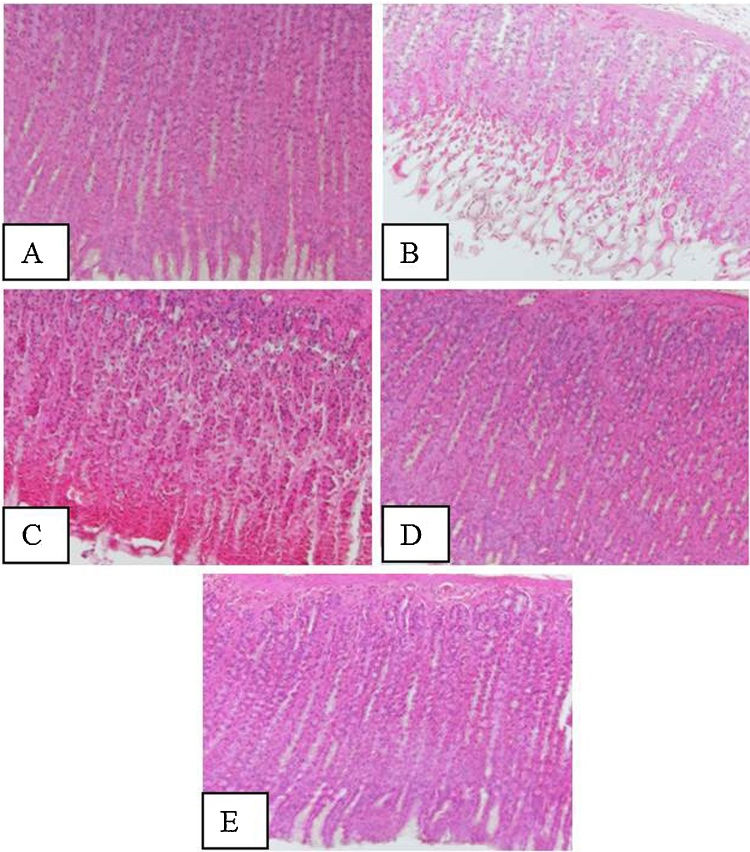
Histological assessment of gastric mucosa showed that treatment with ethanol resulted in the marked appearance of hemorrhage, inflammation and mucosal erosions resulting in the formation of gastric lesions and the formation of gastric pits with detachment of the surface epithelium and loss of glandular cells (Fig. 4). Pretreatment with vanillin considerably reduced these changes in the gastric mucosa and provided protection against ethanol induced gastric lesions (Fig. 4).
Fig. 4.
Effect of vanilllin on ethanol induced histological changes of gastric tissue. VLN enhanced the protective mechanism of the gastric mucosa and helped in the restoration of the damaged histology. Representative sections of the gastric mucosa of (A) normal rats (B) ethanol treated rats. (C) Pretreated with vanillin 50 mg/kg + ethanol. (D) Pretreated with 100 mg/kg vanillin + ethanol. (E) Pretreated with 200 mg/kg vanillin + ethanol.
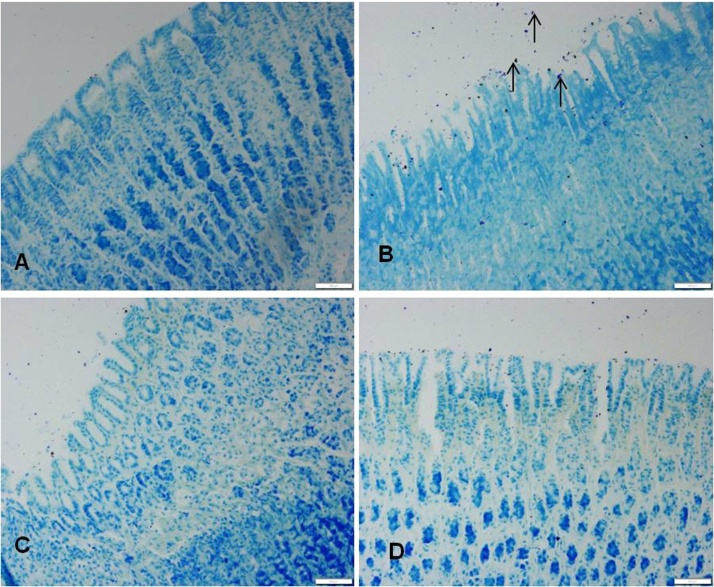
Ethanol administration also resulted in an increase in the infiltration of mast cells in gastric mucosa and sub mucosa regions, whereas in the vanillin pretreated rats the infiltration of mast cells was considerably suppressed (Fig. 5).
Fig. 5.
Effect of vanilllin on ethanol induced changes on mast cell distribution in the gastric mucosa. VLN pretreatment attenuated the ethanol induced increase in the number of mast cells in the gastric mucosa. Representative sections of the gastric mucosa (A) normal rats (B) ethanol treated rats. (C) Pretreated with VLN 50 mg/kg + ethanol. (D) Pretreated with 200 mg/kg vanillin + ethanol.
3.4. Effect of vanillin on ethanol-induced changes in gastric wall mucus
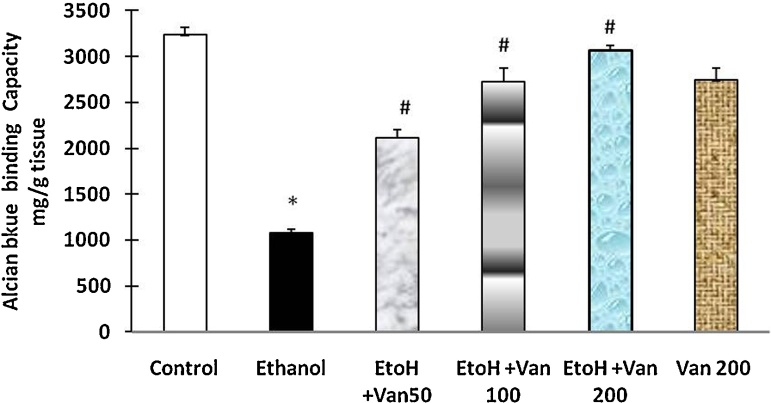
Alcian blue binding is a good indicator for the amount of mucus in the gastric mucosa. Treatment of rats with ethanol resulted in a significant decrease in the Alcian blue binding capacity of gastric wall mucosa (1075.51 ± 43.78 μg/g of tissue) as compared to control rats (3233 ± 82.4 μg/g tissue). Pretreatment of rats with vanillin significantly enhanced the Alcian blue binding capacity of gastric mucosa in a dose dependent manner (ANOVA F = 137.05 P < 0.0001) (Fig. 6).
Fig. 6.
Effect of vanillin on ethanol induced changes in Alcian blue binding capacity (mucus) in gastric mucosa of rats. Values are ±SEM. *P < 0.001 as compared to control (untreated) animals. #P < 0.0001 as compared to ethanol treated animals. EtoH—ethanol; Van—vanillin 50, 100, and 200 mg/kg respectively.
3.5. Effect of vanillin on ethanol-induced changes in the level of gastric mucosal malondialdehyde (MDA)
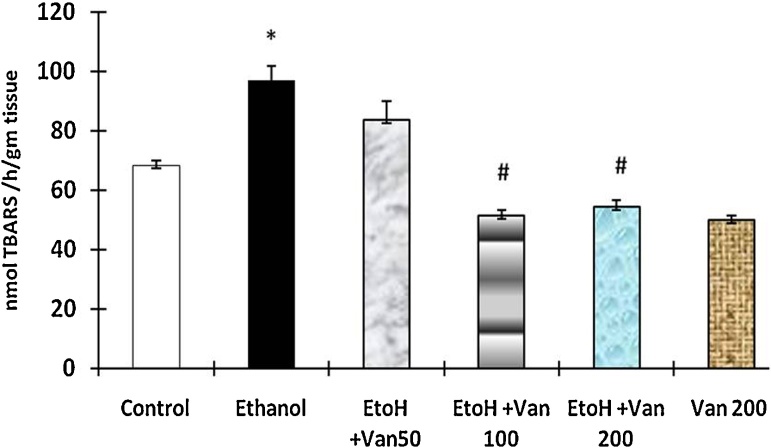
The levels of malondialdehyde an indicator of oxidative stress were significantly increased in ethanol treated animals as compared to control animals (Fig. 7). Pretreatment with vanillin at 50 mg/kg, 100 mg/kg and 200 mg/kg resulted in a dose dependent inhibition of the ethanol induced increase of MDA. However the levels were significantly reduced in the median (100 mg/kg) and high (200 mg/kg) dose of vanillin treated rats only (ANOVA F = 28.76 P < 0.001).
Fig. 7.
Effect of vanillin on ethanol induced changes in malondialdehyde levels in gastric mucosa of rats. Values are ±SEM. *P < 0.0001 as compared with control (untreated) animals. #P < 0.0001 as compared to ethanol treated animals. EtoH—ethanol; Van—vanillin 50, 100, and 200 mg/kg respectively.
3.6. Effect of vanillin on ethanol-induced changes in the gastric mucosal myeloperoxidase (MPO) activity and NF-κB p65 expression
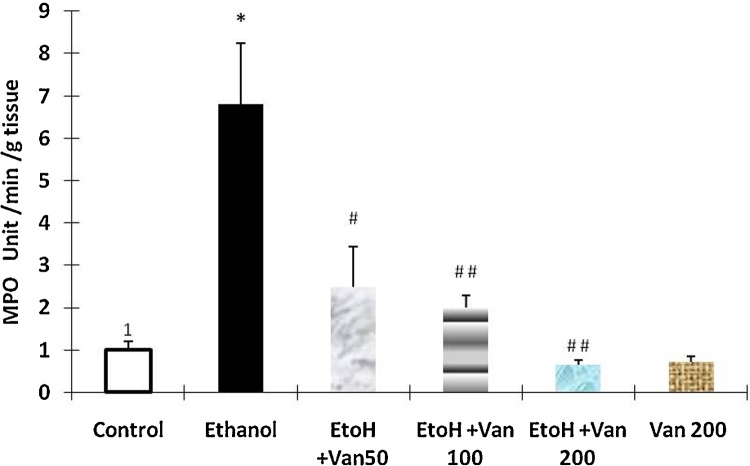
Myeloperoxidase activity a marker for the infiltration of neutrophils was assessed in the gastric tissue of rats. Administration of ethanol resulted in a significant increase in MPO activity as compared to that of control rats (Fig. 8) indicating an increase in the infiltration of neutrophils. Pretreatment of rats with vanillin at 50 mg/kg, 100 mg/kg and 2000 mg/kg resulted in a significant and dose dependent attenuation of the ethanol induced increased MPO activity (ANOVA F = 10.837 P < 0.0001), (Fig. 8).
Fig. 8.
Effect of vanillin on ethanol induced changes in myeloperoxidase activity of gastric mucosa rats. Values are ±SEM. *P < 0.0001 as compared with control (untreated animals. #P < 0.001 and ##P < 0.0001 as compared with ethanol treated animals. EtoH—ethanol; Van—vanillin 50, 100 and 200 mg/kg respectively.
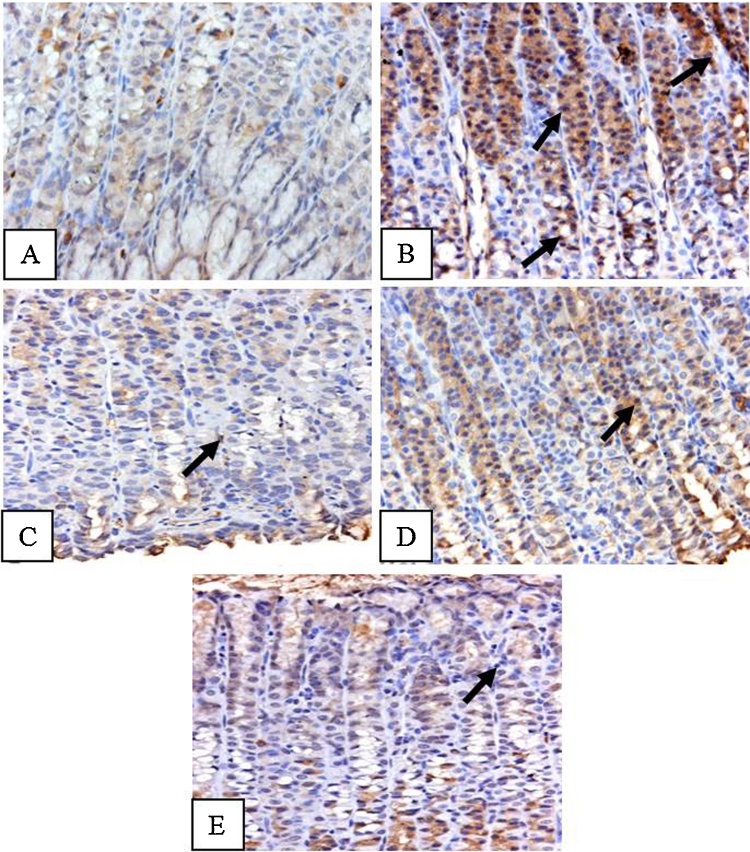
Immunohistochemical staining for NF-κB showed an elevated expression of NF-κB in ethanol treated rats as compared to control animals (Fig. 9A & B), which was markedly reduced in vanillin pretreated animals (Fig. 9C–E).
Fig. 9.
Effect of vanillin on ethanol induced changes in the activation of NF-κB in gastric tissue of rats. Photomicrographs showing immunohistochemical activation of NF-κB. (A) Control animals showing very less staining. (B) Ethanol treated animals showing intense staining as depicted by arrows. (C–E) Vanillin pre treated animals showing a comparatively less staining.
4. Discussion
The present study highlights the gastroprotective effects of vanillin a polyphenolic flavoring agent against ethanol induced gastric ulcers in rats. The gastroprotective effects were evident in both pylorus ligated rats and in ethanol induced gastric ulcerogenesis. The protective action was associated with a reduction in the gastric ulcer index, gastric secretion and acidity and inflammation through the inhibition of NF-κB, recruitment of neutrophils and reduction in ROS through its antioxidant properties.
Normal gastric secretion and acidity are essential for the proper functioning of the digestive system and checks the overgrowth of bacteria and enteric infections [40], whereas an increase in gastric secretion and acidity contribute to gastric ulcerogenesis [56]. On the other hand compounds that inhibit gastric secretion and reduce acidity have strong gastro protective effects [4], [47]. Pretreatment with vanillin significantly reduced the gastric secretion and acidity in pylorus ligated rats (Table 1). Gastric secretion is a dynamic process and regulated by multiple mechanisms that involve neural, paracrine, and hormonal, pathways [41]. One of the principal stimulants and regulator of gastric secretion is histamine released from paracrine cells in the stomach [42]. Histamine stimulates gastric secretion through two different mechanisms; one by directly binding to H2 receptors [40], [46] and the other is indirectly by binding to the H3 receptors coupled to the inhibition of somatostatin [53]. The attenuating effect of vanillin on gastric secretion in pylorus ligated rats may partly be assigned to its inhibitory action on histamine release [31] which is substantiated by our results of a reduction in the histamine secreting mast cells (Fig. 4). Moreover consumption of reductive polyphenols bearing a catechol group effectively enhances the conversion of gastric nitric acid to nitric oxide (NO) in the stomach and helps in relaxation of the smooth muscles of the stomach [13], [35]. The anti ulcerative action of vanillin a polyphenol may therefore be attributed to its ability to attenuate the increased gastric acid secretion and acidity.
Gastric mucus is an important component of the primary defense mechanism against gastro ulcerative agents. It helps in the buffering of the gastric secretions and also reduces friction during peristalsis and contraction of the stomach [51]. Ethanol administration to fasting rats resulted in damage to the gastric mucosa leading to the formation and distribution of hemorrhagic bands all over the glandular part of the stomach (Fig. 2), and an increase in the ulcer index (Fig. 3). The damage to the gastric tissue was accompanied by a significant depletion of the gastric mucus (Fig. 6). Ethanol induced gastric ulceration is associated with a reduction in gastric mucosal blood flow, gastric mucus, vascular permeability, enhanced release of histamine and the production of free radicals [14]. In addition ulcerogenic agents cause dispersal of the gastro protective mucus gel and the associated phospholipids layers leading to acid back diffusion and injury to the gastric mucosa [23], [5]. On the other hand replenishment of the compromised mucosal defense capacity through restoration of the depleted mucus levels exerts a gastro protective effect against gastric ulcerations [3], [6], [7]. Pre treatment with VLN protected the gastric mucosa against ethanol induced gastric ulceration (Figs. 2–4) and also replenished the depleted levels of the gastric wall mucus (Fig. 6). Our results are in agreement with earlier reports. Vanillin consumption helps in the generation of NO [35]. Nitric oxide and its donors reduce the severity of gastric damage in rats [8], [25]. Furthermore NO is a strong gastroprotective agent and serves as a mediator of gastric mucosal defense and augments the resistance of the mucosa to injury [54], enhances mucosal blood flow, and stimulates the production of gastric mucus and reduces infiltration of activated neutrophils [33], [27]. Therefore the ameliorative effect of vanillin against gastric ulceration may be ascribed to the restoration of the depleted gastric mucus levels.
The results of our histological studies also showed the anti ulcerative properties of vanillin (Fig. 4, Fig. 5). VLN pretreatment decreased the damage to the gastric mucosa as seen in both macroscopical and microscopical observations (Figs. 2, 4 and 5). The treated groups showed a better histological architecture with less loss of gastric epithelium, glandular cells, and reduction in inflammation and hemorrhage, and comparatively a more compact mucosa, with less distribution of activated mast cells (Fig. 4, Fig. 5). Our results support the earlier findings wherein vanillin was found to improve the macroscopic and microscopic damage in ulcerative colitis [60]. Oral vanillin not only prevented chemically induced colitis but was also effective in the amelioration of established colitis through its anti inflammatory activity [60].
The gastroprotective effect of vanillin was also observed in the distribution of the activated mast cells in the stomachs of rats. Mast cells are the key inflammatory cells with strong structural and functional relation to gastric ulceration induced by stress and ethanol [38]. An increase in mast cell population was also observed in ulcerated patients [29]. Mast cells upon activation by different stimuli regulate inflammation by releasing the stored secretory granular mediators including histamine, leukotrienes, and platelet activating factor [28], [54]. Vanillin treatment reduced the ethanol induced activation and infiltration of mast cells in the stomach of rats (Fig. 5). Therefore the gastroprotective effect of vanillin may also be attributed to the reduction of mast cell degranulation.
Gastric mucosal damage induced by ethanol has been strongly suggested to be mediated through enhanced oxidative stress [36], [49], [7], [3], [4], [58]. Oxidative stress results in lipid peroxidation that causes damage to the wall of the stomach resulting in hemorrhagic lesions. Malondialdehyde (MDA) is one of the common markers for oxidative stress and its levels are indicative of the intensity of the oxidative stress [16]. Oxidative stress is also increased by the infiltration of activated neutrophils which generate reactive oxygen species. A significant rise in the MDA levels following ethanol administration (Fig. 7) are in agreement with earlier studies as mentioned above and suggest a massive release of reactive oxygen species (ROS) which in turn may damage the cell membrane [10]. Pre treatment with vanillin significantly decreased the ethanol induced oxidative stress (Fig. 7). Our findings are in accordance with earlier reports and strongly suggest the anti oxidative properties of vanillin to be involved in the amelioration of ethanol induced gastric injury. Like other polyphenols of its genre vanillin is a very potent free radical scavenger with an ability for direct and specific scavenging of free radicals [26]. Oral administration of vanillin results in an increase in both vanillin concentration and antioxidant activity in the plasma and helps in the prevention of free radical induced lipid peroxidation in the membranes [48]. Furthermore the neuroprotective action of vanillin has also been shown to be associated with decreasing free radical generation and the resulting oxidative and nitrosative stress [15]. Therefore vanillin induced cytoprotection and anti-ulcerogenic effects observed in our study may be ascribed to the direct antioxidant and free radical scavenging activity of vanillin.
In addition to the generation of free radicals, gastric ulcers are characterized by inflammation that results in an increase in migration and adherence of macrophages and leukocyte in the ulcer and surrounding areas and the release of proinflammatory mediators [21]. Proinflammatory mediators are presumably regulated by NF-κB, a widely distributed redox sensitive transcription factor. Under normal physiological conditions NF-κB is contained in the cytoplasm in an inactivated state. Upon activation by different triggers such as ROS and inflammatory cytokines it translocates to the nucleus and through transcription regulation alters the level of downstream targets of different genes resulting in the accumulation of proinflammatory mediators that may cause damage to the gastrointestinal tissue [58], [2], [7], [37]. The increased expression of NF-κB (Fig. 9) and the concomitant rise in MPO activity (an indicator of neutrophil infiltration) (Fig. 8) observed in our study are in line with these findings. On the other hand agents that down regulate the expression of NF-κB have been observed to be helpful in the amelioration of gastric inflammation and ulcers [20], [45], [21]. Pretreatment with vanillin resulted in the down regulation of the ethanol induced increase in NF-κB expression (Fig. 9) and inhibition of the increased infiltration of activated neutrophils (Fig. 8) suggesting a potent anti inflammatory action of vanillin.
In conclusion the results of the present study strongly indicate the gastroprotective effects of vanillin in ethanol induced gastric ulcers. The ameliorating effect of vanillin against gastric ulcers may be assigned to the observed antisecretory, mucosal strengthening, anti-oxidative and anti-inflammatory properties. However further studies are warranted to examine the gastro protective efficacy of vanillin in a clinical setup.
Transparency document
Acknowledgements
The authors express their appreciation and thanks to the administration of Prince Sultan Military Medical City for their encouragement and support. The support and help of our colleagues Dr. Abdulquaiyoom Khan, Ms. Biju Prasad, Mr. Dhaya Sankar, Mr. Tariq Al Zamil and Ms. Ruba Fahad Al Mohini is thankfully acknowledged.
References
- 1.Abdel-Sater K.A., Abdel-Daiem W.M., Bakheet M.S. The gender difference of selective serotonin reuptake inhibitor, fluoxetine in adult rats with stress-induced gastric ulcer. Eur. J. Pharmacol. 2012;688:42–48. doi: 10.1016/j.ejphar.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Al-Asmari A.K., Khan A.Q., Al-Qasim A.M., Al-Yousef Y. Ascorbic acid attenuates antineoplastic drug 5-fluorouracil induced gastrointestinal toxicity in rats by modulating the expression of inflammatory mediators. Toxicol. Rep. 2015;2:908–916. doi: 10.1016/j.toxrep.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Asmari A., Al Omani S., Al Otaibi M., Al Abdulaaly A.-A., Elfaki I., Al Yahya K., Arshaduddin M. Gastroprotective effect of minocycline in experimentally induced gastric ulcers in rats. Int. J. Clin. Exp. Med. 2014;7:586–596. [PMC free article] [PubMed] [Google Scholar]
- 4.Al Asmari A.K., Al Omani S., Elfaki I., Tariq M., Al Malki A., Al Asmary S. Gastric antisecretory and antiulcer activity of bovine hemoglobin. World J. Gastroenterol. 2013;19:3291–3299. doi: 10.3748/wjg.v19.i21.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen A., Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am. J. Physiol. Cell Physiol. 2005;288:C1–19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- 6.Alqasoumi S., Al-Sohaibani M., Al-Howiriny T., Al-Yahya M., Rafatullah S. Rocket Eruca sativa: a salad herb with potential gastric anti-ulcer activity. World J. Gastroenterol. 2009;15:1958–1965. doi: 10.3748/wjg.15.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arab H.H., Salama S.A., Omar H.A., Arafa E.-S.A., Maghrabi I.A. Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions. PLoS One. 2015;10:e.0122417. doi: 10.1371/journal.pone.0122417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandarage U., Janero D.R. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs novel gastrointestinal-sparing drugs. Mini Rev. Med. Chem. 2001;1:57–70. doi: 10.2174/1389557013407160. [DOI] [PubMed] [Google Scholar]
- 9.Bradley P., Erdman S., Christensen R., Rothstein G. Supply and release of storage neutrophils a developmental study. Biol. Neonate. 1984;41:132–137. doi: 10.1159/000241541. [DOI] [PubMed] [Google Scholar]
- 10.Cadirci E., Suleyman H., Aksoy H., Halici Z., Ozgen U., Koc A., Ozturk N. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem. Biol. Interact. 2007;170:40–48. doi: 10.1016/j.cbi.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Corne S., Morrissey S., Woods R. Proceedings: a method for the quantitative estimation of gastric barrier mucus. J. Physiol. 1974;242:116P–117P. [PubMed] [Google Scholar]
- 12.El-Maraghy S.A., Rizk S.M., Shahin N.N. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem. Biol. Interact. 2015;229:26–35. doi: 10.1016/j.cbi.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira P.G., Lima M.A.S.S., Bernedo-Navarro R.A., Conceicao R.A., Linhares E., Sawaya A.C.H.F., Yano T., Salgado I. Stimulation of acidic reduction of nitrite to nitric oxide by soybean phenolics: possible relevance to gastrointestinal host defense. J. Agric. Food Chem. 2011;59:5609–5616. doi: 10.1021/jf201229x. [DOI] [PubMed] [Google Scholar]
- 14.Glavin G.B., Szabo S. Experimental gastric mucosal injury: laboratory models reveal mechanisms of pathogenesis and new therapeutic strategies. FASEB J. 1992;6:825–831. doi: 10.1096/fasebj.6.3.1740232. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S., Sharma B. Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington’s disease. Pharmacol. Biochem. Behav. 2014;122:122–135. doi: 10.1016/j.pbb.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 17.Ho K., Yazan L.S., Ismail N., Ismail M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009;33:155–160. doi: 10.1016/j.canep.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Ho K., Yazan L.S., Ismail N., Ismail M. Toxicology study of vanillin on rats via oral and intra-peritoneal administration. Food Chem. Toxicol. 2011;49:25–30. doi: 10.1016/j.fct.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Hoogerwerf W.A., Pasricha P.J. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. McGraw-Hill Medical; New York, NY: 2006. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease; pp. 967–981. [Google Scholar]
- 20.Hossen M.J., Baek K.-S., Kim E., Yang W.S., Jeong D., Kim J.H., Kweon D.-H., Yoon D.H., Kim T.W., Kim J.-H. In vivo and in vitro anti-inflammatory activities of Persicaria chinensis methanolic extract targeting Src/Syk/NF-κB. J. Ethnopharmacol. 2015;159:9–16. doi: 10.1016/j.jep.2014.10.064. [DOI] [PubMed] [Google Scholar]
- 21.Kang J.-W., Yun N., Han H.-J., Kim J.-Y., Kim J.-Y., Lee S.-M. Protective effect of flos lonicerae against experimental gastric ulcers in rats: mechanisms of antioxidant and anti-Inflammatory action. Evid. Based Complement. Altern. Med. 2014;2014:56920. doi: 10.1155/2014/596920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.-J., Kim M.-C., Um J.-Y., Hong S.-H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules. 2010;15:7208–7217. doi: 10.3390/molecules15107208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laine L., Takeuchi K., Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y., Kwon J., Khang G., Lee D. Reduction of inflammatory responses and enhancement of extracellular matrix formation by vanillin-incorporated poly (lactic-co-glycolic acid) scaffolds. Tissue Eng. Part A. 2012;18:1967–1978. doi: 10.1089/ten.tea.2012.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Belmonte J., Whittle B.J., Moncada S. The actions of nitric oxide donors in the prevention or induction of injury to the rat gastric mucosa. Br. J. Pharmacol. 1993;108:73–78. doi: 10.1111/j.1476-5381.1993.tb13442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makni M., Chtourou Y., Garoui E., Boudawara T., Fetoui H. Carbon tetrachloride-induced nephrotoxicity and DNA damage in rats Protective role of vanillin. Hum. Exp. Toxicol. 2012;31:844–852. doi: 10.1177/0960327111429140. [DOI] [PubMed] [Google Scholar]
- 27.McCarty M.F. Dietary nitrate and reductive polyphenols may potentiate the vascular benefit and alleviate the ulcerative risk of low-dose aspirin. Med. Hypotheses. 2013;80:186–190. doi: 10.1016/j.mehy.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Molderings G. Mast cell function in physiology and pathophysiology. Biotrend Rev. 2010;5:1–9. [Google Scholar]
- 29.Nakajima S., Krishnan B., Ota H., Segura A., Hattori T., Graham D.Y., Genta R.M. Mast cell involvement in gastritis with or without Helicobacter pylori infection. Gastroenterology. 1997;113:746–754. doi: 10.1016/s0016-5085(97)70167-7. [DOI] [PubMed] [Google Scholar]
- 30.Natale G., Lazzeri G., Blandizzi C., Gherardi G., Lenzi P., Pellegrini A., Del Tacca M. Seriate histomorphometry of whole rat stomach: an accurate and reliable method for quantitative analysis of mucosal damage. Toxicol. Appl. Pharmacol. 2001;174:17–26. doi: 10.1006/taap.2001.9193. [DOI] [PubMed] [Google Scholar]
- 31.Niazi J., Kaur N., Sachdeva R., Bansal Y., Gupta V. Anti-inflammatory and antinociceptive activity of vanillin. Drug Dev. Ther. 2014;5:145–147. [Google Scholar]
- 32.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 33.Ohta Y., Nishida K. Protective effect of l-arginine against stress-induced gastric mucosal lesions in rats and its relation to nitric oxide-mediated inhibition of neutrophil infiltration. Pharmacol. Res. 2001;43:535–541. doi: 10.1006/phrs.2001.0812. [DOI] [PubMed] [Google Scholar]
- 34.Pedroso L.S., Fávero G.M., de Camargo L.E.A., Mainardes R.M., Khalil N.M. Effect of the o-methyl catechols apocynin, curcumin and vanillin on the cytotoxicity activity of tamoxifen. J. Enzyme Inhib. Med. Chem. 2013;28:734–740. doi: 10.3109/14756366.2012.680064. [DOI] [PubMed] [Google Scholar]
- 35.Rocha B.S., Gago B., Barbosa R.M., Laranjinha J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology. 2009;265:41–48. doi: 10.1016/j.tox.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Rozza A., Cesar D., Pieroni L., Saldanha L., Dokkedal A., De-Faria F., Souza-Brito A., Vilegas W., Takahira R., Pellizzon C. Antiulcerogenic activity and toxicity of Bauhinia holophylla hydroalcoholic extract. Evid. Based Complement. Altern. Med. 2015;2015:439506. doi: 10.1155/2015/439506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sangiovanni E., Vrhovsek U., Rossoni G., Colombo E., Brunelli C., Brembati L., Trivulzio S., Gasperotti M., Mattivi F., Bosisio E. Ellagitannins from Rubus berries for the control of gastric inflammation: in vitro and in vivo studies. PLoS One. 2013;8:e71762. doi: 10.1371/journal.pone.0071762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar N., Purkayastha S., Sarkar B., Guha D. Modulation of gastric mucosal mast cell population: role of vestibulo cerebellar lesion. Indian J. Exp. Biol. 2006;44:627–634. [PubMed] [Google Scholar]
- 39.Schiantarelli P., Cadel S., Folco G. Gastroprotective effects of morniflumate, an esterified anti-inflammatory drug. Arzneimittelforschung. 1983;34:885–890. [PubMed] [Google Scholar]
- 40.Schubert M., Shamburek R. Control of acid secretion. Gastroenterol. Clin. North Am. 1990;19:1–25. [PubMed] [Google Scholar]
- 41.Schubert M.L. Gastric secretion. Curr. Opin. Gastroenterol. 2007;23:595–601. doi: 10.1097/MOG.0b013e3282f03462. [DOI] [PubMed] [Google Scholar]
- 42.Schubert M.L., Peura D.A. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Shay H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology. 1945;5:43–61. [Google Scholar]
- 44.Shay J., Elbaz H.A., Lee I., Zielske S.P., Malek M.H., Hüttemann M. Molecular mechanisms and therapeutic effects of (−)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longev. 2015;2015:181260. doi: 10.1155/2015/181260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha K., Sadhukhan P., Saha S., Pal P.B., Sil P.C. Morin protects gastric mucosa from nonsteroidal anti-inflammatory drug, indomethacin induced inflammatory damage and apoptosis by modulating NF-(B pathway. Biochim. Biophys. Acta (BBA): Gen. Subj. 2015;1850:769–783. doi: 10.1016/j.bbagen.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Soll A.H. Potentiating interactions of gastric stimulants on [14C] aminopyrine accumulation by isolated canine parietal cells. Gastroenterology. 1982;83:216–223. [PubMed] [Google Scholar]
- 47.Sumbul S., Ahmad M.A., Mohd A., Mohd A. Role of phenolic compounds in peptic ulcer: an overview. J. Pharm. Bioallied Sci. 2011;3:361–367. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tai A., Sawano T., Yazama F., Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta (BBA): Gen. Subj. 2011;1810:170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Thomas D., Govindhan S., Baiju E.C., Padmavathi G., Kunnumakkara A.B., Padikkala J. Cyperus rotundus L. prevents non-steroidal anti-inflammatory drug-induced gastric mucosal damage by inhibiting oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2015;26:485–490. doi: 10.1515/jbcpp-2014-0093. [DOI] [PubMed] [Google Scholar]
- 50.Thomson A.B., Sauve M.D., Kassam N., Kamitakahara H. Safety of the long-term use of proton pump inhibitors. World J. Gastroenterol. 2010;16:2323–2330. doi: 10.3748/wjg.v16.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripathi K. JP Medical Ltd.; 2013. Essentials of Medical Pharmacology. [Google Scholar]
- 52.Verma M. A review on peptic ulcer: a global threat. J. Pharm. Res. 2010;3:2088–2091. [Google Scholar]
- 53.Vuyyuru L., Harrington L., Arimura A., Schubert M. Reciprocal inhibitory paracrine pathways link histamine and somatostatin secretion in the fundus of the stomach. Am. J. Physiol. Gastrointest. Liver Physiol. 1997;273:G106–G111. doi: 10.1152/ajpgi.1997.273.1.G106. [DOI] [PubMed] [Google Scholar]
- 54.Wallace J.L. Cooperative modulation of gastrointestinal mucosal defence by prostaglandins and nitric oxide. Clin. Invest. Med. 1996;19:346–351. [PubMed] [Google Scholar]
- 55.Wallace J.L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- 56.Wallace J.L. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn't the stomach digest itself? Physiol. Rev. 2008;88:1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 57.Walton N.J., Mayer M.J., Narbad A. Vanillin. Phytochemistry. 2003;63:505–515. doi: 10.1016/s0031-9422(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y., Su W., Zhang C., Xue C., Chang Y., Wu X., Tang Q., Wang J. Protective effect of sea cucumber (Acaudina molpadioides) fucoidan against ethanol-induced gastric damage. Food Chem. 2012;133:1414–1419. [Google Scholar]
- 59.Wu C.-Y., Wu M.-S., Kuo K.N., Wang C.-B., Chen Y.-J., Lin J.-T. Effective reduction of gastric cancer risk with regular use of nonsteroidal anti-inflammatory drugs in Helicobacter pylori—infected patients. J. Clin. Oncol. 2010;28:2952–2957. doi: 10.1200/JCO.2009.26.0695. [DOI] [PubMed] [Google Scholar]
- 60.Wu S.-L., Chen J.-C., Li C.-C., Lo H.-Y., Ho T.-Y., Hsiang C.-Y. Vanillin improves and prevents trinitrobenzene sulfonic acid-induced colitis in mice. J. Pharmacol. Exp. Ther. 2009;330:370–376. doi: 10.1124/jpet.109.152835. [DOI] [PubMed] [Google Scholar]
- 61.Xu J., Xu H., Liu Y., He H., Li G. Vanillin-induced amelioration of depression-like behaviors in rats by modulating monoamine neurotransmitters in the brain. Psychiatry Res. 2015;225:509–514. doi: 10.1016/j.psychres.2014.11.056. [DOI] [PubMed] [Google Scholar]