Abstract
Introduction
Several studies towards the development of an effective treatment for intestinal mucositis have been reported, since this condition represents a major problem in clinical oncology practice due to cytotoxic effects of chemotherapy. However standardized protocols and universally accepted treatment options are yet to be established.
Objectives
Given above, this study evaluated the protective effects of a mucoadhesive formulation containing both Bidens pilosa L. (Asteraceae) (BP) and curcuminoids from Curcuma longa L. (Zingiberaceae) (CL) on intestinal mucositis induced by 5-fluoruoacil (5-FU) in mice.
Results
As expected, animals only treated with 5-FU (200 mg/kg) showed a significant reduction of 60.3 and 42.4% in villi and crypts size, respectively, when compared to control. On the other hand, the proposed therapeutic/prophylactic treatment with mucoadhesive formulations managed to reduce histopathologic changes in mice bearing mucositis, especially at 125 mg/kg BP + 15 mg/kg CL dose. The formulation promoted an increase of 275.5% and 148.7% for villi and crypts size, respectively. Moreover, chemotherapy-related weight loss was reduced by 7.4% following the treatment. In addition, an increase of 10 and 30.5% in red and white blood cells was observed when compared to 5-FU group. Furthermore, treatments with the mucoadhesive formulation containing BP/CL up modulated Ki-67 and Bcl-2 expression while reduced pro-apoptotic regulator Bax. The formulation also modulated inflammatory response triggered by 5-FU through reduction of 68% of myeloperoxidase activity and a 4-fold increase in anti-inflammatory IL-10 levels. In parallel, the oxidative stress via lipid peroxidation was reduced as indicated by decrease of 63% of malondialdehyde concentrations. Additionally, the new formulation presented low acute oral systemic toxicity, being classified in the category 5 (2000 mg/kg < LD50 < 5000 mg/kg) of the Globally Harmonized Classification System.
Conclusions
This study showed an interesting potential of the mucoadhesive formulation of BP/CL for the treatment of 5-FU-induced intestinal mucositis. Given the perspectives for the development of a new medicine, clinical studies are in progress to better understand the protective effects of this innovative formulation in treating mucositis.
Keywords: Bidens pilosa L. (Asteraceae), Curcuma longa L. (Zingiberaceae), Mucoadhesion, Tri-block copolymer, Intestinal damage, Mucositis
1. Introduction
Intestinal mucositis represents a major problem in clinical oncology practice due to cytotoxic effect associated with chemotherapy [1]. This pathological condition is correlated with events such as apoptosis, epithelial hypoproliferation, crypts/villi size decreasing, inflammatory infiltrate, increased expression of TNF-α, IL-1β and IL-6, with consequent changes in the intestinal absorptive capacity and bacterial colonization [2]. Mucositis is also frequently associated with abdominal pain, diarrhea, vomiting and nausea [3], [4]. Additionally, mucositis is the cause of patient longer hospitalization, raising the total cost of the treatment which becomes an economical and public health problem [5]. Although several studies have already been conducted in attempts to treat mucositis, there are still no universally accepted and standardized protocols for the treatment. Substances of natural origin have been recently studied in this context, especially those with anti-inflammatory and anti-oxidant properties such as Bidens pilosa L. (Asteraceae) (BP) and Curcuma longa L. (Zingiberaceae) (CL).
BP is a plant present in tropical and sub-tropical regions, widely used in folk medicine with an extensive phytochemical constitution [6], [7]. In addition to anti-inflammatory and anti-oxidant effects, several pharmacological activities related to BP have been reported such as anti-hypertensive, anti-hyperglycemic, antiulcer, immunosuppressive, hepatoprotective, antileukemic, antimalarial and antibacterial [8], [9]. Its efficacy has been recently demonstrated in vivo on gastric mucosal injury induced by hydrochloric acid/ethanol in rats. Oral administration of BP exerted a protective effect in these lesions, which probably contributed to the suppression of oxidative stress, prostaglandin production and inflammation [10]. Recently, Ávila et al. [11], demonstrated that a mucoadhesive formulation based on BP reduced intestinal injury in mice caused by 5-fluorouracil, in special the formulation led to a reduction in the local inflammatory infiltrate.
CL and its compounds, especially curcumin, have shown important anti-inflammatory and anti-oxidant properties, and have been widely studied in cancer chemoprevention and suppression of tumor growth [12], [13]. CL also showed immunomodulatory capacity, exerting its effects by regulating the expression of several genes and proteins [14]. Moreover, previous study has demonstrated that a formulation containing curcumin, α-tocopherol and sunflower oil was effective in reducing radiation-induced ulceration of the oral mucosa in rats [15]. Furthermore, a pilot study with pediatric patients undergoing chemotherapy has highlighted the promising use of topical curcumin for prevention of oral mucositis [16]. Previous work from our group showed that a mucoadhesive formulation with curcuminoids from CL positively modulated Ki-67 protein in villi and crypts epithelium of mucositis-bearing mice, acting positively on the rate of intestinal cell proliferation [17].
In spite of the wide spectrum of biological effects of the curcumin, its low oral bioavailability and poor water solubility represent a limiting factor for its therapeutic use. These limitations have led to the development of various formulation strategies to improve curcumin effects, such as mucoadhesive products. Mucoadhesion systems are based on polymeric compounds, which have the ability to adhere to the surface of cells, or to the mucus layer that covers the epithelium. These formulations are of great pharmaceutical interest due to their ability in prolonging the residence time of the drug at the absorption site. In addition, they can promote a more intimate contact with the mucosa providing better topical treatment [18], [19]. In this regarding, previous work from our group showed that a mucoadhesive formulation of CL also attenuated body weight loss and protected intestinal mucosa from villus shortening and crypt deepening induced by 5-FU [17].
Previous data indicate that BP or CL alone treated intestinal mucositis through different mechanisms, reducing the local inflammatory infiltrate and/or increasing the rate of intestinal cell proliferation, respectively. Based on these findings, this study evaluated the protective effects of a mucoadhesive formulation based on poloxamer 407, a tri-block copolymer with mucoadhesive properties, containing both BP/CL extracts in mice bearing intestinal mucositis induced by 5-fluorouracil (5-FU).
2. Materials and methods
2.1. Chemicals
C. longa (>95% curcuminoid content) and B. pilosa glycolic extracts (Ecobidens®) were obtained from GAMMA (São Paulo, SP, Brazil) and CHEMYUNION (Sorocaba, SP, Brazil), respectively. Transcutol HP® (diethylene glycol monoethyl ether) was kindly donated by Gattefossé (Lyon, France). Soluplus® HS15 (Macrogol-15-hydroxystearate) was kindly donated by Pharma Ingredients and Services BASF (São Paulo, SP, Brazil). Polyethylene glycol 400 and sodium azide were acquired from Labsynth (Diadema, SP, Brazil). Butylated hydroxytoluene and 3,3diaminobenzidine (DAB) were obtained from Mapric (São Paulo, SP, Brazil) and Dako (Carpinteria, CA, USA), respectively. 5-Fluorouracil, hexadecyltrimethylammonium bromide, tri-block copolymer poloxamer 407, bovine serum albumin (BSA), EDTA, phenylmethylsulfonyl fluoride (PMSF), kallikrein inhibitor units of aprotinin A, n-butanol and ortho-dianisidine were purchased from Sigma–Aldrich (St. Louis, MO, USA). ImmunoCruz™ mouse ABC staining systems (sc-2017 and 2018), monoclonal mouse anti-mouse p53 (clone 3H2820), monoclonal mouse anti-human Bcl2 (clone C-2) and polyclonal rabbit anti-mouse Bax (clone P-19) antibodies were acquired from Santa Cruz Biotechnology (Paso Robles, CA, USA); whereas monoclonal mouse anti-human Ki-67 (clone 124) antibody were obtained from Novacastra (Newcastle, UK). Mouse IL-10 BD Cytometric Bead kit was purchased from BD Biosciences (San Diego, CA, USA). Trichloroacetic acid, NaCl, Tween 20, benzethonium chloride, H2O2, tris, HCl, methanol and xylene were obtained from Vetec (Rio de Janeiro, RJ, Brazil). Thiobarbituric acid, hematoxylin and eosin stainings were acquired from Merck (Darmstadt, HE, Germany). Xylazine and ketamine were purchased from Syntec (Cotia, SP, Brazil) and König (Santana de Parnaíba, SP, Brazil), respectively.
2.2. Preparation of mucoadhesive and non-mucoadhesive formulations
The mucoadhesive formulation was composed by CL (1%, m/m) and BP (40%, v/v) extracts, tri-block copolymer poloxamer 407 (15%, m/m), Surplus® HS15 (3.2%, m/m), Transcutol HP (10%, v/v), citric acid (to pH 4.5–6.0) and polyethylene glycol 400 as a liquid vehicle. The non-mucoadhesive formulation was prepared without poloxamer, which was replaced by PEG 400.
Formulations were prepared by mixing constituents in a heated reactor (65–70 °C) under mechanical stirring. The pH of the final preparation was adjusted using citric acid. Once prepared, formulations were placed in ambar flasks and stored at room temperature protected from light until use. The total polyphenol content of the BP extract determined by the Folin Ciocalteau method was 88.2 ppm of total polyphenols, which was within the range (20–200 ppm) of the analysis certificate issued by the Ecobidens® manufacturer. The total curcuminoid content of the formulations, based on curcumin levels, was evaluated by UV spectrophotometry (λ = 425 nm).
2.3. Animals
Male Swiss mice (age: 8–10 weeks; weight: 35–40 g) were obtained from the Bioterium at Federal University of Goiás, and all efforts were conducted to ensure the animal welfare. Mice were acclimatized for a week prior to the beginning of experiments and kept under steady conditions with light–dark cycles and controlled temperature, while water and food were provided ad libitum. The experimental protocol (UFG no. 036/2012) was approved by the Research Ethics Committee at this University. At the end of each assay, animals were previously anesthetized by 10 mg/kg of xylazine and 100 mg/kg of ketamine hydrochloride administered intraperitoneally and euthanized by cervical dislocation [20].
2.4. Experimental design
Mice (n = 5/group) were treated orally (gavage) with three different doses of mucoadhesive or non-mucoadhesive formulations containing BP/CL for 6 days (days 1–6). The following doses of both preparations were administered to the animals: 75 mg/kg BP + 3.75 mg/kg CL; 100 mg/kg BP + 7.5 mg/kg CL; 125 mg/kg BP + 15 mg/kg CL. Additionally, two groups were treated with each formulation without BP/CL (blank formulations). On days 4–6, intestinal mucositis was induced by intraperitoneal administration of 5-FU (200 mg/kg), as described by Wu et al. [3]. On the 7th day, animals were anesthetized to perform blood collection by cardiac puncture followed by euthanasia. A duodenal portion of about 10 cm was then harvested from each animal at approximately 3 cm beyond the pyloric sphincter.
2.5. Histological and morphometric analysis
The histomorphometric evaluation was conducted as described by Ávila et al. [11]. Briefly, the duodenum of each animal was fixed in 10% neutral buffered formalin solution for 24 h, sectioned, automatically processed (OMA DM-20, M20090257, São Paulo, Brazil), embedded in paraffin and then sectioned with a microtome (Leica RM2165, Gottingen, Germany) in 5 μm slices. The sections were stained using routine haematoxylin/eosin technique and then embedded in paraffin. Sections of 5 mm thick were deparaffinized, stained with hematoxylin-eosin (H&E) and examined under an optical microscope (Zeiss Axioskop 40, Carl Zeiss, Gottingen, Germany) to carry out the measuring of villi and crypts lengths of ten random fields of each slide using AxioVision 40 software, version 4.7.2.0 (Carl Zeiss, Jena, TH, Germany). To assess damage severity of the intestinal mucositis, a semi-quantitative histopathologic evaluation was performed covering parameters obtained from the blades. The evaluation was conducted in a triple-blinded assay and results indicate the mean scores of the three assessments. The criteria used were as follows: inflammatory infiltration, epithelial vacuolization, muscle layer integrity, epithelial integrity and edema. These changes were then classified according to the following scores: 0—normal, 1—mild (changes limited at basal third of the lining epithelium), 2—moderate (when the changes represented two-thirds of the lining epithelium), 3—severe (more than two-thirds of the epithelium affected). The final score was obtained by summing the scores of each characteristic in order to obtain a final score called histological damage index (HDI) and then a comparison among groups were performed. From these analyzes, the most effective dose of the formulations was chosen for subsequent studies.
2.6. Mice body weight evaluation
Animals were weighed daily during 7 days, immediately before the administration of chemotherapy or treatment. The percentage of the variation in body mass of each group in this period was then evaluated day-to-day in relation to the initial weight.
2.7. Hematological analysis
Hematological examination was performed using an ABX Micros 60 apparatus (Horiba, São Paulo, SP, Brazil) by red and white blood cells counting.
2.8. Immunohistochemistry
Analyses were carried out to evaluate the expression of Bcl2, Bax, p53 and Ki-67 as described elsewhere [11]. Briefly, 3 μm thick sections were prepared from different animal groups and immunohistochemistry was performed using Santa Cruz Biotechnology (Paso Robles, CA, USA) kits. Primary antibodies were used as follows: Ki-67 at 1:100, Bcl-2 at 1:50, Bax at 1:50 and p53 at 1:50. The labeled cells were then visualized by light microscopy (Zeiss Axioskop 40, Carl Zeiss, Göttingen, Germany) at 20× and counted in the epithelial layer and bowel infiltration. The percentages of positive cells for the proteins studied were determined by the analysis of 10 consecutive fields (100 cells/field).
2.9. Measurement of IL-10 cytokine
About 250 mg of intestinal tissue was homogenized in 10 mL of antiprotease solution (PBS-BSA buffer containing 0.1 mM PMSF, 0.1 mM benzethonium chloride, 10 mM EDTA, 20 kallikrein inhibitor units of aprotinin A and 0.05% Tween 20) in an ultra-Turrax® T 25 digital (IKA, Baden-Württemberg, Germany) at 10,000 rpm for 5 min. Then, homogenates were centrifuged at 2000 rpm for 15 min at 4 °C and supernatant was used for IL-10 quantification by BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA) using Mouse IL-10 BD Cytometric Bead kit (BD Biosciences) in accordance with the manufacturer’s instructions.
2.10. Myeloperoxidase (MPO) activity
MPO is an enzyme found primarily in neutrophil azurophilic granules and it has been extensively used as a biochemical marker for granulocyte infiltration. The extent of neutrophil accumulation in the intestinal tissue was measured by MPO activity evaluation as previously described [21]. Briefly, 250 mg of tissue was homogenized in 5 mL of potassium phosphate buffer (pH 7.2) with hexadecyltrimethylammonium bromide (HTAB, 0.5%) in an ultra-Turrax® T 25 digital (IKA, Baden-Württemberg, Germany) at 10,000 rpm for 5 min. Then, homogenates were centrifuged at 2000 rpm for 15 min at 4 °C. MPO activity in the resuspended pellet was assayed by measuring the change in absorbance at 450 nm using o-dianisidine dihydrochloride and 1% hydrogen peroxide. Results were reported as MPO units/100 mg of tissue × 1000.
2.11. Determination of lipid peroxidation
Lipid peroxidation products in the intestinal tissue were determined by measuring of malondialdehyde (MDA), thiobarbituric acid reactive substance (TBARS), based on the method described by Marques et al. [22] with some modifications. Briefly, 250 mg of intestinal tissue was homogenized in 5 mL of potassium phosphate buffer (pH 7.2) with hexadecyltrimethylammonium bromide (HTAB, 0.5%) in an ultra-Turrax® T 25 digital (IKA, Baden-Württemberg, Germany) at 10,000 rpm for 5 min. Then, homogenates were centrifuged at 2000 rpm for 15 min at 4 °C. The supernatant obtained was used to determine the activity of MDA. Then, 250 μL of supernatant plus 25 μL BHT butylated 4% in methanol were homogenized in a vortex. Then 1 mL of 12% trichloroacetic acid (TCA), 1 mL of 0.73% thiobarbituric acid (TBA) and 750 μL of Tris–HCl buffer were added and the reaction mixture was heated at 95 °C for 60 min. After, the tubes were placed on ice to block the reaction. After, 5 mL of n-butanol was added and the mixture was shaken vigorously. After centrifugation at 5000 rpm for 10 min, the n-butanol layer was taken and optical density reading was performed at 532 and 453 nm. MDA concentrations were obtained by subtracting 20% of the absorbance at 453 nm from the absorbance at 532 nm using a molar extinction coefficient of 1.56 × 105 M−1 cm−1. Data were reported as nmol MDA/mg of tissue.
2.12. Acute oral systemic toxicity
The analysis of acute oral toxicity was performed according to OECD Guideline 420 – Acute Oral Toxicity – Fixed Dose Procedure [23]. Briefly, after two hours of fasting, mice were randomly separated and a single 2000 mg/kg dose of mucoadhesive formulation was administrated orally (gavage) in each animal. After treatment, clinical observations were conducted at 5, 15 and 30 min, and each hour up to the twelfth hour of the first day. Posteriorly, mice were examined once a day for an additional 13 days. From the results obtained, the mucoadhesive formulation was then categorized according to the Globally Harmonized Classification (GHS) System.
2.13. Statistical analysis
Data are expressed as mean ± standard deviation. The inter group variation was measured by one or two-way Analysis of Variance (ANOVA) followed by Bonferroni test using a GraphPad Prism version 5.01 software for Windows (San Diego, CA, USA). Statistical significance was considered when p < 0.05.
3. Results
3.1. Effects of the mucoadhesive formulation and non-mucoadhesive of BP/CL on intestinal histomorphometric analysis in mice
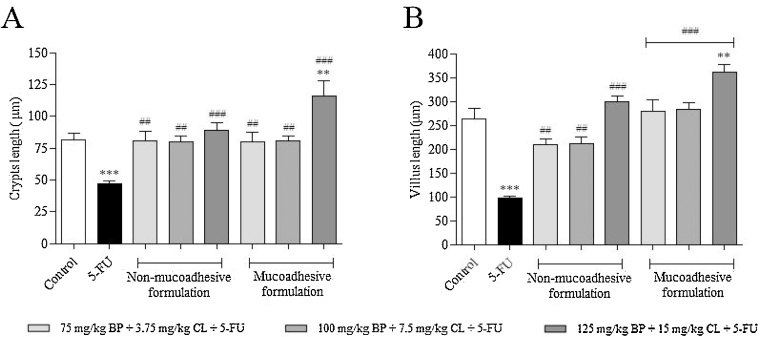
As previously demonstrated, animals exposed only to 5-FU (200 mg/kg) showed a significant reduction of 60.3 and 42.4% in villi and crypts size, respectively, when compared to non-exposed animals (p < 0.0001), according to Fig. 1. In contrast, prophylactic/therapeutic treatments with both, non-mucoadhesive and mucoadhesive formulations, for 6 days protected against 5-FU-induced intestinal damage. When compared to 5-FU group, there was a significant increase of crypts size of 72.6 (p < 0.001), 71.5 (p < 0.001) and 90.5% (p < 0.0001) for non-mucoadhesive formulation at 75 mg/kg BP + 3.75 mg/kg CL, 100 mg/kg BP + 7.5 mg/kg CL and 125 mg/kg BP + 15 mg/kg CL + 5-FU, respectively; while for mucoadhesive formulation it was 71.6 (p < 0.001), 72.8 (p < 0.001) and 148.7% (p < 0.0001), in the same concentrations. In relation to villi size, it was was observed a significant increase of 116.7 (p < 0.001), 120.2 (p < 0.001) and 210.5% (p < 0.0001) for non-mucoadhesive formulation and 190.3 (p < 0.0001), 193.2 (p < 0.0001) and 275.5% (p < 0.0001) for mucoadhesive formulation at 75 mg/kg BP + 3.75 mg/kg CL, 100 mg/kg BP + 7.5 mg/kg CL and 125 mg/kg BP + 15 mg/kg CL + 5-FU, respectively, in comparison to 5-FU group. As noted, mucoadhesive formulation resulted in better protection of intestinal structures, especially at 125 mg/kg + BP 15 mg/kg CL dose, highlighting the potential of this technological preparation against 5-FU-induced intestinal toxicity.
Fig. 1.
Morphometric evaluation of crypts (A) and villi (B) in small intestine of mucositis-bearing mice induced by 5-FU and its treatment with non-mucoadhesive or mucoadhesive formulations containing BP/CL. Formulation doses or blank formulation were administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) were administered intraperitoneally from 4th to 6th day. On day 7, animals (n = 5 mice/group) were euthanized and the small intestine of each mice was collected for morphometric analysis. (*p < 0.05 and ***p < 0.0001 vs. control; #p < 0.05 and ###p < 0.0001 vs. 5-FU. One-way ANOVA and Bonferroni test).
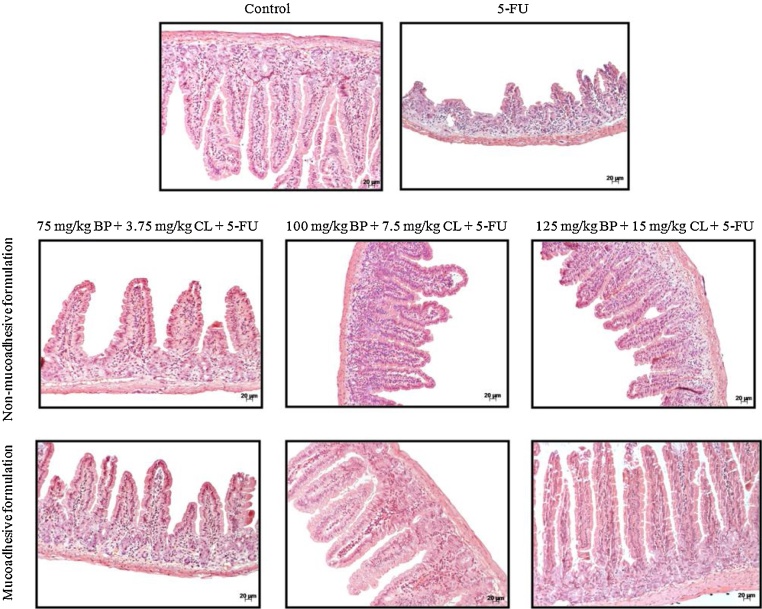
Corroborating these findings, histological analysis of the villi and crypts showed protective effects of formulations, particularly mucoadhesive formulation at 125 mg/kg + BP 15 mg/kg CL dose (Fig. 2). This dose had greater efficacy compared to non-mucoadhesive formulations and showed histological damage index (HDI) of 0.9, similar to control (HDI = 0.5), which was 56.2% lower than 5-FU group (HDI = 1.6) (Table 1). Therefore, the mucoadhesive formulation at 125 mg/kg + BP 15 mg/kg CL dose was used for subsequent assays.
Fig. 2.
Effects of non-mucoadhesive or mucoadhesive formulations containing BP/CL on small intestinal histological damage of mucositis-bearing mice induced by 5-FU. Formulation doses or blank formulation were administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) were administered intraperitoneally from 4th to 6th day. On day 7, animals (n = 5 mice/group) were euthanized and the small intestine of each mice was collected for morphometry analysis. Photomicrographs are representative of each treatment group.
Table 1.
Histological damage index (HDI) of the small intestinal samples of mucositis-bearing mice (n = 5 per group) induced by 5-FU and its treatment with the mucoadhesive formulation containing 125 mg/kg BP + 15 mg/kg CL. Pathological changes were classified according to the following scores: 0–normal (no change), 1–mild, 2–moderate and 3–severe changes.
| Criteria | Treatment groups |
||
|---|---|---|---|
| Control | 5-FU | 125 mg/kg BP + 15 mg/kg CL + 5-FU | |
| Inflammatory infiltrate | 1.1 ± 0.3 | 2.9 ± 0.3c | 2.0 ± 0.6c, e |
| Vacuolation | 1.1 ± 0.3 | 2.0 ± 0.8a | 1.8 ± 0.9 |
| Muscular layer integrity | 0.3 ± 0.5 | 1.4 ± 1.2a | 0.1 ± 0.3b |
| Epithelium integrity | 0.1 ± 0.3 | 1.7 ± 1.0c | 0.6 ± 0.5d |
| Edema | 0 | 0 | 0 |
| HDI | 0.5 ± 0.5 | 1.6 ± 0.9 | 0.9 ± 0.8 |
All data are expressed as mean ± SD of five animals per group. Significantly different at p < 0.05.
p < 0.05.
p < 0.01.
p < 0.001 vs. control.
p < 0.01.
p < 0.001 vs. 5-FU. One way ANOVA and Bonferroni test.
3.2. Effects of the mucoadhesive formulation of BP/CL in body weight variation
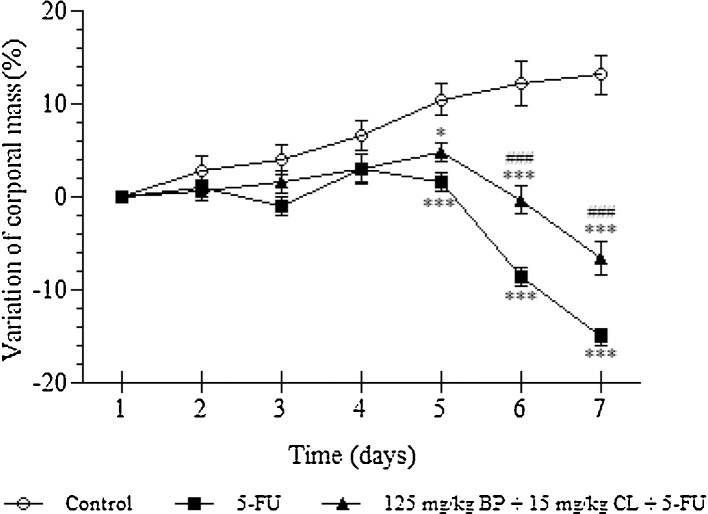
According to previous results, control group showed an increase of weight of about 13.1% throughout the experimental period. However, after 5-FU exposure, animals showed a significant weight loss, reaching a reduction about 15% of initial body weight in the seventh day (p < 0.001). Treatment based on the mucoadhesive formulation at 125 mg/kg + BP 15 mg/kg CL dose resulted in a protection against weight loss triggered by chemotherapy. In these animals, the weight loss was reduced in 7.4% (p < 0.001) (Fig. 3).
Fig. 3.
Effects of the mucoadhesive formulation at 125 mg/kg + BP 15 mg/kg CL dose on the variation of corporal mass of mucositis-bearing mice induced by 5-FU. Formulation doses or blank formulation were administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) were administered intraperitoneally from 4th to 6th day. Mice were weighed throughout 7 days of the experiment to obtain the variation in body mass (%). Each point presents mean ± SD of animals (n = 5). (*p < 0.05 and ***p < 0.001 vs. control; #p < 0.05 and ###p < 0.001 vs. 5-FU. Two-way ANOVA and Bonferroni test).
3.3. Effects of the mucoadhesive formulation of BP/CL in the 5-FU-induced hematotoxicity
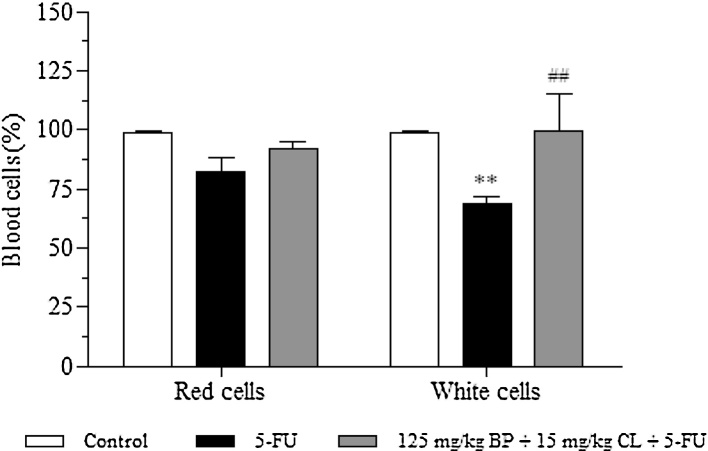
As shown in Fig. 4, 5-FU group showed a reduction of 18 and 31.2% (p < 0.01) in red and white blood cells, respectively, in comparison to control. Contrasting, treatment with the mucoadhesive formulation at 125 mg/kg + BP 15 mg/kg CL dose was able to promote an increase of 10 and 30.5% (p < 0.01), when compared to 5-FU group, demonstrating the hematoprotective potential of the mucoadhesive formulation.
Fig. 4.
Effects of the mucoadhesive formulation at 125 mg/kg + BP 15 mg/kg CL dose in blood cells. Formulation doses or blank formulation were administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) were administered intraperitoneally from 4th to 6th day. On day 7, animals (n = 5 mice/group) were anesthetized and the blood collected by cardiac puncture. (**p < 0.01 vs. control; ##p < 0.01 vs. 5-FU. Two-way ANOVA and Bonferroni test).
3.4. Effects of the mucoadhesive formulation of BP/CL on intestinal cell proliferation and apoptosis markers
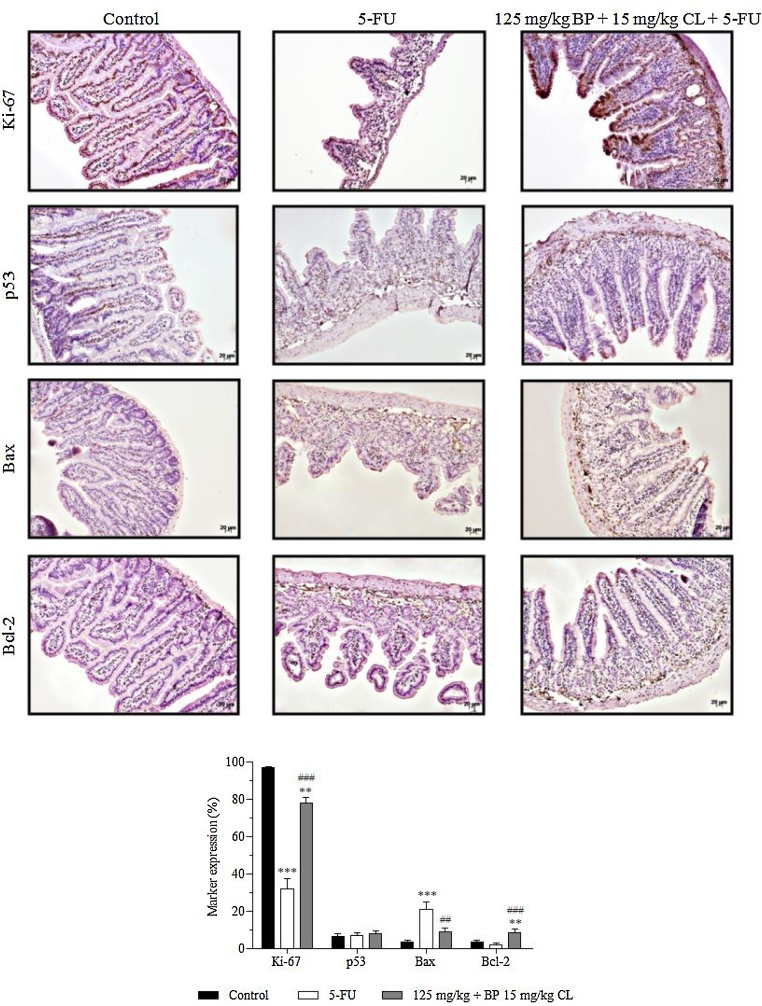
In the immunohistochemistry analysis, the expression of markers Ki-67 (cell proliferation) and p53, Bax and Bcl-2 (apoptosis) were evaluated in mucositis bearing-mice, treated and non-treated with the test formulation (Fig. 5). In all groups, a predominant expression of nuclear staining for p53 and cytoplasmic staining for Bax and Bcl-2 in intestinal villi and crypts was observed; while both nuclear and cytoplasmic expression for Ki-67 was noted. In control animals, normal levels of 96.6, 3.5 and 3.6% for Ki-67, Bax and Bcl-2 markers, respectively, were present. An intense proliferative activity in the intestine of healthy animals was observed, probably due to a high intestinal tissue turnover. On the other hand, mucositis-bearing mice without treatment showed a reduced expression of 32% in Ki-67 expression (p < 0.001) associated with a high apoptosis ratio due to increased marking of 20.8% in pro-apoptotic regulator Bax (p < 0.001) versus a reduced expression of 2% in anti-apoptotic Bcl-2 expression. Contrasting with these findings, treatment with the mucoadhesive formulation was able to modulate the rate of apoptosis and restore proliferative activity of intestinal tissue in mucositis-bearing animals, since Ki-67, Bax and Bcl-2 expressions were 78 (p < 0.001), 8.7 (p < 0.01) and 8.5% (p < 0.001), respectively. Regarding to p53 levels, no statistically significant difference between groups was observed.
Fig. 5.
Effects of non-mucoadhesive or mucoadhesive formulations containing BP/CL on the expression of apoptosis and cell proliferation markers in the intestinal tissue of mucositis-bearing mice induced by 5-FU. Formulation doses or blank formulation were administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) were administered intraperitoneally from 4th to 6th day. On day 7, animals (n = 5 mice/group) were euthanized and the small intestine of each mice was collected for Ki-67, p53, Bax and Bcl-2 detection by immunohistochemistry. Photomicrographs are representative of each treatment group. (**p < 0.01 and ***p < 0.001 vs. control; ##p < 0.01 and ###p < 0.001 vs. 5-FU. ANOVA and Bonferroni test).
3.5. Effects of the mucoadhesive formulation of BP/CL on the inflammatory and oxidative stress response-induced by 5-FU
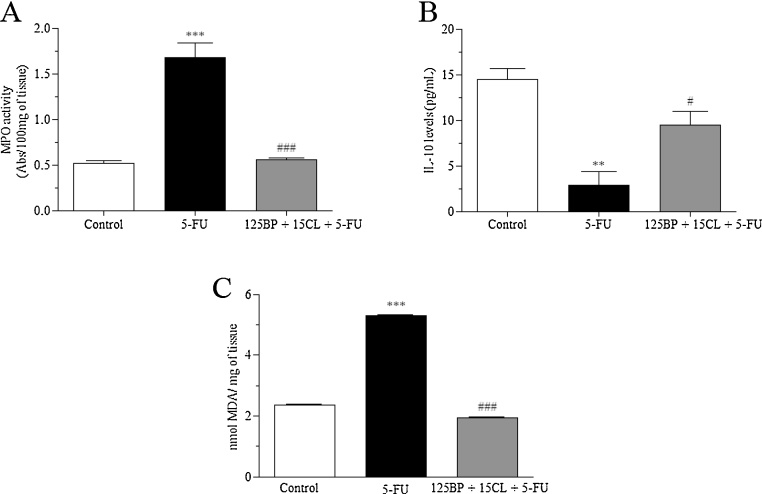
In the inflammatory response evaluation, animals exposed to only 5-FU showed a significant increase of 191.7% (p < 0.0001) for MPO activity associated with a decrease of 80.2% for IL-10 levels (p < 0.001), when compared to control (Fig. 6A, B). In parallel, 5-FU also promoted an increase of 124.6% of the oxidative stress via lipid peroxidation as demonstrated by MDA activity (p < 0.0001). In contrast, values found for mucositis-bearing mice, treated with mucoadhesive formulation at 125 mg/kg BP + 15 mg/kg CL dose, were close to those found in the control group. Therefore, mucoadhesive formulation was significantly able to restore these parameters in relation to 5-FU group (p < 0.05) (Fig. 6A, B).
Fig. 6.
Effects of the mucoadhesive formulation of BP/CL on the inflammatory and oxidative stress response-induced by 5-FU. Formulation doses or blank formulation were administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) were administered intraperitoneally from 4th to 6th day. On day 7, animals (n = 5 mice/group) were euthanized and the small intestine of each mice was collected for MPO activity (A), measurement of IL-10 levels (B) and determination of lipid peroxidation (C). (**p < 0.01 and ***p < 0.0001 vs. control; #p < 0.05 and ###p < 0.0001 vs. 5-FU. One-way ANOVA and Bonferroni test).
3.6. Evaluation of acute oral systemic toxicity of the mucoadhesive formulation of BP/CL
The exposure of animals to a single dose of 2000 mg/kg mucoadhesive formulation of BP/CL was not able to trigger death or clinical signs of toxicity throughout the experimental period (14 days). In addition, macroscopic and histopathological analysis revealed no abnormality induced by the formulation in the organs studied. Consequently, the mucoadhesive formulation of BP and CL was classified in the category 5 (2000 mg/kg < LD50 < 5000 mg/kg) of GHS System. Moreover, this formulation did not increase the frequency of micronucleus (data not shown).
4. Discussion
Many experimental therapeutic protocols for mucositis are being investigated [24], [25]. Among them, the use of natural products has stood out, given the range of phytochemicals that act in association and in multi-targeted manner to achieve the desired prophylactic/therapeutic effect. In this study, we showed that a mucoadhesive formulation containing BP/CL was able to protect mice from 5-FU-induced intestinal injury by through modulating of pro- and anti-apoptotic regulators, increasing of cell proliferation rate in association with reduction of oxidative stress and inflammatory responses. The mucoadhesive property of the formulation induced better protection of intestinal structures.
As mentioned before, the use of mucoadhesive formulation based on poloxamer 407 seems an interesting pharmaceutical strategy. Due to its rheological properties [26], this polyoxyethylene copolymer can decrease the mucociliary clearance and increase the contact time of the formulation with the intestinal mucosa. Recently, it was demonstrated that the incorporation of BP glycolic extract [11] or curcuminoids from CL [17] in a liquid formulation based on poloxamer 407 efficiently reduced 5-FU-induced intestinal injuries in mice, especially by anti-inflammatory/antioxidant effects and/or restoration of intestinal proliferative activity. Therefore, each of these preparations seems to act on key cellular mechanisms of mucositis, inflammation and overproduction of reactive oxygen species (ROS), through different pathways. In this sense, the combined use of these plants in a mucoadhesive preparation appears to promote better results in the treatment/prevention of cytotoxic effects associated with chemotherapy, as demonstrated here.
Azevedo et al. [27] observed that apolipoprotein E COG 133 mimetic peptide also restored the intestinal proliferative activity in the 5-FU-induced mucositis. In addition, the treatment of mucositis-bearing mice with curcumin also modulated 5-FU-induced apoptosis via reduction of Bax and caspase-3 together with increase of Bcl-2 expression [28]. In another study, it was shown that treatment of mucositis-bearing mice with a molecule chemokine (C-X-C motif) ligand 9 (CXCL9), produced by interferon-stimulated mononuclear cells, was also able to attenuate the 5-FU-induced weight loss as well as capable to promote the recovery of the weight [29]. In this respect, treatment with the mucoadhesive formulation containing BP/CL up modulated Ki-67 and Bcl-2 expression while reduced pro-apoptotic regulator Bax. In parallel, the inflammatory response triggered by 5-FU was diminished by reducing 68% of myeloperoxidase activity and increasing IL-10 levels. The formulation also reduced MDA activity.
The activation of transcription nuclear factor-ĸB (NF-ĸB) exerts a critical role in the increased production of pro-inflammatory cytokines, responsible for amplification in pathological pathways of the chemotherapy-induced intestinal toxicity [30], [31]. In addition to its role in immune response, NF-ĸB is also involved in the regulation of key cellular genes of inflammation and cell proliferation signaling pathways [32]. In this respect, Bo et al. [33] showed that flavonoids from Bidens bipinnata L. also promote anti-inflammatory effects through inhibiting production of pro-inflammatory cytokines (IL-8, TNF-α) and nitric oxide levels as well as suppression of NF-κB and fractalkine expression.
Several natural products have drawn attention due to their ability to attenuate oxidative stress by inhibiting NF-ĸB activation as demonstrated by the activity of curcumin in rat liver [34]. Furthermore, the use of curcumin has been proposed in combination with anticancer treatment to prevent the chemotherapy-induced intestinal toxicity [35]. Thus, antioxidant and anti-inflammatory properties of BP or CL were, at least in part, verified here corroborated with the findings widely reported in the literature [36], [37].
Combined with their interesting protective effects, the low oral systemic toxicity of the present mucoadhesive formulation reaffirms its therapeutic potential in the treatment of intestinal mucositis in a safe manner. In vivo studies have highlighted the safe use of BP or CL in acute and subchronic toxicity studies in rodents [38], [39]. Moreover, in a clinical trial, the use of curcuminoids showed no adverse effects in patients with oral lichen planus, an autoimmune mucocutaneous condition [40]. In another clinical study, Elad et al. [16] have shown the use of a curcumin mouthwash was also safe and well-tolerated. Furthermore, Corren et al. [41] demonstrated that a botanical product containing BL did not promote laboratory abnormalities and no adverse events were reported in a clinical study. It has also been reported that BP protects normal human erythrocytes from oxidative damage [42]. Other study has highlighted that essential oil from CL did not change the hematological parameters of rats as well as any chromosome aberration, DNA damage or micronuclei were observed in their bone marrow cells [43]. In this context, no changes in the frequency of micronucleus induced by the formulation was observed.
In conclusion, this study showed important potential of the mucoadhesive formulation of BP/CL for the treatment of 5-FU-induced intestinal mucositis. Results showed that antioxidant and anti-inflammatory activities are involved in the protective pathways of BP/CL as well as their ability to modulate the proliferative and apoptotic activity of intestinal framework compared to the injury triggered by 5-FU. Given the perspectives for the development of a new medicine, clinical studies are in progress to better understand the protective effects of this innovative formulation involved in the treatment of mucositis of patients under chemotherapy.
Conflict of interest
The authors declare that there are no conflicts of interest in this study.
Transparency document
Acknowledgments
The authors acknowledge the support of Fundação de Apoio à Pesquisa da Universidade Federal de Goiás (FUNAPE), Fundo de Amparo à Pesquisa do Estado de Goiás (FAPEG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- 1.Soares P.M.G., Lima-Júnior R.C.P., Mota J.M.S.C., Justino P.F.C., Brito G.A.C., Ribeiro R.A., Cunha F.Q., Souza M.H.L.P. Inflammatory intestinal damage induced by 5-fluorouracil requires IL-4. Cytokine. 2013;61:46–49. doi: 10.1016/j.cyto.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Bowen J.M., Gibson R.J., Cummins A.G., Keefe D.M. Intestinal mucositis: the role of the Bcl-2 family: p53 and caspases in chemotherapy-induced damage. Support. Care Cancer. 2006;14:713–731. doi: 10.1007/s00520-005-0004-7. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z., Han X., Qin S., Zheng Q., Wang Z., Xiang D., Zhang J., Lu H., Wu M., Zhu S., Yu Y., Wang Y., Han W. Interleukin 1 receptor antagonist reduces lethality and intestinal toxicity of 5-fluorouracil in a mouse mucositis model. Biomed. Pharmacother. 2010;64:589–593. doi: 10.1016/j.biopha.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Xian C.J., Couper R., Howarth G.S., Read L.C., Kallincos N.S. Increased expression of HGF and c-met in rat small intestine during recovery from methotrexate-induced mucositis. Br. J. Cancer. 2000;82:945–952. doi: 10.1054/bjoc.1999.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elting L.S., Cooksley C., Chambers M., Cantor S.B., Manzullo E., Rubenstein E.B. The burdens of cancer therapy clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98:1531–1539. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 6.Valdés L.H.A.L., Rego L.H.P.L. Bidens pilosa Linné. Ver. Cubana Plant. Med. 2001;1:28–33. [Google Scholar]
- 7.Grombone-Guarantini M.T., Silva-Brandão K.L., Solferini V.N., Semir J., Trigo J.R. Sesquiterpene and polyacetylene profile of the Bidens pilosa complex (Asteraceae: Heliantheae) from Southeast of Brazil. Biochem. Syst. Ecol. 2005;33:479–486. [Google Scholar]
- 8.Chiang Y.-M., Chuang D.-Y., Wang S.-Y., Kuo Y.-H., Tsai P.-W., Shyur L.-F. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J. Ethnopharmacol. 2004;95:409–419. doi: 10.1016/j.jep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Wu J., Wan Z., Yi J., Wu Y., Peng W., Wu J. Investigation of the extracts from Bidens pilosa Linn. var. radiata Sch. Bip. for antioxidant activities and cytotoxicity against human tumor cells. J. Nat. Med. 2013;67:17–26. doi: 10.1007/s11418-012-0639-x. [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi M., Wachi H., Seyama Y. Effects of Bidens pilosa L. var. radiata Scherff: on experimental gastric lesion. J. Nat. Med. 2010;64:430–435. doi: 10.1007/s11418-010-0426-5. [DOI] [PubMed] [Google Scholar]
- 11.Ávila P.H.M., Ávila R.I., Santos Filho E.X., Bastos C.C.C., Batista A.C., Mendonça E.F., Serpa R.C., Marreto R.N., Cruz A.F., Lima E.M., Valadares M.C. Mucoadhesive formulation of Bidens pilosa L. (Asteraceae) reduces intestinal injury from 5-fluorouracil-induced mucositis in mice. Toxicol. Rep. 2015;2:563–573. doi: 10.1016/j.toxrep.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ç1kr1kç1 S., Mozioglu E., Yilmaz H. Biological activity of curcuminoids isolated from Curcuma longa. Rec. Nat. Prod. 2008;2:19–24. [Google Scholar]
- 13.López-Lázaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008;52:103–127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 15.Rezvani M., Ross C.A. Modification of radiation-induced acute oral mucositis in the rat. Int. J. Radiat. Biol. 2004;80:177–182. doi: 10.1080/09553000310001654693. [DOI] [PubMed] [Google Scholar]
- 16.Elad S., Meidan I., Sellam G., Simaan S., Zeevi I., Waldman E., Weintraub M., Revel-Vilk S. Topical curcumin for the prevention of oral mucositis in pediatric patients: case series. Altern. Ther. Health Med. 2013;19:21–24. [PubMed] [Google Scholar]
- 17.Santos Filho E.X., Ávila P.H.M., Bastos C.C.C., Batista A.C., Naves L.N., Marreto R.N., Lima E.M., Mendonça E.F., Valadares M.C. Curcuminoids from Curcuma longa L. reduced intestinal mucositis induced by 5-fluorouracil in mice: bioadhesive, proliferative, anti-inflammatory and antioxidant effects. Toxicol. Rep. 2015 doi: 10.1016/j.toxrep.2015.10.010. (Article accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagpal M., Sood S. Role of curcumin in systemic and oral health: an overview. J. Nat. Sci. Biol. Med. 2013;4:3–7. doi: 10.4103/0976-9668.107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadi-Samani S., Bahri-Najafi R., Yousefi G. Formulation and in vitro evaluation of prednisolone buccoadhesive tablets. Farmaco. 2005;60:339–344. doi: 10.1016/j.farmac.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Hubrecht R., Kirkwood J. 8th ed. Wiley-Blackwell; United Kingdom: 2010. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. [Google Scholar]
- 21.Victoni T. Dissertação (Mestrado) Universidade de São Paulo; São Paulo: 2008. Avaliação dos mecanismos indutores da inflamação pulmonar decorrente da isquemia e reperfusão intestinal em camundongos geneticamente selecionados. [Google Scholar]
- 22.Marques L.H.S., Silva C.M.G., Lameiro T.M.M., Almeida M.G., Cunha F.L., Pereira J.A., Martinez C.A.R. Avaliação dos níveis de peroxidação lipídica em células da mucosa cólica após aplicação de enemas com peróxido de hidrogênio. Estudo experimental em ratos. Rev. Bras. Colo-proctol. 2010;30:272–280. [Google Scholar]
- 23.OECD (Organization for Economic Cooperation and Development), Acute oral toxicity: fixed dose procedure, Guideline for The Testing of Chemicals. n.420, 2001.
- 24.Scully C., Epstein J., Sonis S. Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Part 2: diagnosis and management of mucositis. Head Neck. 2004;26:77–84. doi: 10.1002/hed.10326. [DOI] [PubMed] [Google Scholar]
- 25.Reinke D., Kritas S., Polychronopoulos P., Skaltsounis A.L., Aligiannis N., Tran C.D. Herbal substance, acteoside, alleviates intestinal mucositis in mice. Gastroenterol. Res. Pract. 2015;2015 doi: 10.1155/2015/327872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho F.C., Bruschi M.L., Evangelista R.C., Gremião M.P. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010;46:1–17. [Google Scholar]
- 27.Azevedo O.G.R., Oliveira R.A.C., Oliveira B.C., Zaja-Milatovic S., Araújo C.V., Wong D.V.T., Costa T.B., Lucena H.B.M., Lima-Júnior R.C.P., Ribeiro R.A., Warren C.A., Lima A.A.M., Vitek M.P., Guerrant R.L., Oriá R.B. Apolipoprotein E COG 133 mimetic peptide improves 5-fluorouracil-induced intestinal mucositis. BMC Gastroenterol. 2012;12 doi: 10.1186/1471-230X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao Q., Ye X., Wang L., Gu J., Fu T., Wang Y., Lai Y., Wang Y., Wang X., Jin H., Guo Y. Protective effect of curcumin on chemotherapy-induced intestinal dysfunction. Int. J. Clin. Exp. Pathol. 2013;6:2342–2349. [PMC free article] [PubMed] [Google Scholar]
- 29.Han X., Wu Z., Di J., Pan Y., Zhang H., Du Y., Cheng Z., Jin Z., Wang Z., Zheng Q., Zhang P., Wang Y. CXCL9 attenuated chemotherapy-induced intestinal mucositis by inhibiting proliferation and reducing apoptosis. Biomed. Pharmacother. 2011;65:547–554. doi: 10.1016/j.biopha.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Logan R.M., Stringer A.M., Bowen J.M., Yeoh A.S., Gibson R.J., Sonis S.T., Keefe D.M. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 2007;33:448–460. doi: 10.1016/j.ctrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Sonis S.T. The biologic role for nuclear factor-jB in disease and its potential involvement in mucosal injury associated with antineoplastic therapy. Crit. Rev. Oral Biol. Med. 2002;13:380–390. doi: 10.1177/154411130201300502. [DOI] [PubMed] [Google Scholar]
- 32.Hoesel B., Schmid J.A. The complexity of NF-ĸB signaling in inflammation and cancer. Mol. Cancer. 2013;12 doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bo Y., Yuan L.P., Zhang J.J., Meng D.D., Jing H., Dai H.J. Total flavonoids of Bidens bipinnata L. a traditional chinese medicine inhibits the production of inflammatory cytokines of vessel endothelial cells stimulated by sera from Henoch-Schönlein purpura patients. J. Pharm. Pharmacol. 2012;64:882–887. doi: 10.1111/j.2042-7158.2012.01480.x. [DOI] [PubMed] [Google Scholar]
- 34.Chuang S.E., Cheng A.L., Lin J.K., Kuo M.L. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem. Toxicol. 2000;38:991–995. doi: 10.1016/s0278-6915(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 35.van't Land B., Blijlevens N.M., Marteijn J., Timal S., Donnelly J.P., de Witte T.J., M'Rabet L. Role of curcumin and inhibition of NF-KappaB in the onset of chemotherapy-induced mucosal barrier injury. Leukemia. 2004;18:276–284. doi: 10.1038/sj.leu.2403233. [DOI] [PubMed] [Google Scholar]
- 36.Bartolome A.P., Villaseñor I.M., Yang W.-C. Bidens pilosa L. (Asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. Evid. Based Complement. Alternat. Med. 2013;2013 doi: 10.1155/2013/340215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramadan G., Al-Kahtani M.A., El-Sayed W.M. Anti-inflammatory and anti-oxidant properties of Curcuma longa (turmeric) versus Zingiber officinale (ginger) rhizomes in rat adjuvant-induced arthritis. Inflammation. 2011;34:291–301. doi: 10.1007/s10753-010-9278-0. [DOI] [PubMed] [Google Scholar]
- 38.Liju V.B., Jeena K., Kuttan R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L.) Food Chem. Toxicol. 2013;53:52–61. doi: 10.1016/j.fct.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Yang W.-C. Botanical pharmacological phytochemical and toxicological aspects of the antidiabetic plant Bidens pilosa L. Evid. Based Complement. Alternat. Med. 2014;2014 doi: 10.1155/2014/698617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chainani-Wu N., Madden E., Lozada-Nur F., Silverman S. High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J. Am. Acad. Dermatol. 2012;66:752–760. doi: 10.1016/j.jaad.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Corren J., Lemay M., Lin Y., Rozga L., Randolph R.K. Clinical and biochemical effects of a combination botanical product (ClearGuard) for allergy: a pilot randomized double-blind placebo-controlled trial. Nutr. J. 2008;7 doi: 10.1186/1475-2891-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H.L., Chen S.C., Chang N.W., Chang J.M., Lee M.L., Tsai P.C., Fu H.H., Kao W.W., Chiang H.C., Wang H.H., Hseu Y.C. Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food Chem. Toxicol. 2006;44:1513–1521. doi: 10.1016/j.fct.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Liju V.B., Jeena K., Kuttan R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L.) Food Chem. Toxicol. 2013;53:52–61. doi: 10.1016/j.fct.2012.11.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.