Abstract
Lymphatic malformations (LMs; especially those involving the central conducting lymphatic channels) are characterized by dysplastic and incompetent lymphatic channels in multiple tissues and organs. The major cause of mortality and morbidity in patients with thoracic LM is deterioration of pulmonary function due to chronic chylous effusions and progressive interstitial lung disease. The etiology of these pulmonary processes is unknown, although lymphatic involvement is certain. Understanding of the changes in the lymphatic anatomy in patients with LM has been hindered by difficulty of imaging of the lymphatic system. Recently developed dynamic contrast-enhanced magnetic resonance lymphangiography (DCMRL) allows dynamic MR imaging of the lymphatic system by injecting gadolinium contrast agent in the groin lymph nodes. Using this technique, pathological lymphatic flow from the central lymphatic system and/or retroperitoneal and mediastinal masses into lung parenchyma (“pulmonary lymphatic perfusion syndrome”) has been demonstrated in patients with LM. This abnormal lymphatic perfusion overflows pulmonary parenchyma and results in deterioration of pulmonary function due to interstitial process and/or compression effect of chylous effusions. Percutaneous thoracic duct embolization or lymphatic interstitial embolization of the lymphatic masses results in cessation of the pulmonary lymphatic overflow and significant improvement in pulmonary symptoms in these patients.
Keywords: lymphangiography, lymphatic malformations, embolization, pulmonary function, interventional radiology
Objectives : Upon completion of this article, the reader will be able to identify that pulmonary symptoms of the patients with lymphatic malformations are result of the abnormal pulmonary lymphatic flow from the thoracic duct and retroperitoneal masses. Cessation of this abnormal lymphatic flow using embolization techniques can result in improvement of the patient symptoms.
Accreditation : This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Lymphatic malformations (LMs) encompass a group of developmental anomalies including cystic LM, conductive lymphatic disorders, generalized lymphatic anomaly (GLA), and Gorham-Stout disease (GSD), as well as a proliferative disorder, kaposiform lymphangiomatosis (KLA). 1 These conditions range in severity and extent, and are characterized by the presence of abnormal lymphatic tissue in multiple organs, including lungs, soft tissue, and bones. 2
The main cause of morbidity and mortality in patients with thoracic LM or KLA is deterioration of pulmonary function, due to chylothorax and interstitial lung disease (Cameron Trenor, 2nd International Conference on Generalized Lymphatic Anomaly and Gorham-Stout Disease, Atlanta, GA). The mechanism by which LM cause these pathological processes is unknown.
Methods of imaging the central lymphatic system have lagged behind vascular imaging, primarily due to difficulty in delivering contrast material into the lymphatic vessels. Until recently, the only two contrast-enhanced imaging methods of the lymphatic system were direct cannulation of the lymphatic vessels (pedal lymphangiography) and interstitial injection of contrast agents that are absorbed into the lymphatic system (lymphoscintigraphy and lower extremities magnetic resonance [MR] lymphangiography). 3 Pedal lymphangiography, however, is technically tedious, is associated with a significant number of complications, and is limited in its ability to image the central lymphatic system. Lymphoscintigraphy, on the other hand, has poor anatomical resolution; however, it can provide some information regarding flow in the lymphatic system.
The introduction of intranodal lymphangiography (IL) a few years ago, which is based on simple ultrasound (US)-guided access of inguinal lymph nodes using a small gauge needle, allowed any interventionalist to perform lymphangiography and image the lymphatic system. 4 This technique further broke the barrier to entry to perform image-guided lymphatic interventional procedures, such as thoracic duct embolization (TDE). 5
Application of a similar intranodal contrast delivery technique as for MR imaging resulted in the development of the dynamic contrast MR lymphangiography (DCMRL). DCMRL is a novel MR technique in which gadolinium-based contrast material is injected into the groin lymph nodes through 25-G needles positioned under US and fluoroscopy guidance. 6 MR is then used to track the advancement of the contrast through the central lymphatic system. This technique allows dynamic imaging of the flow in the lymphatic system as well as imaging of the lymphatic masses and vessels ( Fig. 1 ).
Fig. 1.
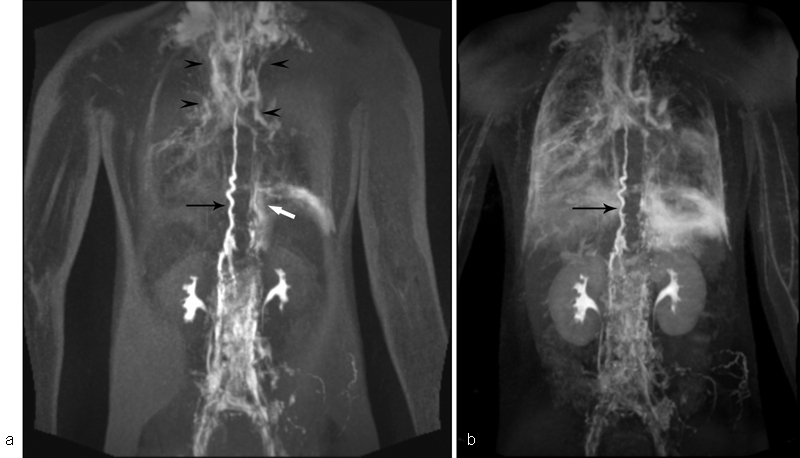
DCRML of the patient with KL and progressive deterioration of pulmonary function. ( a ) Selected image of the dynamic part of the DCMRL demonstrated dilated and tortuous TD (black arrow) and abnormal pulmonary lymphatic flow that originates from the upper part of the TD (black arrowheads) and from the left retroperitoneal mass (white arrow). ( b ) Delayed, high-resolution part of the DCRML, demonstrating dilated and tortuous TD (black arrow) and lymphatic perfusion of the lungs and mediastinum.
Over the past 3 years, the Lymphatic Imaging and Interventions Program at HUP/CHOP utilized DCMRL to demonstrate that there is a pathological pulmonary lymphatic flow from the lymphatic branches that originate in TD or retroperitoneum toward pulmonary parenchyma in patients with LM who have chylothorax. Similar findings were described in idiopathic chylothorax, plastic bronchitis, and congenital lymphatic dysplasia and was termed pulmonary lymphatic perfusion syndrome (PLPS). The anatomical substrate of PLPS is a congenital malformation of the thoracic lymphatic ducts and retroperitoneal and mediastinal lymphatic masses. 7 8 9
Magnetic Resonance Imaging Technique
Imaging and interventions are performed in an XMR suite that combines an MR scanner with a cardiac catheterization laboratory (Siemens, Erlangen, Germany). The main feature of this system is the operational table that moves between the catheterization laboratory and MR scanner. This table allows the transfer of patients between the two units with minimal patient movements, thus preventing dislodgement of the needle positioned in the inguinal lymph nodes (see below). All procedures are performed under general anesthesia in children and under local anesthesia in adults. Initially, the patient is positioned on the table in the catheterization laboratory and the inguinal lymph nodes are accessed under ultrasound guidance, using a 25-gauge spinal needle (BD Medical, Franklin Lakes, NJ) attached to a short connector tubing (BD Medical). A small amount of water-soluble contrast agent (Omnipaque, GE Healthcare) is injected under fluoroscopy guidance to confirm the correct position of the needles inside the lymph nodes. To minimize needle movement, the needles are fixed to the skin with Tegaderm (3M, St. Paul, MN). After stabilizing the needle, the patients are transported to the adjacent MR suite equipped with a 1.5-T Siemens Magnetom Avanto scanner (Siemens, Erlangen, Germany) for heavy T2W and DCMRL imaging.
Heavy T2W Imaging
Heavy T2W MR imaging is very similar to MRCP and MR urography techniques and is exceptionally sensitive for detection of fluid-filled lymphatic structures. This technique has been explored in the past to visualize the anatomy of the TD and its tributaries. 10 11 However, it is also very sensitive in the identification of the fluid-filled soft tissue and bone lymphatic masses and areas of lymphangiectasia. Dori et al used this technique to identify the size of the TD and lymphangiectasia in patients with significant right-sided heart failure. 12 Using T2W, Malone et al were able to demonstrate dilation of the TD and widespread dilation of the aberrant retroperitoneal and mediastinal lymphatic ducts in patients with lymphangiectasia. 13
Heavy T2W MR lymphatic imaging is performed using a respiratory-navigated and cardiac-gated three-dimensional (3D) turbo spin echo sequence. Scan time varies from 2 to 5 minutes depending on the size of the patient. 12
Dynamic Contrast MR Lymphangiography
Following completion of the heavy T2W lymphatic imaging (see above), weight-based dose of undiluted gadopentetate dimeglumine (Gadavist, Bayer Healthcare Pharmaceuticals Inc., Wayne, NJ) is injected by hand simultaneously into each LN at a rate of 0.5 to 1 mL/min. Two types of the DCRML imaging are performed: dynamic over the period of 10 to 15 minutes and delayed, high-resolution at the end of the study.
Dynamic imaging is started 1 minute after the injection, using high spatial and temporal resolution MR angiography (syngo time-resolved angiography with stochastic trajectories [TWIST]) sequence. The sequence parameters are adjusted with a time delay such that a complete volume is acquired approximately every 20 to 60 seconds. This is followed by delayed high-resolution imaging that utilizes a high-resolution navigator-gated 3D flash IR sequence. In all patients, the scan area covered the neck, chest, and upper- to mid-abdomen.
Imaging Findings on MR Lymphangiography in Patients with LM
Utilizing T2W MR lymphangiography, we are able to demonstrate the abnormal lymphatic masses in the typical lymphatic pathways, pelvis, retroperitoneum, mesentery, and mediastinum.
The lymphatic involvement of the other organs, such as bones, liver spleen, and intestine outside the lymphatic system, allows for differentiation between lymphatic conductive disorders (lymphangiectasia) and LM that involve multiple organs, such as KL, GLA, and GSD ( Fig. 2 ). Retroperitoneal and mediastinal masses can be a potential source of the chylous leaks in patients with chylothorax and chylous ascites. T2W MR is also exceptionally sensitive for identifying and quantifying lymphatic effusions. One of the important findings is the presence of increased interstitial lung markings, which often reflects the dilated lymphatic vessels in the lung parenchyma due to PLPS ( Fig. 3 ).
Fig. 2.
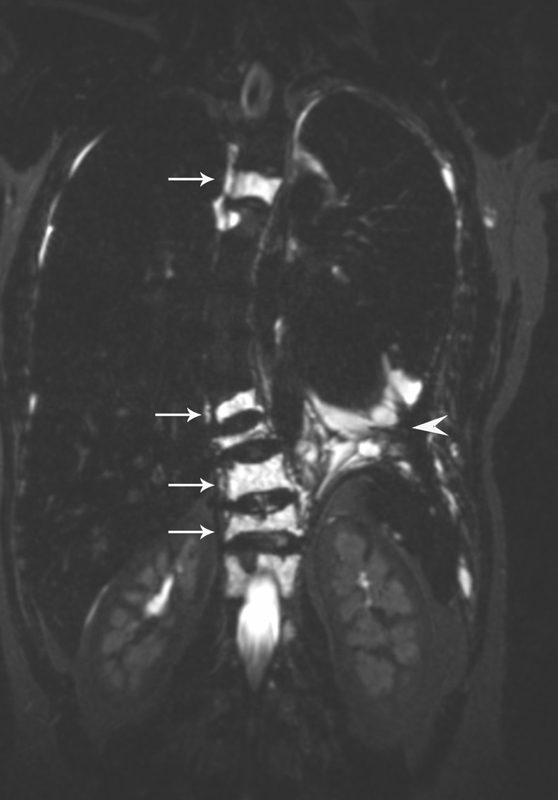
Heavy T2W imaging of the chest of the patient with GLA and vertebral compression fracture demonstrating lymphatic masses in the vertebrae (white arrows) and small amount of pleural effusion (white arrowhead).
Fig. 3.
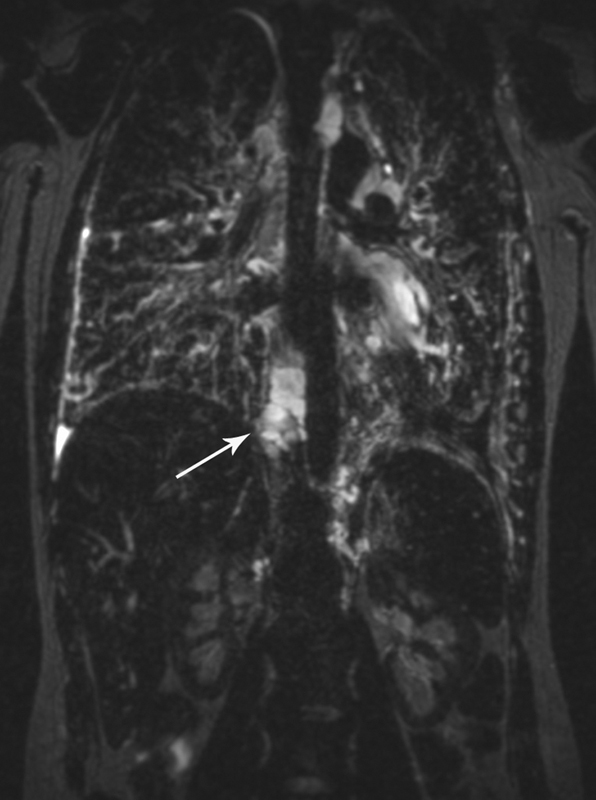
Heavy T2W imaging of the chest of the patient with GLA and progressive deterioration of pulmonary function, demonstrating dilated TD duct (white arrow) and increased T2 signal of the lung interstitium.
As mentioned earlier, DCRML is composed of dynamic and high-resolution imaging components. In the dynamic part of study (TWIST), the dynamic acquisition of images over a period of 10 to 15 minutes is performed, generating the map of the lymphatic flow. Understanding the flow patterns of the lymph allows for determination of the pathway and the sources of pathological lymphatic perfusion of the lung parenchyma and mediastinum ( Fig. 1 ). It is critically important to understand if the abnormal pulmonary lymphatic perfusion originates from the TD or from retroperitoneal/mediastinal masses (see below; Figs. 4 and 5 ). This abnormal lymphatic flow is a cause of chylothorax and deterioration of the pulmonary function, due to progressive interstitial lung disease. Delayed high-definition imaging (3D flash IR) further allowed us to confirm and understand the extent of the PLPS.
Fig. 4.
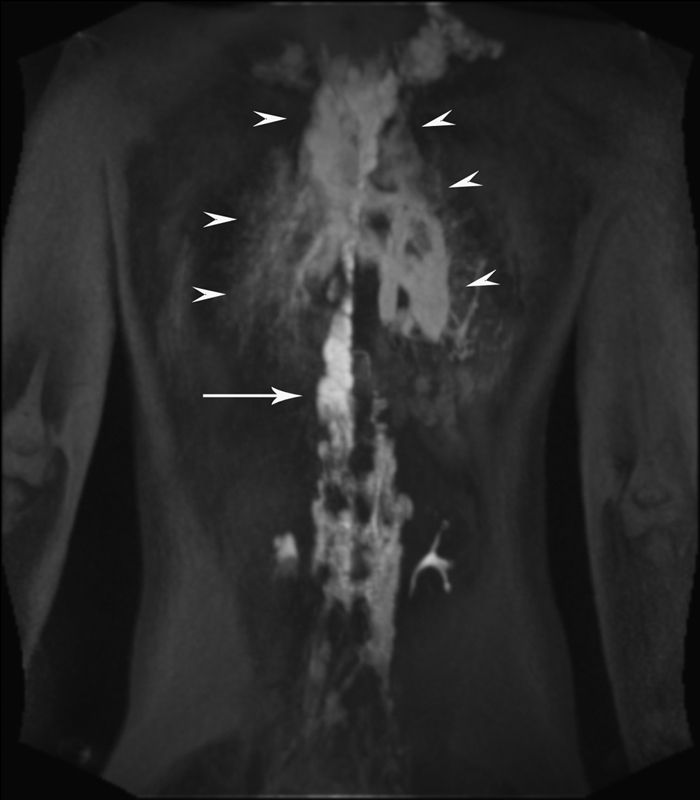
DCRML imaging of the patient with GLA, and progressive deterioration of pulmonary function and hemoptysis demonstrated dilated TD (white arrow) and abnormal pulmonary lymphatic perfusion that originates in the distal TD toward lung parenchyma (white arrowheads).
Fig. 5.
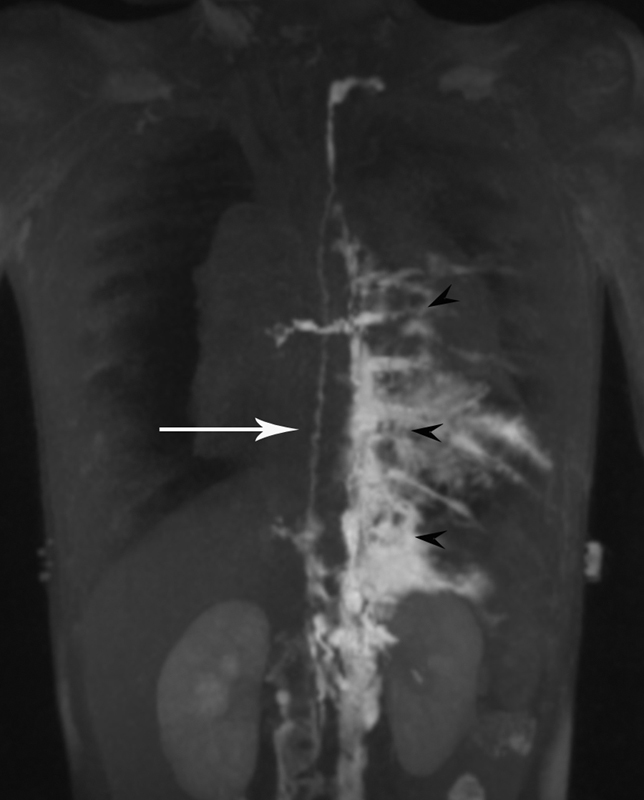
DCRML imaging of the patient with GLA and bilateral pleural effusion demonstrates normal size TD (white arrow) and abnormal pulmonary lymphatic perfusion that originates in the left retroperitoneum and extends into the mediastinum and left pleural cavity (black arrowheads).
In addition, the TD is commonly dilated and tortuous on DCRML, a sign of increased flow in the lymphatic system ( Fig. 4 ).
Flow Physiology of the Lymphatic System in Patients with LM
Eighty to ninety percent of the lymph in the body is generated below the diaphragm in the liver and intestinal lymphatic systems. 14 Under normal conditions, abdominal and lower body lymph is collected in the area of cisterna chyli and then is channeled to the TD that crosses into the mediastinum and ultimately drains lymph into the subclavian vein. PLPS is a syndrome that was described in several conditions such as cardiac and lymphatic plastic bronchitis and neonatal chylothorax. 8 15 16 In these conditions, there is an abnormal pulmonary lymphatic flow that caries the lymph from the TD toward lung parenchyma. Clinical presentations of PLPS depend on the type of lung surface that is in proximity to the abnormal lymphatic ducts. If the closest surface to the lymphatic vessels is bronchial mucosa, the clinical presentation of PLPS is plastic bronchitis; if the surface is visceral, parietal pleura, or pericardium, the presentation will be chylothorax or chylopericardium.
The anatomical substrate of PLPS is probably a congenital lymphatic variant, and can present clinically spontaneously (neonatal chylothorax), or as a result of injury to the lung surface such as trauma (postcardiac surgery chylothorax) or a sequela of severe upper respiratory disease (lymphatic plastic bronchitis) or result from significant increase of the flow of the lymphatic system in CHF (cardiac plastic bronchitis). One of the most prominent findings on DCRML in most patients with LM is a significant dilation and tortuosity of the TD ( Figs. 1 and 4 ). This finding indicates that the lymphatic flow in patients with LM is significantly increased. We hypothesize that the pulmonary symptoms in patients with LM are a result of a combination of the congenitally abnormal pulmonary lymphatic anatomy and significant increase in the lymphatic production that result in overdistention of these abnormal lymphatic vessels and weeping or leakage of the lymph.
Treatment Algorithm for Pulmonary Presentation of the LM
The treatment goal in these patients is an interruption of the abnormal pulmonary lymphatic flow from the abdomen toward the lungs, thus improving pulmonary symptoms and potentially increasing the life expectancy. To achieve this goal, we utilize two embolization techniques: TDE and lymphatic interstitial embolization (LIE). 17
The choice of techniques is dictated by the imaging findings on the DCMRL and IL. If abnormal pulmonary lymphatic flow originates from the TD, TDE would resolve PLPS. If the abnormal pulmonary flow originates from the retroperitoneal and mediastinal lymphatic masses, LIE of the lymphatic masses would stop the lymphatic flow.
In some cases, identification of the source of the abnormal pulmonary lymphatic flow—TD versus retroperitoneal masses—is difficult even on DCMRL. Embolization of normal TD that serves as a main conduit of lymphatic drainage into the venous system in patients with leakage from retroperitoneal or mediastinal lymphatic masses can be devastating. For that reason, in cases where the source of the abnormal pulmonary flow is uncertain, temporary occlusion and/or diversion of the flow from the TD is performed. In these cases, a 4- to 5F vascular sheath is placed through the left arm vein retrograde into TD, with the tip of the sheath positioned in the proximal TD close to cisterna chyli. The TD can then be occluded temporarily for the period of a few days to 1 week using the occlusion balloon. If this temporary occlusion is successful in resolution of the pleural effusion or improvement of the pulmonary function, TDE can be performed through the same vascular sheath. In cases of deterioration of the pulmonary function, TDE should be avoided and LIE should be attempted.
Both TDE and LIE start with IL to identify the anatomical landmarks that were identified on the DCMRL. The IL is performed as described by Nadolski and Itkin. 4
The technique of the TDE has been described extensively in the literature and generally is not different from the one that is performed for traumatic chylothorax. 18 In brief, after identification of the target lymphatic vessel in the abdomen (the cisterna chyli or its lumbar tributaries), it is accessed transabdominally using a 21- to 22-G Chiba needle. Microwire is then advanced into the TD, followed by a microcatheter. The microcatheter is then injected with water-soluble contrast to confirm the lymphatic anatomy ( Fig. 6 ). After identification of the anatomy of the TD and the source of the abnormal pulmonary lymphatic flow, we perform the embolization of the TD using a combination of the n-butyl cyanoacrylate glue (Trufill; Cordis Corporation, Warren, NJ) and endovascular coils.
Fig. 6.
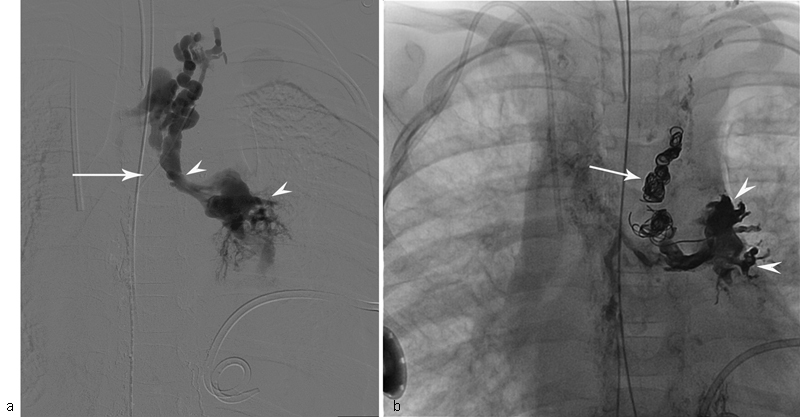
Fluoroscopic image of the injection of the contrast into TD through microcatheter (white arrow) of the patient with KL, and progressive deterioration of pulmonary function and left pleural effusion. ( a ) Image demonstrates abnormal pulmonary lymphatic flow from the TD into the lung parenchyma (white arrowheads). ( b ) Fluoroscopic image of the same patient shows endovascular coils (white arrow) and glue cast (white arrowheads) in the pulmonary lymphatics.
The technique of LIE has been recently described and is used in cases where catheterization of the lymphatic vessels is not possible due to their small size. 17 19 20 21 After identification of the target that is either lymph node or in the lymphatic mass using IL and fluoroscopic guidance, a 25- to 22-G needle is positioned percutaneously using fluoroscopy guidance. Water-soluble contrast is then injected to confirm proper position of the needle, followed by lipiodol to evaluate the extent of the flow. The needle and the mass are then flushed with DW5 and nBCA glue diluted 1:2 to 1:4 with lipiodol injected into the mass ( Fig. 7 ). In most of the cases, several LIEs need to be performed in different lymphatic areas.
Fig. 7.
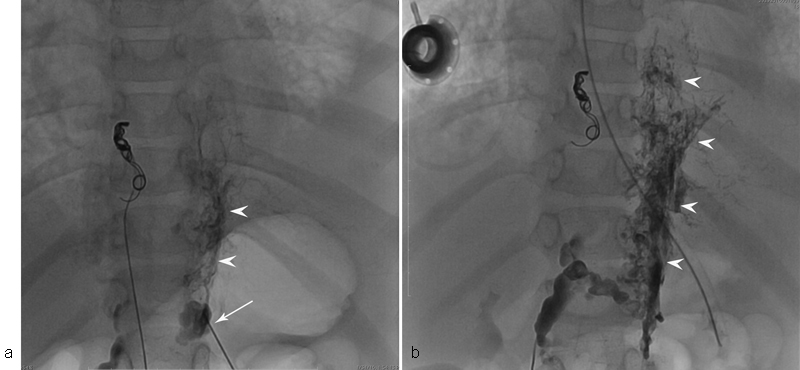
( a ) Fluoroscopic image of the patient with KL and progressive deterioration of pulmonary function demonstrates opacification of the retroperitoneal lymphatic masses (white arrowheads) opacified through 22-G needle (white arrow). ( b ) Glue cast in the retroperitoneal lymphatic mass (white arrowheads) immediately after glue injection.
Extravasation of the glue in the tissue is common; however, usually it has no clinical consequences. nBCA glue is very irritating to the lymphatic system and the patient has significant pain after the procedure for the period of 2 to 3 days.
Conclusion
Malformations of the central lymphatic conducting channels can involve the pulmonary system, leading to pulmonary lymphedema, plastic bronchitis, or chylothorax. This pulmonary involvement in turn is associated with significant mortality and morbidity. The main cause of this pulmonary deterioration is abnormal pulmonary lymphatic flow from the TD and retroperitoneal and mediastinal masses toward lung parenchyma. Embolization of these networks results in significant improvement of the pulmonary function and can potentially result in reduction of the mortality and morbidity.
References
- 1.Wassef M, Blei F, Adams D et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136(01):e203–e214. doi: 10.1542/peds.2014-3673. [DOI] [PubMed] [Google Scholar]
- 2.Faul J L, Berry G J, Colby T Vet al. Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome Am J Respir Crit Care Med 2000161(3, Pt 1):1037–1046. [DOI] [PubMed] [Google Scholar]
- 3.Mitsumori L M, McDonald E S, Wilson G J, Neligan P C, Minoshima S, Maki J H. MR lymphangiography: how I do it. J Magn Reson Imaging. 2015;42(06):1465–1477. doi: 10.1002/jmri.24887. [DOI] [PubMed] [Google Scholar]
- 4.Nadolski G J, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23(05):613–616. doi: 10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 5.Kerlan R K, Jr, Laberge J M. Intranodal lymphangiography: coming soon to a hospital near you. J Vasc Interv Radiol. 2012;23(05):617. doi: 10.1016/j.jvir.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Dori Y, Zviman M M, Itkin M. Dynamic contrast-enhanced MR lymphangiography: feasibility study in swine. Radiology. 2014;273(02):410–416. doi: 10.1148/radiol.14132616. [DOI] [PubMed] [Google Scholar]
- 7.Dori Y, Keller M S, Rychik J, Itkin M. Successful treatment of plastic bronchitis by selective lymphatic embolization in a Fontan patient. Pediatrics. 2014;134(02):e590–e595. doi: 10.1542/peds.2013-3723. [DOI] [PubMed] [Google Scholar]
- 8.Gray M, Kovatis K Z, Stuart T et al. Treatment of congenital pulmonary lymphangiectasia using ethiodized oil lymphangiography. J Perinatol. 2014;34:720–722. doi: 10.1038/jp.2014.71. [DOI] [PubMed] [Google Scholar]
- 9.Itkin M. Interventional treatment of pulmonary lymphatic anomalies. Tech Vasc Interv Radiol. 2016;19(04):299–304. doi: 10.1053/j.tvir.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Okuda I, Udagawa H, Takahashi J, Yamase H, Kohno T, Nakajima Y. Magnetic resonance-thoracic ductography: imaging aid for thoracic surgery and thoracic duct depiction based on embryological considerations. Gen Thorac Cardiovasc Surg. 2009;57(12):640–646. doi: 10.1007/s11748-009-0483-4. [DOI] [PubMed] [Google Scholar]
- 11.Erden A, Fitoz S, Yagmurlu B, Erden I. Abdominal confluence of lymph trunks: detectability and morphology on heavily T2-weighted images. AJR Am J Roentgenol. 2005;184(01):35–40. doi: 10.2214/ajr.184.1.01840035. [DOI] [PubMed] [Google Scholar]
- 12.Dori Y, Keller M S, Fogel M A et al. MRI of lymphatic abnormalities after functional single-ventricle palliation surgery. AJR Am J Roentgenol. 2014;203(02):426–431. doi: 10.2214/AJR.13.11797. [DOI] [PubMed] [Google Scholar]
- 13.Malone L J, Fenton L Z, Weinman J P, Anagnost M R, Browne L P. Pediatric lymphangiectasia: an imaging spectrum. Pediatr Radiol. 2015;45(04):562–569. doi: 10.1007/s00247-014-3191-x. [DOI] [PubMed] [Google Scholar]
- 14.Brauer R W. Liver circulation and function. Physiol Rev. 1963;43:115–213. doi: 10.1152/physrev.1963.43.1.115. [DOI] [PubMed] [Google Scholar]
- 15.Dori Y, Keller M S, Rome J J et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016;133(12):1160–1170. doi: 10.1161/CIRCULATIONAHA.115.019710. [DOI] [PubMed] [Google Scholar]
- 16.Itkin M G, McCormack F X, Dori Y. Diagnosis and treatment of lymphatic plastic bronchitis in adults using advanced lymphatic imaging and percutaneous embolization. Ann Am Thorac Soc. 2016;13(10):1689–1696. doi: 10.1513/AnnalsATS.201604-292OC. [DOI] [PubMed] [Google Scholar]
- 17.Itkin M. Lymphatic intervention techniques: look beyond thoracic duct embolization. J Vasc Interv Radiol. 2016;27(08):1187–1188. doi: 10.1016/j.jvir.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Itkin M, Krishnamurthy G, Naim M Y, Bird G L, Keller M S. Percutaneous thoracic duct embolization as a treatment for intrathoracic chyle leaks in infants. Pediatrics. 2011;128(01):e237–e241. doi: 10.1542/peds.2010-2016. [DOI] [PubMed] [Google Scholar]
- 19.Baek Y, Won J H, Chang S J et al. Lymphatic embolization for the treatment of pelvic lymphoceles: preliminary experience in five patients. J Vasc Interv Radiol. 2016;27(08):1170–1176. doi: 10.1016/j.jvir.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Chick J FB, Reddy S N, Nadolski G J, Dori Y, Itkin M. Single-session endolymphatic glue embolization of lymphocele after heart transplantation. J Vasc Interv Radiol. 2016;27(06):929–930. doi: 10.1016/j.jvir.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Hur S, Shin J H, Lee I J et al. Early experience in the management of postoperative lymphatic leakage using lipiodol lymphangiography and adjunctive glue embolization. J Vasc Interv Radiol. 2016;27(08):1177–11860. doi: 10.1016/j.jvir.2016.05.011. [DOI] [PubMed] [Google Scholar]


