Abstract
Background
Xylan, the major constituent of hemicellulose, is composed of β-(1,4)-linked xylopyranosyl units that for the backbone, with side chains formed by other chemical moieties such as arabinose, galactose, mannose, ferulic acid and acetyl groups. Acetyl xylan esterases and α-l-arabinofuranosidases are two important accessory enzymes that remove side chain residues from xylan backbones and may act in synergy with other xylanolytic enzymes. Compared with enzymes possessing a single catalytic activity, multifunctional enzymes can achieve lignocellulosic biomass hydrolysis using a less complex mixture of enzymes.
Results
Here, we cloned an acetyl xylan esterase (PcAxe) from Penicillium chrysogenum P33 and expressed it in Pichia pastoris GS115. The optimal pH and temperature of the recombinant PcAxe (rPcAxe) for 4-nitrophenyl acetate were 7.0 and 40 °C, respectively. rPcAxe is stable across a broad pH range, retaining 100% enzyme activity om pH 6–9 after a 1 h incubation. The enzyme tolerates the presence of a wide range of metal ions. Sequence alignment revealed a GH62 domain exhibiting α-l-arabinofuranosidase activity with pH and temperature optima of pH 7.0 and 50 °C, in addition to the expected esterase domain. rPcAxe displayed significant synergy with a recombinant xylanase, with a degree of synergy of 1.35 for the hydrolysis of delignified corn stover. Release of glucose was increased by 51% from delignified corn stover when 2 mg of a commercial cellulase was replaced by an equivalent amount of rPcAxe, indicating superior hydrolytic efficiency.
Conclusions
The novel bifunctional enzyme PcAxe was identified in P. chrysogenum P33. rPcAxe includes a carbohydrate esterase domain and a glycosyl hydrolase family 62 domain. This is the first detailed report on a novel bifunctional enzyme possessing acetyl xylan esterase and α-l-arabinofuranosidase activities. These findings expand our current knowledge of glycoside hydrolases and pave the way for the discovery of similar novel enzymes.
Electronic supplementary material
The online version of this article (doi:10.1186/s12934-017-0777-7) contains supplementary material, which is available to authorized users.
Keywords: Penicillium chrysogenum, Acetyl xylan esterase, α-l-Arabinofuranosidase, Hydrolysis, Synergy
Background
Xylan, the major constituent of hemicellulose, is composed of β-(1,4)-linked xylopyranosyl units that for the backbone, with side chains formed by other chemical moieties such as arabinose, galactose, mannose, ferulic acid and acetyl groups [1]. Because of the structural diversity and complexity of xylan, its complete hydrolysis requires a combination of main chain degrading enzymes including xylanases and β-xylosidases, and side chain degrading enzymes comprising α-l-arabinofuranosidases, acetyl xylan esterases, feruloyl esterases and α-glucuronidases [2, 3]. Among these accessory enzymes, acetyl xylan esterases catalyze the cleavage of acetyl substituents from acetylated xylan, and α-l-arabinofuranosidases hydrolyze the glycosidic bonds that link α-l-arabinofuranoyl side chains to the backbone. Therefore, acetyl xylan esterases and α-l-arabinofuranosidases are two important accessory enzymes that remove side chain residues from the hemicellulose backbone and function synergistically with other xylanolytic enzymes [4, 5]. These well-characterized degradative enzymes usually only catalyze a certain reaction.
In order to achieve efficient hydrolysis of lignocellulose, a series of enzymes are required to work in combination, which increases the cost of enzymes used in a biotechnological context. Compared with enzymes possessing a single function, multifunctional enzymes can hydrolyze a variety of different substrates simultaneously, which reduces the amount of enzyme types needed for the hydrolysis of lignocellulose, decreases their cost in biotechnological processes, and has additional advantages. In this sense, multifunctional enzymes are more intriguing than traditional enzymes, especially those involved in heteroxylan hydrolysis [6, 7]. β-d-Xylosidase/α-l-arabinofuranosidase [8], xylanase/α-l-arabinofuranosidase [5, 6] and xylanase/acetyl xylan esterase [9–12] bifunctional systems have been reported. Although the bifunctional α-l-arabinofuranosidase/acetyl xylan esterase was reported in Arthrobacter sp. MTCC5214 and Lactobacillus sp. based on the elution profile and zymogram analysis [13], a bifunctional enzyme possessing acetyl xylan esterase and α-l-arabinofuranosidase activities has not been studied in detail, and would be potentially useful for hydrolyzing lignocellulose.
Aspergillus and Trichoderma are two fungal genera that have been widely studied due to their cellulase and hemicellulase activities [14–16]. By contrast, research on P. chrysogenum is lacking. The cellulase, xylanase and mannanase activities of P. chrysogenum have received some attention [17, 18], but there has been no report of an acetyl xylan esterase from this fungus. We previously isolated the P. chrysogenum P33 strain, which is able to degrade lignocellulosic biomass efficiently, and secrete abundant acetyl xylan esterases (unpublished data). In the present study, a bifunctional enzyme from P33 displaying both acetyl xylan esterase and α-l-arabinofuranosidase activities was cloned and expressed in Pichia pastoris GS115, and its enzymatic characteristics studied for the first time. The bifunctional enzyme exhibited significant synergy with xylanase, and promoted the hydrolysis of cellulose.
Methods
Strains, culture conditions and plasmids
The P. chrysogenum P33 (CGMCC 3.15539) used in this study has been maintained in our laboratory since its discovery. Stock cultures were stored at 4 °C on potato dextrose agar (PDA) slants. For enzyme production, the strain was grown in modified Mandels’ salt solution medium supplemented with 1% (w/v) cellulose and 1% (w/v) wheat bran (MMSWC) [19]. The composition of Mandels’ salt solution was as follows: 3 g L−1 KH2PO4, 2.6 g L−1 NaNO3, 0.5 g L−1 urea, 0.5 3 g L−1 MgSO4·7H2O, 0.5 3 g L−1 CaCl2, 0.0075 g L−1 FeSO4·7H2O, 0.0025 g L−1 MnSO4·H2O, 0.0036 g L−1 ZnSO4·7H2O, 0.0037 g L−1 CoCl2·6H2O, and 1 g L−1 peptone.
Escherichia coli DH5α (Biomed, Beijing, China) was used as the host for gene cloning, and P. pastoris GS115 (Invitrogen, MA, USA) was used for protein expression and was cultured on YPD agar medium (1% yeast extract, 2% peptone, 2% dextrose and 2% agar) at 28 °C. MD medium (2% dextrose, 1.34% YNB, 4 × 10−5% biotin and 2% agar) was used to screen the recombinant P. pastoris. YPD agar medium containing different concentrations of G418 (geneticin) was used to screen the copy number of the inserted target gene. rPcAxe expression was carried out by inoculating recombinant P. pastoris in BMMY medium (1% yeast extract, 2% peptone, 1.34% YNB, 100 mM phosphate buffer pH 6.0, 4 × 10−5% biotin and 2% methanol). The pcaxe gene was amplified by PCR and the product ligated into the pGEM-T easy vector (Promega, Madison, USA). The pPIC9K vector (Invitrogen, MA, USA) was used for pcaxe expression in P. pastoris.
Purification of acetyl xylan esterase
P33 was cultivated in MMSWC medium and fermentation broth was collected on the third day and centrifuged at 4 °C, 8000 rpm for 20 min. The supernatant was salted out with 85% (NH4)2SO4 for 1 h, and the mixture centrifuged at 4 °C, 8000 rpm for 30 min. The pellet was suspended in 10 mM TRIS–HCl buffer (pH 7.5) and dialyzed to remove (NH4)2SO4. The resultant protein solution was loaded onto various columns to isolate the acetyl xylan esterase. The protein solution was loaded on a 1 mL Q Bestarose Fast Flow column (Bestchrom, Shanghai, China) equilibrated in 10 mM TRIS–HCl buffer (pH 7.5), and adsorbed proteins were eluted with a linear concentration gradient of 0–1 M NaCl. The fraction containing acetyl xylan esterase was collected and dialyzed prior to further purification on a Ezload Chromdex 75 Prep Grade column (Bestchrom, Shanghai, China) equilibrated in 10 mM TRIS–HCl buffer (pH 7.5). The active fraction was collected and loaded on a phenyl-sepharose fast flow column (Bestchrom, Shanghai, China), and the adsorbed protein was eluted with a linear concentration gradient of 2–0 M NaCl in 50 mM sodium phosphate (pH 7.5). The active fraction was collected and used for subsequent experiments.
Amino acid sequencing of the acetyl xylan esterase by LC–MS/MS
The single protein band on the SDS-PAGE gel was cut into small pieces and subjected to tryptic digestion as previously described [20]. The residue was reconstituted with 0.1% formic acid for nanoLC–MS/MS analysis to elucidate the amino acid sequence. The acquired rPcAxe amino acid sequence was used for identification of the signal peptide using the online SignalP tool (http://www.cbs.dtu.dk/services/SignalP/). The sequence was also used in BLAST searches (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to identify related sequences in the database. The rPcAxe sequence was aligned with the extracted reference sequences using MEGA 6.06 software [21] and a neighbor-joining phylogenetic tree was constructed [22]. Domains in rPcAxe were also identified by comparing with the aligned reference sequences.
Construction of the recombinant plasmid and transformation
The nucleotide sequence of P. chrysogenum pcaxe was obtained from the sequence of the most similar protein Pc20g11110. The cDNA of pcaxe including the signal peptide was amplified by PCR using forward primer 5′-ATGATGATCCTCCCTGTCC-3′ and reverse primer 5′-TCAAGCCGCCATGAAAAACT-3′ by denaturation at 98 °C for 2 min followed by 30 cycles at 98 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, and a final elongation step at 72 °C for 10 min, using Phusion DNA polymerase (New England BioLabs, MA, USA) and the suggested PCR mixture (25 μL). PCR products were purified using a Universal DNA Purification Kit (Tiangen, Beijing, China) and A-tailed using Taq DNA polymerase (Thermo Scientific, CA, USA) at 72 °C for 30 min. The A-tailed PCR product was purified using the same method and inserted into the pGEM-T easy vector and transformed into E. coli DH5α competent cells. The pcaxe gene excluding the signal peptide was amplified by PCR using forward primer 5′-AAGAATTCCATCATCATCATCATCATGCGGCATCTTCGGGCTGCGGC-3′ and reverse primer 5′-AAGCGGCCGCTCAAGCCGCCATGAAAAACTCCCAGGTCGC-3′, in which the underlined sequences indicate restriction sites EcoRI and NotI that were used for ligation, and letters in italics denoted the His tag for purification of the recombinant protein by affinity chromatography. PCR products were ligated with the plasmid pPIC9K using T4 DNA ligase (Promega, Madison, USA) and transformed into E. coli DH5α competent cells.
Expression and production of recombinant PcAxe
The recombinant pPIC9K-axe construct was linearized with the restriction endonuclease SalI, transformed into P. pastoris GS115 competent cells by electroporation, and cells were cultured on MD agar medium at 28 °C for 3–4 days. All transformants were scraped and resuspended in sterile water, and grown on YPD agar medium containing different concentrations of G418. The inoculation amount was ~ 105 cells per agar plate, and cultivation proceeded at 28 °C for 2–5 days. The level of protein expression in the transformants was validated in BMMY medium following growth for 72 h with 2% methanol as the inducer.
Purification of rPcAxe by affinity chromatography
For purification of rPcAxe expressed by P. pastoris, the cell-free supernatant was collected by centrifugation at 4 °C, 8000 rpm for 10 min, and filtered through a 0.45 μm filter. The Ni2+ His-tag column was equilibrated with binding buffer (20 mM sodium phosphate, 0.5 M NaCl, pH 7.4) and the filtered supernatant was loaded and nonspecific binding proteins removed by wash buffer (20 mM sodium phosphate, 0.5 M NaCl, 20 mM imidazole, pH 7.4). The bound protein was eluted with elution buffer (20 mM sodium phosphate, 0.5 M NaCl, 300 mM imidazole, pH 7.4).
Enzymatic assays
Acetyl xylan esterase activity was determined using 4-nitrophenyl acetate as the substrate (pNPA) [23]. α-l-Arabinofuranosidase activity was assayed using 4-nitrophenyl-α-l-arabinofuranoside (pNPAF) as the substrate [24]. And β-xylosidase activity measurement was performed using 4-nitrophenyl-β-d-xylopyranoside (pNPX) as the substrate [25]. One unit of enzyme activity was defined as the amount of enzyme required to release 1 μmol of p-nitrophenol per min under the conditions assayed.
To determine the optimal pH for acetyl xylan esterase activity, assays were performed at 37 °C in buffers ranging from pH 4–9 using mixtures of 0.1 M citric acid and 0.2 M sodium hydrogen phosphate. The optimal temperature was investigated in the range of 20–70 °C at the optimal pH. The stability of the acetyl xylan esterase at different pH values was determined by measuring the residual activity in standard conditions after incubation of the enzyme in different buffers as described above at room temperature for 1 h. Thermal stability was assessed by measuring the residual activity in standard conditions after incubation of the enzyme at different temperatures for 1 h. Enzyme not pre-incubated was used as a control.
To determine the optimal pH for α-l-arabinofuranosidase activity, the enzyme activity was measured at between pH 5 and 11 using mixtures of 0.1 M citric acid and 0.2 M sodium hydrogen phosphate. The optimal temperature was determined in the range of 20–60 °C at the optimal pH value.
The effect of metal ions (Na+, K+, Ca2+, Fe3+, Mg2+, Al3+, Zn2+, Cu2+, Pb2+, Mn2+ and Fe2+) and sodium dodecyl sulfate (SDS) on the acetyl xylan esterase activity was determined by incubating the enzyme with the agent at a final concentration of 1, 2, 5 and 10 mM for 1 h at 4 °C. The residual activity was then measured under standard conditions. Enzyme without any added agent was used as a control.
Substrate specificity and kinetic parameters of the acetyl xylan esterase
The substrate preferences of the purified recombinant acetyl xylan esterase were investigated with 4-hydroxy-3-methoxycinnamic acid methyl ester (a substrate for ferulic acid esterase) and ethyl-4-hydroxy-3-methoxycinnamate and 4-nitrophenyl acetate (pNPA; a substrate for acetyl xylan esterase) using the method described previously [26]. The Michaelis-Menten constant (K m) and maximal velocity (V max) were assessed based on pNPA concentrations of 0.2, 0.25, 0.5, 1, 1.25 and 2 mM. K m and V max were calculated based on the Lineweaver–Burk plot constructed by plotting the reciprocal of the substrate concentration on the x-axis and the reciprocal of the enzyme reaction velocity on the y-axis. All experiments were conducted in triplicate at pH 7 and 40 °C.
Enzymatic hydrolysis
Corn stover was delignified with sodium chlorite before enzymatic hydrolysis according to the procedure in the Pulp and Paper Technical Association of Canada (PAPTAC) Useful methods G10.U [27]. Enzyme preparations used in the hydrolysis assay consisted of recombinant xylanase from Schizophyllum commune (Additional file 1: Figure S1) and cellulase cocktails from Trichoderma longibrachiatum (C9748, Sigma, St Louis, MO, USA). Hydrolysis experiments were carried out with 2% (w/v) delignified corn stover in 50 mM sodium acetate buffer (pH 5) in a final reaction volume of 1 mL [20]. For hydrolysis by xylanase, 5 mg protein/g cellulose of rPcAxe was added to the recombinant xylanase (10 mg protein/g cellulose). Reactions were performed in an orbital shaker incubator at 50 °C (the optimal temperature for recombinant xylanase). For hydrolysis by cellulase, varying amounts of commercial cellulase were replaced with the equivalent amount of rPcAxe at a fixed total protein loading (10 mg protein/g cellulose). Reactions were performed in an orbital shaker incubator at 37 °C. Control assays containing enzyme without substrate, and substrate without enzyme, were included under the same conditions. All hydrolysis experiments were conducted in triplicate. Protein concentration was determined using the Bradford Protein Assay Kit (GenStar, Beijing, China) according to the manufacturer’s instructions.
After incubation for the indicated time, hydrolysis was terminated by heating the reaction mixture at 100 °C for 10 min to inactivate the enzymes. The content of total reducing sugar was determined by the DNS method [28]. The individual concentration of glucose and xylose in the supernatants was determined by Essentia LC-15C high performance liquid chromatography (HPLC) (Shimadzu, Kyoto, Japan) using a Remex ROA-organic acid H+ (8%) column (Phenomenex, Los Angeles, CA, USA) and a RID-10A refractive index detector (Shimadzu, Kyoto, Japan). The content of acetic acid was quantified by HPLC using the aforementioned column but with a SPD-15C Essentia UV/VIS detector (Shimadzu, Kyoto, Japan) and the UV wavelength was set at 210 nm. Datasets were tested for statistical significance using ANOVA, and P values < 0.05 were deemed significant.
The hydrolysis of wheat arabinoxylan (P-EDWAX30, Megazyme, Wicklow, Ireland) was performed as follows: 400 μL of 1% (w/v) water-soluble wheat arabinoxylan and 100 μL of rPcAxe was reacted in an orbital shaker incubator at 37 °C in buffer mixtures of 0.1 M citric acid and 0.2 M sodium hydrogen phosphate (pH 7.0). After 24 h incubation, reactions were terminated in boiling water for 10 min. Blank controls containing substrate alone were included under the same conditions. The hydrolysates were determined using HPLC according to the method described previously [29].
To examine the synergism between rPcAxe and xylanase on degradation of arabinoxylan, hydrolysis experiments were carried out by adding 2.5 mg protein/g cellulose of rPcAxe into the recombinant xylanase (5 mg protein/g cellulose), with a final reaction volume of 1 mL and a concentration of 1% (w/v) wheat arabinoxylan in 50 mM sodium acetate buffer (pH 5). Reactions were performed in an orbital shaker incubator at 50 °C for 24 h. The contents of total reducing sugar, arabinose and acetic acid were determined by the method described above.
Results
Purification and identification of a novel acetyl xylan esterase in P. chrysogenum P33
The acetyl xylan esterase was successively purified using strong anion exchange, gel filtration and hydrophobic interaction chromatographic steps. The resultant PcAxe protein ran as a single band on SDS-PAGE with a molecular mass of 31.16 kDa (Additional file 2: Figure S2). The specific activities of purified PcAxe were 4.8 and 0.9 U/mg, with pNPA and pNPAF as the substrates respectively. According to the LC–MS/MS spectrum and database search, PcAxe peptides (Fig. 1a) matched most closely with Pc20g11110, which is annotated as a hypothetical protein in the NCBI database and belongs to CE (carbohydrate esterase) family 1 in the CAZy database.
Fig. 1.

Sequence of PcAxe and SDS-PAGE analysis of purified rPcAxe. a Sequence of PcAxe based on LC–MS/MS analysis (the PcAxe band is highlighted in red). b SDS-PAGE analysis of purified rPcAxe. Lanes: M standard protein molecular weight markers; 1 rPcAxe purified by affinity chromatography
Expression and purification of rPcAxe
After induction with methanol at 28 °C for 72 h, the supernatant contained recombinant acetyl xylan esterase activity of 87.46 U/L. Following one-step immobilized metal-affinity chromatography (IMAC), the purified rPcAxe migrated as a single band with an apparent molecular mass of 33.92 kDa in SDS-PAGE (Fig. 1b). The specific activity of purified rPcAxe was 5.4 U/mg with 4-nitrophenyl acetate as the substrate, and 1.2 U/mg with pNPAF as the substrate, which were respectively 12.5 and 33.3% higher than that of the native enzyme.
Sequence analysis
The nucleotide sequence of pcaxe is 804 bp long (Accession Number: KY882362) and corresponds to a protein composed of 267 amino acids. The deduced amino acid alignment showed 99% identity with the Pc20g11110 protein. Although there were three amino acids in rPcAxe that differ from Pc20g11110, the conserved domains of rPcAxe and Pc20g11110 protein were the same. It indicated that rPcAxe and Pc20g11110 protein were identical and performed the same function. A signal peptide of 18 amino acids was also identified in the rPcAxe sequence. By comparing with the reference sequences, it was found that rPcAxe contains an esterase domain and a GH62 domain, which is annotated as α-l-arabinofuranosidase in the CAZy database (Fig. 2). In addition, the Gly-Xaa-Ser-Xaa-Gly consensus motif that is characteristic of the active site of serine peptidases and members of the α/β-hydrolase enzyme family [12] was identified in rPcAxe (Fig. 2). rPcAxe shares high sequence identity with esterases from Aspergillus flavus AF70 (77%), Aspergillus fischeri NRRL_181 (75%), Aspergillus fumigatus Af293 (75%), Aspergillus clavatus NRRL_1 (74%) and Aspergillus nomius NRRL_13137 (75%). These results imply that rPcAxe is a carboxylesterase belonging to the serine hydrolase superfamily. The sequence identities between rPcAxe and GH62 from Aspergillus clavatus NRRL_1, Magnaporthe oryzae Y34, Magnaporthe oryzae 70–15 and Penicillium chrysogenum 31B were 71, 52, 51 and 32% respectively. The neighbor-joining phylogenetic tree containing rPcAxe and the reference sequences (Additional file 3: Figure S3) indicated that rPcAxe is a novel bifunctional enzyme with both esterase and α-l-arabinofuranosidase activities.
Fig. 2.
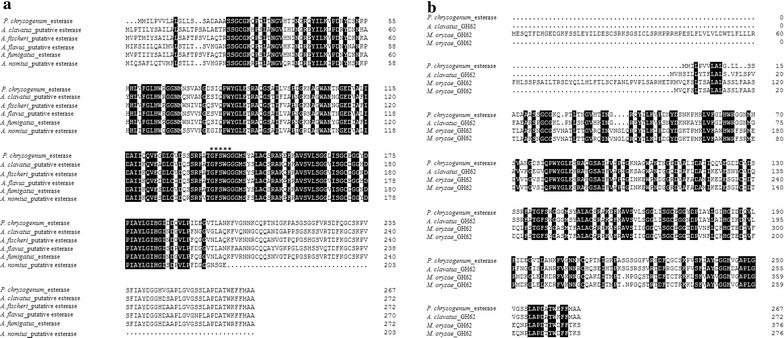
The deduced amino acid sequence alignments of rPcAxe and other esterases and family 62 glycoside hydrolases. a The alignment of rPcAxe and other esterases. The consensus motif of the active site of serine peptidases and α/β hydrolases (Gly-Xaa-Ser-Xaa-Gly) was marked with an asterisk. b The alignment of rPcAxe and other glycoside hydrolases 62. Aspfi, Aspergillus fischeri; Aspfu, Aspergillus fumigatus; Aspcl, Aspergillus clavatus; Aspfl, Aspergillus flavus; Aspno, Aspergillus nomius; Magor, Magnaporthe oryzae
Enzymatic properties of rPcAxe
The rPcAxe enzyme was unable to hydrolyze 4-hydroxy-3-methoxycinnamic acid methyl ester or ethyl-4-hydroxy-3-methoxycinnamate, but could hydrolyze 4-nitrophenyl acetate and release 4-nitrophenol. The K m and the V max values for 4-nitrophenyl acetate were 0.465 mM and 0.035 μmol/min/mg, respectively. The purified rPcAxe activity was highest at 40 °C (Fig. 3a), and activity was stable at a temperature range below 40 °C, but the residual activity decreased rapidly at temperature higher than 40 °C (Fig. 3b). The rPcAxe activity was optimal at a pH of 7 (Fig. 3c) and 100% activity was maintained from pH 6 to pH 9 after a 1 h incubation. It could maintain a respective activity of 86 and 58% at pH 10 and pH 11 after a 1 h incubation, indicating strong pH stability (Fig. 3d).
Fig. 3.
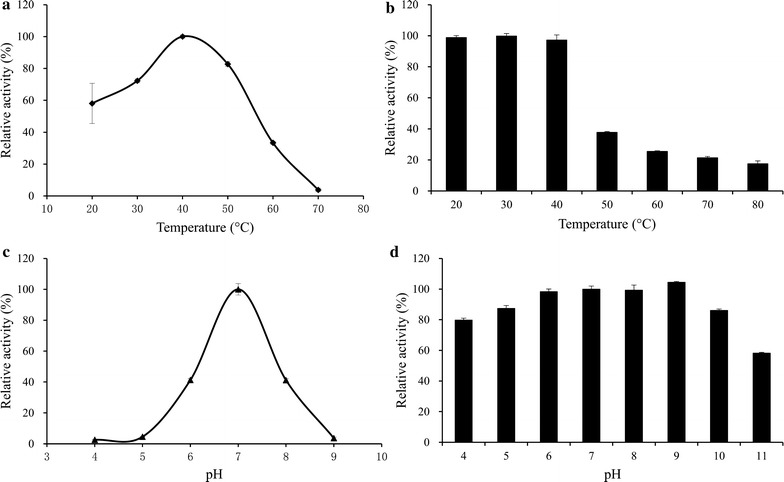
Characterization of purified rPcAxe with 4-nitrophenyl acetate as the substrate. a The effect of temperature on enzyme activity. b Thermostability of purified rPcAxe. c The effect of pH on enzyme activity. Thermal stability was assessed by measuring the residual activity after incubation of rPcAxe at different temperatures for 1 h. d pH stability of purified rPcAxe. pH stability was determined by measuring the residual activity after incubation of rPcAxe in different buffers for 1 h. The relative activity was determined, and the maximum activity was defined as 100% (a, c). The initial activity of rPcAxe not pre-incubated in different buffers was defined as 100% (b, d). Values are the means and standard deviations of triplicate experiments
The rPcAxe activity was slightly enhanced in the presence of Mg2+, Cu2+ and Pb2+ at a concentration of 5 mM, and by Mn2+ and Fe2+ at 10 mM, with relative activities ranging from 102.1 to 109.0%. The relative activity of rPcAxe was maintained at 95.8 and 92.7% in the presence of 5 mM Mn2+ and Fe2+. The ions Na+, K+, Ca2+, Fe3+, Al3+ and Zn2+ had a minor inhibitory effect under different concentrations, as did the detergent SDS (Table 1). These results indicate that rPcAxe is relatively stable in the presence of these metal ions.
Table 1.
Effect of metal ions and chemical reagents on the enzyme activity of rPcAxe
| Metal ions | Relative activity (%)a | |||
|---|---|---|---|---|
| 1 mM | 2 mM | 5 mM | 10 mM | |
| Na+ | 94.1 ± 1.0 | 98.9 ± 2.1 | 97.9 ± 3.5 | 87.2 ± 4.5 |
| K+ | 91.0 ± 1.1 | 91.0 ± 1.4 | 93.3 ± 0.7 | 88.6 ± 2.1 |
| Ca2+ | 89.9 ± 2.0 | 95.5 ± 1.6 | 96.5 ± 1.5 | 86.7 ± 2.9 |
| Fe3+ | 89.1 ± 1.5 | 89.7 ± 0.7 | 87.9 ± 2.3 | 76.9 ± 1.0 |
| Mg2+ | 89.8 ± 1.6 | 90.9 ± 1.9 | 102.1 ± 1.6 | 93.6 ± 5.9 |
| Al3+ | 92.6 ± 2.1 | 93.7 ± 2.2 | 87.8 ± 5.0 | 82.6 ± 1.6 |
| Zn2+ | 88.4 ± 3.2 | 90.5 ± 1.3 | 91.3 ± 3.5 | 89.7 ± 3.8 |
| Cu2+ | 98.6 ± 1.8 | 97.4 ± 1.7 | 108.8 ± 1.4 | 96.8 ± 2.0 |
| Pb2+ | 94.0 ± 1.3 | 95.4 ± 2.1 | 109.0 ± 4.2 | 102.3 ± 0.8 |
| Mn2+ | 86.4 ± 1.4 | 92.1 ± 3.1 | 95.8 ± 2.0 | 103.3 ± 3.0 |
| Fe2+ | 82.5 ± 4.2 | 93.5 ± 2.2 | 92.7 ± 6.5 | 105.7 ± 2.2 |
| SDS | 85.0 ± 1.7 | 90.6 ± 2.5 | 88.7 ± 1.5 | 72.7 ± 2.3 |
aValues represent the mean ± SD (n = 3) relative to untreated control samples
α-l-Arabinofuranosidase activity of rPcAxe
Based upon its high level of sequence identity shared with GH62 (Fig. 2), the α-l-arabinofuranosidase activity of rPcAxe was tested using 5 mM pNPAF as the substrate. The optimal pH and temperature were 7.0 and 50 °C, respectively (Fig. 4). The relative activity for arabinofuranosidase was 87% at 40 °C, which was the optimal temperature for acetyl xylan esterase.
Fig. 4.
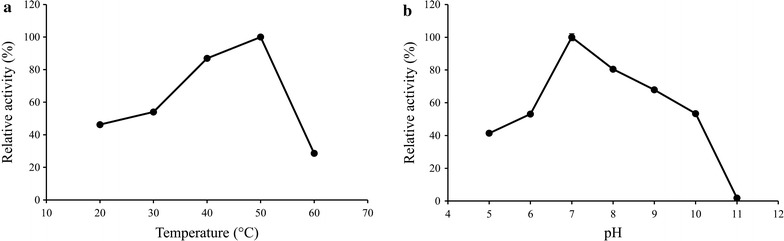
Effects of pH and temperature on the enzyme activity of purified rPcAxe with 4-nitrophenyl-α-l-arabinofuranoside. a Effect of temperature on enzyme activity. The optimal temperature was determined in the range of 20–60 °C. b Effect of pH on enzyme activity. Enzyme activity was measured between pH 5 and 11 using mixtures of 0.1 M citric acid and 0.2 M sodium hydrogen phosphate. The maximum activity was defined as 100%, and the relative activity was determined. Values are the means and standard deviations of triplicate experiments
In order to confirm the α-l-arabinofuranosidase activity of rPcAxe, we examined the action of rPcAxe on wheat arabinoxylan. The optimal temperature for the α-l-arabinofuranosidase activity towards pNPAF of rPcAxe was 50 °C (Fig. 4). However, the thermostability of the α-l-arabinofuranosidase activity of rPcAxe at 37 °C was higher than that at 50 °C (Additional file 4: Figure S4). The relative α-l-arabinofuranosidase activity was 80% at 37 °C (Fig. 4), hence the hydrolysis of wheat arabinoxylan was performed at this temperature. After 24 h of hydrolysis, rPcAxe released 3.16 μg/mL arabinose from wheat arabinoxylan, which was 0.13% of the total arabinose in the substrate. Surprisingly, 21.22 μg/mL xylose was also released in addition to arabinose (Fig. 5), indicating that rPcAxe could cleave the β-1,4 bonds in the xylan backbone. Purified rPcAxe was therefore used to further validate the β-xylosidase activity, and a specific activity of 0.512 U/mg with 4-nitrophenyl-β-d-xylopyranoside as the substrate was observed.
Fig. 5.
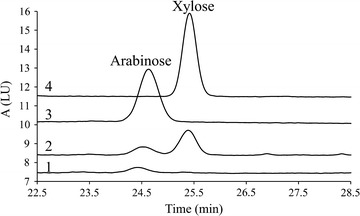
Hydrolysis of water-soluble wheat arabinoxylan by purified rPcAxe. 1, Substrate incubated without enzyme for 24 h (control); 2, hydrolysate treated with rPcAxe for 24 h; 3, arabinose standard; 4, xylose standard. Hydrolysis reactions were performed at 37 °C in buffer consisting of mixtures of 0.1 M citric acid and 0.2 M sodium hydrogen phosphate (pH 7.0) for 24 h with 1% (w/v) water-soluble wheat arabinoxylan loading. The amount of arabinose and xylose released was determined using HPLC. Values are the means and standard deviations of triplicate experiments
Action of rPcAxe in enzymatic hydrolysis
In order to investigate the effects of rPcAxe on the hydrolysis of lignocellulose, hydrolysis of delignified corn stover was performed in combination with recombinant xylanase or commercial cellulase. After 24 h of hydrolysis, the released reducing sugar content was 0.278 and 0.408 mg/mL with recombinant xylanase alone and with recombinant xylanase supplemented with rPcAxe (Fig. 6). The degree of synergy between rPcAxe and xylanase was calculated as 1.35 at 24 h.
Fig. 6.
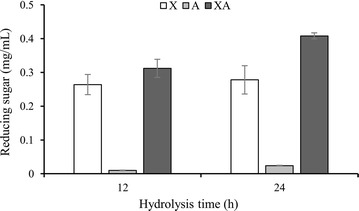
Total reducing sugars released from delignified corn stover by recombinant xylanase, rPcAxe and the enzymatic mix. Hydrolysis reactions were performed at 50 °C for 12 or 24 h with 2% (w/v) biomass loading. The amount of reducing sugars released was determined using the DNS method. X (white bars), recombinant xylanase from S. commune; A (light gray bars), rPcAxe; XA (dark gray bars), mixtures of recombinant xylanase from S. commune and rPcAxe. Values are the means and standard deviations of triplicate experiments
The effects of rPcAxe on cellulose hydrolysis were also investigated and are presented in Fig. 7. The amount of released reducing sugars was 2.5% higher with a mixture containing 8 mg of commercial cellulase and 2 mg of rPcAxe than with 10 mg of commercial cellulase alone. When 4 or 5 mg of commercial cellulase was replaced by the equivalent amount of rPcAxe, the released reducing sugar content was 2.52 and 2.43 mg/mL, respectively, which was not significantly different from that obtained using 10 mg of commercial cellulase alone (P < 0.05; Fig. 7a). HPLC analysis revealed that the amount of glucose released by a mixture of 8 mg cellulase and 2 mg rPcAxe was 51% higher than that released by cellulase alone. The enzyme mixture of 8 mg cellulase and 2 mg rPcAxe released 14.1% of the total glucose in the substrate. Meanwhile, the amount of cellobiose released was 45% lower in the mixture. In the treatment containing 5 mg cellulase and 5 mg rPcAxe, the amount of glucose released was significantly higher (P < 0.05) than that released by cellulase alone (Fig. 7b). An increase in the concentration of acetic acid was also observed when a higher proportion of commercial cellulase was replaced by purified rPcAxe, and the highest concentration of acetic acid was obtained when up to 60% of commercial cellulase was replaced. These results confirmed that rPcAxe could act on the acetyl esters of delignified corn stover (Fig. 7c).
Fig. 7.
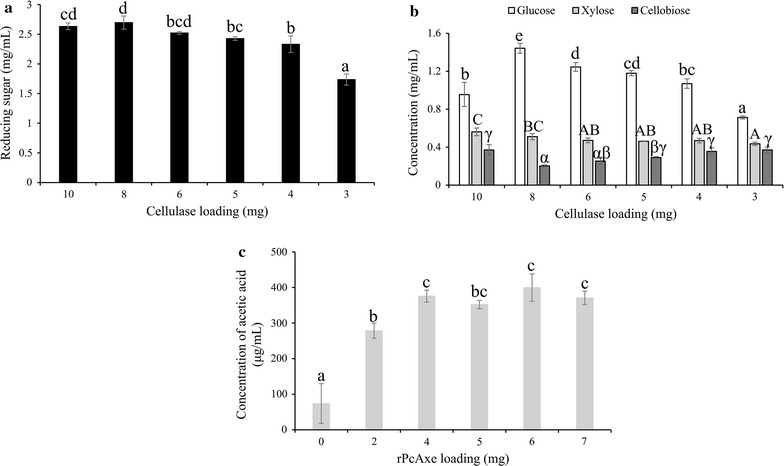
Hydrolysis of delignified corn stover using cellulase and rPcAxe at a fixed total protein dosage. a Amount of released reducing sugars determined by the DNS method. b Amount of glucose (white bars), xylose (light gray bars) and cellobiose (dark gray bars) determined by HPLC. c Concentration of acetic acid in the hydrolysate determined by HPLC. Hydrolysis reactions were performed at 37 °C for 24 h with 2% (w/v) biomass loading. Values are the means and standard deviations of triplicate experiments. Statistical significance is indicated by different letters on columns based on ANOVA (P < 0.05)
The synergism between rPcAxe and the recombinant xylanase on degradation of arabinoxylan was also examined (Additional file 5: Table S1). After 24 h of hydrolysis, the reducing sugar contents released by recombinant xylanase alone and by its synergy with rPcAxe were 0.423 and 0.665 mg/mL respectively, and the degree of synergism was 1.454. When arabinoxylan was hydrolyzed by rPcAxe alone, 0.13% of the total arabinose in the substrate was released (0.003 mg/mL). In comparison, by the synergistic hydrolysis of rPcAxe and recombinant xylanase, the released arabinose increased by 200%, and the released acetic acid went up by 25% (0.057–0.071 mg/mL).
Discussion
Cellulose and hemicellulose chains form an alternating layered structure in the cell wall, and the presence of hemicellulose hinders the hydrolysis of cellulose [4]. Acetyl xylan esterase is an important auxiliary enzyme that remove acetyl groups from the side chains of hemicellulose, promoting the hydrolysis of hemicellulose and cellulose [30]. In the present study, the K m of rPcAxe was 0.465 mM, which was higher than that of the acetyl xylan esterase from P. chrysosporium [12], but lower than that from Aspergillus awanori [31]. The V max of rPcAxe with pNPA as the substrate was lower than that of the acetyl xylan esterase from Coprinopsis cinerea [32]. rPcAxe displayed optimal acetyl xylan esterase activity at pH 7, similar to other fungal acetyl xylan esterases such as those from P. chrysosporium [12], Aspergillus ficuum [33] and A. awamori [31]. However, the stable activity between pH 4 and pH 9, especially the retention of full enzyme activity after incubation between pH 6 and 9 for 1 h, marked rPcAxe as different from all other previously described fungal acetyl xylan esterases. During incubation of strain P33, the pH value of the culture increased gradually and reached ~ 8.1, during which the acetyl xylan esterase activity remained high (data not shown), in accordance with the basophilic properties of rPcAxe. Industrial processes often include steps at different pH, and the majority of known enzymes need to be stabilized under such conditions. Therefore, enzymes that are inherently stable under these conditions have great potential in industrial applications [34, 35]. It was reported that the rPcAxe2 enzyme from P. chrysosporium lost more than half of its relative activity in the presence of Mn2+ and Fe2+ (5 mM), and activity dropped to 1.6% when incubated with 5 mM Fe2+ [12]. In our study, the relative activity of rPcAxe was maintained at 95.8 and 92.7% in the presence of 5 mM Mn2+ and Fe2+, respectively. rPcAxe could maintain a relative activity of more than 80% with the presence of most of the tested metal ions except Fe3+ (10 mM). This resistance to metal ions is also advantageous in industrial applications, since metal ions are inevitably present in industrial production. rPcAxe displayed optimal α-l-arabinofuranosidase activity at pH 7, which is typical for bacterial arabinofuranosidases [24, 36], and the same was true for its acetyl xylan esterase activity, which differs from PcAxe2 from P. chrysosporium [12] and most other fungal arabinofuranosidases (optimal pH less than 5.0) [37]. These characteristics make rPcAxe a promising enzyme for biotechnological use.
In the hydrolysis of delignified corn stover, a significant synergistic effect (P < 0.05) was observed in the mixture of rPcAxe and the recombinant xylanase (degree of synergism = 1.35). This suggested that rPcAxe promoted the hydrolytic activity of xylanase. Although the total reducing sugar production was almost the same in a mixture of 8 mg of commercial cellulase and 2 mg of rPcAxe as that with 10 mg of cellulase alone, the release of glucose was increased by 51%, and the amount of cellobiose in the mixture was reduced, suggesting rPcAxe markedly promoted the hydrolysis of cellulose. It was speculated that the removal of ester groups by rPcAxe enhanced the β-glucosidase activity in the commercial cellulase cocktail, which hydrolyzed the cellobiose to liberate more glucose, although it was not directly involved in the hydrolysis of cellulosic component. Previous studies showed similar results. The amount of glucose released from pretreated wheat straw by the combination of cellulase and xylanase plus acetyl xylan esterase was higher than that released by the combination of cellulase and xylanase, while the amount of cellobiose was lower [4]. When the dosage of commercial cellulase was decreased, the decrease in released xylose was not significant. Because rPcAxe cleaves β-1,4 bonds in the xylan backbone (Fig. 5), it is likely that rPcAxe releases xylose, which presumably made up for the decrease in xylose resulting from the use of less commercial cellulase. This showed that rPcAxe could facilitate the hydrolytic activity of xylanase in the commercial cellulase cocktail [20]. It is likely that the content of arabinose in delignified corn stover was too low to be detected in the enzymatic hydrolysis assays. Partially replacing commercial cellulase with an equivalent amount of rPcAxe markedly increased the release of glucose, and promoted the hydrolysis of cellulose. These results suggest increasing the dosage of cellulase was not necessary for effective lignocellulose hydrolysis, and hemicellulolytic enzymes appeared to play an important role in biomass degradation. Zhu et al. [38] reported a high degree of synergism (up to 75%) between a commercial fungal cellulase and a hemicellulase-enriched mixture derived from a bacterial consortia upon replacement of the commercial enzymes. Synergy between cellulolytic and hemicellulolytic enzymes and acetyl xylan esterase has been studied widely [4, 12, 30, 39, 40], and it is a useful way to increase the sugar yield and reduce the enzyme cost. The presence of acetyl groups prevents cellulase from accessing cellulose [4]. It was speculated that rPcAxe hydrolyzes the ester groups connected to hemicellulose, thereby creating new hydrolytic sites for xylanase and increasing the accessibility of xylan to xylanase, and subsequently increasing the accessibility of cellulose to cellulase [4, 41].
In the present study, we observed activity against water-soluble wheat arabinoxylan, as demonstrated by the release of arabinose and acetic acid after 24 h of hydrolysis, proving that rPcAxe possessed α-l-arabinofuranosidase activity in addition to acetyl xylan esterase activity. rPcAxe in this study was active on pNPAF and arabinoxylan. It mainly displayed acetyl xylan esterase activity, and the activity towards arabinoxylan was low. Bifunctional enzymes showed one main enzyme activity, which was similar to what had been reported by Yang et al. [5]. The structure and composition of lignocellulose is complicated. Therefore, to effectively degrade lignocellulose, fungi usually secrete a set of extracellular lignocellulolytic enzymes [42], and anaerobic bacteria have evolved cell-associated multiprotein complexes known as the cellulosome [43] or xylosome [44]. Another important strategy in lignocellulolytic microbes is the induction of multifunctional enzymes to degrade different substrates [7], and this has received significant attention of late [5, 6, 45]. To our knowledge, the present work is the first detailed report of a bifunctional enzyme possessing acetyl xylan esterase and α-l-arabinofuranosidase activities. Acetyl xylan esterase and α-l-arabinofuranosidase are both important auxiliary enzymes. This bifunctional enzyme must be combined with cellulolytic/hemicellulolytic enzymes (e.g. cellulases or hemicellulases) to effectively degrade lignocellulose. Due to the bifunctionality of rPcAxe, the mixture of enzymes needed for effective lignocellulose hydrolysis can be reduced, which can lower the enzyme cost. Additionally, to a certain extent, the problems associated with the complex interactions and the difficulty in controlling different glycoside hydrolases can be avoided, and the efficiency of enzymatic hydrolysis improved. We anticipate being able to design simpler and lower-cost xylanolytic cocktails in the future based on the discovery of rPcAxe.
Conclusions
The novel bifunctional enzyme PcAxe was identified in P. chrysogenum P33. rPcAxe includes a carbohydrate esterase domain and a glycosyl hydrolase family 62 domain. rPcAxe is stable across a broad pH range, retaining 100% enzyme activity om pH 6–9 after a 1 h incubation. The enzyme tolerates the presence of a wide range of metal ions, and a significant synergy with xylanase was apparent; rPcAxe enhanced the hydrolysis of cellulose by hydrolyzing side groups, and improved the glucose yield. These findings expand our current knowledge of glycoside hydrolases and pave the way for the discovery of similar novel enzymes.
Additional files
Additional file 1: Figure S1. Recombinant xylanase from S. commune. Lanes: M, standard protein molecular weight markers; rScXYL, recombinant xylanase from S. commune purified by affinity chromatography. The arrow indicates recombinant xylanase.
Additional file 2: Figure S2. Elution profiles of acetyl xylan esterase from P. chrysogenum P33 and SDS-PAGE analysis of purified PcAxe. a, Ion exchange chromatography. b, Gel filtration chromatography. c, Hydrophobic interaction chromatography. d, SDS-PAGE analysis of purified PcAxe. Lanes: M, standard protein molecular weight markers; AXE, purified PcAxe. The arrow indicates purified PcAxe.
Additional file 3: Figure S3. Phylogenetic tree of rPcAxe. The phylogenetic tree was constructed by the neighbor-joining method using MEGA 6.06 software. Aspfi, Aspergillus fischeri; Aspfu, Aspergillus fumigatus; Aspcl, Aspergillus clavatus; Aspfl, Aspergillus flavus; Aspno, Aspergillus nomius; Magor, Magnaporthe oryzae.
Additional file 4: Figure S4. Thermostability of purified rPcAxe with 4-nitrophenyl-α-L-arabinofuranoside as the substrate. Thermal stability was assessed by measuring the residual activity after incubation of rPcAxe at different temperatures for 1 h. The initial activity of rPcAxe not pre-incubated in different buffers was defined as 100%. Values are the means and standard deviations of triplicate experiments.
Additional file 5: Table S1. Hydrolysis of water-soluble wheat arabinoxylan by recombinant xylanase, rPcAxe and the enzymatic mix.
Authors’ contributions
YY and HY conceived and designed the experiments. YY performed the majority of the laboratory work, analyzed the results and wrote the manuscript. NZ and JY contributed to the interpretation of the results and revision of the manuscript. YL prepared the recombinant xylanase. JL and RW carried out the material pretreatment and determination of chemical compositions. FW helped to revise the manuscript. HY supervised the overall work, discussed the results, and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the native English speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the National High Technology Research and Development Program of China (863 Program) [Grant Number 2011AA10A206] and the Project of State Key Laboratory of Agrobiotechnology (2017SKLAB7-15).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CAZy
carbohydrate-active enzyme database
- CE
carbohydrate esterase
- DNS
3,5-dinitrosalicylic acid
- GH
glycoside hydrolase
- PDA
potato dextrose agar
- HPLC
high performance liquid chromatography
- IMAC
immobilized metal-affinity chromatography
- Km
Michaelis–Menten constant
- Vmax
maximal velocity
- MMSWC
modified Mandels’ salt solution supplemented with 1% (w/v) cellulose plus 1% (w/v) wheat bran
- nanoLC–MS/MS
nano liquid chromatography–tandem mass spectrometry
- PcAxe
acetyl xylan esterase from Penicillium chrysogenum P33
- rPcAxe
recombinant PcAxe
- pNPA
4-nitrophenyl acetate
- pNPAF
4-nitrophenyl-α-l-arabinofuranoside
- pNPX
4-nitrophenyl-β-d-xylopyranoside
- SDS
sodium dodecyl sulfate
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12934-017-0777-7) contains supplementary material, which is available to authorized users.
Contributor Information
Yi Yang, Email: yang11yi@aliyun.com.
Ning Zhu, Email: joylign@yeah.net.
Jinshui Yang, Email: yangjsh1999@163.com.
Yujian Lin, Email: closed12345@126.com.
Jiawen Liu, Email: 1070184179@qq.com.
Ruonan Wang, Email: 1181439829@qq.com.
Fengqin Wang, Email: w_fengqin@163.com.
Hongli Yuan, Email: hlyuan@cau.edu.cn.
References
- 1.Saha BC. Hemicellulose bioconversion. J Ind Microbiol Biotechnol. 2003;30:279–291. doi: 10.1007/s10295-003-0049-x. [DOI] [PubMed] [Google Scholar]
- 2.Jørgensen H, Kristensen JB, Felby C. Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefining. 2007;1:119–134. doi: 10.1002/bbb.4. [DOI] [Google Scholar]
- 3.Girio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Lukasik R. Hemicelluloses for fuel ethanol: a review. Bioresour Technol. 2010;101:4775–4800. doi: 10.1016/j.biortech.2010.01.088. [DOI] [PubMed] [Google Scholar]
- 4.Zhang JH, Siika-Aho M, Tenkanen M, Viikari L. The role of acetyl xylan esterase in the solubilization of xylan and enzymatic hydrolysis of wheat straw and giant reed. Biotechnol Biofuels. 2011;4:60. doi: 10.1186/1754-6834-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang WX, Bai YG, Yang PL, Luo HY, Huang HQ, Meng K, Shi PJ, Wang Y, Yao B. A novel bifunctional GH51 exo-α-l-arabinofuranosidase/endo-xylanase from Alicyclobacillus sp. A4 with significant biomass-degrading capacity. Biotechnol Biofuels. 2015;8:197. doi: 10.1186/s13068-015-0366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouraoui H, Desrousseaux ML, Ioannou E, Alvira P, Manai M, Remond C, Dumon C, Fernandez-Fuentes N, O’Donohue MJ. The GH51 α-l-arabinofuranosidase from Paenibacillus sp. THS1 is multifunctional, hydrolyzing main-chain and side-chain glycosidic bonds in heteroxylans. Biotechnol Biofuels. 2016;9:140. doi: 10.1186/s13068-016-0550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khandeparker R, Numan MT. Bifunctional xylanases and their potential use in biotechnology. J Ind Microbiol Biotechnol. 2008;35:635–644. doi: 10.1007/s10295-008-0342-9. [DOI] [PubMed] [Google Scholar]
- 8.Jordan DB, Braker JD. Opposing influences by subsite −1 and subsite +1 residues on relative xylopyranosidase/arabinofuranosidase activities of bifunctional β-d-xylosidase/α-l-arabinofuranosidase. Biochim Biophys Acta. 2011;1814:1648–1657. doi: 10.1016/j.bbapap.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Kosugi A, Murashima K, Doi RH. Xylanase and acetyl xylan esterase activities of XynA, a key subunit of the Clostridium cellulovorans cellulosome for xylan degradation. Appl Environ Microbiol. 2002;68:6399–6402. doi: 10.1128/AEM.68.12.6399-6402.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pai CK, Wu ZY, Chen MJ, Zeng YF, Chen JW, Duan CH, Li ML, Liu JR. Molecular cloning and characterization of a bifunctional xylanolytic enzyme from Neocallimastix patriciarum. Appl Microbiol Biotechnol. 2010;85:1451–1462. doi: 10.1007/s00253-009-2175-5. [DOI] [PubMed] [Google Scholar]
- 11.Čepeljnik T, Rincón MT, Flint HJ, Marinšek-Logar R. Xyn11A, a multidomain multicatalytic enzyme from Pseudobutyrivibrio xylanivorans Mz5T. Folia Microbiol. 2006;51:263–267. doi: 10.1007/BF02931809. [DOI] [PubMed] [Google Scholar]
- 12.Huy ND, Thiyagarajan S, Kim DH, Park SM. Cloning and characterization of a novel bifunctional acetyl xylan esterase with carbohydrate binding module from Phanerochaete chrysosporium. J Biosci Bioeng. 2013;115:507–513. doi: 10.1016/j.jbiosc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Khandeparker R, Jalal T. Xylanolytic enzyme systems in Arthrobacter sp. MTCC 5214 and Lactobacillus sp. Biotechnol Appl Biochem. 2015;62:245–254. doi: 10.1002/bab.1253. [DOI] [PubMed] [Google Scholar]
- 14.Florencio C, Cunha FM, Badino AC, Farinas CS, Ximenes E, Ladisch MR. Secretome analysis of Trichoderma reesei and Aspergillus niger cultivated by submerged and sequential fermentation processes: enzyme production for sugarcane bagasse hydrolysis. Enzyme Microb Technol. 2016;90:53–60. doi: 10.1016/j.enzmictec.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Singhania RR, Sukumaran RK, Patel AK, Larroche C, Pandey A. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb Technol. 2010;46:541–549. doi: 10.1016/j.enzmictec.2010.03.010. [DOI] [Google Scholar]
- 16.de Vries RP, Visser J. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev. 2001;65:497–522. doi: 10.1128/MMBR.65.4.497-522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nwodo-Chinedu S, Okochi VI, Smith HA, Okafor UA, Onyegeme-Okerenta BM, Omidiji O. Effect of carbon sources on cellulase (EC 3. 2. 1. 4) production by Penicillium chrysogenum PCL501. Afr J Biochem Res. 2007;1:6–10. [Google Scholar]
- 18.Zhang H, Sang Q. Production and extraction optimization of xylanase and β-mannanase by Penicillium chrysogenum QML-2 and primary application in saccharification of corn cob. Biochem Eng J. 2015;97:101–110. doi: 10.1016/j.bej.2015.02.014. [DOI] [Google Scholar]
- 19.Liu G, Zhang L, Wei XM, Zou G, Qin YQ, Ma L, Li J, Zheng HJ, Wang SY, Wang CS, Xun LY, Zhao GP, Zhou ZH, Qu YB. Genomic and secretomic analyses reveal unique features of the lignocellulolytic enzyme system of Penicillium decumbens. PLoS ONE. 2013;8:e55185. doi: 10.1371/journal.pone.0055185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu N, Liu JW, Yang JS, Lin YJ, Yang Y, Ji L, Li M, Yuan HL. Comparative analysis of the secretomes of Schizophyllum commune and other wood-decay basidiomycetes during solid-state fermentation reveals its unique lignocellulose-degrading enzyme system. Biotechnol Biofuels. 2016;9:42. doi: 10.1186/s13068-016-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, Shi HZ, Guo QS, Lv F, Yu YB, Lv LL, Shen WB, Zhao WH, Zhang MM. Analysis of the genetic diversity and population structure of Perinereis aibuhitensis in China using TRAP and AFLP markers. Biochem Syst Ecol. 2015;59:194–203. doi: 10.1016/j.bse.2015.01.002. [DOI] [Google Scholar]
- 22.Abuelhassan NN, Mutalib SA, Gimba FI, Yusoff WM. Molecular characterization and phylogeny of shiga toxin-producing E. coli (STEC) from imported beef meat in Malaysia. Environ Sci Pollut Res. 2016;23:17553–17562. doi: 10.1007/s11356-016-6954-0. [DOI] [PubMed] [Google Scholar]
- 23.Biely P, Puls J, Schneider H. Acetyl xylan esterases in fungal cellulolytic systems. FEBS Lett. 1985;186:80–84. doi: 10.1016/0014-5793(85)81343-0. [DOI] [Google Scholar]
- 24.Margolles A, de los Reyes-Gavilan CG. Purification and functional characterization of a novel α-l-arabinofuranosidase from Bifidobacterium longum B667. Appl Environ Microbiol. 2003;69:5096–5103. doi: 10.1128/AEM.69.9.5096-5103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilda KH, Marc C, Elisa VD, Eric S, De Bruyne CK. β-d-Xylosidase from Baccilus pumilus. Method Enzymol. 1982;83:631–639. doi: 10.1016/0076-6879(82)83062-0. [DOI] [PubMed] [Google Scholar]
- 26.Yang SQ, Tang L, Yan QJ, Zhou P, Xu HB, Jiang ZQ, Zhang P. Biochemical characteristics and gene cloning of a novel thermostable feruloyl esterase from Chaetomium sp. J Mol Catal B Enzym. 2013;97:328–336. doi: 10.1016/j.molcatb.2013.06.011. [DOI] [Google Scholar]
- 27.Park J, Shin H, Yoo S, Zoppe JO, Park S. Delignification of lignocellulosic biomass and its effect on subsequent enzymatic hydrolysis. Bioresources. 2015;10:2732–2743. [Google Scholar]
- 28.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 29.Meyer A, Raba C, Fischer K. Ion-pair RP-HPLC determination of sugars, amino sugars, and uronic acids after derivatization with p-aminobenzoic acid. Anal Chem. 2001;73:2377–2382. doi: 10.1021/ac001402s. [DOI] [PubMed] [Google Scholar]
- 30.Varnai A, Costa TH, Faulds CB, Milagres AM, Siika-Aho M, Ferraz A. Effects of enzymatic removal of plant cell wall acylation (acetylation, p-coumaroylation, and feruloylation) on accessibility of cellulose and xylan in natural (non-pretreated) sugar cane fractions. Biotechnol Biofuels. 2014;7:153. doi: 10.1186/s13068-014-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koseki T, Miwa Y, Fushinobu S, Hashizume K. Biochemical characterization of recombinant acetyl xylan esterase from Aspergillus awamori expressed in Pichia pastoris: mutational analysis of catalytic residues. Biochim Biophys Acta. 2005;1749:7–13. doi: 10.1016/j.bbapap.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Juturu V, Aust C, Wu JC. Heterologous expression and biochemical characterization of acetyl xylan esterase from Coprinopsis cinerea. World J Microbiol Biotechnol. 2013;29:597–605. doi: 10.1007/s11274-012-1215-y. [DOI] [PubMed] [Google Scholar]
- 33.Chung HJ, Park SM, Kim HR, Yang MS, Kim DH. Cloning the gene encoding acetyl xylan esterase from Aspergillus ficuum and its expression in Pichia pastoris. Enzyme Microb Technol. 2002;31:384–391. doi: 10.1016/S0141-0229(02)00122-9. [DOI] [Google Scholar]
- 34.Kumar L, Singh B, Adhikari DK, Mukherjee J, Ghosh D. A thermoalkaliphilic halotolerant esterase from Rhodococcus sp. LKE-028 (MTCC 5562): enzyme purification and characterization. Process Biochem. 2012;47:983–991. doi: 10.1016/j.procbio.2012.03.020. [DOI] [Google Scholar]
- 35.Bull AT, Bunch AW, Robinson GK. Biocatalysts for clean industrial products and processes. Curr Opin Microbiol. 1999;2:246–251. doi: 10.1016/S1369-5274(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 36.Degrassi G, Vindigni A, Venturi V. A thermostable α-arabinofuranosidase from xylanolytic Bacillus pumilus: purification and characterisation. J Biotechnol. 2003;101:69–79. doi: 10.1016/S0168-1656(02)00304-8. [DOI] [PubMed] [Google Scholar]
- 37.Saha BC. α-l-Arabinofuranosidases: biochemistry, molecular biology and application in biotechnology. Biotechnol Adv. 2000;18:403–423. doi: 10.1016/S0734-9750(00)00044-6. [DOI] [PubMed] [Google Scholar]
- 38.Zhu N, Yang JS, Ji L, Liu JW, Yang Y, Yuan HL. Metagenomic and metaproteomic analyses of a corn stover-adapted microbial consortium EMSD5 reveal its taxonomic and enzymatic basis for degrading lignocellulose. Biotechnol Biofuels. 2016;9:243. doi: 10.1186/s13068-016-0658-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selig MJ, Knoshaug EP, Adney WS, Himmel ME, Decker SR. Synergistic enhancement of cellobiohydrolase performance on pretreated corn stover by addition of xylanase and esterase activities. Bioresour Technol. 2008;99:4997–5005. doi: 10.1016/j.biortech.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 40.Selig MJ, Adney WS, Himmel ME, Decker SR. The impact of cell wall acetylation on corn stover hydrolysis by cellulolytic and xylanolytic enzymes. Cellulose. 2009;16:711–722. doi: 10.1007/s10570-009-9322-0. [DOI] [Google Scholar]
- 41.Moriyoshi K, Koma D, Yamanaka H, Sakai K, Ohmoto T. Expression and characterization of a thermostable acetylxylan esterase from Caldanaerobacter subterraneus subsp. tengcongensis involved in the degradation of insoluble cellulose acetate. Biosci Biotechnol Biochem. 2013;77:2495–2498. doi: 10.1271/bbb.130568. [DOI] [PubMed] [Google Scholar]
- 42.Wilson DB. Microbial diversity of cellulose hydrolysis. Curr Opin Microbiol. 2011;14:259–263. doi: 10.1016/j.mib.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Bayer EA, Shoham Y, Lamed R. Lignocellulose-decomposing bacteria and their enzyme systems. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The prokaryotes-Prokaryotic Physiology and Biochemistry. Berlin: Springer; 2013. pp. 215–66.
- 44.Lin LL, Thomson JA. An analysis of the extracellular xylanases and cellulases of Butyrivibrio fibrisolvens H17c. FEMS Microbiol Lett. 1991;84:197–204. doi: 10.1111/j.1574-6968.1991.tb04596.x. [DOI] [PubMed] [Google Scholar]
- 45.Peng XW, Su H, Mi SF, Han YJ. A multifunctional thermophilic glycoside hydrolase from Caldicellulosiruptor owensensis with potential applications in production of biofuels and biochemicals. Biotechnol Biofuels. 2016;9:98. doi: 10.1186/s13068-016-0509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Recombinant xylanase from S. commune. Lanes: M, standard protein molecular weight markers; rScXYL, recombinant xylanase from S. commune purified by affinity chromatography. The arrow indicates recombinant xylanase.
Additional file 2: Figure S2. Elution profiles of acetyl xylan esterase from P. chrysogenum P33 and SDS-PAGE analysis of purified PcAxe. a, Ion exchange chromatography. b, Gel filtration chromatography. c, Hydrophobic interaction chromatography. d, SDS-PAGE analysis of purified PcAxe. Lanes: M, standard protein molecular weight markers; AXE, purified PcAxe. The arrow indicates purified PcAxe.
Additional file 3: Figure S3. Phylogenetic tree of rPcAxe. The phylogenetic tree was constructed by the neighbor-joining method using MEGA 6.06 software. Aspfi, Aspergillus fischeri; Aspfu, Aspergillus fumigatus; Aspcl, Aspergillus clavatus; Aspfl, Aspergillus flavus; Aspno, Aspergillus nomius; Magor, Magnaporthe oryzae.
Additional file 4: Figure S4. Thermostability of purified rPcAxe with 4-nitrophenyl-α-L-arabinofuranoside as the substrate. Thermal stability was assessed by measuring the residual activity after incubation of rPcAxe at different temperatures for 1 h. The initial activity of rPcAxe not pre-incubated in different buffers was defined as 100%. Values are the means and standard deviations of triplicate experiments.
Additional file 5: Table S1. Hydrolysis of water-soluble wheat arabinoxylan by recombinant xylanase, rPcAxe and the enzymatic mix.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional files.


