Highlights
-
•
t(14;18)(q32;q21) occurs in about one tenth of middle aged Jordanian males.
-
•
Occupational exposure to pesticides increased the frequency of t(14:18) in farmers.
-
•
Age, smoking or personal protective equipment use did not influence t(14;18) frequency.
Keywords: Pesticides, Translocation t(14;, 18), Follicular lymphoma
Abstract
Background
An increased incidence of non-Hodgkin’s lymphoma (NHL) has been reported in farmers and other occupational groups working with pesticides. In these individuals, an increased prevalence of the chromosomal translocation t(14;18)(q32;q21), one of the most common chromosomal abnormalities in NHL, has been detected in peripheral blood lymphocytes. This translocation juxtaposes the antiapoptotic BCL2 protein to the immunoglobulin heavy chain gene locus (IGH) leading to overexpression of BCL2. This causes an increase in cell survival, paving the way for malignant transformation.
Aim of the study
The present study aimed to evaluate the association between the occurrence of the chromosomal translocation t(14;18) and occupational exposure to pesticides among a group of Jordanian farmers.
Methods
A total of 192 male subjects including 96 agricultural workers and 96 control subjects participated in this study. BCL2-IGH t(14;18) fusions were detected by a nested polymerase chain reaction (PCR) assay targeting the major breakpoint region (MBR).
Results
We found that occupational exposure to pesticides in open-field farming and insecticide used on animals increased the frequency of the chromosomal translocation t(14;18). Farmers occupationally exposed to pesticides and insecticide were 13.5 times more likely to harbor t(14;18). 63.5% (61 of 96) of farmers compared to 11.5% (11 of 96) of controls carried the translocation (odds ratio: 13.5; 95% confidence interval (CI) = 6.3–28.6). We ruled out the influence of possible confounding factors such as age, duration of sun exposure, alcohol intake, smoking, and use of personal protective equipment.
Conclusion
Our results indicate that pesticides increased the frequency of chromosomal translocation in the 14q32 region. Accordingly, the presented data agrees with previous suggestions from the literature that pesticides might be involved in the development of NHL through the t(14;18) pathway.
1. Introduction
Non-Hodgkin’s lymphoma (NHL) is a heterogeneous group of Lymphoproliferative malignancies that can arise from B- or T-lymphocytes. B-cell NHLs are primarily most abundant in adulthood. Follicular lymphoma is one of the most common types of B-cell derived NHL in western countries [1]. However, in Jordan, it only accounts for 8.1% of cases of diagnosed B-cell lymphoma, with the majority being attributed to Diffuse Large B-cell lymphoma [2].
The chromosomal translocation t(14;18)(q32;q21) is one of the most common chromosomal abnormalities in NHL, which occurs in 70–90% of cases of follicular lymphoma (FL), 20–30% of diffuse large B-cell lymphoma, and 5–10% of other less common subtypes [3]. This translocation involves 2 specific loci; the immunoglobulin heavy chain (IgH) locus on chromosome 14q32 and the B-cell leukemia/lymphoma-2 (BCL-2) locus on chromosome 18q2l [4]. During the typical translocation process, the BCL2 gene located on chromosome 18 is juxtaposed to the transcriptionally active IgH gene on chromosome 14 resulting in over expression of the former. Consequently, the heightened anti-apoptotic function of BCL2 increases cell survival, which represents an early step in the malignant process of NHL [4], [5], [6].
Pesticides are substances or mixture of substances used for preventing, destroying, repelling, or mitigating pests, which include insects, rodents, weeds, as well as other unwanted organisms. Most commonly, they are classified relying on the target species they act on such as insecticides, herbicides, or fungicides [7]. Pesticides may cause a variety of acute and delayed adverse health effects in those who are exposed. Acute effects range from simple skin and eyes irritation to general malaise, while chronic long-term effects encompass cancer and adverse reproductive outcomes [8]. Carcinogenic pesticides exist among insecticides, herbicides, and fungicides as well as in several chemical classes such as triazines, organophosphates and organochlorines [8], [9], [10]. Pesticides may exert their carcinogenic effects through a variety of mechanisms including genotoxicity [9].
An increased incidence of NHL has been reported among farmers and other occupational groups working with pesticides [9]. Furthermore, an increased prevalence of the chromosomal translocation t(14;18)(q32;q21) has been detected in peripheral blood lymphocytes from individuals occupationally exposed to pesticides [11]. To date, the relationship between pesticide exposure and occurrence of t(14;18) chromosomal translocation in farmers from Jordan, where FL is not common, has not been investigated.
The aim of this study is to evaluate if occupational exposure to pesticides among farmers in Jordan may contribute as a risk factor to the occurrence of the chromosomal translocation t(14;18)(q32;q21). We detected the translocation t(14;18) by a nested polymerase chain reaction (PCR) assay targeting the major breakpoint region (MBR) from peripheral blood lymphocytes of a group of farmers. We assessed pesticide exposure in detail taking into account potential confounding factors including smoking, sunlight, and age. To the best of our knowledge, this is the first study in Jordan and the Middle East region to address this issue.
2. Materials and methods
2.1. Study subjects
Study subjects were selected from the Jordanian Ghor region, the northern part of the Jordan Valley, a key agricultural area in the country. 96 farmers occupationally exposed to pesticides and 96 control individuals not exposed to pesticides were recruited from the town of Deir Alla. All subjects signed informed consent forms to participate in this study. Two farms were included: The Jordan University Agricultural Research Center Farm and The National Center for Agricultural Research and Extension (NCARE). In Both farms, agricultural activities consisted of open field farming, green house farming as well as cattle and poultry breeding. Inclusion criteria for cases were as follows: Jordanian males, age ≥ 18 years, direct exposure to pesticides including one or more of the following: using pesticides on open field crop, using insecticides on animals, or using herbicides. Agricultural workers with less than 6 months of pesticide exposure, or indirect exposure, were excluded. Inclusion criteria for controls were as follows: Jordanian males, age ≥ 18 years, never undertaken work related to farming. Subject were interviewed face-to-face and given a comprehensive questionnaire detailing age, tobacco use, alcohol consumption, medical history—especially haemolymphatic cancer, agricultural practices, personal use pesticides, type(s) of pesticides used, years of exposure, use of protective equipment, and sunlight exposure.
2.2. Sample collection and DNA preparation
Peripheral blood samples (10 ml/subject) were collected in Vacutainer tubes EDTA (K2) collection tubes (BD Bioscience). DNA was extracted from buffy coat peripheral blood lymphocytes using Wizzard Genomic DNA Purification Kit (Promega, USA) according to the manufacturer’s protocol. DNA concentration and purity was measured using UV spectrophotometry (Bio-Rad, SmartSpec plus, USA). All samples had OD260/OD280 ratio values of 1.7–2.0. A working dilution for DNA samples of 100 ng/μl was prepared in nuclease-free water and stored at −20 °C for analysis.
2.3. PCR and nested-PCR
The ß-globin gene was used an internal gene control for DNA integrity (primer sequences are shown in Table 1) [12]. The PCR mixture contained 300 ng of genomic DNA, 1X Green GoTaq® Reaction Buffer (Promega, USA), 2.5 U GoTaq® DNA Polymerase (Promega, USA), 10 mM dNTPs Mix (Promega, USA) and 20 pmol oligonucleotide primer (Invitrogen, USA) in a final volume of 50 μl. The PCR conditions were an initial denaturation step at 94 °C for 1 min, followed by 35 cycles of 94 °C for 30 s, 57 °C for 45 s and 72 °C for 60 s, then a final extension step at 72 °C for 5 min. A negative control was included in each run, whereby no DNA template was added.
Table 1.
Primer sequences.
| ß-Globin primers | |
|---|---|
| ß-Globin F | Forward: 5′-CAA CTT CAT CCA CGT TCA CC-3′ |
| ß-Globin R | Reverse: 5′-GAA GAG CCA AGG ACA GGT AC-3′ |
| t(14;18) MBR, first round primers | |
| MBR-1 | Forward: 5′- CAGCCTGAAACATTGATGG-3′ |
| JH-1 | Reverse: 5′- ACCTGAGGAGACGGTGACC-3′ |
| t(14;18) MBR, second round primers | |
| MBR-2 | Forward: 5′-TATGGTGGTTTGACCTTTAG-3′ |
| JH-2 | Reverse: 5′- ACCAGGGTCCCTTGGCCCCA-3′ |
For BCL2-IGH t(14;18) fusions nested PCR amplification, we used specific primers for the major breakpoint region (MBR) in combination with JH consensus primers as described in Gribben et al. [12], [13] (Table 1). For each individual, 2–3 technical replicates were utilized in both rounds of the nested-PCR. Almost all replicates were homogeneous with respect to the observed PCR products, showing either a band of the expected size or not displaying a PCR product. Where replicates did not show that same result, be it positive or negative, the reaction was repeated. The PCR mixture for each sample contained 1 μg of genomic DNA, 1X Green GoTaq® Flexi Buffer (Promega, USA), 2.5 U GoTaq® Hot Start Polymerase (Promega, USA), 2.5 mM MgCl2 (Promega, USA), 10 mM dNTPs Mix (Promega, USA) and 20 pmol oligonucleotide primer (Midland oligos, USA) in a final volume of 50 μl. The reaction conditions for the first round of nested PCR were as follows: an initial denaturation step at 94 °C for 7 min, followed by 35 cycles of 94 °C for 30 s, 58 °C for 45 s, 72 °C for 60 s, with a final extension step at 72 °C for 5 min. One micro liter of the first round PCR product was re-amplified under the same conditions for 30 additional cycles in the second round. Both negative (no template) and positive controls were added in each PCR round. The positive controls used were plasmid DNA (Nanogen Advance Diagnostic, Italy) containing the MBR region within the BCL2-IGH rearrangement. Amplified PCR products, t(14;18) fusions, were analyzed on 3% agarose gel electrophoresis containing ethidium bromibe (1 μg/ml) and visualized under UV light, using gel documentation system (UVP, USA).
2.4. Statistical analysis
Data were summarized as average ± SD and range for continuous variables (age, duration of pesticides use and sunlight exposure) or counts (percentages) for categorical variables (smoking, alcohol consumption, pesticides use, insecticides use, herbicides use and protective clothing). Normality was checked by Kolmogorov–Smirnov test (K–S test) and homogeneity of variance by Levene’s test. Normally distributed continuous variables were analyzed using independent t-test, otherwise Mann–Whitney U test was used for variables with skewed distribution. Pearson Chi-square or Fisher’s exact test were used as appropriate to determine occupational exposure to pesticides and risk of developing t(14;18) translocation: the occurrence of BCL2-IGH t(14;18) translocation among farmers compared to the control group and within separate groups. Logistic regression was utilized to discern the true association between exposure to pesticides and development of genetic translocation in the presence of unequal distribution of confounders (age, smoking, sunlight exposure, and alcohol consumption). Mantel Hanzel statistics were utilized to calculate odds ratio and 95% confidence interval (OR, 95% CI). p values < 0.05 were considered significant. All the statistical analyses were performed using Statistical Package for Social Sciences (SPSS Inc., Chicago, Illinois) version 16.0 software for windows. Figures were compiled in GraphPad Prism 6.0 and Microsoft Powerpoint.
3. Results
3.1. Characteristics of the study subjects
Baseline characteristics of the study cohort; age, smoking and alcohol consumption are shown in Table 2. Farmers were slightly older than controls (mean age 31.8 vs 36.1 years old, respectively; p = 0.003) and had a higher alcohol consumption percentage (p = 0.023). No difference was detected between the two groups in smoking status (67.7% vs 64.6%, respectively; p = 0.65). Table 3 illustrates agricultural practices and exposures for farmers recruited in the study. Whereas 80.2% of farmers used pesticides in open fields (77 out of 96) and 95.8% used herbicides (92 out of 96), only 47.9% of them used insecticides on animals (46 out of 96). The average duration of pesticide use 10.9 years, with a range of 1–40 years. Only a minority used protective clothing.
Table 2.
General characteristics of farmers and controls.
| Characteristics | Farmers (n = 96) | Controls (n = 96) | p Value |
|---|---|---|---|
| Mean age ± SD [min–max] | 31.8 ± 8.8 [19–69] | 36.1 ± 10.7 [18–67] | 0.003* |
| Smoking (n) | 67.70% (65) | 64.60% (62) | 0.65** |
| Alcohol consumption (n) | 16.70% (16) | 6.30% (6) | 0.023** |
<0.05.
<0.01.
Table 3.
Occupational characteristics of farmers.
| Characteristics | Farmers (n = 96) |
|---|---|
| Mean duration of pesticide use (years ± SD) [min–max] | 10.9 ± 7.9 [1–40] |
| Pesticide use on open fields | 80.2% (77) |
| Insecticide use on animals | 47.9% (46) |
| Herbicide use | 95.8% (92) |
| Protective clothing | |
| Mask only (n) | 2.1% (2) |
| Mask + gloves (n) | 27.1% (26) |
| Mean of sunlight exposure (hours/day ± SD) [min–max] | 5.9 ± 1.6 [2–9] |
3.2. The frequency of t(14;18) translocation in farmers and controls
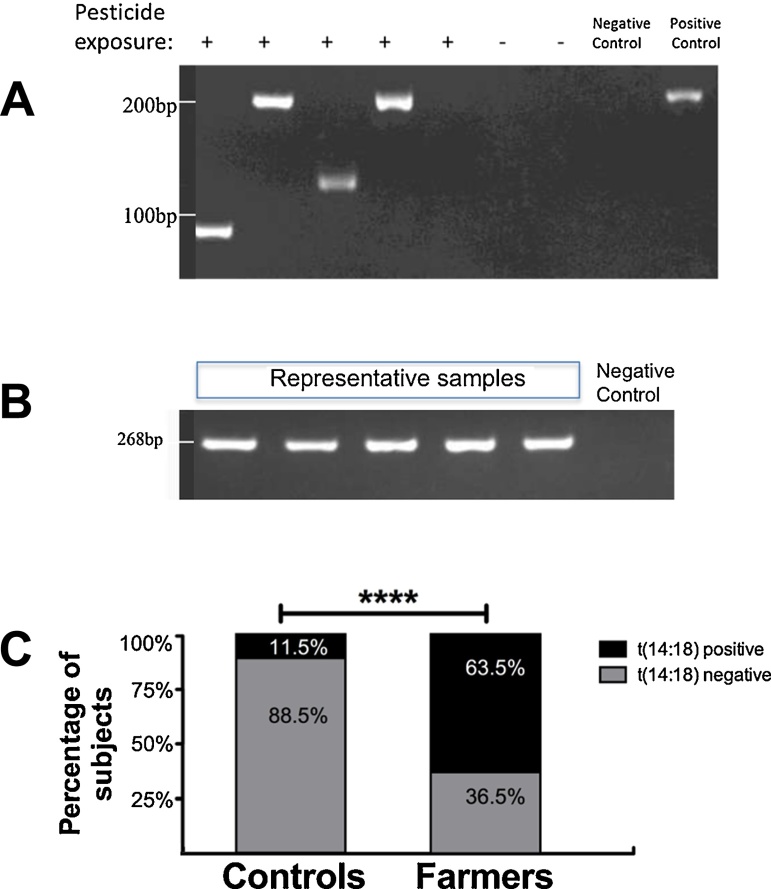
BCL2-IGH t(14;18) fusions were detected using nested PCR targeting the MBR region, with specific products measuring from 80 to 300 base pairs as shown in Fig. 1A. Representative PCR reactions for the internal control β-Globin are shown in Fig. 1B. The frequency of BCL2-IGH t(14;18) translocation in farmers occupationally exposed to pesticides was 63.5% (61 of 96) vs 11.5% (11 of 96) in the control group. Farmers occupationally exposed to pesticides were 13.5 times more likely to bear the chromosomal translocation t(14;18) compared to the control group (p < 0.0001; odds ratio (OR) = 13.5; 95% confidence interval (CI) = 6.3–28.6) Fig. 1C. In sum, these results show a significant association between occupational exposure to pesticides and an increased frequency of the chromosomal translocation BCL2-IGH t(14;18) in farmers.
Fig. 1.
Nested polymerase chain reaction (n-PCR) detection of t(14:18) IGH:BCL2 in DNA from farmers and controls. (A) Representative electrophoretic separation of PCR products from the second round of n-PCR using primers specific to the major break point (MBR) region. The primers detect products ranging from 80 to 300 base pairs (bp) in the MBR region. Products are seen in 4 out of 5 samples from farmers and the positive control, but not in the negative control or control samples. (B) Representative electrophoretic separation of PCR products from participant samples and water (negative) control for internal control β-Globin. A band measuring 268 bp is seen for the participant samples but not the negative control. (C) Quantification of the percentage of t(14:18) IGH:BCL2 positivity showing a significant increase in the percentage of t(14:18) IGH:BCL2 positivity in farmers (63.5%) compared to controls (11.5%), with an odds ratio (OR) = 13.5; 95%, and a confidence interval (CI) = 6.3–28.6. ****p < 0.0001 calculated using Pearson Chi-Square test.
Next, we assessed the frequency of t(14;18) in relation to the type of pesticide used, duration of pesticide use, and individual protective clothing within the farmers group. We found significant associations with pesticide use on open field crops (OR = 3.0, 95% CI = 1.1–8.5, p = 0.03) and insecticides on animals (OR = 2.4, 95% CI = 1.02–5.7, p = 0.043). No significant associations were observed with herbicides use (OR = 0.57, 95% CI = 0.06–5.7, p = 0.627), individual protective clothing (Mask only; OR = 0.7, 95% CI = 0.04–0.7, p = 0.99. Mask and gloves; OR = 2.3, 95% CI = 0.8–6.6, p = 0.15), and mean duration of pesticide use (mean ± standard deviation (SD); BCL2-IGH t(14:18) positive 10.6 ± 7.9 years vs negative 11.7 ± 8.2 years, p = 0.51).
3.3. The occurrence of t(14;18) and the influence of confounding factors
We assessed the effect of age, smoking, and alcohol consumption—factors known to influence the risk of NHL—as confounding factors on the frequency of BCL2-IGH t(14;18) in all study subjects. Initially, we examined whether there was a difference in age between t(14;18) positive and negative cases in the pooled population of farmers and control. The mean age of the t(14;18) positive and negative cases was not significantly different (mean ± SD: 32.3 ± 8.9 in positive cases; 34.9 ± 10.5 in negative cases; p = 0.077). Additionally, the proportion of t(14;18) positive cases who smoked was not different from that in t(14;18) negative cases (66.7% in positive cases; 65.8% in negative cases; p = 0.9). Similarly, alcohol intake was not significantly different between positive and negative cases (16.7% in positive cases; 8.3% in negative cases; p = 0.08). Finally, we examined the influence of the aforementioned confounders in farmers and controls separately. We did not detect any significant associations between BCL2-IGH t(14;18) frequency and age, sunlight exposure, smoking status, or alcohol intake, in the farmers group (Fig. 2) or the control group (data not shown). Therefore, we convincingly show that the risk of BCL2-IGH t(14;18) is related to pesticide exposure in open fields and insecticide use on animals, but not to age, smoking, alcohol intake, or sunlight exposure.
Fig. 2.
Evaluation of the contribution of confounding factors to t(14:18) IGH:BCL2 detection in farmers. t(14:18) IGH:BCL2 positivity was tested against age (A), sunlight exposure (B), smoking (C) and alcohol intake (D) in the farmers group (n = 96). There was no significant difference between t(14:18) IGH:BCL2 positive and negative groups with respect to age (p = 0.92), sunlight exposure (p = 0.43), smoking (OR = 1.2, 95% CI = 0.5–2.8, p = 0.752), or alcohol intake (OR = 1.3, 95% CI = 0.4—4.2, p = 0.78). Significance was tested for using Independent t-test (A, B), and Pearson Chi-Square test for (C, D).
4. Discussion
Follicular lymphoma is closely associated with BCL2-IGH t(14;18) translocation [14] which increases survival of B-cells that harbor the translocation [5], paving the way for malignant transformation [15]. We previously found that BCL2-IGH t(14;18) can be detected in 95% of FL cases using a very nested PCR assay, and that it occurs at the major breakpoint region (MBR) in 81% of cases, whereas only 3.5% and 10.5% of cases are attributed to the minor cluster region (mcr) and intermediate cluster region (icr), respectively [6]. We therefore focused on MBR detection in healthy individuals for this study.
We note the limitations of our study; first, our method discounts detection of icr and mcr positive translocations. Second, we did not quantify the levels of t(14;18) detected in peripheral blood and therefore cannot infer the frequency of t(14;18) positive lymphocytes. Finally, it is difficult to comment on potential dose-response relationships, since information relating to accurate amounts of pesticide used or post-exposure blood levels is lacking.
Despite the aforementioned limitations, our findings support the association between pesticide exposure and increased frequency of BCL2-IGH t(14;18) translocation detection described by others [11], [16], [17]. The association was evident for exposure to pesticides in open-field farming and use of insecticides on animals, but not for the use of herbicides, possibly due to the low number of farmers who used herbicides (4 out 96). The use of protective gloves and masks did not show a reduction in detection frequency. This might be explained by the fact that only 2.1% of farmers used masks, and 27.1% used masks and gloves, reducing the power of the study to detect statistical differences. This is an alarming fact that puts the health of Jordanian farmers at risk, and warrants immediate action by the Ministry of Agriculture to protect farmers against potential pesticide-related ill effects. Further studies are required to assess whether personal protective equipment can reduce the detection frequency of t(14;18) in farmers exposed to pesticides.
We assessed the effects of potential confounding factors on the frequency of BCL2-IGH t(14;18) translocation detection. Firstly, alcohol consumption did not contribute to the frequency of detection, potentially due to the low rate of alcohol consumption in our study group (16.7% for the farmers group and 6.3% for the control group). Additionally, we did not detect an association between the age participants and the frequency of BCL2-IGH t(14;18) translocation detection, consistent with Roulland et al. [11], and contrary to what was described by others [18], [19]. Both our study and Roulland et al. [11] included subjects with median ages less than 50 years old, where as association with age was detected only in samples older than 60 [19] and 70 years [18] of age. Moreover, cigarette smoking did not increase the frequency of BCL2-IGH t(14;18) translocation detection in our study sample, consistent with a previous report [18]. Of note, cigarette smoking was not found to be associated with t(14:18)-positive and negative NHL in men [20], [21]. Furthermore, sunlight exposure did not show an effect on t(14:18) detection frequency, consistent with what others have shown [11]. Finally, we did not find a correlation between the duration of pesticide exposure and detection frequency. It was shown than cumulative exposure to pesticides increases the frequency of t(14:18) bearing cells in the blood [17], we did not, however, quantify the frequency of t(14:18) positive cells in this study. It is therefore possible that individuals with longer exposure periods might have more translocation bearing cells.
Previous studies detected the BCL2-IGH t(14;18) translocation in the peripheral blood and reactive lymph nodes of healthy cancer-free individuals [11], [16], [17], [21], [22], [23], [24]. A recent study by Roulland et al. [25] incriminates positivity for t(14:18) as a predictive marker of and a risk factor for FL. The authors found increased risk for FL with high frequency of t(14:18) positive cells (>1 × 104 cells). Despite the relatively low frequency of FL in Jordanians, pesticide exposure increased t(14;18) detection in farmers, making the link between pesticide exposure and lymphoma-genesis stronger. Therefore, we posit that pesticides contribute to promoting t(14;18) translocation regardless of the population of study. Finally, given the low cost, rapidity, high sensitivity and specificity of our nested-PCR assay in detecting t(14;18), coupled with the aforementioned evidence incriminating the translocation in lymphoma-genesis, it may therefore be important and feasible to screen and monitor farmers occupationally exposed to pesticides for the presence and levels of t(14:18) bearing cells over the period of exposure.
5. Conclusion
Our study is the first in Jordan and the Middle East to document the association between occupational exposure to pesticides and insecticides, and increased detection frequency of BCL2-IGH t(14;18) translocation in healthy farmers. We show that pesticides are important contributing factors to chromosomal translocations in the region 14q32, which is directly relevant to lymphoma-genesis.
Conflict of interest
Authors declares that there is no conflict of interest.
Transparency document
Acknowledgement
We thank to the Deanship for scientific research at the University of Jordan for funding this work.
References
- 1.Muller A.M., Ihorst G., Mertelsmann R., Engelhardt M. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Annal. Hematol. 2005;84:1–12. doi: 10.1007/s00277-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 2.Almasri N.M., Habashneh M.A., Khalidi H.S. Non-Hodgkin lymphoma in Jordan—types and patterns of 111 cases classified according to the WHO classification of hematological malignancies. Saudi Med. J. 2004;25:609–614. [PubMed] [Google Scholar]
- 3.Janz S., Potter M., Rabkin C.S. Lymphoma- and leukemia-associated chromosomal translocations in healthy individuals. Genes Chromosomes Cancer. 2003;36:211–223. doi: 10.1002/gcc.10178. [DOI] [PubMed] [Google Scholar]
- 4.Nadel B., Marculescu R., Le T., Rudnicki M., Bocskor S., Jager U. Novel insights into the mechanism of t(14;18)(q32;q21) translocation in follicular lymphoma. Leukemia Lymphoma. 2001;42:1181–1194. doi: 10.3109/10428190109097743. [DOI] [PubMed] [Google Scholar]
- 5.Hockenbery D., Nunez G., Milliman C., Schreiber R.D., Korsmeyer S.J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 6.Ismail S.I., Sughayer M.A., Al-Quadan T.F., Qaqish B.M., Tarawneh M.S. Frequency of t(14;18) in follicular lymphoma patients: geographical or technical variation. Int. J. Lab. Hematol. 2009;31:535–543. doi: 10.1111/j.1751-553X.2008.01075.x. [DOI] [PubMed] [Google Scholar]
- 7.Costa L. Toxic effects of pesticides. In: Klaassen C., editor. Casarett and Doull’s Toxicology—The Basic Science of Poisons. 7th ed. McGraw-Hill Medical Publishing Division; New York: 2008. pp. 883–930. [Google Scholar]
- 8.Sanborn M., Kerr K.J., Sanin L.H., Cole D.C., Bassil K.L., Vakil C. Non-cancer health effects of pesticides: systematic review and implications for family doctors. Can. Fam. Physician. 2007;53:1712–1720. [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu B.C., Blair A. Pesticides, chromosomal aberrations, and non-Hodgkin’s lymphoma. J. Agromed. 2009;14:250–255. doi: 10.1080/10599240902773140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dich J., Zahm S.H., Hanberg A., Adami H.O. Pesticides and cancer. Cancer Causes Control: CCC. 1997;8:420–443. doi: 10.1023/a:1018413522959. [DOI] [PubMed] [Google Scholar]
- 11.Roulland S., Lebailly P., Lecluse Y., Briand M., Pottier D., Gauduchon P. Characterization of the t(14;18) BCL2-IGH translocation in farmers occupationally exposed to pesticides. Cancer Res. 2004;64:2264–2269. doi: 10.1158/0008-5472.can-03-3604. [DOI] [PubMed] [Google Scholar]
- 12.Taspinar M., Aydos S.E., Comez O., Elhan A.H., Karabulut H.G., Sunguroglu A. CYP1A1 GST gene polymorphisms and risk of chronic myeloid leukaemia. Swiss Med. Wkly. 2008;138:12–17. doi: 10.4414/smw.2008.12036. [DOI] [PubMed] [Google Scholar]
- 13.Gribben J.G., Freedman A.S., Neuberg D. Immunologic purging of marrow assessed by PCR before autologous bone marrow transplantation for B-cell lymphoma. New Engl. J. Med. 1991;325:1525–1533. doi: 10.1056/NEJM199111283252201. [DOI] [PubMed] [Google Scholar]
- 14.Tsujimoto Y., Finger L.R., Yunis J., Nowell P.C., Croce C.M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 15.Staudt L.M. A closer look at follicular lymphoma. New Engl. J. Med. 2007;356:741–742. doi: 10.1056/NEJMcibr067155. [DOI] [PubMed] [Google Scholar]
- 16.Garry V.F., Tarone R.E., Long L., Griffith J., Kelly J.T., Burroughs B. Pesticide appliers with mixed pesticide exposure: G-banded analysis and possible relationship to non-Hodgkin’s lymphoma. Cancer Epidemiol. Biomarker Prev. 1996;5:11–16. [PubMed] [Google Scholar]
- 17.Agopian J., Navarro J.M., Gac A.C. Agricultural pesticide exposure and the molecular connection to lymphomagenesis. J. Exp. Med. 2009;206:1473–1483. doi: 10.1084/jem.20082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirt C., Weitmann K., Schuler F. Circulating t(14;18)-positive cells in healthy individuals: association with age and sex but not with smoking. Leukemia Lymphoma. 2013;54:2678–2684. doi: 10.3109/10428194.2013.788177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Hernandez A.M., Shibata D., Cortopassi G.A. BCL2 translocation frequency rises with age in humans. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8910–8914. doi: 10.1073/pnas.91.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder J.C., Olshan A.F., Baric R. Agricultural risk factors for t(14;18) subtypes of non-Hodgkin’s lymphoma. Epidemiology. 2001;12:701–709. doi: 10.1097/00001648-200111000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Chiu B.C., Dave B.J., Blair A. Cigarette smoking, familial hematopoietic cancer, hair dye use, and risk of t(14;18)-defined subtypes of non-Hodgkin’s lymphoma. Am. J. Epidemiol. 2007;165:652–659. doi: 10.1093/aje/kwk044. [DOI] [PubMed] [Google Scholar]
- 22.Schuler F., Hirt C., Dolken G. Chromosomal translocation t(14;18) in healthy individuals. Semi. Cancer Biol. 2003;13:203–209. doi: 10.1016/s1044-579x(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 23.Bell D.A., Liu Y., Cortopassi G.A. Occurrence of bcl-2 oncogene translocation with increased frequency in the peripheral blood of heavy smokers. J. Natl. Cancer Inst. 1995;87:223–224. doi: 10.1093/jnci/87.3.223. [DOI] [PubMed] [Google Scholar]
- 24.Tellier J., Menard C., Roulland S. Human t(14;18) positive germinal center B cells: a new step in follicular lymphoma pathogenesis? Blood. 2014;123:3462–3465. doi: 10.1182/blood-2013-12-545954. [DOI] [PubMed] [Google Scholar]
- 25.Roulland S., Kelly R.S., Morgado E. t(14;18) translocation: a predictive blood biomarker for follicular lymphoma. J. Clin. Oncol. 2014;32:1347–1355. doi: 10.1200/JCO.2013.52.8190. [DOI] [PubMed] [Google Scholar]