Abstract
Tetrabromobisphenol A (TBBPA), a nongenotoxic flame retardant, causes uterine tumors in female rats. A proposed mode of action (MoA) for these tumors involves an increase in the bioavailability of estradiol as a result of TBBPA inhibiting estrogen sulfotransferases (ES), the enzymes responsible for inactivating and enhancing the elimination of estradiol. The objective of this study was to evaluate the effect of dose and repeated administration of TBBPA on the level of TBBPA, TBBPA-glucuronide (GA) and TBBPA-sulfate (S) conjugates in plasma, liver and uterus of female Wistar Han rats administered TBBPA (50, 100, 250, 500 or 1000 mg/kg) for 28 consecutive days. In accordance with this objective, TBBPA sulfation was used as a surrogate for evaluating the potential for estradiol sulfation to be limited at high dose levels of TBBPA. Blood samples were collected at 4 and 8 h post-dosing on study day 7, 14, and 28, while liver and uterus were collected at the same time points following 28 days of dosing. Tissue samples were analyzed for TBBPA, TBBPA-GA and TBBPA-S by LC–MS/MS. A dose-related increase in the concentration of all three analytes occurred in plasma (day 7, 14, and 28) as well as liver and uterus tissue (day 28) at both 4 and 8 h post dose. The plasma concentration of TBBPA-GA and TBBPA-S was higher in animals dosed for 28 days compared to those dosed for 7 or 14 days showing an increase in systemic circulation of these conjugates with repeated administration. The balance of these conjugates was also different in tissues with TBBPA-S > TBBPA-GA at high doses in the liver and TBBPA-GA > TBBPA-S in both plasma and uterus. In all three tissues the ratio of TBBPA-S/TBBPA-GA showed a decreasing trend with dose, suggesting that at high TBBPA dose levels sulfation of TBBPA becomes limited. This effect was most apparent in the liver and plasma at 28 days of administration. Together these data show that administration of high doses of TBBPA associated with the induction of uterine tumors, results in a disruption in the balance of conjugates reflected by a decrease in the TBBPA-S/TBBPA-GA ratio. A limitation in the sulfation of TBBPA in vivo supports in vitro data defining TBBPA as an inhibitor of ES activity, thus providing further support that the proposed MoA occurs under conditions of high dose, chronic TBBPA administration to Wistar Han rats. Given that the uterine tumors observed in rats (250–1000 mg/kg-day) only occur at very high doses that perturb homeostatic control, it is unlikely such effects would occur in humans given that current TBBPA exposure levels are approximately eight orders of magnitude lower than these doses that are associated with exceeding the capacity of conjugation pathways in animal studies.
Keywords: TBBPA, Estrogen sulfotransferases, Glucuronidation, Uterus, Liver
1. Introduction
Tetrabromobisphenol A (TBBPA) is the most widely produced brominated flame retardant, primarily because of its effectiveness and low hazard profile (http://www.bsef.com). It is used in epoxy, polycarbonate and phenolic resins in circuit boards, as well as in acrylonitrile–butadiene–styrene (ABS). Examples of products containing TBBPA include printed circuit boards, communications and electronics equipment, appliances, transportation devices, sports and recreation equipment, automotive parts, pipes and fittings [2]. Humans can be exposed to TBBPA during manufacturing and production, via use of TBBPA-containing products, and via recycling of TBBPA-containing products. TBBPA has been detected in the ppb range in human serum in cases of exposure in occupational and non-occupational settings, as well as in breast milk, demonstrating that TBBPA is in fact absorbed in exposed human populations [19], [34], [32], [33].
The acute oral toxicity of TBBPA in rats is low (LD50 > 5 g/kg) [18], with little toxicity identified overall in a number of robust standard 28- and 90-day toxicology studies [8], [28], [38], [29]. In a recent cancer bioassay conducted by the National Toxicology Program (NTP), TBBPA was administered to Wistar Han rats at dose levels of 250, 500, and 1000 mg/kg for 2 years. The NTP reported an increased incidence of uterine epithelial tumors in female rats (combined adenoma, adenocarcinoma, or malignant mixed Mullerian tumor) at the mid- and high dose levels of 500 mg/kg-day (32%) and 1000 mg/kg-day (38%), when compared to the vehicle control group (12%) [28]. Given that TBBPA has been shown not to be mutagenic or genotoxic [15], [8], several investigators have proposed a mode of action (MoA) for uterine tumors that involves endocrine mediated events that would result in a disruption of estrogenic activity [6], [26]. A review of both the published literature and high throughput screening (HTS) data (http://actor.epa.gov/edsp21/) has shown that TBBPA does not directly interact with the estrogen receptor (ER) [42]. Based on the lack of ability of TBBPA to be an ER agonist, the focus of this current investigation has been on the ability of TBBPA to directly disrupt the metabolism of estrogen.
To date, toxicokinetic data on TBBPA are limited to relatively traditional kinetic studies. Existing data reflect administration of varying dose levels of TBBPA for different durations in order to characterize TBBPA’s major metabolites in multiple strains of rats and humans [12], [25], [31], [20], [24]. Overall, TBBPA is rapidly absorbed and efficiently metabolized by glucuronyltransferase and sulfotranferase enzymes in the liver. The major route of elimination of administered TBBPA is in feces, with minor elimination in urine resulting in low bioavailability [12], [25], [24]. Together the data from these studies support the conclusion that TBBPA is cleared via bile for fecal elimination with the two major metabolites identified as a TBBPA-sulfate and TBBPA-glucuronide conjugate.
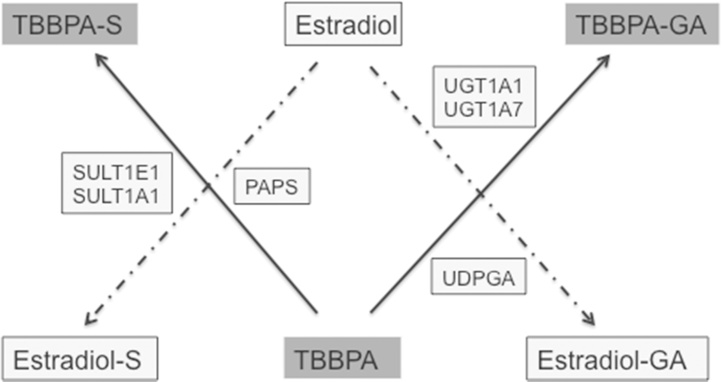
Similar to TBBPA, estrogen and its metabolites are conjugated to both sulfate and glucuronic acid, with sulfation serving as the main pathway for the inactivation of estrogen [43]. Kester et al. [21] reported that TBBPA inhibits estrogen sulfotransferase (ES) in vitro with IC50 values ranging from 12 to 33 nM. Another study conducted by Hamers et al. [13] confirmed that TBBPA acts as a potent inhibitor of ES in vitro (IC50 = 16 nM), with it being approximately 13 times more potent than pentachlorophenol (PCP), a known inhibitor of sulfotransferase. A QSAR study evaluated binding to ES and found that TBBPA fulfilled the chemical structure criteria that would predict potent inhibition of ES [14]. A more recent evaluation of the crystal structure of ES involving computation modeling found that TBBPA binds to the same site as estradiol [10]. Taken together, these studies provide evidence to support the hypothesis that TBBPA inhibits ES, and more specifically the isoforms SULT1E1 and SULT1A1. When these data are considered, and metabolic pathways for TBBPA and estrogen compared (Fig. 1), it appears that TBBPA could compete in vivo with the same enzyme systems as estrogens. However, currently there are no data available to characterize such a relationship in vivo—and, importantly, to characterize the doses at which ES inhibition may occur and affect the sulfation of estrogens. These data are critical for determining the feasibility for TBBPA to influence estrogenic potential through a disruption of its metabolism. Several studies [6], [26] proposed that the TBBPA-inhibition of ES, a key enzyme in the inactivation and elimination of estrogens, would potentially result in an increased bioavailability of estrogens, which, in both humans and rats, is associated with endometrial tumors [16].
Fig. 1.
Schematic to illustrate the competition of TBBPA with estradiol for sulfation and potentially glucuronidation in vivo. SULT1E1 and SULT1A1 are estrogen sulfotransferases with 3′-phosphoadenosine 5′-phosphosulfate (PAPS) being the cofactor that donates sulfate. Members of the glucuronyltransferase family, UGT1 have preferential recognition for estrogens and have been identified in human and rat uterine tissue [11], [27]. UGT1A1 is identified as the isoform involved with the conjugation of estradiol and its metabolites using uridine diphosphate glucuronic acid (UDPGA) to donate glucuronic acid. UGT1A7 has broad specificity and glucuronidates both planar and nonplanar compounds, polycyclic aromatics and compounds with bulky side chain ring substitutions [40], [27].
Kinetic data are not yet available to determine if the administration of TBBPA at dose levels used in the NTP study that were associated with uterine tumors in rats, inhibits ES in vivo, and subsequently, if such an inhibition could result in an increased bioavailability of “unconjugated” estradiol in the uterus (an estrogen responsive tissue). Based on the challenges associated with directly measuring estrogens and its metabolites in tissues (e.g., assay sensitivity, specificity, and variability), the objective of this study was to determine if conjugation of TBBPA to sulfate would be limited at dose levels associated with the development of pre-neoplastic and neoplastic changes in the uterus of rats following chronic administration of TBBPA. TBBPA sulfation can be used as a surrogate for indirectly measuring the potential disruption in estradiol conjugation since both are metabolized through the same enzyme systems. Investigating the dose level at which the sulfation of TBBPA exceeds its capacity would provide further support that the sulfation of estradiol in vivo would be impacted following repeated high dose levels of TBBPA.
2. Materials and methods
2.1. Chemicals
Tetrabromobisphenol A (TBBPA) [CAS No. 79-94-7] was obtained from Albemarle Corporation (Baton Rouge, LA) with a purity of 98.83%. Corn oil [CAS no. 8001-30-7], used as the vehicle, was obtained from MP Biomedical, LLC (Solon, OH). All reagents used for LC–MS/MS analysis were HPLC grade. 13C12-TBBPA was purchased from Cambridge Isotope Laboratories and control rat plasma was purchased from Innovation Research Inc.
2.2. TBBPA dose formulation
TBBPA dose formulations were prepared in corn oil at concentrations of 0, 10, 20, 50, 100, and 200 mg/mL to administer dose levels of 0, 50, 250, 500, and 1000 mg TBBPA/kg body weight, respectively, at a volume of 5 mL/kg. TBBPA dose formulations were reported to be stable in corn oil at room temperature for up to 40 days [28]. As such, dose formulations were prepared once every 2 weeks (2×) during this 28-day study. Dose concentration and homogeneity were confirmed by sampling the top, middle and bottom of each formulation and analyzing by HPLC-UV (285 nm) using a Shiseido Capcell Pak C18 column, 3 × 100 mm, 3 μm with a flow of 0.6 mL/min. Briefly, the HPLC method consisted of a mobile phase of 0.1 mL of trifluoroacetic acid (TFA) in deinonized water (A) and (B) 0.1 mL of TFA in acetonitrile. The gradient was 0 to 40% B over 4 min with a linear increase to 100% B up to 6 min. Approximate retention time for TBBPA was 5.6 min. Concentration and homogeneity results were acceptable if the mean concentration was within 15% of the target and the coefficient of variation in the homogeneity was less than 15%.
2.3. Animal model
Female Wistar (CRL:W(Han)) rats were obtained from Charles River Laboratory International, Inc (Kingston, NY) at approximately 9 weeks of age and supplied with NTP-2000 wafer diet (Zeigler Bros., Gardners, PA) and reverse-osmosis-treated tap water (City of Durham, NC) ad libitum. Animals were acclimated for 8 days prior to the initiation of the study and housed 3 per cage in polycarbonate cages with absorbent heat-treated hardwood bedding. The temperature and humidity of the animal rooms were held between 23.4–25.1°C and 41–64.9%, respectively, with a 12/12 h light/dark cycle. All animal procedures were in compliance with the Animal Welfare Act Regulations, 9CRF 1–4 with animals being handled and treated according to the Guide for the Care and Use of Laboratory Animals [17].
2.4. Study design
Strain of rats, animal vendor, diet and dose formulation (corn oil as vehicle) were identical to the specifications used in the TBBPA chronic NTP study [28]. Female Wistar Han rats (10 weeks of age) were allocated to one of six designated dose groups using a procedure that stratifies animals across groups by body weight such that the mean body weight of each group was not statistically different from any other group. Within each dose group animals were designated for tissue collection at either 4 or 8-h post dosing (n = 6 per group). Animals were administered corn oil (vehicle control- 0 mg/kg TBBPA) or one of the five dose levels of TBBPA (50, 100, 250, 500, and 1000 mg/kg) by oral gavage daily for 28 consecutive days. Body weight measurements and clinical observations were performed within 2 days upon animal arrival, prior to dose group allocation and daily prior to dose administration. Blood was collected in EDTA containing tubes from animals via the retro-orbital plexus (under isoflurane anesthesia) 4 and 8 h following dose administration on study days 7 and 14 and processed to plasma. At study termination on day 28, blood was collected (processed to plasma) under isoflurane anesthesia by cardiac puncture at 4 and 8 h following the final dose and the liver and uterus collected, weight recorded and tissue specimens were flash frozen in liquid nitrogen prior to storing at/or below −70 °C. Plasma was prepared by centrifugation of blood at 10,000 × g for 5 min at 4 °C and stored at or below −70 °C.
2.5. Liquid chromatography/mass spectrometry (LC–MS/MS)
In preparation for analysis, liver and uterine tissues were thawed on ice and homogenized in deionized water at a ratio of 2 mL per 1 g of tissue using a PowerGen 125 (∼15,000–20,000 rpm) for 30 s pulses to minimize heat. For uterine tissue homogenates, an additional 1 mL of ice-cold deionized water was added and further homogenized using a Retsch Mixer Mill with four steel beads per sample for 5 min at 30 cps. Homogenates were stored frozen at or below –70 °C until analyzed.
Plasma and liver homogenate (20 μL) samples were fortified with the internal standard 13C12-TBBPA in acetonitrile. Precipitated proteins were removed by centrifugation. Approximately 200 mg of uterine tissue homogenate was extracted (2x) with methyl tert-butyl ether (MTBE). MTBE layers were combined into a single clean tube, evaporated to dryness under nitrogen and samples dissolved in 50% methanol containing the internal standard 13C12-TBBPA.
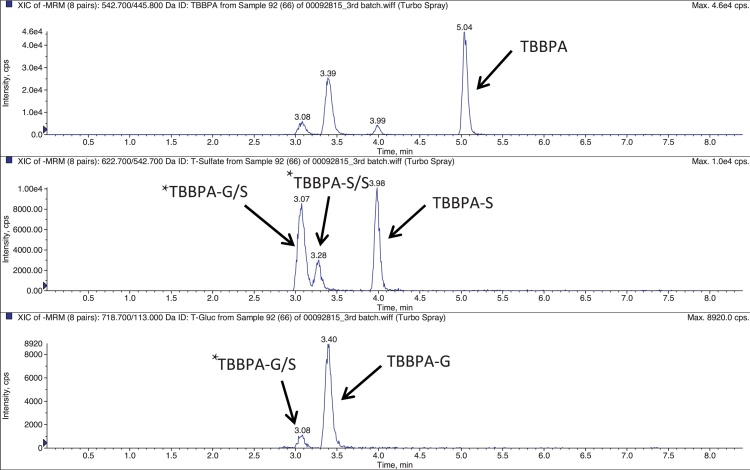
TBBPA, TBBPA-GA and TBBPA-S were quantitated by LC-MS/MS using electrospray ionization. Samples were separated on a Capcell Pak C18 HPLC column (3 × 100 mm; 3 μm; Shiseido, Japan) using an Agilent 1100 autosampler and an Agilent 1100HPLC pump. Separation was performed by gradient elution with 8 mM ammonium acetate (solvent A) and acetonitrile/8 mM ammonium acetate (solvent B) using the following conditions: 90% A, 10% B, linear increase to 100% B within 3 min, isocratic for 2 min at a flow rate of 500 μL/min. The HPLC system was directly coupled to a triple-stage quadrupole mass spectrometer (API 4000, Applied Biosystems) using a 3:1 split. Analytes were detected in the negative-ion mode at a vaporizer temperature of 450 °C and a TurboIon Spray voltage of 4.5 kV. Analysis was performed using multiple reaction monitoring (MRM) mode using two individual transitions for each analyte. The transitions were 542.700/445.800 m/z, 622.700/542.700 m/z, and 718.700/113.00 m/z for TBBPA, TBBPA-S and TBBPA-GA, respectively similar to those previously reported by [31]. Fig. 2 provides a representative extracted ion chromatograph for TBBPA, TBBPA-S and TBBPA-GA in plasma. Tentative assignments for conjugates other than TBBPA-S and TBBPA-G are provided in Fig. 2. These were based on the previous evidence provided by Schauer et al. [31] and supported by their detection in the respective MRM channels for TBBPA-S or TBBPA-G and relative chromatographic retention.
Fig. 2.
Representative extracted ion chromatogram (EIC) for TBBPA, TBBPA-S and TBBPA-GA conjugates quantitated in plasma, with similar scans observed in the liver and uterus. The x-axis is time (min) and the y-axis is intensity (CPS). * denotes putative conjugates, TBBPA-GA/S (diconjugate of glucuronide and sulfate) and TBBPA-S/S (sulfate diconjugate) as described in Section 2.5.
2.6. Quantitation of TBBPA, TBBPA-GA, and TBBPA-S in tissues
Quantitation of TBBPA was based on the responses of the internal standard and on calibration curves obtained by fortifying plasma and liver samples from control rats with TBBPA over the concentration range of ∼2–40,000 ng/mL. Based on limitation in sufficient uterine tissue to prepare calibration curves, recovery of analyte from the uterus was determined by spiking TBBPA at low, mid- and high levels of the solvent calibration curve (0.2–4,000 ng/mL) to determine the extraction efficiency (EE). The EE averaged 30% and was used to calculate analyte concentration.
Since TBBPA-GA and TBBPA-S reference standards were not commercially available, the relative response ratios were determined for these TBBPA conjugates against TBBPA. TBBPA conjugates were extracted from feces collected from rats administered 1000 mg/kg TBBPA as previously described by [31]. The extract was analyzed by HPLC-UV and molar ratio of TBBPA to conjugate calculated by determining the molar ratio by HPLC-UV and ionization response using the LC/MS MRM method described above. The ratio of the responses of conjugates to TBBPA was then used as a correction factor in calculating levels of conjugates from the TBBPA calibration curves.
The concentration of TBBPA was calculated by direct comparison to the calibration curve, and the concentration of each conjugate was calculated by applying the corrected molar ratio to the response ratio of the conjugate and TBBPA in the sample. Calibration curves were calculated from eight to twelve calibration concentrations using Analyst, 1.5 processing software (Applied Biosystems, Waltham, MA). The LOD for TBBPA ranged from 0.02 to 2 ng/mL depending on analyte and matrix with the lower limit of quantitation (LLOQ), determined as the lowest point on the calibration curve that achieved ± 15% back-calculated recovery, ranging from ∼0.2 ng/mL (uterus) to 4.1 ng/mL (plasma and liver) for TBBPA and from 0.1 (uterus) to 2.73 ng/mL (plasma and liver) for TBBPA-S or TBBPA-GA.
2.7. Statistical analysis
Descriptive statistics (mean and standard deviation) of initial body weight, final body weight, body weight gain, and absolute liver and uterus weights were calculated and analyzed using SAS version 9.2 (SAS Institute, Cary, NC). Homogeneity of variance on these weights was analyzed using Levene’s test. Homogenous data were analyzed using a one-way analysis of variance and treated groups were compared to the control group using Dunnett’s t-test (two-tailed). Descriptive statistics (mean and standard deviation) of TBBPA, TBBPA-GA, and TBBPA-S tissues levels were calculated and analyzed using GraphPad Prism® (GraphPad Software, Inc., San Diego, CA). If the analyte concentrations were below the LLOQ the values were set to LLOQ/2 to evaluate dose-related trends. Analyte concentrations in each group were evaluated for outliers prior to statistical analysis using a two-sided Grubb’s test. To assess dose-related changes of analytes, a trend test was conducted and statistical significance reported if p < 0.05. To compare levels of conjugates at specific dose groups and time points, Students t-test was performed. Statistically-significance was reported when p < 0.05.
3. Results
3.1. Dose formulation analysis
The two highest dose formulation concentrations (100 and 200 mg/mL) were suspensions. Homogeneity was evaluated in all dose formulations as described in the method sections with all formulations administered to rats found to be within 15% of the target concentration with the coefficient of variation less than 15% for homogeneity. The actual concentrations prepared over the course of the study are listed in Table 1 of the supplemental materials.
3.2. Clinical Observations
There were no abnormal observations related to the administration of TBBPA during the course of the study. One animal in the 500 mg/kg group was found dead following dose administration on study day 21 due to suspected dosing error. No other instances of mortality or morbundity were observed.
3.3. Terminal body, liver and uterine weights
Body weight gain did not change with dose of TBBPA (data not shown), nor was there any significant difference in the final terminal body weight on day 28 at any TBBPA dose level compared to the vehicle control group (Table 2 of the supplemental materials). There were no significant changes noted in the absolute liver or uterine weights at any dose level of TBBPA compared to vehicle control rats, nor were there any dose-related trends in liver or uterine weights at either 4- or 8-h post dose on day 28 (Table 2 of the Supplemental materials).
3.4. Liver concentrations of TBBPA, TBBPA-GA and TBBPA-S
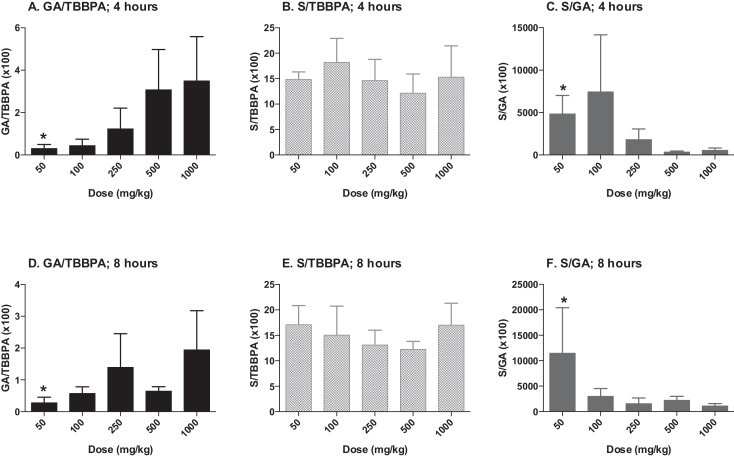
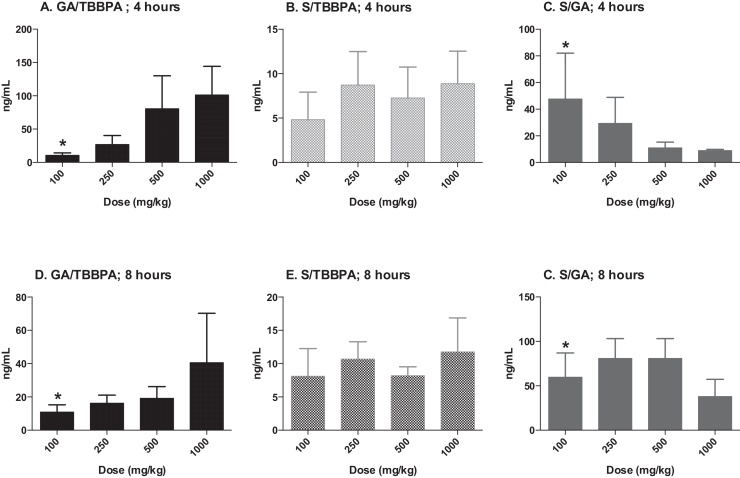
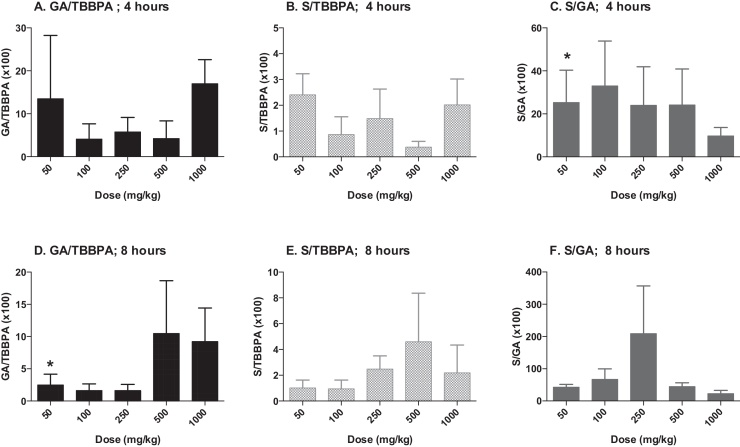
The concentrations of TBBPA, TBBPA-GA and TBBPA-S measured in liver tissue collected from rats administered TBBPA for 28 consecutive days increased with dose of TBBPA (trend test; p < 0.01) at 4- and 8-h following the last dose (Fig. 3). The concentration of TBBPA at 8-h was significantly lower than at 4-h at the two highest dose levels (t-test, p < 0.05). The concentration of TBBPA-S was significantly higher (t-test, p < 0.05) than the concentration of TBBPA-GA at all dose levels (Fig. 4), demonstrating that sulfation is the major pathway for conjugation of TBBPA in liver. To evaluate the balance of these conjugates with dose and time, the ratio of the conjugates to the parent compound (TBBPA), as well as with each other, were evaluated at 4- and 8-h following the last dose on day 28. The GA/TBBPA ratio increased with dose (trend test, p < 0.05) (Fig. 5A and D) with no change in the S/TBBPA ratio (Fig. 5B and E) at both the 4- and 8-h time points. The proportion of TBBPA sulfated vs. glucuronidated, as reflected in S/GA ratio, decreased with dose at both the 4- and 8-h time points (trend test, p < 0.05) (Fig. 5C and F), suggesting that the sulfate pathway becomes limited with administration of high doses levels of TBBPA.
Fig. 3.
Liver; the concentrations (ng/gram of tissue) of A TBBPA, B TBBPA-GA, and C TBBPA-S with dose at 4- and 8-h following dosing on day 28. Each bar represents the mean ± SD (n = 4–6 per group). (*) indicates a significant dose-related increasing trend (p < 0.01). (^) indicates a significant decrease in the analyte at each dose level at 8 h compared to the 4-h time point (p < 0.05).
Fig. 4.
Liver; the concentrations (ng/gram of tissue) of TBBPA-GA and TBBPA-S at A 4 and B 8 h= following dosing on day 28. Each bar represents the mean ± SD (n = 4–6 per group). (^) indicates a significantly higher concentration of TBBPA-S compared to TBBPA-GA at each dose level (t-test, p < 0.05).
Fig. 5.
Liver; ratio of TBBPA-GA (GA)/TBBPA (A and D), TBBPA-S (S)/TBBPA (B and E), and S/GA (C and F) with dose at 4- and 8-h following dosing on day 28. Each bar represents the mean ± SD (n = 4–6 per group). (*) indicates a significant dose-related increasing (A and D) or decreasing (C and F) trend (p < 0.05).
3.5. Plasma Concentrations of TBBPA, TBBPA-GA and TBBPA-S
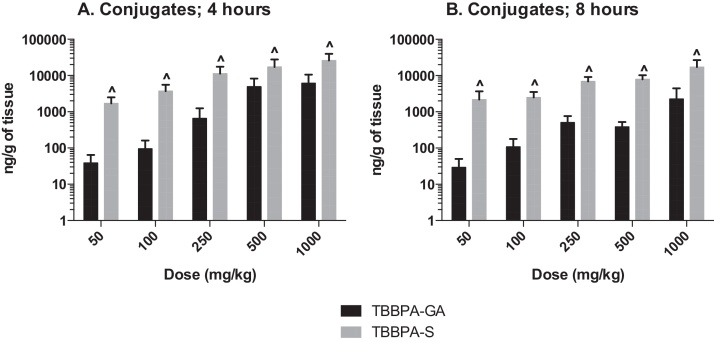
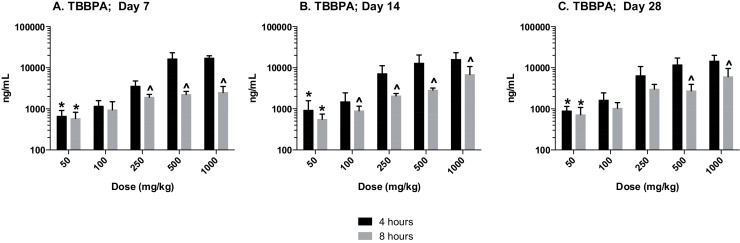
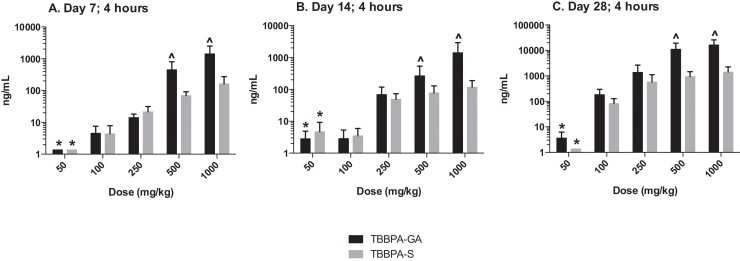
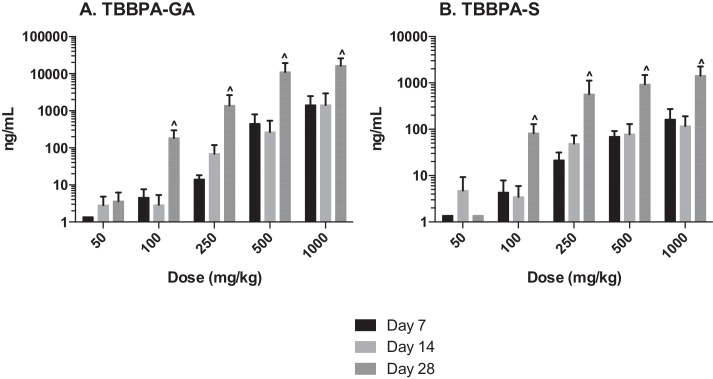
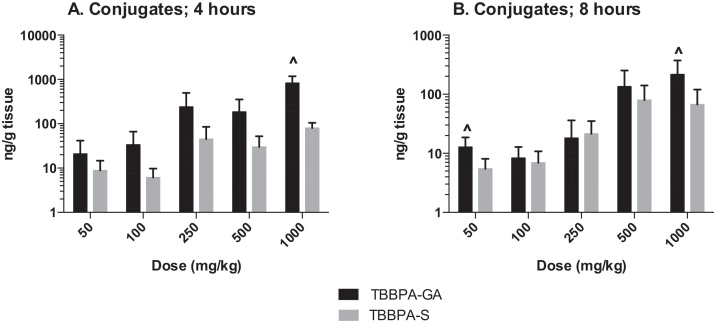
The plasma concentration of TBBPA in rats administered TBBPA for 7, 14, and 28 days increased with TBBPA dose level (trend test, p < 0.01) at both 4- and 8-h following the last dose on each respective day, with lower levels measured at 8 h compared to the 4-h time point at the higher dose levels (t-test, p < 0.05) (Fig. 6). Similar to the parent TBBPA, the conjugates increased with TBBPA dose level (trend test, p < 0.01) at both 4- (Fig. 7) and 8-h (data not shown) following the last dose on each respective day. TBBPA-GA plasma levels were higher compared to levels of TBBPA-S at dose levels >250 mg/kg (t-test, p < 0.05) (Fig. 7) at 4-h post dose. TBBPA-GA and TBBPA-S plasma levels were significantly higher following 28-days of dosing compared to either 7 or 14 days at dose levels above 50 mg/kg (p < 0.05) (Fig. 8), suggesting increased conjugation with repeated administration of TBBPA. Similar to what was observed in the liver, the GA/TBBPA ratio evaluated in plasma increased with dose (trend test, p < 0.01) with no change in the S/TBBPA ratio at either 4- or 8-h following dosing on day 28 (Fig. 9). Since the levels of conjugates were at/or below the LLOQ in rats administered 50 mg/kg TBBPA, these trends were evaluated at the dose level of 100 mg/kg and above. The proportion of TBBPA sulfated vs. glucuronidated decreased with dose (trend test, p < 0.01 at 4 h and p < 0.05 at 8 h) as reflected in the S/GA ratio, again similar to the changes observed in the liver.
Fig. 6.
Plasma; The concentration (ng/mL) of TBBPA at 4- and 8-h following A 7, B 14, and C 28-days of dosing. Each bar represents the mean ± SD (n = 4–6 per group). (*) indicates a significant dose-related increasing trend (p < 0.01). (^) indicates a significant decrease in TBBPA concentration at each dose level at 8-h compared to the 4-h time point (p < 0.05).
Fig. 7.
Plasma; The concentration of TBBPA-S and TBBPA-GA at 4 h following 7, 14, and 28 days of consecutive dosing. Each bar represents the mean ± SD (n = 4–6 per group). (*) indicates a significant dose-related increasing trend in TBBPA-GA and TBBPA-S at A 7, B 14, and C 28-days of dosing (p < 0.01). (^) indicates a significantly higher concentration of TBBPA-GA compared to TBBPA-S at each dose level (p < 0.05).
Fig. 8.
The concentration of A TBBPA-GA and B TBBPA-S in plasma 4 h following dosing on study day 7, 14, and 28. Each bar represents the mean ± SD (n = 4–6 per group). (^) indicates a higher concentration of conjugate at day 28 compared to day 7 or day 14 (p < 0.05).
Fig. 9.
Plasma; ratio of TBBPA-GA (GA)/TBBPA (A and D), TBBPA-S (S)/TBBPA (B and E), and S/GA (C and F) with dose at the 4- and 8-h time points following 28 days of consecutive dosing. Each bar represents the mean ± SD (n = 4–6 per group). (*) indicates a significant dose-related increasing (A and D) (p < 0.01) or decreasing (C; p < 0.01) (F; p < 0.05) trend.
3.6. Uterine concentrations of TBBPA, TBBPA-GA and TBBPA-S
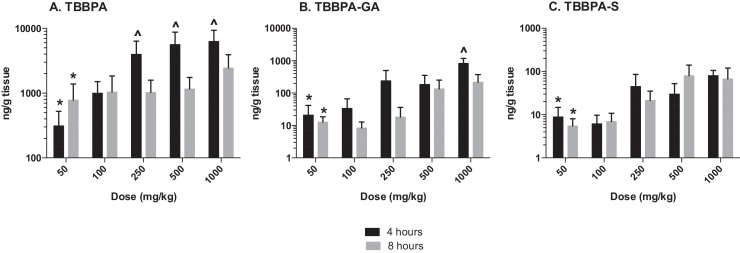
Similar to liver and plasma, the concentration of TBBPA and conjugates in the uterus increased with dose of TBBPA at both 4- and 8-h following dosing on day 28 (trend test, p < 0.01) (Fig. 10). Again, higher levels of TBBPA were measured at 4-h compared to 8-h post dose at dose levels of 250 mg/kg and above (t-test, p < 0.05). The concentration of TBBPA-GA was higher at 4-h compared to 8-h but only statistically significant following administration of the highest dose level of 1000 mg/kg dose (Fig. 10). Similar to plasma, the concentrations of TBBPA-GA in the uterus were higher compared to TBBPA-S at both 4- and 8-h post dosing, however, statistical significant in this tissue was only detected at the highest dose of 1000 mg/kg (t-test, p ≤ 0.05) (Fig. 11). The dose-related trends in the ratio of conjugates to parent and each other that were observed in liver and plasma were also observed in the uterus, however due to the higher variability and lower concentration of these analytes in this tissue statistical significance was only observed for in the S/GA ratio at the 4-h time point (decreasing trend) and in the GA/TBBPA ratio (increasing trend) at the 8-h time point (trend test, p < 0.05) (Fig. 12).
Fig. 10.
Uterus; the concentrations (ng/g of tissue) of A TBBPA, B TBBPA-GA, and C TBBPA-S with dose at 4- and 8-h following dosing on day 28. Each bar represents the mean ± SD (n = 4–6 per group). (*) indicates a significant dose-related increasing trend (p < 0.01). (^) indicates a significantly higher concentration of analyte at 4 h compared to 8 h at each dose level (p < 0.05).
Fig. 11.
Uterus; the concentration of TBBPA-GA and TBBPA-S at A 4 and B 8 h following 28 consecutive days of dosing. Each bar represents the mean ± SD (n = 4–6 per group). (^) indicates a higher concentration of TBBPA-GA compared to TBBPA-S at each respective dose (p ≤ 0.05).
Fig. 12.
Uterus; ratio of TBBPA-GA (GA)/TBBPA (A and D), TBBPA-S (S)/TBBPA (B and E), and S/GA(C and F) with dose at 4 and 8 h following 28 days of consecutive dosing. Each bar represents the mean ± SD (n = 4–6 per group). (*) indicates a significant dose-related increasing (D) or decreasing (C) trend (p < 0.05).
3.7. Comparison of analytes in all three tissues
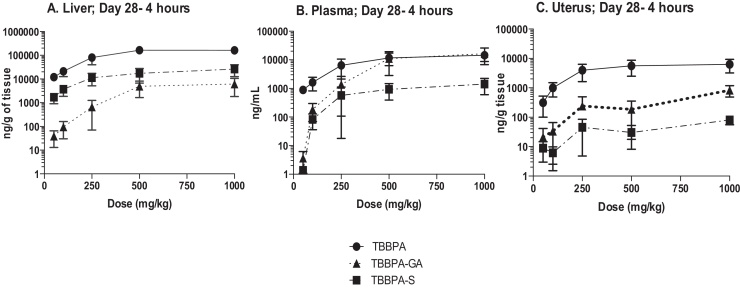
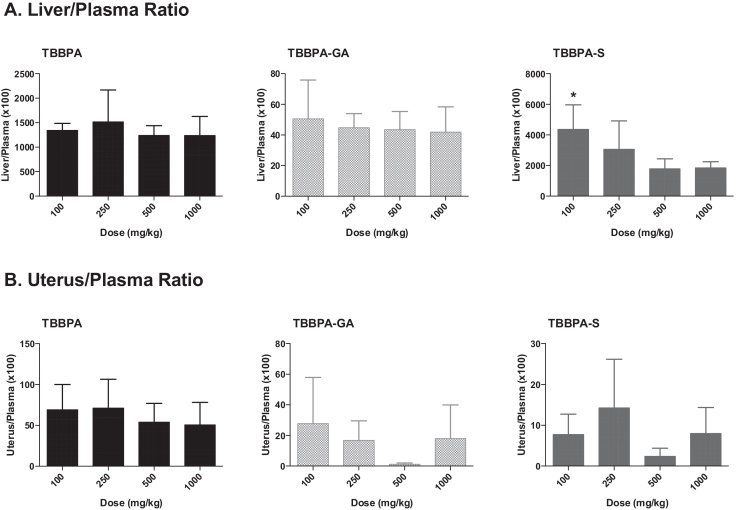
The overall changes in TBBPA, TBBPA-GA and TBBPA-S, measured in all three tissues at 4 h following dosing on study day 28, are shown in Fig. 13. These dose-related changes in the concentration of all three analytes suggest saturation of conjugation of TBBPA at doses approximately ≥250 mg/kg in liver, plasma, and uterus. To evaluate the liver or uterus/plasma ratio for all three analytes, the dose-related trends were evaluated starting at the 100 mg/kg dose level. The 100 mg/kg dose level was chosen as the cutoff because the levels of conjugates in the plasma samples at 50 mg/kg were at/or below the LLOQ. Fig. 14 shows that overall these ratios are the same with dose except for the TBBPA-S liver/plasma ratio which decreases with dose of TBBPA (trend test, p < 0.5).
Fig. 13.
The concentration of TBBPA, TBBPA-GA, and TBBPA-S in A liver, B plasma, and C uterus tissue 4 h post dosing on day 28. Each symbol is the mean ± SD (n = 4–6 per group).
Fig. 14.
The concentration of TBBPA, TBBPA-GA, and TBBPA-S in A liver, B plasma, and C uterus tissue 4 h post dosing on day 28. Each symbol is the mean ± SD (n = 4–6 per group). (*) indicates a significant dose-related decreasing trend (p < 0.05).
4. Discussion
In this study we evaluated the effect of repeated dosing, dose level, and time post dosing on the concentrations of TBBPA and its glucuronide and sulfate conjugates in liver, plasma and uterus of female Wistar Han rats administered TBBPA for up to 28 days. Following 28 days of dosing at levels up to 1000 mg TBBPA/kg, there were dose-related increases in the concentration of TBBPA and its major conjugates, TBBPA-GA and TBBPA-S in liver, plasma, and uterine tissue. The concentration of TBBPA-S was higher in liver compared to TBBPA-GA, while an inverse relationship was observed in the plasma and uterus at high dose levels. Overall, the ratio of the TBBPA-S to TBBPA-GA in all three tissues decreased with increasing dose level of TBBPA, suggesting the sulfation pathway becomes limited with increased dose of TBBPA. When considering the published in vitro data, which demonstrate both binding and inhibition of ES by TBBPA [21], [13], the data from this current in vivo study provides an additional line of evidence supporting the hypothesis that high doses of TBBPA limit estradiol sulfation serving as the molecular initiating event in the MoA associated with development of uterine tumors in Wistar Han rats.
The data in this study are consistent with other kinetic investigations of TBBPA [25], [31], [20], [24]. Most notably, Knudsen et al. [24] reported that the elimination pathways of TBBPA in female Wistar Han rats become saturated following even a single oral dose of 1000 mg/kg, which resulted in a slower excretion. The authors attributed this delay to a potential competition for metabolism with endogenous substrates in extrahepatic tissues, which these authors noted could result in endocrine disruption. Although [24] did not observe a delay in elimination at lower doses (25 or 250 mg/kg), kinetics were only evaluated after a single dose. The data presented in the current study build on these findings, demonstrating that after 28 days of administration, sulfation was limited at doses of ∼250 mg/kg-day and above.
As noted by Kester et al. [21] the competitive or noncompetitive inhibition of estradiol sulfation may be explained by a chemical’s preferential affinity for the active or the allosteric site, respectively. Although not determined specifically for TBBPA, Kester et al. [21] showed that 3,3,5,5′-tetrachlorobisphenol A (not TBBPA) inhibits SULT1E1 by a noncompetitive mechanism. Gosavi et al. [10] demonstrated in a crystallization experiment that in the presence of both estradiol and TBBPA, binding of estradiol to SULT1E1 did not permit the binding of TBBPA at another position. The observed electron density was consistent with both molecules completing for binding at the same catalytic site. The potential inhibition of sulfation may also be influenced by the ability of TBBPA to inhibit the binding of the cofactor PAPS to SULT1E1. Although there is no direct evidence in the literature concerning such, Gosavi et al. [10] described that the specific inhibition of TBBPA may be a result of high-affinity binding to the SULT1E1-PAP postcatalytic complex. An understanding of the mechanism by which TBBPA inhibits sulfotransferase would be useful to understand the potential tissue and species specificity of the overall limitation in this metabolic pathway in vivo.
Evaluation of changes in the concentration of TBBPA and both the TBBPA glucuronide and sulfate conjugates in liver was important given that it is the key organ system following oral administration (first pass effect) having a major role in the metabolism of TBBPA. TBBPA was also metabolized to diconjugates that were only putatively identified, but not quantitated in this study or in a study described by Schauer et al. [31] since only the monoconjugates were isolated and extracted from the feces of rats administered TBBPA. Measuring the level of TBBPA and its conjugates in plasma provided an overall assessment of the systemic exposure to TBBPA and its conjugates, that appears to be the the source of target tissue (e.g., uterus) exposure.
It is also critical to note that the liver is largely responsible for the synthesis of estrogens and is involved in its subsequent metabolism. Estrogens can undergo both phase I and phase II metabolism with conjugation by both sulfotransferases and glucuronosyltransferases. Conjugation of estrogen in turn increases its elimination and decreases the ability of estrogens to bind to the estrogen receptor [30]. Though levels of estradiol and its conjugate were not obtained in this study, these data demonstrated, using TBBPA conjugation patterns as a surrogate, that sulfation is limited in the liver as well as in the target tissue (uterus) following administration of high dose levels of TBBPA. These findings are critical for characterizing the ability of TBBPA at high dose levels to limit the sulfation pathway in vivo and subsequently limiting estrogen sulfation due to its ability to inhibit these enzymes and potentially overwhelm the capacity of this pathway. Factors that would play a role in the regulation of local estrogen levels in the uterus, the target tissue of interest, will ultimately depend on the potential for TBBPA to inhibit ES and glucuronosyltransferases in vivo, along with the reversal of these conjugates by local tissue levels of sulfatases or glucuronidases. In the case of sulfation, since this enzyme pathway is a high-affinity, low-capacity process, the binding of 3′-phosphoadenosine 5′-phosphosulfate (PAPS) (the cofactor that donates sulfate to the enzyme outlined in Fig. 1), along with its availability, is also critical in determining the level of sulfate conjugate formed, whether it is with TBBPA or estradiol. Klaassen and Boles [22] demonstrated that in rats, the limited sulfation capacity is due to availability of sulfate, and that there are differences in sulfation capacity by tissue. These factors (e.g., sulfotransferase and sulfatase activity and PAPS availability) together determine the levels of sulfate conjugates formed in tissues, whether the substrate is TBBPA or estradiol.
Based on the proposed MoA that TBBPA induces rat uterine tumors through its ability to inhibit ES and increase the bioavailability of estrogens, it is important to compare the metabolism of estrogens in both humans and rats. Wang and James [39] provide information on the tissue distribution of sulfotransferases in humans, with identification of the major substrates including phenols, catecholamines, thyroid hormone, estrogens and hydroxysteroids. The endometrium is an estrogen responsive tissue in both rats and humans and is known to express ES in human tissue [37]. The ES isoform, SULT1E1, has been shown to exhibit a particularly high affinity for estradiol and is primarily responsible for estrogen metabolism in humans [9], [4], [43]. However, much less information is available in other species. With respect to estrogen metabolism, one study found that estradiol glucuronidation was more active in the uterus of Wistar Han rats compared to estradiol sulfation suggesting that the rat uterus is not a source of sulfotransferase enzymes [1]; however these animals were ovariectomized, and activity of SULT1E1 was not specifically evaluated. Dunn and Klaassen [5] did report the lack of detection of sulfotransferase mRNA in the rat uterus. As such, homeostatic characterization of estrogen metabolism in the Wistar Han rat uterus remains a data gap. This is particularly notable given that this strain of rats appears to have a higher incidence of uterine tumors compared to other strains reviewed [23], [42]. The data collected in this current study suggest that the concentration of TBBPA and conjugates measured in the uterus are most likely due to its translocation from plasma since the balance of conjugates was the same in the plasma and uterus. It is of interest to note that at 4–8 h following administration of 50 mg/kg TBBPA (a dose that is 10× lower than a dose associated with uterine tumors), the mean TBBPA plasma level was approximately 1478 nM, an estimated 45–123× higher than the IC50 value associated with the inhibition of estrogen sulfotransferase by TBBPA reported in vitro (12–33 nM) [21] demonstrating that the concentration of TBBPA in the plasma is well above the concentration shown to inhibit ES activity in vitro.
Perhaps the most critical finding from this current study is the observation that metabolism of TBBPA at high doses appears to be nonlinear. It is well established that one should proceed with caution when extrapolating findings from animals to humans when the adverse effects only occur in animals at high doses and the evidence suggest that such findings are the result of perturbed normal physiological functions and protective mechanisms to humans [35]. The adverse effect of concern in the NTP study, uterine tumors, only occurred following chronic administration of TBBPA at very high doses (500 and 1000 mg/kg-day). As suggested by Wikoff et al. [42], several lines of evidence support that these tumors occur via an initial event involving inhibition of ES (e.g., perturbed normal physiological functions). As such, there are clearly challenges with respect to characterizing and extrapolating the uterine tumors observed in rats to potential effects to humans, as even the lowest dose tested by the NTP was orders of magnitude higher than estimates of human exposure reported in the scientific literature [8], [15], [7], [3], [41]. In the case of TBBPA, dose levels of TBBPA in the NTP bioassay ranged from 250 to 1000 mg/kg-day, whereas human exposure has been estimated by multiple entities to be <0.00008 mg/kg-day [8], [15], [7], [41].
Data from the current study demonstrate that the sulfation pathway at repeated TBBPA dose levels of ∼250 mg/kg-day and greater becomes limited in its capacity, an observation that is consistent with the incidence of atypical endometrial hyperplasia reported in the National Toxicology Program (NTP) study (e.g., 4, 26, 22, and 26% at 0, 250, 500, and 1000 mg/kg-day, respectively) (NTP, 2014). The statistically significant increase in uterine tumors (combined) however, observed in the 500 and 1000 mg/kg-day dose groups, highlights that it is most likely the combined influence of high dose of TBBPA at levels that inhibit ES and prolonged (chronic) exposure to unconjugated estradiol together are critical for the development of uterine tumors. Additionally, the data from this current study demonstrates the need for lower-dose toxicokinetic studies that evaluate the toxicokinetic behavior of TBBPA after longer exposures, a data gap recently identified by the [36].
In conclusion, though there are a number of studies in the literature that have evaluated the toxicokinetics of TBBPA in rats and humans, none specifically focus on the influence of dose- and repeated administration of TBBPA on selected tissue levels of TBBPA and its conjugates. This approach provided the opportunity to evaluate the sulfation of TBBPA as a surrogate for understanding the potential disruption in estradiol sulfation. Findings from this current study demonstrate that metabolic capacities are altered in female Wistar Han rats following repeated, high dose administration of TBBPA; specifically in key enzyme pathways involved in the metabolism of both TBBPA and estradiol that exceed its capacity at high TBBPA doses levels (i.e., at doses that result in perturbation of homestatic conditions). Therefore, given that the uterine tumors observed in rats in the NTP study (250–1000 mg/kg-day) only occur at very high doses that perturb homeostatic control, it is unlikely such effects would occur in humans given that current TBBPA exposure levels are approximately eight orders of magnitude (or more) lower than these doses that are associated with exceeding the capacity of key enzyme pathways in animal studies.
Conflict of interest
The conduct of this study and the oversight and the preparation of this manuscript was funded by the North American Flame Retardant Alliance (NAFRA) Panel of the American Chemistry Council (ACC). The funders were given the opportunity to review the draft manuscript. The purpose of such review was to allow input on the clarity of the science presented, but not in interpretation of the research findings. The researcher’s scientific conclusions and professional judgments were not subject to the funders’ control; contents of this manuscript reflect solely the view of the authors. The authors employment affiliation is as shown on the cover page. ToxStrategies is a private consulting firm providing services to private and public organizations on toxicology and risk assessment.
Acknowledgements
The in-life portion of this study was conducted at Integrated Laboratory Systems, Inc. (ILS) in Research Triangle Park, NC. Samples were prepared at ILS and transferred to Alera Labs, also located in Research Triangle Park, NC, where analyzed by LC–MS/MS. The raw data was transferred to ToxStrategies for analysis and interpretation.
References
- 1.Blom M.J., Wassink M.G., Kloosterboer H.J., Ederveen A.G., Lambert J.G., Goos H.J. Metabolism of estradiol, ethynylestradiol, and moxestrol in rat uterus, vagina, and aorta: influence of sex steroid treatment. Drug Metab. Dispos. 2001;29:76–81. [PubMed] [Google Scholar]
- 2.Bromine Science and Environmental forum (BSEF) Bromine Science and Environmental forum; 2012. TBBPA Factsheet Brominated Flame Retardant In Tetrabromobisphenol A for Printed Circuit Boards and ABS Plastics.http://www.bsef.com [Google Scholar]
- 3.Colnot T., Kacew S., Dekant W. Mammalian toxicology and human exposures to the flame retardant 2,2′,6,6′-tetrabromo-4,4′-isopropylidenediphenol (TBBPA): implications for risk assessment. Arch. Toxicol. 2014;88(3):553–573. doi: 10.1007/s00204-013-1180-8. [DOI] [PubMed] [Google Scholar]
- 4.Coughtrie M.W.H. Sulfation through the looking glass-recent advances in sulfotransferase research for the curious. Pharmacogenomics J. 2002;2:297–308. doi: 10.1038/sj.tpj.6500117. [DOI] [PubMed] [Google Scholar]
- 5.Dunn R.T., Klaassen C.D. Tissue-specific expression of rat sulfotransferase messenger RNAs. Drug Metab. Dispos. 1998;26:598–604. [PubMed] [Google Scholar]
- 6.Dunnick J.K., Sanders J.M., Kissling G.E., Johnson C.L., Boyle M.H., Elmore S.A. Environmental chemical exposure may contribute to uterine cancer development: studies with tetrabromobisphenol A. Toxicol. Pathol. 2015;43:464–473. doi: 10.1177/0192623314557335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Food Safety Administration (EFSA) Scientific opinion on tetrabromobisphenol A (TBBPA) and its derivatives in food. EFSA J. 2011;9(12):2477. [Google Scholar]
- 8.European Union (EU) Vol. 63. European Union; 2006. (Risk Assessment Report: 2,2′,6,6′-Tetrabromo-4,4′-Isopropylidenediphenol (Tetrabromobisphenol-a or TBBP-A), Part II—Human Health). [Google Scholar]
- 9.Falany J.L., Azziz R., Falany C.N. Identification and characterization of cytosolic sulfotransferases in normal human endometrium. Chem. Biol. Interact. 1998;109:329–339. doi: 10.1016/s0009-2797(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 10.Gosavi R.A., Knudsen G.A., Birnbaum L.S., Pedersen L.C. Mimicking of estradiol binding by flame retardants and their metabolites: a crystallographic analysis. Environ. Health Perspect. 2013;121(10):1194–1199. doi: 10.1289/ehp.1306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillemette C., Belanger A., Lepine J. Metabolic inactivation of estrogens in breast tissue by UDP-glucuronosyltransferase enzymes: an overview. Breast Cancer Res. 2004;6(6):246–254. doi: 10.1186/bcr936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakk H., Larsen G., Bergman A., Orn U. Metabolism, excretion and distribution of the flame retardant tetrabromoobisphenol-A in conventional and bile-duct cannulated rats. Xenobiotica. 2000;30(9):881–890. doi: 10.1080/004982500433309. [DOI] [PubMed] [Google Scholar]
- 13.Hamers T., Kamstra J.H., Sonneveld E., Murk A.J., Kester M.H., Andersson P.L., Legler J., Brouwer A.J. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 14.Harju M., Hamers T., Kamstra J.H., Sonneveld E., Boon J.P., Tysklind M., Andersson P.L. Quantitative structure-activity relationship modeling on in vitro endocrine effects and metabolic stability involving 26 selected brominated flame retardants. Environ. Toxicol. Chem. 2007;26:816–826. doi: 10.1897/06-308r.1. [DOI] [PubMed] [Google Scholar]
- 15.Health Canada/Environment Canada Screening Assessment Report: Phenol, 4,4′-(1-methylethylidene) bis[2,6-dibromo- (Chemical Abstracts Service Registry Number 79-94-7), Ethanol, 2,2′-[(1-methylethylidene) bis[(2,6-dibromo-4,1-phenylene) oxy]]bis (Chemical Abstracts Service Registry Number 4162-45-2), Benzene, 1,1′-(1methylethylidene) bis[3,5-dibromo-4-(2-propenyloxy)-(Chemical Abstracts Service Registry Number 25327-89-3) Health Canada/Environment Canada, 2013; ISBN: 978-1-100-22898-3
- 16.International Agency for Research on Cancer (IARC) vol. 100 A. International Agency for Research on Cancer; 2012. (Pharmaceuticals: A Review of Human Carcinogens IARC Monographs). Available at: http://monographs.iarc.fr/ENG/Monographs/vol100A/mono100A.pdf. [Google Scholar]
- 17.Institute for Laboratory Animal Research (IIAR) The National Academies Press; Washington, DC 20001: 2011. Guide for the Care and Use of Laboratory Animals. ISBN: 978-0-309-15401-7. [Google Scholar]
- 18.IPCS/WHO . World Health Organization; Geneva: 1995. Tetrabromobisphenol A and Derivatives. Available at: http://www.inchem.org/documents/ehc/ehc/ehc172.htm. [Google Scholar]
- 19.Jakobsson K., Thuresson K., Rylander L., Sjodin A., Hagmar L., Bergman A. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere. 2002;46(5):709–716. doi: 10.1016/s0045-6535(01)00235-1. [DOI] [PubMed] [Google Scholar]
- 20.Kang M.J., Kim J.H., Shin S., Choi J.H., Lee S.K., Kim H.S., Kim N.D., Kang G.W., Jeong H.G., Kang W., Chun Y.J., Jeong T.C. Nephrotoxic potential and toxicokinetics of tetrabromobisphenol A in rat for risk assessment. J. Toxicol. Environ. Health A. 2009;72:1439–1445. doi: 10.1080/15287390903212907. [DOI] [PubMed] [Google Scholar]
- 21.Kester M.H.A., Bulduck S., van Toor H., Tibboel D., Meinl W., Glatt H., Falany C.N., Coughtrie M.W.H., Schuur A.G., Brouwer A., Visser T.J. Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J. Clin. Endocrinol. Metab. 2002;87:1142–1150. doi: 10.1210/jcem.87.3.8311. [DOI] [PubMed] [Google Scholar]
- 22.Klaassen C.D., Boles J.W. The importance of 3′-phosphoadenosine 5′-phophosulfate (PAPS) in the regulation of sulfation. FASEB J. 1997;11(6):404–418. doi: 10.1096/fasebj.11.6.9194521. [DOI] [PubMed] [Google Scholar]
- 23.Klaunig J.E., Dekant W., Plotzke K., Scialli A.R. Biological relevance of decamethylcyclopentasiloxane (D5) induced rat uterine endometrial adenocarcinoma tumorigenesis: mode of action and relevance to humans. Regul. Toxicol. Pharmacol. 2015 doi: 10.1016/j.yrtph.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Knudsen G.A., Sanders J.M., Sadik A.M., Birnbaum L.S. Disposition and kinetics of tetrabromobisphenol A in female Wistar Han rats. Toxicol. Rep. 2014;1:214–223. doi: 10.1016/j.toxrep.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuester R., Solyom A.M., Rodriguez V.P., Sipes G.I. The effects of dose, route, and repeated dosing on the disposition and kinetics of tetrabromobisphenol A in male F-344 rats. Toxicol. Sci. 2007;96:237–245. doi: 10.1093/toxsci/kfm006. [DOI] [PubMed] [Google Scholar]
- 26.Lai D.Y., Kacew S., Dekant W. Tetrabromobisphenol A (TBBPA): possible modes of action of toxicity and carcinogenicity in rodents. Food Chem. Toxicol. 2015;80:206–214. doi: 10.1016/j.fct.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto J., Iwano H., Inoue H., Iwano N., Yamashiki N., Yokota H. Metabolic barrier against bisphenol A in a rat uterine endometrium. Toxicol. Sci. 2007;99(1):118–125. doi: 10.1093/toxsci/kfm148. [DOI] [PubMed] [Google Scholar]
- 28.National Toxicology Program (NTP) National Institutes of Health; Research Triangle Park, NC: 2014. Technical Report on the Toxicology Studies of Tetrabromobisphenol A (CAS no. 79-94-7) in F344/NTac Rats and B6C3F1/N Mice and Toxicology and Carcinogenesis Studies of Tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] Rats and B6C3F1/N Mice (Gavage Studies) [Google Scholar]
- 29.Osmitz Subchronic toxicology of tetrabromobisphenol-A (TBBPA) in rats. Hum. Exp. Toxicol. 2016 doi: 10.1177/0960327115627684. (accepted for publication) [DOI] [PubMed] [Google Scholar]
- 30.Rizner T.L. Estrogen biosynthesis, phase I and phase II metabolism, and action in endometrial cancer. Mol. Cell. Endocrinol. 2013;381(1–2):124–139. doi: 10.1016/j.mce.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Schauer U.M., Volkel W., Dekant W. Toxicokinetics of tetrabromobisphenol A in humans and rats after oral administration. Toxicol. Sci. 2006;91:49–58. doi: 10.1093/toxsci/kfj132. [DOI] [PubMed] [Google Scholar]
- 32.Shi Z.X., Wu Y.N., Li J.G., Zhao Y.F., Feng J.F. Dietary exposure assessment of Chinese adults and nursing infants to tetrabromobisphenol-A and hexabromocyclododecanes: occurrence measurements in foods and human milk. Environ. Sci. Technol. 2009;43(12):4314–4319. doi: 10.1021/es8035626. [DOI] [PubMed] [Google Scholar]
- 33.Shi Z., Jiao Y., Hu Y., Sun Z., Zhou X., Feng J., Li J., Wu Y. Levels of tetrabromobisphenol A, hexabromocyclododecanes and polybrominated diphenyl ethers in human milk from the general population in Beijing, China. Sci. Total Environ. 2013;452–453:10–18. doi: 10.1016/j.scitotenv.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 34.Sjodin A., Patterson D.G., Jr., Bergman A. A review on human exposure to brominated flame retardants—particularly polybrominated diphenyl ethers. Environ. Int. 2003;29(6):829–839. doi: 10.1016/S0160-4120(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 35.Slikker W., Anderson M.E., Bogdanffy M.S., Bus J.S., Cohen S.D., Conolly R.B., David R.M., Doerrer H.G., Dorman D.C., Gaylor D.W., Hattis D., Rogers J.M., Setzer R.W., Swenbery J.A., Wallace K. Dose-dependent transitions in mechanisms of toxicity: case studies. Toxicol. Appl. Pharmacol. 2004;201(3):226–294. doi: 10.1016/j.taap.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 36.U. S. Environmental Protection Agency (USEPA) U.S. Environmental Protection Agency; 2015. TSCA Work Plan Chemical Problem Formulation and Initial Assessment: Tetrabromobisphenol A and Related Chemicals Cluster Flame Retardants. EPA Document #740-R1-4004. [Google Scholar]
- 37.Utsunomiya H., Ito K., Suzuki T., Kitamura T., Kaneko C., Nakata T., Niikura H., Okamura K., Yaegashi N., Sasano H. Steroid sulfatase and estrogen sulfotransferase in human endometrial carcinoma. Clin. Cancer Res. 2004;10:5850–5856. doi: 10.1158/1078-0432.CCR-04-0040. [DOI] [PubMed] [Google Scholar]
- 38.Van der Ven L.T., Van de Kuil T., Verhoef A., Verwer C.M., Lilienthal H., Leonards P.E., Schauer U.M., Canton R.F., Litens S., De Jong F.H., Visser T.J., Dekant W., Stern N., Hakansson H., Slob W., Van den Berg M., Vos J.G., Piersma A.H. Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproduction study and a subacute toxicity study. Toxicology. 2008;245(1–2):76–89. doi: 10.1016/j.tox.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Wang L.Q., James M.O. Inhibition of sulfotransferases by xenobiotics. Curr. Drug Metab. 2006;7:83–104. doi: 10.2174/138920006774832596. [DOI] [PubMed] [Google Scholar]
- 40.Webb L.J., Miles K.K., Auyeung D.J., Kessler F.K., Ritter J.K. Analysis of substrate specificities and tissue expression of rat UDP-glucuronosyltransferases UGT1A7 and UGT1A8. Drug Metab. Dispos. 2005;33:77–82. doi: 10.1124/dmd.104.001321. [DOI] [PubMed] [Google Scholar]
- 41.Wikoff D., Thompson C., Perry C., White M., Borghoff S., Fitzgerald L., Haws L.C. Development of toxicity values and exposure estimates for tetrabromobisphenol A: application in a margin of exposure assessment? J. Appl. Toxicol. 2015;35(11):1292–1308. doi: 10.1002/jat.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wikoff D., Rager J., Haws L.A., Borghoff S.J. A high dose mode of action for tetrabromobisphenol A-induced uterine adenocarcinomas in Wistar Han rats: a critical evaluation of key events in an adverse outcome pathway framework. Regul. Toxicol. Pharmacol. 2016 doi: 10.1016/j.yrtph.2016.01.018. (accepted for publication) [DOI] [PubMed] [Google Scholar]
- 43.Xu Y., Liu X., Gui F., Ning Y., Zhi X., Wang X., Chen S., Yin L., Li X. Effect of estrogen sulfation by SULT1E1 and PAPSS on the development of estrogen-dependent cancers. Cancer Sci. 2012;103:1000–1009. doi: 10.1111/j.1349-7006.2012.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]