Abstract
Background
Human papillomavirus (HPV) infections cause approximately 30,700 cancers annually among US men and women, cervical cancer being the most common. Human papillomavirus vaccination is recommended routinely for US girls and boys at age 11 to 12 years, and for those not previously vaccinated, through age 26 and 21 years for women and men, respectively. Our objective was to assess current cervical cancer screening and HPV vaccination practices among sexually transmitted disease (STD) clinics in the United States.
Methods
We surveyed a geographically diverse convenience sample of US STD clinics identified by members of the National Coalition of STD Directors within 65 state, territorial, and local jurisdictions. An online multiple-choice survey about clinical services was administered to clinic directors or designees during October 2014 to February 2015.
Results
Survey respondents included 78 clinics from 46 states and territories. Of these clinics, 31 (39.7%) offered both cervical cancer screening and HPV vaccination, 6 (7.7%) offered cervical cancer screening only, 21 (26.9%) offered HPV vaccination only, and 20 (25.6%) offered neither cervical cancer prevention service. Among those not offering the service, the most commonly reported barrier to cervical cancer screening was time constraints (25/41, 61.0%); for HPV vaccination it was reimbursement (11/26, 42.3%).
Conclusions
By early 2015, in a geographically diverse group of 78 STD clinics, 39.7% provided nationally recommended HPV vaccination and cervical cancer screening, whereas 25.6% provided neither. Further research could identify strategies for STD clinics to reduce HPV-associated cancers by increasing provision of HPV vaccination and cervical cancer screening services, particularly among medically underserved populations.
Approximately 30,700 cases of cancer attributed to infection with human papillomavirus (HPV) occur annually among men and women in the United States.1 The most common of these are cervical cancers, with an average of 11,771 cases annually, and oropharyngeal cancers with 15,738 cases annually (3100 among females and 12,638 among males).1 Many of these cancers would likely be prevented through programs providing HPV vaccination as well as early screening and treatment for cervical cancer.
The first HPV vaccine was licensed for use in the United States in 2006, and routine vaccination has been recommended by the Advisory Committee on Immunization Practices for girls since 2006 and boys since 2011.2,3 At the time of this evaluation, 2 HPV vaccines were available in the United States: a quadrivalent vaccine (Gardasil, Merck and Co., Inc) and a bivalent HPV vaccine (Cervarix, GlaxoSmithKline); more recently, a 9-valent vaccine (Gardasil 9, Merck and Co., Inc) was also approved and recommended for routine use.4 Early evidence from many countries has shown decreases in vaccine-type HPV infections and genital warts after HPV vaccine implementation.5 However, HPV vaccine coverage in the United States remains below target levels.6 Among adolescents aged 13 to 17 years in 2015, estimated coverage for at least 1 dose of HPV vaccine was 62.8% among girls and 49.8% among boys; for 3 doses of HPV vaccine, it was 41.9% among girls and 28.1% among boys.7 These coverage estimates lag substantially behind those for adolescent vaccines to prevent pertussis and meningococcal disease.7 Contributing factors have included lack of strong recommendations for HPV vaccines from health care providers discussing adolescent vaccines with patients and parents.8
In the United States, cervical cancer screening via cervical cytology (eg, Pap testing) is recommended every 3 years, or up to 5 years if done in conjunction with HPV testing in women older than 30 years.9–11 However, identifying women who need cervical cancer screening is often challenging, and both overscreening and underscreening occur.12–14 Referrals to another institution for cervical cancer screening can result in loss to follow-up.15 Furthermore, timely follow-up and treatment are required for screening to be effective, and racial, socioeconomic, and other disparities occur.16,17 Evidence-based guidance from the United States Preventive Services Task Force and others recommends against using HPV testing alone when screening for cervical cancer,9,10 and there are no recommendations to conduct screening for anal cancer or oropharyngeal cancer.18 Human papillomavirus testing is not a prerequisite for HPV vaccination.
Although vaccination and cervical cancer screening often occur in primary care settings, medically underserved or vulnerable individuals with limited access to primary care may seek care at sexually transmitted disease (STD) clinics, family planning clinics, or other publicly-funded sites. For this reason, STD clinics have been described as safety net clinics that can provide sexual health care and family planning services. Elements of quality family planning services include (a) on-site HPV vaccination; (b) Pap testing, and (c) HPV testing in accordance with United States Preventive Services Task Force and other national recommendations.19 HPV prevalences between 27% and 49% have been measured among female STD clinic patients,20,21 yet little is known about the cervical cancer screening and prevention services currently offered in STD clinics. We surveyed STD clinics to assess whether available clinical services included HPV vaccination, Pap tests, and HPV testing.
METHODS
For this evaluation, we surveyed a large and geographically diverse convenience sample of US STD clinics nominated by members of the National Coalition of STD Directors (NCSD) representing 65 jurisdictions, encompassing state, territorial, and local health department STD programs offering STD prevention and control services. Each NCSD member was asked to nominate the largest sexual health safety net clinic/s providing STD and other sexual health services within their jurisdiction. At least 3 attempts were made to contact directors at each nominated clinic including email and telephone calls. Nonresponding clinics received additional email and phone requests. In addition, NCSD members leveraged personal networks to reach out to clinic directors and staff in their jurisdictions.
An online multiple-choice survey with limited open-ended response options was administered to clinic directors or designees during October 2014 through February 2015. Qualitative data from open-ended questions were coded and analyzed using Microsoft Excel to identify themes that were then recoded as dichotomous variables for quantitative analysis. Statistical analyses were conducted using SPSS for descriptive and bivariate comparisons. To assess clinic characteristics associated with offering specific clinical services,χ2 and odds ratios (ORs) were calculated and variables with a P value of <0.05 were considered significant.
Survey questions addressed clinic area and population served, cervical cancer screening practices, HPV vaccination practices, and barriers. Clinic level variables included clinic type, HPV-related services and business capacities (electronic health records, insurance billing), patient characteristics, and perception of barriers to provision of services. Community-level variables included: location, rurality, funding for services, per capita state public health funding, and adolescent consent policies. Clinic service areas were identified as “mostly urban,” “mostly suburban,” “mostly rural,” “unsure/don’t know,” or “other kind of area.” Four census regions were used to group jurisdictions: Northeast, Midwest, South, and West.22 HPV vaccination financing was described as public funding, public or private insurance, patient financing, and/or grants and donations.
Clinics offering any cervical cancer screening services were classified as “screening clinics” and clinics offering HPV vaccination were classified as “vaccinating clinics.” Clinics offering cervical cancer screening and vaccination services were compared with their counterparts not offering these services.
Each participant was offered a nominal gift card for completing the survey. This analysis was limited to public health program evaluation and therefore was not subject to review by an institutional review board.
RESULTS
In total, 143 clinics were nominated to participate, including at least 1 clinic from each of 50 states and 3 territories (ie, District of Columbia, Puerto Rico, and U.S. Virgin Islands). Complete responses were received from 78 clinics, located in 46 states and territories, and all census regions. The majority (73, 93.6%) represented a single clinic site. Multi-site respondents were from metropolitan areas distributed across U.S. census regions. Most respondents represented local health departments (56, 71.8%); although some represented state health departments or districts (7, 9.0%), and several other types of sites (5 each, 6.4%): family planning, hospital-affiliated or nonprofit/community health center (Table 1). No information was available on nonresponding clinics.
TABLE 1.
Characteristics of 78 STD Clinics Surveyed—United States, 2014–2015
| Characteristic | n (%) |
|---|---|
| Census region | |
| South | 25 (32.1) |
| Midwest | 19 (24.4) |
| West | 18 (23.1) |
| Northeast | 16 (20.5) |
| Type of clinic | |
| Local health department | 56 (71.8) |
| State health department | 7 (9.0) |
| Other (eg, family planning, hospital, community health centers) | 15 (19.2) |
| Service area | |
| Mostly urban | 56 (71.8) |
| Mostly rural | 12 (15.4) |
| Mostly suburban | 6 (7.7) |
| Combination of urban/suburban | 4 (5.1) |
Of the 78 clinics, 31 (39.7%) offered both cervical cancer screening and HPV vaccination services, 6 (7.7%) offered cervical cancer screening only, 21 (26.9%) offered HPV vaccination only, and 20 (25.6%) offered neither service.
Cervical Cancer Screening
Almost half of the clinics (37, 47.4%) offered any type of screening for cervical cancer. Screening clinics were located in every census region, and most (23, 62.1%) were part of the National Breast and Cervical Cancer Early Detection Program.23 The majority (26, 70.3%) offered simultaneous HPVand Pap testing or “co-testing,” and the others (11, 29.7%) offered Pap testing only. None offered primary HPV testing.
Of screening clinics, 15 (40.1%) also provided cervical screening follow-up, offering additional procedures such as colposcopy. Most of these (12, 80.0%) provided colposcopy with biopsy, 6 provided cryotherapy and 6 provided endocervical curettage.
The vast majority of the 37 screening clinics (30, 81.1%) tracked patients who needed follow-up from abnormal screenings. Most (28, 75.7%) used a referral or reminder system, and many (20, 54.1%) used a reminder system specifically to notify patients when due for cervical screening. Clinics provided abnormal screening results to their patients primarily through phone contact: 16 clinics (43.2%) called patients with their cervical screening results, and 21 clinics (56.8%) reported a wide range of additional activities including notifying by mail or email.
Screening clinics offered cervical screening based on patient age (35, 94.6%), documented medical history of time since last Pap test (32, 86.5%), patient self-report of time since last Pap test (25, 67.6%), patient request (18, 48.6%), and whether patient was new or returning to the clinic (7, 18.9%).
Several differences were noted between screening and nonscreening clinics. Clinics offering cervical cancer screening could be found in every geographic region, but compared with non-screening clinics, they were less likely to be in the Midwest (OR, 4.0; P = 0.01). Clinics offering cervical cancer screening were more likely to be affiliated with local health departments (OR, 1.5; P = 0.01), be located in metropolitan areas (OR, 1.3; P = 0.001), and offer mainly reproductive health services (OR, 1.1; P = 0.05).
Barriers to Cervical Cancer Screening
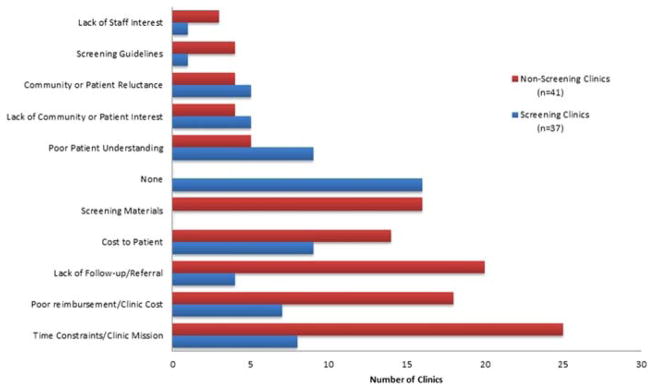
Among all 78 STD clinics, the most commonly reported barriers to offering cervical cancer screening were time constraints related to the competing mission of an STD clinic (33, 42.3%), poor reimbursement or cost (25, 32.1%), lack of follow-up to provide results and referrals if needed (24, 30.8%), and cost to patient (23, 29.5%).
Among 41 clinics not offering cervical cancer screening, the most commonly reported barriers were time constraints (25, 61.0%), lack of follow-up to provide results and referrals if needed (20, 48.8%), and poor reimbursement or cost (18, 43.9%) (Fig. 1).
Figure 1.
Barriers to offering cervical cancer screening reported by 78 STD clinics—United States, 2014–2015.
Among 37 clinics providing cervical cancer screening, most reported no barriers (16, 43.2%); however, others reported barriers including cost to patient (9, 24.3%), poor understanding (9, 24.3%), and time constraints given the competing needs of an STD clinic (8, 21.6%).
HPV Vaccination
Among the 78 STD clinics, a total of 52 (66.7%) offered any HPV vaccination. Most of these (47, 90.4%) offered quadrivalent HPV vaccine, and 5 offered bivalent HPV vaccine. Most vaccinating clinics (33, 63.5%) offered HPV vaccine to consenting patients through age 26 years. Most reported offering HPV vaccine to female patients (50, 96.2%) and to male patients (45, 86.5%). These STD clinics also offered other vaccinations, including hepatitis B, hepatitis A, influenza, combined tetanus, diphtheria and pertussis (Tdap), and meningococcal vaccines (Table 2). A total of 37 clinics participated in the Vaccines for Children program (VFC)24; among vaccinating clinics, 19 (36.5%) only offered HPV vaccine to patients eligible for this program. Many vaccinating clinics (35, 67.3%) reported relying on multiple sources of funding for HPV vaccine, such as private insurance, public insurance, grants, donations, and/or federally funded programs.
TABLE 2.
Vaccination Practices Among 78 STD Clinics—United States, 2014–2015
| Characteristics | n (%) |
|---|---|
| Vaccine offered | |
| Hepatitis B | 60 (76.9) |
| Hepatitis A | 54 (69.2) |
| HPV | 52 (66.7) |
| Influenza | 44 (56.4) |
| Tdap | 36 (46.2) |
| Meningococcal | 34 (43.6) |
| Vaccine funding | |
| Vaccines for Children | 37 (47.4) |
| 317 funding | 9 (11.5) |
| Grants/donations | 20 (25.6) |
| Insurance mechanisms (private and/or public) | 26 (33.3) |
| Patient out-of-pocket for entire cost | 17 (21.8) |
| Other/none | 29 (37.2) |
Insurance billing (private or public) was not significantly associated with clinic type; however, clinics in the Northeast region tended not to bill insurance for HPV vaccination (χ2 9.35, P = 0.003), and clinics in the Midwest region did (χ2 9.51, P = 0.002).
Barriers to HPV Vaccination
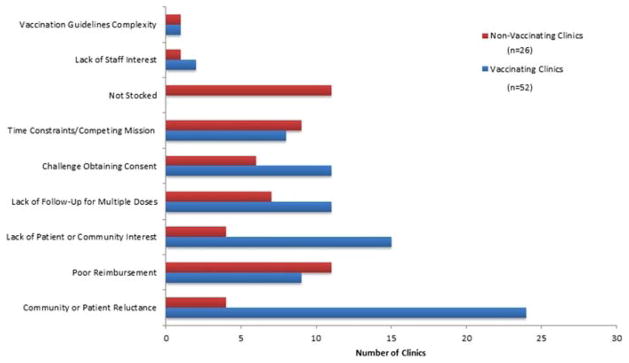
Among 78 STD clinics, the most commonly reported barriers to offering HPV vaccine were community or patient reluctance (28, 35.9%), lack of patient or community interest (19, 24.4%), poor reimbursement (20, 25.6%), lack of follow-up for multiple dose completion (18, 23.1%), challenge obtaining consent (17, 21.8%), and time constraints (17, 21.8%).
Among 26 clinics not offering HPV vaccine, the most commonly reported barriers were poor reimbursement (11, 42.3%), vaccine not being stocked in the clinic (11, 42.3%), and time constraints (9, 34.6%).
Among 52 vaccinating clinics, the most commonly reported barrier to expanding this practice was patient or community reluctance (24, 46.2%) followed by lack of patient and community interest (15, 28.8%) (Fig. 2).
Figure 2.
Barriers to offering HPV vaccination reported by 78 STD clinics—United States, 2014–2015.
DISCUSSION
This evaluation of cervical cancer screening and prevention among a diverse group of 78 STD clinics in the United States found that in early 2015, less than half (39.7%) were providing both of the most important prevention services related to HPV-associated cancers: HPV vaccination and cervical cancer screening. Almost half (47.4%) of STD clinics offered cervical cancer screening, and two thirds (66.7%) offered HPV vaccination. Although these are promising findings, we also observed that 25.6% of STD clinics provided neither HPV vaccination nor cervical cancer screening.
Barriers to cervical cancer screening were similar for screening and nonscreening clinics: time, reimbursement and lack of follow-up for abnormal screening results ranked among the most common. Notably, 80% of the screening clinics offered follow-up evaluation testing within the clinic for abnormal screening results. Reimbursement was cited as an issue, yet only 62% of screening clinics were providers within their state’s Breast and Cervical Cancer Early Detection Program. Given this program’s emphasis on reaching rarely and never screened women, improving links with STD clinics could be beneficial for women and to help underwrite such services in these clinics.
Barriers to offering HPV vaccination included reimbursement for nonvaccinating clinics, and reluctance, lack of follow-up, interest, and time constraints for vaccinating clinics. Also, consent issues were commonly reported as a barrier. Although most states allow minors to consent for STD services, consent age requirements for vaccination can differ in some states,25 impacting ability to deliver vaccine to adolescents in this setting. Some barriers due to reimbursement might be mitigated by increasing STD clinic participation in the Vaccines for Children program.26
These findings are subject to at least four limitations. First, although our convenience sample was selected to identify geographically diverse sites offering a wide range of sexual health-related clinical services, results may not be generalizable to other practice settings, and a variety of sexual health safety net providers were included as no formal definition of an STD clinic has been established. Second, reported data were not verified by external reviews and could contain biases. Third, it is not known how many STD clinics have the capability to bill insurers, or whether this might result in reduction of financial barriers. Finally, since there are no national recommendations to screen for anal cancers or oro-pharyngeal cancers, even among men who have sex with men or HIV-infected individuals, this study did not address this issue.
There are few previous studies of cervical cancer prevention services in STD clinics. A 2011 study of 42 STD clinics participating in the CDC STD Surveillance Network, found that before HPV vaccine was routinely recommended for males, only 7 (17%) clinics were offering HPV vaccine, 22 (52%) clinics routinely referred patients elsewhere for HPV vaccination and 13 (31%) clinics did not routinely refer patients for HPV vaccination.27 These clinics identified barriers to HPV vaccine delivery as cost, staff time, and follow-up.27 Another prior study from 2007 reported that 20% to 49% of STD clinics were providing Pap or HPV testing.28 The percentage of clinics offering HPV vaccination in our study (66.7%) was somewhat higher than identified previously, which could be attributable to different survey methodology, the impact of broader national recommendations on HPV vaccination, and a changing health care delivery landscape in which public health STD clinics are working to diversify financing; in some jurisdictions, these pressures have resulted in a wider array of clinical services being offered in STD clinics.
Even with national guidelines in place recommending cervical cancer screening and HPV vaccination as key elements of quality care, barriers still exist to cervical cancer screening and HPV vaccination in STD clinics. Given that not all of the STD clinics in our survey offered HPV vaccination and cervical cancer screening services, future studies could identify opportunities to prevent HPV-associated cancers by implementing or expanding these services in this setting.29 In a rapidly evolving health care delivery system, STD clinics may be subject to increasing financial pressure to provide nationally recommended services. Understanding uptake of HPV vaccine and cervical cancer screening services, as well as insurance billing capabilities and financial partnerships, will be helpful. In addition, STD clinics may be uniquely positioned to reach vulnerable patients with limited access to care in other settings.
Footnotes
Conflict of interest: none declared.
Disclosures: The National Coalition of STD Directors has received support from Merck Vaccines, Hologic, and Roche Diagnostics. Dr. Meyerson has received research support from GlaxoSmithKline and Roche Diagnostics. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus–associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661–666. doi: 10.15585/mmwr.mm6526a1. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- 3.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–1408. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 4.Petrosky E, Bocchini JA, Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 5.Drolet M, Benard E, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Healthy People 2020 [Internet] Washington, DC: U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion; [Accessed May 1, 2017]. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. [Google Scholar]
- 7.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 8.Kasting ML, Wilson S, Dixon BE, et al. A qualitative study of health-care provider awareness and informational needs regarding the nine-valent HPV vaccine. Vaccine. 2016;34:1331–1334. doi: 10.1016/j.vaccine.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis M, Feldman S. Making sense of cervical cancer screening guidelines and recommendations. Curr Treat Options Oncol. 2015;16:55. doi: 10.1007/s11864-015-0373-1. [DOI] [PubMed] [Google Scholar]
- 10.Sawaya GF, Smith-McCune K. Cervical cancer screening. Obstet Gynecol. 2016;127:459–467. doi: 10.1097/AOG.0000000000001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JW, Baldwin LM, Matthews B, et al. Physicians' beliefs about effectiveness of cancer screening tests: A national survey of family physicians, general internists, and obstetrician-gynecologists. Prev Med. 2014;69:37–42. doi: 10.1016/j.ypmed.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida CM, Rodriguez MA, Skootsky S, et al. Cervical cancer screening overuse and underuse: Patient and physician factors. Am J Manag Care. 2013;19:482–489. [PubMed] [Google Scholar]
- 14.Kepka D, Breen N, King JB, et al. Demographic factors associated with overuse of Pap testing. Am J Prev Med. 2014;47:629–633. doi: 10.1016/j.amepre.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Cooper CP, Saraiya M. Opting out of cervical cancer screening: Physicians who do not perform pap tests. Am J Prev Med. 2014;47:315–319. doi: 10.1016/j.amepre.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akers AY, Newmann SJ, Smith JS. Factors underlying disparities in cervical cancer incidence, screening, and treatment in the United States. Curr Probl Cancer. 2007;31:157–181. doi: 10.1016/j.currproblcancer.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Meyerson BE, Sayegh MA, Davis A, et al. Cervical cancer screening in a sexually transmitted disease clinic: Screening adoption experiences from a midwestern clinic. Am J Public Health. 2015;105(Suppl 2):e8–e14. doi: 10.2105/AJPH.2014.302272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moyer VA U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–891. W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 19.Gavin L, Moskosky S, Carter M, et al. Providing quality family planning services: Recommendations of CDC and the U.S. Office of Population Affairs MMWR Recomm Rep. 2014;63(RR-04):1–54. [PubMed] [Google Scholar]
- 20.Datta SD, Koutsky LA, Ratelle S, et al. Human papillomavirus infection and cervical cytology in women screened for cervical cancer in the United States, 2003–2005. Ann Intern Med. 2008;148:493–500. doi: 10.7326/0003-4819-148-7-200804010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Brown DR, Legge D, Qadadri B. Distribution of human papillomavirus types in cervicovaginal washings from women evaluated in a sexually transmitted diseases clinic. Sex Transm Dis. 2002;29:763–768. doi: 10.1097/00007435-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 22.United States Census Bureau. [Accessed May 1, 2017];Geographic Terms and Concepts—Census Divisions and Census Regions. https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html.
- 23.Lantz PM, Mullen J. The national breast and cervical cancer early detection program: 25 years of public health service to low-income women. Cancer Causes Control. 2015;26:653–656. doi: 10.1007/s10552-015-0565-9. [DOI] [PubMed] [Google Scholar]
- 24.Whitney CG, Zhou F, Singleton J, et al. Centers for Disease Control and Prevention. Benefits from immunization during the vaccines for children program era—United States, 1994–2013. MMWR Morb Mortal Wkly Rep. 2014;63:352–355. [PMC free article] [PubMed] [Google Scholar]
- 25.Guttmacher Institute. Minors' Access to STI Services. [Accessed May 1, 2017];State Policies in Brief. 2016 https://www.guttmacher.org/state-policy/explore/minors-access-sti-services.
- 26.Walsh B, Doherty E, O'Neill C. Since the start of the vaccines for children program, uptake has increased, and most disparities have decreased. Health Aff (Millwood) 2016;35:356–364. doi: 10.1377/hlthaff.2015.1019. [DOI] [PubMed] [Google Scholar]
- 27.Meites E, Llata E, Hariri S, et al. HPV vaccine implementation in STD clinics—STD Surveillance Network. Sex Transm Dis. 2012;39:32–34. doi: 10.1097/OLQ.0b013e3182315584. [DOI] [PubMed] [Google Scholar]
- 28.Selvam N, Barrow R, Shlay J, et al. Should STD clinics participate in cervical cancer screening: (1) measurement of Pap test abnormalities and HPV infection among women attending STD clinics and (2) a survey of cervical cancer screening practices; Seattle, WA. Paper presented at: International Society for Sexually Transmitted Diseases Research (ISSTDR), 17th Meeting; 2007. [Google Scholar]
- 29.Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: A systematic review. Hum Vaccin Immunother. 2016;12:1566–1588. doi: 10.1080/21645515.2015.1125055. [DOI] [PMC free article] [PubMed] [Google Scholar]