Abstract
In vitro cell proliferation, cell cycle arrest and induction of apoptosis were investigated, using three human head and neck squamous cell carcinoma (HNSCC) cell lines (OSCC-3, SCC-61, and SQ-20B). Aqueous extracts of Camellia sinensis, Ilex paraguariensis, and Ardisia compressa were tested and (−) epigallocatechin-3-gallate (EGCG) was used for comparison. For EGCG the IC50 values were between 80 and 166 μM and for the extracts among 75 and 505 μM eq. (+) catechin, with C. sinensis demonstrating dominant cytotoxicity. There was not a correlation between antioxidant capacity and cytotoxicity. Flow cytometry analysis revealed similarities in response for EGCG and C. sinensis. The A. compressa extract altered DNA distribution (P < 0.05) and was the most effective in induction of apoptosis via caspases (P < 0.05). Not all HNSCC cells tested responded to the same preventive agents. The fact that A. compressa inhibits HNSCC cell proliferation makes this aqueous extract a potential source of chemopreventive agents.
Keywords: Camellia sinensis, Ardisia compressa, Ilex paraguariensis, Polyphenols, Chemoprevention, Head and neck squamous carcinoma cells
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) is an aggressive epithelial malignancy and accounts for about 640,000 new cases of cancer worldwide and approximately 355,000 deaths annually [22]. In the United States approximately 45,780 new cases are expected in 2015 with an estimated of 8650 deaths for HNC of the oral cavity, pharynx, and larynx. Both morbidity and mortality rate are higher in men than women [1]. Exposure to tobacco and its smoke, and excessive alcohol consumption are the primary risk factors associated with HNSCC carcinogenesis [17]. Various other factors including infections with Epstein-Barr virus, past infections with human papilloma viruses (HPV16 and HPV18), chewing betel nut or shamma, dietary factors, poor oral hygiene, occupational hazards and possibly infections with human immunodeficiency virus, etc., have been also shown to be associated with etiology of HNSCC [25]. The 5-year survival rate (have remained approximately 50% for decades) and quality of life have not been improved significantly because these cancers arise in physically compact and anatomically complex sites (larynx, pharynx, oral cavity and tongue), the so frequent presentation with advanced stage disease (Stage I cancers have an ∼80% 5-year survival while Stage IV have an ∼20%), the diversity of histologic types of tumors, the high recurrence rate after surgical removal, and additionally the extremely frequent development of multiple additional second primary tumors (STPs) further worsen the prognosis of patients with HNSCC [41], [40]. In HNSCC multiple genetic and epigenetic events are highly associated with its pathogenesis, including the dysregulation of the cell growth, cell cycle, apoptosis and angiogenesis. The mutation of the p53 tumor suppressor gene (occur in 47% to 62% of HNSCC), inactivation of the cyclin-dependent kinase 4 (CDK4) inhibitor p16 and overexpression (80–90% of HNSCC) of epidermal growth factor receptor (EGFR), have been considered as critical transforming events of the disease [43], [27], [2]. The numerous genetic mutations in regulatory genes provide a strong rationale to targeting specific molecular pathways for a chemopreventive approach to the control of the HNSCC carcinogenesis.
Of all strategies involving chemoprevention, the induction of cell cycle arrest and cancer-cell-selective apoptosis by polyphenols has been receiving considerable attention as an approach to elimination of malignant cells [20]. Polyphenols may derive their preventive effect against HNSCC by coming into direct contact with tissues before being absorbed or metabolized. For instance, Camellia sinensis (green tea, GT) contains a high amount of antioxidant polyphenols, most notably (−) epigallocatechin-3-gallate (EGCG), and regular GT consumption is linked to a reduced risk for various types of cancer including colorectal [11], prostate [23] and HNSCC [5], [19] cancer. Yerba mate (MT), a tea-like infusion of Ilex paraguariensis consumed regularly in many parts of South America, is sold in commercial herbal preparations as antirheumatic with anticancerigen properties [36], [38], [15]. Aqueous extracts of Ardisia compressa, a native plant of the Pacific Coast of Mexico have been used to treat liver cancer [39]. Medicinal properties of ardisia tea (AT) including antigenotoxicity, anticytotoxicity, and antioxidant activities have been previously summarized [24]. Despite the widespread consumption of these herbal extracts, very little is known of their chemopreventive properties against HNSCC. The objective of this investigation was to compare the in vitro antitumor capacity of C. sinensis, I. paraguariensis and A. compressa extracts and EGCG against human head and neck squamous carcinoma cells, by assessing their cytotoxicity, cell proliferation, antioxidant capacity, cell cycle distribution and apoptosis induction.
2. Materials and methods
2.1. Preparation of aqueous extracts
A. compressa leaves were collected from pacific coast of Mexico (Michoacan State), while fine dried leaves of I. paraguariensis and C. sinensis (Romance and Lipton brand, respectively) were obtained from local market. Dry leaves (2.7 g) of AT, MT, and GT were soaked separately in 250 ml boiling water and allowed to stand for 10 min. The mixture of each tea was cooled to room temperature and filtered (0.45 μm nylon filter), freeze-dried and kept at −20 °C in a plastic container sealed with Parafilm and protected from light. Previous to use, the freeze-dried materials (FD) or instant teas were dissolved in double distilled water (ddH2O) (1 mg/100 μl), filtered with a 0.22 μl syringe top filter and serially diluted in serum-free medium.
2.2. Total polyphenol content of aqueous extracts
All chemicals and reagents used in this study were purchased from Sigma–Aldrich (St. Louis, MO) unless noted otherwise. The total polyphenol content of the aqueous extracts was measured as described by [35]. This method is based on the reduction of Folin Ciocalteu reagent by the electrons from the phenols. Briefly, 1 ml 1 N Folin-Ciocalteu reagent and 1 ml sample were mixed and allowed to stand for 2–5 min, and then ml of 20% Na2CO3 solution were added and allowed to stand for 10 min before measuring the absorbance at 730 nm using a Beckman DU® 640 spectrophotometer (Coulter Inc., Fullerton, CA). The total polyphenol content was expressed as μg equivalents of (+) catechin per ml of aqueous extract. The equation of the standard curve used was: y = 0.027x − 0.50, r2 = 0.98 (where, y: absorbance at 730 nm; x: polyphenol concentration; r2: correlation coefficient).
2.3. Antioxidant capacity assay
The oxygen radical absorbance capacity (ORAC) assay [37] was used to assess antioxidant capacity by measuring the protection of the extracts and EGCG on β-phycoerythrin (β-PE) fluorescence in the presence of free radicals generated by 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH). The assay was carried out in black-walled 96-well plates (Fisher Scientific, Hanover Park, IL). Each well had a final volume of 200 μl. The following reactants were added in the order: 25 μl of 75 mM phosphate buffer pH 7; either 25 μl Trolox standard (1 mM final concentration) or sample (1.0–3.0 μg eq. (+)-catechin/ml); 100 μl of β-PE (1.52 nM final concentration); and 50 μl of AAPH (41.6 mM final concentration). As a blank, 25 μl of 75 mM phosphate buffer pH 7 was added instead of Trolox or samples. Immediately after addition of AAPH, plates were placed in a FL × 800 fluorescence plate reader (Bio-Tek Instruments, Winooski, VT), set with excitation filter 530/25 nm and emission filter 590/35 nm, and then read every 2 min for 2 h to reach a 95% loss of fluorescence. Final fluorescence measurements were expressed relative to the initial reading. Results were calculated based on the differences in the area under the β-PE decay curve between the blank and a sample, and expressed as micromoles of Trolox equivalents (TE)/g dry leaves (DL). Trolox (1–4 μM) was used as a standard (y = 3.35x + 0.42, r2 = 0.98).
2.4. In vitro-assays of cytotoxicity and proliferation
OSCC-3 and HaCaT cell lines were cultured in Dulbecco’s modified Eagle’s medium plus Ham F12 (DME/F12) mixture (1:1) medium containing 10% fetal bovine serum (FBS). SCC-61 and SQ-20B cells were cultured in the same medium with added hydrocortisone (0.4 μg/ml). All cells were maintained at 37 °C in 5% CO2 atmosphere with 95% humidity. Cultures were passaged as needed, and the culture medium was changed every other day. SQ-20B, HaCaT, SCC-61 and OSCC-3 cells were used because one of our long-term objectives was to determine the role of herbal teas in the prevention of HNSCC. All four different cell types were used as a starting point and emphasis was given to SCC-61, SQ-20B and OSCC-3 for the study of apoptosis.
Cells were plated onto 96-well plates during exponential growth at a density of 5 × 104 cells/ml, 100 μl/well. After 24 h, cells were treated with 100 μl serial concentrations of EGCG (AG Scientific, San Diego, CA), or GT, MT and AT. After incubating for 48 h, the medium containing the extracts or EGCG was discarded and the plates washed twice with phosphate-buffered saline (PBS). PBS was used as a control. One hundred microliters of serum-free medium containing 10 μl of cell counting kit 8 (CCK-8) solution (Dojindo Molecular Technologies, Gaithersburg, MD) were added to each well of the plate. The amount of the formazan dye generated by the activity of dehydrogenases within cells was directly proportional to the number of living cells, ensuring a linear relationship between absorbance at 450 nm and the cell number. The plate was incubated for 2 h and the absorbance measured at 450 nm using a microplate reader interfaced with a computer. At the end of each experiment, cytotoxicity and proliferation were calculated for each extract and EGCG according to the protocol described by [33]. The following parameters were used:
IC50—the concentration of the agent that inhibits growth by 50%, is the concentration at which (T/C) × 100 = 50, where T = number of cells, at time t of treatment; C = control cells at time t of treatment.
GI50—the concentration of the agent that inhibits growth by 50%, relative to untreated cells, is the concentration at which ([T − T0]/[C − T0]) × 100 = 50, where T and C are the number of treated and control cells, respectively, at time t of treatment and T > T0; T0 is the number of cells at time zero.
GI50 considers cells at time zero and IC50 does not.
TGI—the concentration of the agent that completely inhibits cell growth, is the concentration at which T = T0.
LC50—the concentration of the agent that results in a net loss of 50% cells, relative to the number at the start of treatment, is the concentration at which ([T − T0]/T0) × 100 = −50; T < T0.
2.5. Cell cycle distribution
All reagents for cell staining were obtained from Molecular Probes (Eugene, OR). Since fluorescence of propidium iodide-stained cells is directly proportional to their nuclear content, it was used to measure the DNA content of each cell. Flow cytometric analysis of DNA content was conducted on all four cell lines with and without test compounds or extracts at IC50 levels. In addition to EGCG, treatments with etoposide, a known inducer of cell cycle arrest in certain cell types, was included at IC50 levels: 0.79 μM, HaCaT; 0.44 μM, SCC-61; 2.95 μM, SQ-20B; and 3.39 μM, OSCC-3. For flow cytometric analysis, cells were seeded at 1.25 × 105. After 24 h, the cells were treated with the extracts. At 48 h cells were washed with PBS then trypsinized, diluted with twice their volume of culture medium, then centrifuged for 5 min at 2000 × g and the medium discarded. Propidium iodine (PI) cell staining was carried out as described previously [44] using 375 μl of staining solution [300 μl H2O + 37.5 μl sodium citrate (10 mg/ml) + 3.75 μl Triton X-100 (10%, v/v) + 18.75 μl PI (1 mg/ml)], followed by 15 μl deoxyribonuclease-free ribonuclease A (7 units/ml). Cells were briefly vortex and then tilted in staining solution every 3–5 min for 45 min at 4 °C. Subsequently, 625 μl of cold PBS were added to each tube, and the cells filtered through 53 μm nylon mesh, followed by incubation on ice for half an hour. Cells were again filtered through the nylon mesh, prior to flow cytometric analysis. Cell cycle measurements were done using an EPICS XL flow cytometer (Coulter Electronics, Hileah, FL, U.S.A.) with an excitation wavelength of 488 nm and emission at 670 nm. Ten thousand events were analyzed per sample. DNA content was determined by ModFit software (Verity Software House, Topsham, ME).
2.6. Apoptosis
Two types of cell stain, Hoechst 33342 and propidium iodine were used to distinguish apoptotic cells from dead or normal cells. Hoechst 33342 preferentially stains apoptotic cells over normal cells due to the presence of condensed chromatin, whereas propidium iodine stains dead cells, but not apoptotic or normal cells. After treatment, cells were trypsinized, washed and re-suspended in PBS. Cells were stained according to the manufacturer’s instruction. Cells were incubated on ice for 20 min following addition of 50 μl of Hoechst 33342 trihydrochloride, trihydrate (100 μg/ml) and 10 μl of PI (100 μg/ml) to the cell suspension, prior to analysis. Fluorescence of Hoechst and PI was measured by flow cytometry using a MoFlo instrument (Cytomation, Fort. Collins, CO, U.S.A.), equipped with a Coherent Innova 90 C laser with an excitation wavelength of 488 nm (PI) and a Coherent I90 with an excitation wavelength of 351 nm (Hoechst). Fluorescence emission for PI was measured at 670 nm with a 40 nm band pass filter and for Hoechst at 450 nm with a 65 nm band pass filter. A minimum of 10,000 events were acquired per sample. Data were analyzed using Summit V3.1 software (Cytomation).
2.7. Detection of caspase activity
A distinctive feature of apoptosis is the activation of caspase enzymes, the name applied to cysteine aspartic acid-specific proteases. Caspases are key components of the apoptotic machinery of cells, participating in an enzyme cascade that results in cellular disassembly. The Vybrant FAM Poly Caspases Assay Kit for flow cytometry (Molecular Probes, Eugene, OR, U.S.A.) allows one to detect these key apoptotic events. This method detects a fluorescent inhibitor of caspases (FLICA) bound to the enzymatic reactive center of activated caspases. The reagent contains a fluoromethyl ketone (FMK) moiety that covalently associates with a caspase-specific amino acid sequence that includes reactive cysteine at the active site of caspases and inhibits further enzymatic activity. Unbound FLICA reagent diffuses out of the cell and is washed away. A carboxyfluorescein group (FAM) is attached as a reporter.
To further investigate the mechanism of ardisia-induced apoptosis, experiments were conducted using the Vybrant FAM Poly Caspases assay to detect caspase activity in AT-treated cells. Cells were prepared and exposed to AT for 24 or 48 h. Cell detachment was achieved by trypsinization, followed by deactivation of the enzyme with FBS containing medium. Cells were centrifuged for 5 min at 2000 × g, and re-suspended in medium at a density of 1.0 × 106 cells/ml. To 300 μl of the cell suspension, 10 μl of a solution of fluochrome-labeled caspase inhibitors (FAM-VAD-FMK FLICA) was added according to the manufacturer’s instruction. Cells were incubated for 45 min at 37 °C and 5% CO2. After the incubation, 2 ml of washing buffer was added to each tube and cells were centrifuged for 5 min at 2,000 rpm. The supernatant was aspirated immediately after centrifugation, and 1 ml of fresh washing buffer was added before another centrifugation and removal. Finally, 1 ml of washing buffer was added to each tube, and cells were then re-suspended. Prior to analysis, 10 μl of propidium iodine (100 μg/ml) was added. Measurements of cell samples were conducted with the EPICS XL flow cytometer (488 nm excitation wavelength) with 525 nm band pass emission filter for FLICA fluorescence and 670 nm band pass emission filter for PI fluorescence. At least 10,000 events were analyzed per sample. The remaining green fluorescent signal was considered a direct measure of the amount of active caspases that were present at the time the inhibitor was added.
2.8. Statistical analysis
Results are expressed as the mean ± SD of values obtained in at least triplicate measurements from three different experiments. Fisher’s least significant difference test was performed for mean separation of DNA distribution and apoptosis induction. P values less than 0.05 were considered significant. When more than two means were compared, significance was determined by one-way analysis of variance followed by pairwise comparisons using the Tukey test. Analysis on sigmoidal dose-response was performed by non-linear regression (curve fit) using the GraphPad Prism ® software.
3. Results
Endpoint parameters were calculated for each individual cell line including the GI50, which is defined as the extract concentration that inhibits growth by 50%; the TGI, which is the lowest extract concentration that totally inhibits cell growth; and the LC50, which is the lowest concentration that kills 50% of cells. All of the cell lines responded to extract treatments in a dose-dependent manner. Table 1 depicts the cytotoxicity and proliferation values after 48 h exposure of the various HNSCC cell lines to the different extracts. Comparison is made with the positive control EGCG. The IC50 value for EGCG against immortalized keratinocytes (116.1 μM) was intermediate between the oral squamous cell lines studied, SQ-20B (81.3 μM eq. (+) catechin), SCC-61 (123.0 μM eq. (+) catechin) and OSCC-3 (166.0 μM eq. (+) catechin). In general, GT was one of the most potent of all extracts tested, and exhibited cytotoxicity against oral cancer cells in a clearly dose-dependent manner, similar to that of EGCG. GT had the second best IC50 in two of the cell lines tested. GT required an average of 142 μM eq. (+) catechin to obtain IC50 for all the cells tested and EGCG 121 μM.
Table 1.
Cytotoxicity and inhibition of proliferation of HaCaT, SCC-61, SQ-20B and OSCC-3 cells after 48 h exposure to (−) epigallocatechin-3-gallate (EGCG), and hot water extracts of Camellia sinensis (GT), Ilex paraguariensis (MT) and Ardisia compressa (AT).*
| HaCaT | |||||
|---|---|---|---|---|---|
| EGCGm (μM) | GT (μM eq. (+) catechin) | MT (μM eq. (+) catechin) | AT (μM eq. (+) catechin) | ||
| IC50 | 116.1a | 99.6a | 99.9a | 504.5b | |
| GI50 | 100.0a,b | 85.4a | 84.2a | 125.0b | |
| TGI | 177.9b | 191.5b | 94.7a | 239.0c | |
| LC50 | 237.1b | 270.8c | 118.7a | >222.3b | |
| SCC-61 | |||||
|---|---|---|---|---|---|
| EGCG (μM) | GT (μM eq. (+) catechin) | MT (μM eq. (+) catechin) | AT (μM eq. (+) catechin) | ||
| IC50 | 123.0b | 132.6b | 74.8a | 453.4c | |
| GI50 | 79.4a | 107.8a,b | 94.3a | 235.3c | |
| TGI | 133.4 b | 135.0b | 105.8a | >295.9c | |
| LC50 | 177.8 b | 170.9b | 118.7a | >295.9c | |
| SQ-20B | |||||
|---|---|---|---|---|---|
| EGCG (μM) | GT (μM eq. (+) catechin) | MT (μM eq. (+) catechin) | AT (μM eq. (+) catechin) | ||
| IC50 | 81.3a | 152.2b | 213.9c | 355.2d | |
| GI50 | 100.0a | 170.8b | 186.4b | 296.0c | |
| TGI | 199.5a | 303.7b | 209.1a | >592.0c | |
| LC50 | 251.2a | 340.8b | 263.2a | >592.0c | |
| OSCC-3 | |||||
|---|---|---|---|---|---|
| EGCG (μM) | GT (μM eq. (+) catechin) | MT (μM eq. (+) catechin) | AT (μM eq. (+) catechin) | ||
| IC50 | 166.0b | 184.3b | 266.0c | 112.7a | |
| GI50 | 141.3b | 170.9b | 237.1c | 73.0a | |
| TGI | 173.8b | 191.5b | 266.0c | 148.5a | |
| LC50 | 251.1b | 227.7b | 298.7c | 166.4a | |
See Section 2 for details. Values are the average of three independent experiments performed in triplicate. Different letters in a row, per type of cell, are statistically different (P < 0.05).
Potency of MT was in a narrow range across all cell lines tested and for all parameters evaluated. The IC50, GI50, TGI, and LC50 values of MT for HaCaT were 99.9, 84.2, 94.7, and 118.7 μM eq. (+) catechin, respectively. A similar relationship between IC50, GI50, TGI, and LC50 values was seen for all three other cell lines, although SCC-61 required lower concentration of MT (74.8 μM eq. (+) catechin) to achieve reduction in cell growth than did SQ-20B (214 μM eq. (+) catechin), and OSCC-3 (266 μM eq. (+) catechin).
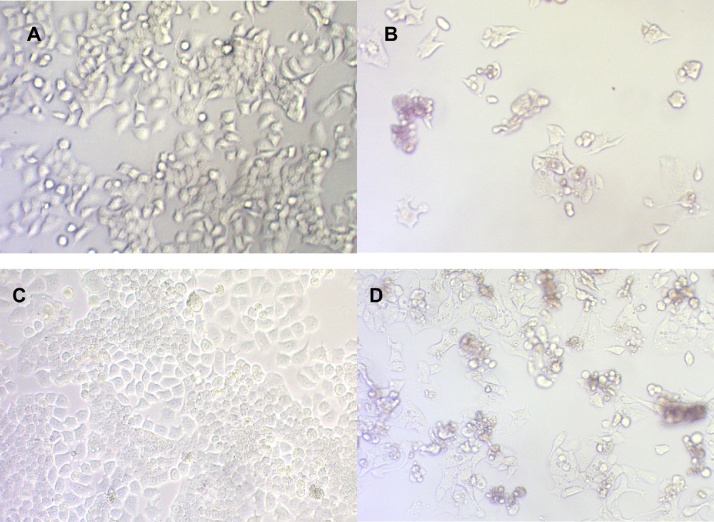
A relatively small amount of AT was necessary to inhibit 50% proliferation of OSCC-3 (112.7 μM eq. (+) catechin), however higher concentrations were required to inhibit proliferation of the other two malignant cells. In contrast to other cell lines, where MT was most potent, AT inhibited proliferation of OSCC-3 cells with the greatest potency. The IC50 for AT was substantially greater for HaCaT, SCC-61 and SQ-20B (504.5, 453.4, 355.2 μM eq. (+) catechin, respectively) than for OSCC-3 cells (112.7 μM eq. (+) catechin). Differences in morphology of OSCC-3 cells were observed among AT-treated (112.7 μM eq. (+) catechin) and control cells by light microscopy. As shown in Fig. 1, the most visible changes observed in AT-treated cells included cell shrinkage, extensive detachment of the OSCC-3 cells from the cell culture substratum, and single cells or small clusters of cells. These changes are characteristics of apoptotic cells [13], and became visible after 24 h of AT-treatment, but were absent in control cells. The morphological changes become more notable with increased time (48 h) of AT-treatment (112.7 μM eq. (+) catechin). These observations suggested that OSCC-3 cells treated with AT detached from the substratum and died by apoptosis.
Fig. 1.
Effect of AT on OSCC-3 cell histomorphology. (A) Control, 24 h; (B) treated with 112.7 μM eq. (+) catechin AT, 24 h; (C) control, 48 h; (D) treated with 112.7 μM eq. (+) catechin AT, 48 h (original magnification, ×32).
The antioxidant capacity and total polyphenol content of GT, MT and AT are summarized in Table 2. When the antioxidant capacity was expressed as μmol TE/ml or as μmol TE/g DL, GT with the highest total polyphenol content, showed the highest antioxidant capacity as ORAC values. AT (333.5 μmol TE/g DL or 4.9 μmol TE/ml) had significantly lower ORAC values than MT (1238.9 μmol TE/g DL or 17.4 μmol TE/ml) and GT (1345.9 μmol TE/g DL or 20.1 μmol TE/ml). The antioxidant values obtained in this study agree with the range of ORAC values reported for other herbal aqueous extracts (235–1526 μmol TE/g DL) [37]. AT, MT, and GT had significantly different total polyphenol contents (P < 0.001). AT showed the lowest value (36.78 ± 1.07 mg eq. catechin/g DL) and GT the highest (137.19 ± 5.79 mg eq. catechin/g DL).
Table 2.
Antioxidant capacity and total polyphenol content of Camellia sinensis (GT), Ilex paraguariensis (MT) and Ardisia compressa (AT), using ORAC.
| Aqueous extract | ORAC valued, e |
Total polyphenol content | |
|---|---|---|---|
| (μmol Trolox/ml) | (μmol Trolox/g DL) | (mg equivalents of (+) catechin/g DL) | |
| GT | 20.1 ± 0.9b | 1345.9 ± 60.0b | 137.19 ± 5.79c |
| MT | 17.4 ± 0.8b | 1238.9 ± 55.7b | 82.13 ± 3.83b |
| AT | 4.9 ± 0.1a | 333.5 ± 8.2a | 36.78 ± 1.07a |
DL = dried leaves.
Antioxidant capacity relative to 1 μM Trolox. Values are the average of three independent extract preparations.
Values with different letters in the same column are statistically different, P < 0.001.
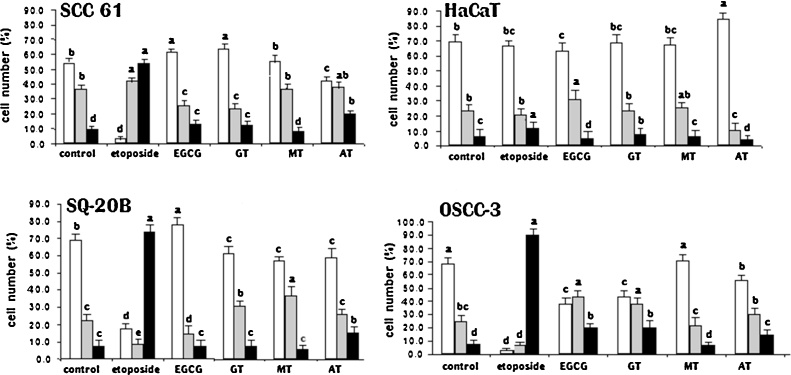
Fig. 2 depicts the effects of the various extracts on cell cycle distribution. Although the overall pattern of cell cycle distribution remained similar, significant differences in DNA distribution were found among treatments. AT caused a small but significant arrest of the cell cycle for all cell lines; there was accumulation of SCC-61, SQ-20B and OSCC-3 cells in the G2/M phase and in phase G0/G1 for the HaCaT cells. Excitingly the treatment of the SCC-61, SQ-20B and OSCC-3 cells with etoposide arrested similarly the cycles in G2/M phase, but in a more marked way. There was little or no effect by MT. Untreated SCC-61 cells revealed a distribution of cells in G0/G1 (54.1%), in S (36.5%), and G2/M (9.4%), EGCG and GT arrested these cells in G0/G1. The cell cycle of the OSCC-3 cells was arrested in phase G2/M by the treatment with either EGCG or GT.
Fig. 2.
Cell cycle distribution (%) of SCC-61, HaCaT, SQ-20B and OSCC-3 exposed to etoposide, EGCG, GT, MT or AT for 48 h; see Section 2 for details. Values are means ± SD from three independent experiments performed in triplicate. Bars with different letters are statistically different from the control (PBS) (P < 0.05) for each cell cycle stage, □ G0/G1;  S; ■ G2/M, within treatments. IC50 concentrations were used and these differed depending on extract and cell line tested (see Table 1).
S; ■ G2/M, within treatments. IC50 concentrations were used and these differed depending on extract and cell line tested (see Table 1).
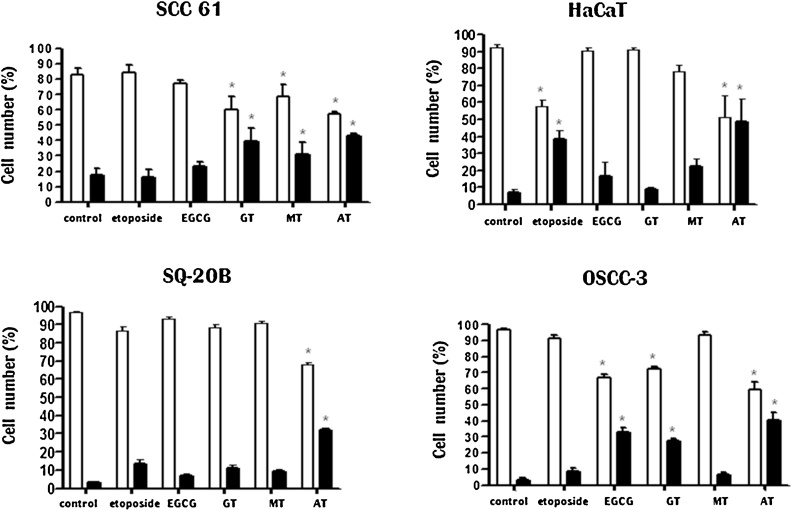
The results of induction of apoptosis on HNSCC by etoposide, EGCG, GT, MT and AT are shown in Fig. 3. Although EGCG caused significant induction of apoptosis, in OSCC-3 cells (33.2%), it had less effect on other cell types with only a small number of apoptotic cells observed in SCC-61 (20%), SQ-20B (10%) and HaCaT (15%). GT was capable of inducing apoptosis in OSCC-3 and SCC-61 cells (27.8 and 42.7% respectively), while little or no effect was observed on SQ-20B and HaCaT cells. MT significantly decreased the percentage of live HaCaT and SCC-61 cells and increased the proportion of apoptotic cells. Of all extracts tested, AT was found to be the most pro-apoptotic, and the percent of apoptotic cells was 44.3% (OSCC-3), 35.1% (SQ-20B), 43.0% (SCC-61), and 48.9% (HaCaT). These results indicated that AT was capable of altering, to various degrees, cell cycle distribution and producing apoptosis.
Fig. 3.
Induction of apoptosis (%) by extracts in HaCaT, immortalized human keratinocytes, and HNSCC cells. Rapidly proliferating HaCaT and three HNSCC cells; SCC-61, SQ-20B and OSCC-3, were exposed to IC50 concentrations of EGCG and etoposide, GT and MT (AT: IC20 for SCC-61 and OSCC-3). Values are means ± SD from three independent experiments performed in triplicate. *Statistically different from PBS (P < 0.05) for either alive or apoptotic cells. □ alive cells, ■ apoptotic cells.
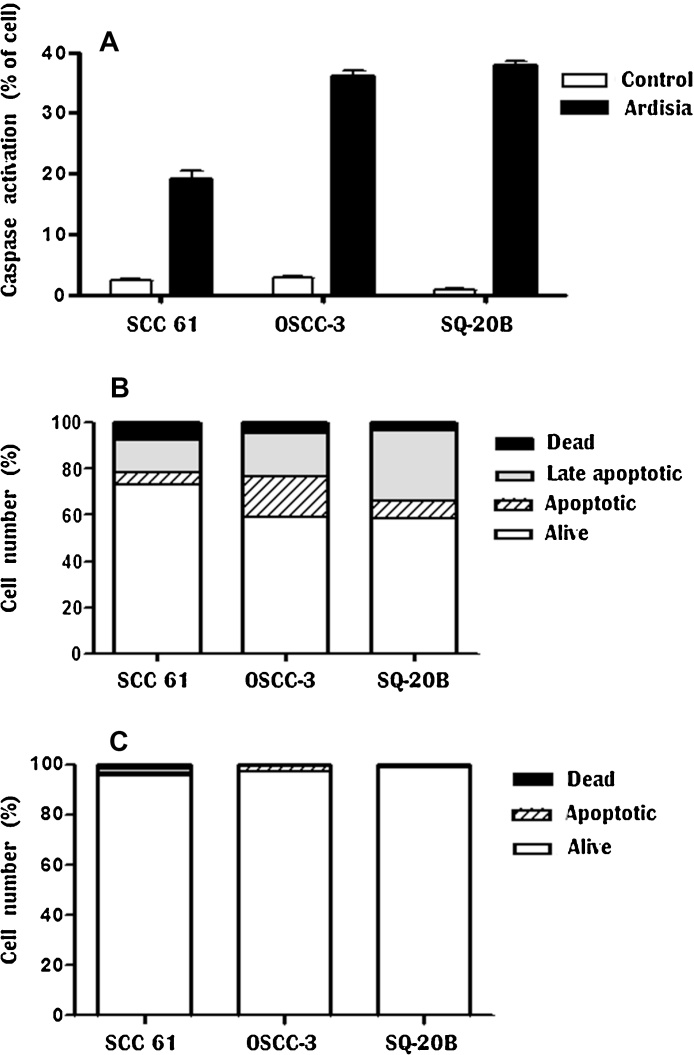
Caspases were activated in HNSCC cells although the difference in the degree of activation varied with treatment. Activation of caspases was observed in 39% and 35% of SQ-20B and OSCC-3 cells respectively, 48 h after exposure to IC50 concentration of AT (Fig. 4A). For both cell lines, the activation of caspases in untreated cells was 1.4 and 1.3%, respectively. In contrast, caspases activation was evident in only 20% of SCC-61 cells and significantly greater than control cells. Fig. 4B presents the number of dead, late apoptotic, apoptotic, and alive cells in AT treated for 48 h, AT significantly decreased the percentage of live OSCC-3, SCC-61 and SQ-20B cells and increased the proportion of late apoptotic and apoptotic cells, in comparison to the untreated cells (Fig. 4C). HaCaT cells were not included in this experiment due to the low AT effect on cytotoxicity.
Fig. 4.
(A) Caspase activation by Ardisia compressa extract in HNSCC cell lines. Cell were treated with AT for 48 h. (B) Effect of AT on cell death. Cells were treated with AT for 48 h, data are shown as percent of the total number of tumorogenic cells (SCC-61, OSCC-3, SQ-20B). (C) Control (cells without treatment). Values are means ± SD from three independent experiments performed in triplicate.
4. Discussion
This investigation evaluated the anticancer potential of GT, MT and AT by assessing their chemical composition and their biological properties in an in vitro model. The cells used were all derived from human oral cavity squamous cell carcinomas (HaCaT, SCC-61, SQ-20B and OSCC-3). As such, they are all histologically very similar, the presumed genetic differences may explain why some are more and some are less responsive. This would be an interesting avenue of investigation, for instance, correlate their response to agents versus genetic/gene expression profile. HaCaT cells are a spontaneously transformed human skin keratinocyte cell line; they are premalignant and therefore are frequently used as a model to compare with malignant cell lines. HaCaT cells are analogous to dysplasia since they have a mutant p53, immortal, but do not form tumors. The SCC-61 line is characterized by its radiosensitivity and is a wild-type p53. The SQ-20B is a squamous cell carcinoma of lineage derived from an explant from the oral cavity, harboring a tp53 mutant allele and has been reported to express a functional p53 protein [31]. The oral squamous cell carcinoma cells (OSCC-3) are representative of a particularly aggressive epithelial malignancy.
EGCG, a major polyphenolic constituent of GT, was used as a positive control in this study, partly because researchers have investigated its role in inducing apoptosis in HNSCC cells while no affecting normal cells [19]. An IC70 for EGCG against YCU-H-891, a nasopharynx carcinoma cell line has been reported to be 21.8 μM after 72 h exposure [32]. Lin et al. treated three HNSCC cell lines (SAS, Cal-27 and Ca9-22) with EGCG during 24 h and reported IC50 values from 6 to 18 μM [29]. In the present study, the IC50 values for EGCG on HSNCC cell lines and immortalized keratinocytes after 48 h were considerably greater (from 82 to 166 μM). However, the present discrepancy might have stemmed from differences in duration of chemical exposure, assay types, different cell number, working volume and above all, cell types and their stage of malignancy [12]. In fact, a wide range for the IC50 for EGCG has been reported for different oral cancer cell types (1–200 μM) [10]. HaCaT cells responded to treatments with EGCG in a dose-dependent manner, and the IC50 value obtained fell in the range of concentrations reported to activate extracellular signal-regulated kinase, p38 and c-Jun NH2-terminal kinase [6]. EGCG is also known to diminish 2,2′-azobis (2-amidinopropane) dihydrochloride cyclooxygenase-2 and p38 activation in HaCaT. Although the concentrations reported previously [8] were lower than the doses having significant effects in this study.
Treatments with GT resulted in loss of cell growth in a dose-dependent manner, and the overall shapes of the curves were very similar to those for EGCG. Indeed, the similarities between the two became even more apparent when the concentration of GT was converted into (+) catechin equivalents. For example, the IC50 values of EGCG and GT against SCC-61 were 123.0 μM, and 132.6 μM eq. (+) catechin, respectively, while the LC50 values were 177.8 μM and 170.9 μM eq. (+) catechin for this cell line. Moreover, GT and EGCG cytotoxicity values against two cell lines, HaCaT and OSCC-3, were also very similar. These findings clearly indicate that EGCG is likely the major constituent of GT responsible for the cytotoxicity reported here on HNSCC cell lines. One exception was the SQ-20B cell line, where roughly twice the amount of GT in catechin equivalents was required compared to EGCG to achieve IC50. Taken together, the current findings suggest that while cytotoxicity of GT against HNSCC cell lines is likely attributable to EGCG or related compounds, the antitumor activity of these compounds may depend on the cell type. Moreover, in comparison to the immortalized cell line, HaCaT, all carcinoma lines required higher concentrations of GT to achieve the IC50, rather than selectively killing the more malignant lines. Although the IC50 for MT against SCC-61 (74.8 μM eq. (+) catechin) was lower than that of HaCaT (99.9 μM eq. (+) catechin), other cytotoxicity values between these two cell lines were similar (Table 1). On the other hand, a higher concentration (266 μM eq. (+) catechin) of MT was necessary to observe 100% growth inhibition of OSCC-3, more than two times greater than that for SCC-61 cells (105.8 μM eq. (+) catechin). These differences in sensitivity to MT indicate that these two cell lines may be at different stages of cancer malignancy. Interestingly, MT-induced cytotoxicity exhibited a narrow range across the cell types studied, regardless of end point. For instance, for HaCat cells, 50% growth inhibition, total growth inhibition, and 50% lethal concentration were between 84.2 and 118.7 μM eq. (+) catechin.
When cytotoxicity of AT was evaluated, OSCC-3 cells were found to be very much more sensitive than other cell lines, including the non-cancerous cell line HaCaT (Table 1). The chemical analysis of AT has shown the presence of relatively little catechins compared to GT, but instead a unique flavonoid identified as ardisin [4]. There may be a relationship between the unique chemical composition of AT and its biological activity showing less cytotoxicity toward the immortalized cells HaCat.
Etoposide is an anticancer drug that inhibits DNA topoisomerase II [21]. Etoposide caused accumulation in G2/M in HaCaT, SCC-61 and SQ-20B, suggesting that the DNA repair process is activated and repairs the DNA damage by arresting the cell cycle in the G2/M phase, confirming a previous report that the p53 gene of both HaCaT and SQ-20B lines express a functional p53 mutant [16] and that SCC-61 cells contain wild p53 [9]. A similar observation was made when OSCC-3 cells were treated with etoposide, resulting in loss of cell number in the G0/G1 phase. Although this cell line has not been well characterized to date, it has a high proliferation rate and our data suggest that p53 is also mutated in this cell line.
Treatments with extracts and ECGC did alter cell cycle distribution although the changes were smaller, compared to etoposide. The effect of the teas on cell cycle distribution varied among the cell lines, although no extract arrested the cell cycle in all cell types tested. Neither GT nor MT significantly altered the cell cycle in HaCaT cells at the concentrations studied. In contrast to their effects on the other cell types, treatments with GT and EGCG resulted in significant cell cycle arrest in OSCC-3 cells (Fig. 2). When OSCC-3 cells were subjected to GT, the percentage of cells at G0/G1 stage declined from 66.1% to 41.3%, while cells at S and G2 phases increased from 26.4 and 7.6 to 39.7 and 19.0, respectively. A similar effect was seen with EGCG, indicating that the GT components that were responsible for this effect are either EGCG or related compounds which function in the same manner. Furthermore, treatments with AT resulted in a small but significant G2/M arrest. The majority of OSCC-3 cells treated with AT detached within 24 h, and a high proportion of cells were necrotic. This was not seen for the other cell lines. While the mechanism is unclear, these cells are clearly more sensitive and future experiments to determine differences in responsiveness based upon genetic similarities/differences is warranted.
When HaCaT cells were treated with AT for 24 h, approximately 87% of cells were at the G0/G1 phase, an approximately 20% increase in the number of cells at this stage compared to untreated cells. This marked increase in the G0/G1 phase indicates two things. First, that the mechanisms of cytotoxicity induced by AT is different from that of the others tested exracts, at least for this particular cell line. Second, HaCaT cells contain two heterozygous p53 mutations (exons 5 and 8) [3] and has a more extended protein half-life than the wild type p53, and despite the mutations the p53 protein is still functional and regulates cell cycle at the G0/G1 check-point [18]. The present finding confirms that HaCaT indeed has a functional p53 protein that arrest the cell cycle at G0/G1 to allow for repair of the damage, and that AT can interfere with regulation of the cell cycle for this particular cell line.
Both GT and EGCG are known to induce apoptosis in several carcinoma cell types, but not in normal cells. For example, GT was pro-apoptotic in SV40 virally transformed WI38 human fibroblasts (WI38VA), while it had little or no effect on its normal counterpart, WI38 cells [7]. EGCG was capable of inducing apoptosis in HNSCC cells, while it protected survival of normal cells [19]. Because of these reported properties of EGCG, this and its related compounds may be effectively used for chemoprevention of HNSCC. Despite the pro-apoptotic properties of reported EGCG [45], apoptosis of cells was observed only when OSCC-3 were treated with this compound (Fig. 3). On the other hand, GT, in which EGCG is accounted for 10% of total polyphenols, was capable of inducing apoptosis in OSCC-3 and SCC-61. Induction of apoptosis was observed in GT-treated SQ-20B cells, but the percent of the apoptotic cells was smaller, compared to that of the two other cell lines. In contrast, treatment with GT resulted in a lower number of apoptotic HaCaT cells, similar to that of untreated cells. A study by Liu et al. has demonstrated that GT and EGCG inhibited cell growth in three squamous cell lines (CAL-27, SCC-25 and KB) via S and G2/M phase cell cycle arrest [30].
MT showed a significant apoptotic activity against HaCaT and SCC-61 cells with approximately 25 and 32% of apoptotic cells respectively. No statistical differences were found between untreated and MT-treated OSCC-3 and SQ-20B cells.
Unlike any other treatments, AT caused apoptosis in all HNSCC and HaCaT cells. In fact, the number of apoptotic cells ranged from 35.1% (SQ-20B) to 48.9% (HaCaT). [34] indicated that malignancy occurred in oral leukoplakia as a result of the avoidance of apoptosis. Hence induction of apoptosis by AT treatment constitutes a protective mechanism against carcinogenesis, via the elimination of damaged cells or abnormal excess of HNSCC cells. Ardisia extract and its constituents caused cytotoxicity, an accumulation of cells in G1 (HaCaT) or G2/M (SCC-61, SQ-20B and OSCC-3), and apoptosis. These results support previous observations linking ardisia aqueous extracts with chemopreventive effects, both in animal studies and in folk medicine [14], [24].
To further elucidate the mechanism of ardisia-induced apoptosis, caspase activation was investigated. Perhaps the most essential finding during the apoptosis of cells exposed to AT was that hinge upon the activation of a family of cysteine proteases called caspases. We used a phenotype analysis of caspases to detect apoptosis, by the method of flurorchrome-labeled inhibitors of caspases (FLICA), which is relatively nontoxic to cells and penetrates through the plasma membrane of live cells, binding to activated caspases in apoptotic cells. FLICA is capable of simultaneously detect induction of multiple caspases-1, 3, 4, 5, 7, 8 and 9, allowing the analysis of apoptosis.
Interestingly, while the degree of caspases activation increased over a period of time for OSCC-3 and SQ-20B treated with AT, it was lower for SCC-61 cell lines after 24 h. Compared to these two cell lines, SCC-61 appears to be at the least malignant stage. Furthermore, the number of cells showing caspases activation was lower than that of apoptotic cells with AT treatment. Several studies have demonstrated that a variety of human malignancies, including HNSC, contain subpopulation of cells [42], [28], for this reason the extent of the intracellular activation of caspases could be different to the number of apoptotic cells. The current findings suggest the presence of caspases-induced apoptotic pathways in HNSCC cell lines treated with AT. Furthermore, the polyphenolic content of AT (36.78 mg eq. of (+) catechin/g DL) and its antioxidant capacity (333.5 μmol Trolox/g DL) did not correlate with the cytotoxicity activity.
In summary, the data indicate that all tested extracts were capable of inhibiting at least one line of HNSCC in a dose-dependent manner. The effects of GT often, but not always, mimicked those of EGCG suggesting a key role for this compound for prevention of OSCC-3 and SCC-61 growth, but not for SQ-20B. EGCG was apparently important for the effect on OSCC-3 and SCC-61 and was no so effective against SQ-20B. Based on previous observations, the inhibitory effect of AT on cell growth of OSCC-3 may be due to the presence of quinone type compounds, such as ardisin [39]. Ardisia extract exerted the greatest effect on apoptosis in all cell lines; most probably by inducing caspases-dependent metabolic pathways. These results are promising, but more studies are needed to better understand the underlying mechanism of action of these extracts, as well as their specific anticarcinogenic potential depending on chemical composition and the cancer cell type. These results suggest that AT has a different mechanism of protection against cytotoxicity that is not related to its antioxidant capacity. Although additional studies on the effects of ardisia extract on cell cycle-associated protein are required in order to know the molecular mechanism behind its anticancer activity and elucidate which of the pathways of apoptosis (extrinsic, intrinsic or the perforin/granzyme pathway) is activated by the complex mixture of compounds present in the extract.
Although bioavailability of aqueous extract constituents is low, the concentrations used in the present study are relevant to normal tea drinking because in addition to the direct contact between oral squamous cells and AT, there may also be a more prolonged cellular interaction via the systemic circulation. In the context of the present findings, under normal preparation conditions the phenolic concentration in A. compressa extract is 36.78 ± 1.07 mg eq. (+) catechin/g DL. Therefore, in practical terms, and based on the effective concentrations of catechin found in the present study, a person will have to ingest an approximate amount of 1 liter of extract/day (400 mg eq. (+) catechin). After the consumption of 10–100 mg of a single phenolic compound, the maximum concentration in plasma rarely exceeds 1 mM [26]. Then it may be possible that the anticancer properties of AT could be due to a synergistic effect of the multiple constituents present in the extract, each of one has its unique bioavailability.
5. Conclusions
The fact that AT inhibits HSNCC cell proliferation makes this botanical product a potential source of still unknown active substances that can be added to the arsenal of compounds that could be used as chemotherapeutic, chemomodulating or chemopreventive agents. Therefore, AT deserves further studies in order to understand the specific mechanism of action.
Acknowledgements
Special thanks to Dr. Anna Keck for her technical support to post-doctoral fellow Hideka Kobayashi. Supported by NIH-Innovative Cancer CAM Initiative in Cancer Centers-University of Chicago Sub-award No. 22883, Paradox No. 23174. The authors are grateful to Barbara Pilas, Elizabeth Jeffery and Mark Lingen for their valuable assistance in this study.
References
- 1.American Cancer Society . ACS; Atlanta: 2015. Cancer Facts & Figs. 2015. [Google Scholar]
- 2.Ang K.K., Berkey B.A., Tu X., Zhang H.Z., Katz R., Hammond E.H., Fu K.K., Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 3.Boukamp P., Petrussevska R.T., Breitkreutz D., Hornung J., Markham A., Fusenig N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandra S., Gonzalez de Mejia E. Comparative study of total polyphenol, antioxidant capacity, quinone reductase activity and phenolic composition of ardisia, mate and green teas. J. Agric. Food Chem. 2004;52:3583–3589. doi: 10.1021/jf0352632. [DOI] [PubMed] [Google Scholar]
- 5.Chen P.N., Chu S.C., Kuo W.H., Chou M.Y., Lin J.K., Hsieh Y.S. Epigallocatechin-3 gallate inhibits invasion, epithelial-mesenchymal transition, and tumor growth in oral cancer cells. J. Agric. Food Chem. 2011;59:3836–3844. doi: 10.1021/jf1049408. [DOI] [PubMed] [Google Scholar]
- 6.Chen W., Dong Z., Valcic S., Timmermann B.N., Bowden G.T. Inhibition of ultraviolet B–induced c-fos gene expression and p38 mitogen-activated protein kinase activation by (−)-epigallocatechin gallate in a human keratinocyte cell line. Mol. Carcinog. 1999;24:79–84. doi: 10.1002/(sici)1098-2744(199902)24:2<79::aid-mc1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z.P., Schell J.B., Ho C.T., Chen K.Y. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129:173–179. doi: 10.1016/s0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y., Kim D.S., Park S.H., Yoon J.A., Kim S.K., Kwon S.B., Park K.C. Involvement of ERK AND p38 MAP kinase in AAPH-induced COX-2 expression in HaCaT cells. Chem. Phys. Lipids. 2004;129:43–52. doi: 10.1016/j.chemphyslip.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Dey S., Spring P.M., Arnold S., Valentino J., Chendil D., Regine W.F., Mohiuddin M., Ahmed M.M. Low-dose fractionated radiation potentiates the effects of Paclitaxel in wild-type and mutant p53 head and neck tumor cell lines. Clin. Cancer Res. 2003;9:1557–1565. [PubMed] [Google Scholar]
- 10.Ding Y., Yao H., Yao Y., Fai L.Y., Zhang Z. Protection of dietary polyphenols against oral cancer. Nutrients. 2013;5:2173–2191. doi: 10.3390/nu5062173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du G.-J., Zhang Z., Wen X.-D., Yu C., Calway T., Yuan C.-S., Wang C.-Z. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4:1679–1691. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbling L., Herbacek I., Weiss R.-M., Gerner C., Heffeter P., Jantschitsch C., Trautinger F., Grusch M., Pangratz H., Berger W. EGCG-meditated cyto- and genotoxicity in HaCat keratinocytes is impaired by cell-mediated clearance of auto-oxidation-derived H2O2: an algorithm for experimental setting correction. Toxicol. Lett. 2011;205:173–182. doi: 10.1016/j.toxlet.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez de Mejıa E., Ramırez-Mares M.V., Arce-Popoca E., Wallig M., Villa-Treviño S. Inhibition of liver carcinogenesis in Wistar rats by consumption of an aqueous extract from leaves of Ardisia compressa. Food Chem. Toxicol. 2004;42:509–516. doi: 10.1016/j.fct.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez de Mejia E., Song Y.S., Ramirez-Mares M.V., Kobayashi H. Effect of Yerba Mate (Ilex paraguariensis) tea on topoisomerase inhibition and oral carcinoma cell proliferation. J. Agric. Food Chem. 2005;53:1966–1973. doi: 10.1021/jf048158g. [DOI] [PubMed] [Google Scholar]
- 16.Hall P.A., Campbell S.J., O’neill M., Royston D.J., Nylander K., Carey F.A., Kernohan N.M. Expression of the p53 homologue p63alpha and deltaNp63alpha in normal and neoplastic cells. Carcinogenesis. 2000;21:153–160. doi: 10.1093/carcin/21.2.153. [DOI] [PubMed] [Google Scholar]
- 17.Hashibe M., Brennan P., Benhamou S., Castellsague X., Chen C., Curado M.P., Dal Maso L., Daudt A.W., Fabianova E., Fernandez L., Wunsch-Filho V., Franceschi S., Hayes R.B., Herrero R., Koifman S., La Vecchia C., Lazarus P., Levi F., Mates D., Matos E., Menezes A., Muscat J., Eluf-Neto J., Olshan A.F., Rudnai P., Schwartz S.M., Smith E., Sturgis E.M., Szeszenia-Dabrowska N., Talamini R., Wei Q., Winn D.M., Zaridze D., Zatonski W., Zhang Z.F., Berthiller J., Boffetta P. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International head and neck. Cancer Epidemiol. Consort. J. Natl. Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 18.Henseleit U., Zhang J., Wanner R., Haase I., Kolde G., Rosenbach T. Role of p53 in UVB-induced apoptosis in human HaCaT keratinocytes. J. Invest. Dermatol. 1997;109:722–727. doi: 10.1111/1523-1747.ep12340708. [DOI] [PubMed] [Google Scholar]
- 19.Hsu S., Singh B., Schuster G. Induction of apoptosis in oral cancer cells: agents and mechanisms for potential therapy and prevention. Oral Oncol. 2004;40:461–473. doi: 10.1016/j.oraloncology.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Hsu Y.-L., Chen C.-Y., Hou M.-F., Tsai E.-M., Jong Y.-J., Hung C.-H., Kuo P.-L. 6-dehydrogingerdione an active constituent of dietary ginger, induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human breast cancer cells. Mol. Nutr. Food Res. 2010;54:1307–1317. doi: 10.1002/mnfr.200900125. [DOI] [PubMed] [Google Scholar]
- 21.Hu T., Gibson D.P., Carr G.J., Torontali S.M., Tiesman J.P., Chaney J.G., Aardema M.J. Identification of a gene expression profile that discriminates indirect-acting genotoxins from direct-acting genotoxins. Mutat. Res. 2004;549:5–27. doi: 10.1016/j.mrfmmm.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. Ca-Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 23.Khan N., Bharali D.J., Adhami V.M., Siddiqui I.A., Cui H., Shabana S.M., Mousa S.A., Mukhtar H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 2014;35:415–423. doi: 10.1093/carcin/bgt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi H., Gonzalez de Mejia E. The genus Ardisia: a novel source of health-promoting compounds and phytopharmaceuticals. J. Ethnopharmacol. 2005;96:347–354. doi: 10.1016/j.jep.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 25.Lacko M., Braakhuis B.J., Sturgis E.M., Boedeker C.C., Suárez C., Rinaldo A., Ferlito A., Takes R.P. Genetic susceptibility to head and neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. 2014;89:38–48. doi: 10.1016/j.ijrobp.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 26.Landete J.M. Updated knowledge about polyphenols functions, bioavailability, metabolism, and health. Food Sci. Nutr. 2012;52:936–948. doi: 10.1080/10408398.2010.513779. [DOI] [PubMed] [Google Scholar]
- 27.Langendijk J.A., Psyrri A. The prognostic significance of p16 overexpression in oropharyngeal squamous cell carcinoma: implications for treatment strategies and future clinical studies. Ann. Oncol. 2010;21:1931–1934. doi: 10.1093/annonc/mdq439. [DOI] [PubMed] [Google Scholar]
- 28.Lim Y.C., Oh S.Y., Cha Y.Y., Kim S.H., Jin X., Kim H. Cancer stem cell traits in squamospheres derived from primary head and neck squamous cell carcinomas. Oral Oncol. 2011;47:83–91. doi: 10.1016/j.oraloncology.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Lin H.-Y., Hou S.-C., Chen S.-C., Kao M.-C., Yu C.-C., Funayama S., Ho C.-T., Way T.-D. (−)-Epigallocatechin gallate induces Fas/CD95-mediated apoptosis through inhibiting constitutive and IL-6-induced JAK/STAT3 signaling in head and neck squamous cell carcinoma cells. J. Agric. Food Chem. 2012;60:2480–2489. doi: 10.1021/jf204362n. [DOI] [PubMed] [Google Scholar]
- 30.Liu X., Zhang D.Y., Zhang W., Zhao X., Yuan C., Ye F. The effect of green tea extract and EGCG on the signaling network in squamous cell carcinoma. Nutr. Cancer. 2011;63:466–475. doi: 10.1080/01635581.2011.532901. [DOI] [PubMed] [Google Scholar]
- 31.Maalouf M., Alphonse G., Colliaux A., Beuve M., Trajkovic-Bodennec S., Battiston-Montagne P., Testard I., Chapet O., Bajard M., Taucher-Scholz G., Fournier C., Rodriguez-Lafrasse C. Different mechanisms of cell death in radiosensitive and radioresistant p53 mutated head and neck squamous cell carcinoma cell lines exposed to carbon ions and x-rays. Int. J. Radiat. Oncol. Biol. Phys. 2009;74:200–209. doi: 10.1016/j.ijrobp.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Masuda M., Suzui M., Weinstein I.B. Effects of epigallocatechin-3-gallate on growth epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin. Cancer Res. 2001;7:4220–4229. [PubMed] [Google Scholar]
- 33.Monks A., Scudiero D., Skehan P., Shoemaker R., Paull K., Vistica D., Hose C., Langley J., Cronise P., Vaigro-Wolff A., Gray-Goodrich M., Campbell H., Mayo J., Boyd M. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991;83:757–765. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 34.Nogami T., Kuyama K., Yamamoto H. Histopathological and immunohistochemical study of malignant transformation of oral leukoplakia, with special reference to apoptosis-related gene products and proliferative activity. Acta Otolaryngol. 2003;123:767–775. doi: 10.1080/00016480310000700b. [DOI] [PubMed] [Google Scholar]
- 35.Nurmi K., Ossipov V., Haukioja E., Pihlaja K. Variation of total phenolic content and individual low-molecular-weight phenolics in foliage of mountain birch trees (Betula pubescens ssp tortuosa) J. Chem. Ecol. 1996;22:2023–2040. doi: 10.1007/BF02040093. [DOI] [PubMed] [Google Scholar]
- 36.Pérez J.M., Maldonado M.E., Rojano B.A., Alzate F., Sáez J., Cardona W. Comparative antioxidant, antiproliferative and apoptotic effects of Ilex laurina and Ilex paraguariensis on colon cancer cells. Trop. J. Pharm. Res. 2014;13:1279–1286. [Google Scholar]
- 37.Prior R.L., Cao G. Antioxidant capacity and polyphenolic components of teas: implications for altering in vivo antioxidant status. Proc. Soc. Exp. Biol. Med. 1999;220:255–261. doi: 10.1046/j.1525-1373.1999.d01-44.x. [DOI] [PubMed] [Google Scholar]
- 38.Puangpraphant S., Berhow M.A., Gonzalez de Mejia E. Yerba Mate (Ilex Paraguariensis St: Hilaire) saponins inhibit human colon cancer cell proliferation. ACS Symp. Ser. 2012;1109:307–332. [Google Scholar]
- 39.Ramirez-Mares M.V., Chandra S., Gonzalez de Mejia E. In vitro chemopreventive activities of Camelia sinensis: Ilex paraguariensis and Ardisia compressa tea extracts and selected polyphenols. Mutat. Res. 2004;554:53–65. doi: 10.1016/j.mrfmmm.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Ries L.A.G., Melbert D., Krapcho M., Stinchcomb D.G., Howlader N., Horner M.J., Mariotto A., Miller B.A., Feuer E.J., Altekruse S.F., Lewis D.R., Clegg L., Eisner M.P., Reichman M., Edwards B.K. N.I.H., N.C.I.; Bethesda, MD, U.S: 2008. SEER Cancer Statistics Review, 1975–2005. [Google Scholar]
- 41.Sannigrahi M.K., Singh V., Sharma R., Panda N.K., Khullar M. Role of autophagy in head and neck cancer and therapeutic resistance. Oral Dis. 2014 doi: 10.1111/odi.12254. online version 1–9. [DOI] [PubMed] [Google Scholar]
- 42.Shackleton M., Quintana E., Fearon E.R., Morrison S.J. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Stransky N., Egloff A.M., Tward A.D., Kostic A.D., Cibulskis K., Sivachenko A., Kryukov G.V., Lawrence M.S., Sougnez C., McKenna A., Shefler E., Ramos A.H., Stojanov P., Carter S.L., Voet D., Cortés M.L., Auclair D., Berger M.F., Saksena G., Guiducci C., Onofrio R.C., Parkin M., Romkes M., Weissfeld J.L., Seethala R.R., Wang L., Rangel-Escareño C., Fernandez-Lopez J.C., Hidalgo-Miranda A., Melendez-Zajgla J., Winckler W., Ardlie K., Gabriel S.B., Meyerson M., Lander E.S., Getz G., Golub T.R., Garraway L.A., Grandis J.R. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taets C., Aref S., Rayburn A.L. The clastrogenic potential of triazine herbicide combinations found in potable water supplies. Environ. Health Perspect. 1998;106:197–201. doi: 10.1289/ehp.98106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto T., Staples J., Wataha J., Lewis J., Lockwood P., Schoenlein P., Rao S., Osaki T., Dickinson D., Kamatani T., Schuster G., Hsu S. Protective effects of EGCG on salivary gland cells treated with gamma-radiation or cis-platinum (II) diammine dichloride. Anticancer Res. 2004;24:3065–3073. [PubMed] [Google Scholar]