Abstract
Human navigation studies show that landmarks are used for orientation, whereas objects contribute to the contextual representation of an environment. What constitutes a landmark? Classic rodent studies show that hippocampal place fields are controlled by distal, polarizing cues. Place fields, however, are also influenced by local cues. One difficulty in examining mechanisms by which distal and local cues influence the activity of hippocampal cells is that distant cues are necessarily processed visually, whereas local cues are generally multimodal. Here, we compared the effects of 90° rotations under different cue conditions in which cues were restricted to the visual modality. Three 2-dimensional visual cue conditions were presented in a square open field: a large vertical cue on one wall, a large floor cue in a corner abutting two walls, and a smaller complex floor cue in a corner adjacent to two walls. We show that rotations of large distal cues, whether on the wall or floor, were equally effective in controlling place fields. Rotations of the smaller floor cues were significantly more likely to result in remapping, whether or not animals were also exposed to the distal polarizing cues. Responses of distal and local cues were affected differently by extended experience. Our data suggest that the hippocampus processes visual cues either as stable landmarks useful for orientation and navigation or as nonstationary objects or features of the local environment available for associative learning or binding items in context. These classifications are determined by perspective, salience of the object, and prior experience.
INTRODUCTION
The hippocampus is critical for learning and memory in the mammalian brain and its role in spatial learning and navigation is well described. Seminal rodent physiology studies discovered hippocampal place cells, later identified as complex spiking CA1 and CA3 pyramidal cells, that exhibit location-specific firing when the animal is exploring an environment (O’Keefe and Dostrovsky, 1971; Fox and Ranck, 1981). The firing patterns of active place cells are specific to a particular environment (O’Keefe and Conway, 1978; Muller and Kubie, 1987), persist over time, and are relatively insensitive to direction, except in linearly organized tasks (Muller et al., 1987; but see Markus et al., 1995). For these reasons, place cells were originally proposed to be the neural correlates of spatial memory. The concert of place cell activity was thought to provide an allocentric representation of the spatial environment (O’Keefe and Nadel, 1978).
Early experiments demonstrated the importance of visual information in defining spatial environments and guiding navigation. In particular, rotations or distortions of distal extramaze or polarizing intramaze visual cues tend to elicit concordant place field rotations or distortions (O’Keefe and Conway, 1978; Muller and Kubie, 1987; Fenton et al., 2000a, b). Local or proximal multimodal cues also modulate place cell activity, but are less likely to be used for orientation or defining space (Shapiro et al., 1997; Tanila et al., 1997; see also Knierim, 2002). One limitation of comparing the effects of distal polarizing cues and local cues on hippocampal cell activity is that local cues are generally multimodal, whereas distal cues are restricted to the visual modality.
Other issues about the use of visual cues for orientation and navigation have to do with size, portability, and perspective. Studies of the parahippocampal and retrosplenial cortices suggest that the spatially-defining properties of visual objects are related at least in part to size and portability (Mullally and Maguire, 2011; Auger et al., 2012; Mullally and Maguire, 2013). Others argue the salience of visual cues, or likelihood to be used for orientation, is not an inherent property, but rather a combination of cue characteristics, the environment, and the observer’s point of view, or perspective (Caduff and Timpf, 2008). Whether such properties of visual stimuli influence hippocampal activity has not been tested.
The present study examined the effect of purely visual cues differing in size and proximity on hippocampal place cells. There is evidence that rats are more prepared to process visual information presented on, or low to, the ground (Lashley, 1938; Minini and Jeffery, 2006; but see Wallace et al., 2013). This notion is consistent with findings that retinal ganglion cells are concentrated in the superior retina of rats, resembling a visual streak (Dreher et al., 1985; Fukuda, 1977; Salinas-Navarro et al., 2009). Indeed, recent research suggests that hippocampal place fields may be more strongly modulated by the appearance of the floors than the appearance of the walls (Jeffery and Anderson, 2003; Anderson et al., 2006; Ji and Wilson, 2007). We compared the responses of hippocampal place fields to 90° rotations of three 2-dimensional (2D) visual cue conditions presented on the wall or floor of a square open field: a large vertical cue on one wall, a large floor cue in a corner abutting two walls, or smaller floor cues in a corner adjacent to two walls. In the real world, landmarks are defined as large features of the landscape that can be easily seen from a distance. Objects can also be large, but are less likely to be classified as landmarks if they are smaller. Thus, we have termed the larger stimuli as Landmarks and the smaller stimuli as Objects. We hypothesized that salience could be dynamically determined depending on the environment. We predicted that larger, distal cues would be used for orientation. Thus 90° rotations of these cues would elicit concordant place field rotations. Smaller, local cues would be less likely to be used for orientation and, instead, would be processed by the hippocampus for other purposes, for example associative or configural learning. As such, 90° rotations of these cues would more likely to elicit place field remapping.
We found that hippocampal place fields responded similarly to rotations of the large polarizing cues whether located on the wall or floor. We also found that place fields responded to rotations of the pair of small stimuli, but not in a way that suggested these stimuli would be utilized for orientation and navigation. In the absence of any experience with large distal cues, small object stimuli were more likely to be used for orientation.
MATERIALS AND METHODS
Subjects
Eleven male Long Evans rats (Charles River Laboratories, Wilmington, MA) used in this experiment and were 2–4 months old (275–325g) at the time of implantation. Eight rats were exposed to all three conditions, and three rats were exposed to a single condition. Prior to behavioral training, rats were brought to 85–90% of free feeding weight. After surgery, rats were housed individually in large Plexiglas® cages on a 12hr light/12hr dark schedule with ad libitum access to water. All testing occurred during the light phase. Experiments were performed with the approval of Brown University’s institutional animal care committee and in accordance with all NIH guidelines for the care and use of animals in research.
Surgery, Electrode Preparation and Localization
Microdriver assemblies housing between 4–8 tetrodes were constructed in-house. Each independently driveable tetrode was constructed from four twisted 12 μm nichrome wires (California Fine Wire Company, Grover Beach, CA). Electrode tips were cut and plated with platinum chloride (Sigma-Aldrich, St. Louis, MO) prior to surgery to reduce impedances to 100–300 kΩ at 1 kHz.
All surgeries were performed under aseptic conditions. Thirty minutes prior to the beginning of surgery, rats were given the anticholinergic glycopyrrolate (0.5 mg/kg SC), the antiepileptic diazepam (2 mg/kg I.P.), and analgesics butorphanol tartrate (0.5 mg/kg SC) and carprofen (5.0 mg/kg SC). Rats were then deeply anesthetized with vaporized isoflurane, placed in a stereotaxic apparatus, and secured with blunt ear bars. Rats were implanted with a microdrive assembly using a stereotaxic apparatus. One or two rows of four tetrodes were inserted above the right hippocampus in the coronal plane with the intention of recording along the proximodistal CA1 subfield axis (AP 3.8 mm, ML 2.5–3.0 mm, DV 2.0–2.5 mm relative to bregma, 2–2.5mm diameter)(Leutgeb et al., 2004; Leutgeb et al., 2005). Jeweler’s screws, grip cement, and dental cement (Henry Schein, Melville, NY) were used to secure the microdrive to the skull. One or two screws in the skull of the contralateral hemisphere were connected to the microdrive ground.
At the end of the experiment, rats were given an overdose of Beuthanasia-D (100 mg/kg, i.p.) and electrode tip placements were marked with a small lesion. Rats were perfused with 0.9% Saline followed by 10% formalin and the brains were extracted and prepared for histology. The locations of electrode tips were reconstructed with a light microscope.
Behavioral Apparatus
The behavioral recording chamber (Figure 1A) was a 1 m square open field arena with modular white Plexiglas® walls (46 cm height, 0.6 cm thick) that rested on top of a Floor Projection Maze (Furtak et al., 2009; Jacobson et al., in press). This apparatus was a custom built 112 x 147.3 x 76 cm aluminum frame tabletop (80/20, Inc., Columbia City, IN) holding a 1.27 cm thick top of clear Plexiglas® covered by flexible fabric projection screen material (Dual Vision Fabric, Da-Lite Screen Company, Warsaw, IN) protected by a thin protective Plexiglas® floor. This design allows for rear projection of images to the maze floor by an ultra-short throw projector (NEC WT615, NEC Display Solutions, Ltd.).
Figure 1.
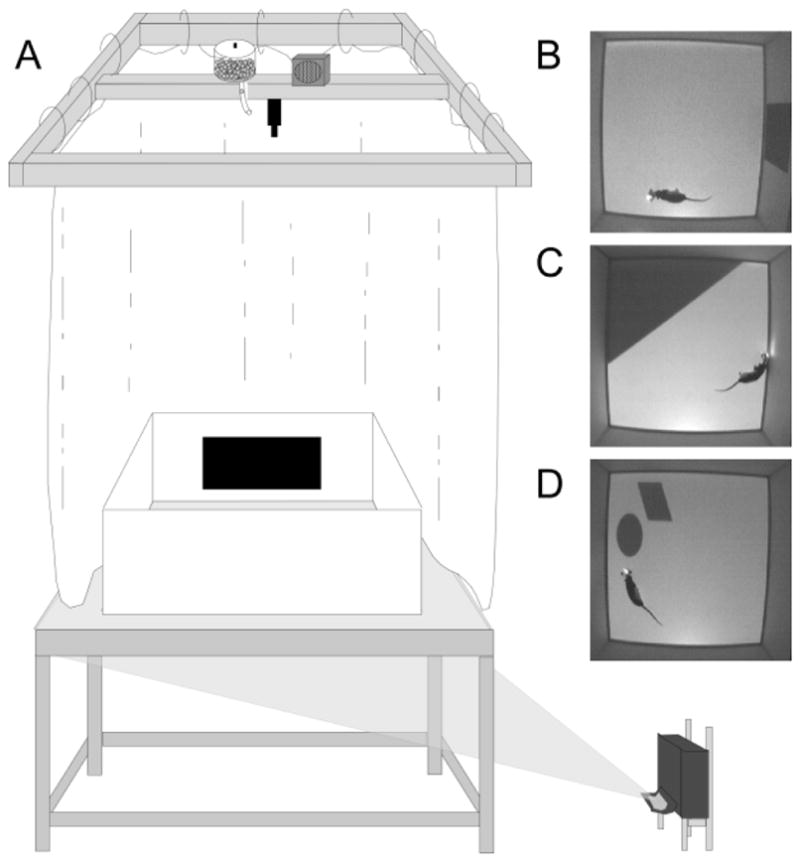
Behavioral apparatus and cue manipulation conditions. (A) Schematic of Floor Projection Maze with a depiction of the Landmark Wall cue. Floor cues are projected to the Plexiglas® maze floor using a short throw projector. The maze was completely enclosed by a white curtain, thereby masking extramaze visual cues. A speaker and automatic food dispenser were located directly above the maze, near the video camera; the speaker played 70db white noise to mask auditory cues. Top down views are shown for an animal in the Landmark Wall Condition (B), the Landmark Floor Condition (C), and the Object Floor Condition (D).
The maze was interfaced with three computers, one for tracking, one for data acquisition, and one for behavioral control. Tracking was accomplished by a CinePlex Digital Video Recording and Tracking System (Plexon, Inc., Dallas, TX) with a StingrayTM camera (640x480 resolution, 40 frames per second) interfaced with a computer running Windows XP or Windows 7. The digital video camera was positioned 140 cm above the maze floor and provided a live-image aerial view of the chamber. Behavioral control, including dropping of pellets and control of screen projection to the floor of the maze, was accomplished by a custom program written in MedState Notation and running on a Windows XP or Windows 7 under the control of MED-PC IV (Med-Associates, Inc., Burlington, VT).
Behavior and Recording Procedures
Prior to implantation, rats were trained to collect randomly scattered 45 mg dustless pellets (BioServ, Frenchtown, NJ) for two 10-minute sessions in an 81 cm square arena located in a separate behavior room. Pellets were dropped from above at randomly selected intervals of 10, 15 or 17 seconds. The habituation arena comprised three white and one black Plexiglas® walls (46 cm tall, 0.6 cm thick) and a white Plexiglas® floor. The habituation arena was not encircled by curtains, thereby allowing the animal to have visual access to distal extramaze cues. By the end of the second session, all rats were continually exploring and foraging the entirety of the arena.
There were three cue conditions in this experiment (Figure 1B–D). Two cue conditions were termed “Landmark” conditions because the cues were large, highly-salient, peripherally located, polarizing cues. For the remaining cue condition we used the term, “Object”, because the cue comprised smaller stimuli, though still highly salient. These cues were located close, but not adjacent, to the walls. The rationale was that properties of size and portability appear to determine whether or not an item is used for navigation (Auger et al., 2012). In the Landmark Wall condition, the walls were opaque white except for a black polarizing rectangle (approximately 1084 cm2) centered on the east wall accompanied by a projected grey floor with no markings. The Landmark Floor condition consisted of a projected grey floor and a dark grey triangle that occupied approximately 1/3 of the maze floor (2080cm2) in the northeast corner abutting two walls. The walls were opaque white. The Object Floor condition consisted of a grey floor with a dark grey polygon (345 cm2) and an ellipse (355 cm2) located in the northeast corner of the maze. These objects were located 5 cm from each other and approximately 4 cm from the wall. Again, the walls were opaque white. Note that each condition included an image projected to the floor under the control of the MedState Notation program, either a plain grey floor for the Landmark Wall condition, or salient cues on a grey background floor for the Landmark Floor and Object Floor conditions.
Following 5–7 days of postsurgical recovery, rats received additional habituation sessions in the recording arena, but no recordings were obtained during these sessions. In each session, rats collected scattered pellets that dropped at randomly selected intervals of 10, 15 or 17 seconds until they were habituated to the recording apparatus, automated food dispenser and white noise. Eight rats received daily habituation sessions to each of the three cue conditions. To address the issue of whether there was interaction among distal and local cues, two additional rats were habituated and tested only in the Object Floor condition.
Rats were transported to and from the recording room during the habitation phase and experimental phase in a black rubber container covered with a towel to minimize any exposure to extramaze cues. Before being placed into the recording chamber and after being removed, subjects were disoriented to minimize the impact of vestibular cues by rotation of the holding container. Habituation sessions were terminated when rats no longer exhibited thigmotaxic behavior and collected all scattered food pellets. Rats generally required 2–3 habituation foraging sessions in each cue condition.
Once habituated, rats were screened daily for evidence of single unit activity. Rats continued to run one 10-minute session per cue condition (3 total) per day until single unit activity appeared at depths of 2.0 mm or lower relative to the cortex. Tetrodes were lowered in steps of 26–53 μm at the end of each screening day if neuronal activity was not observed. Once single unit activity was evident, unit data was recorded and 90 degree rotations were performed in each cue condition. After each 10-minute foraging session the rat was removed from the recording chamber, and placed back in its home cage, located outside the recording room. The wireless headstage was turned off but not unplugged during these intersession intervals in an effort to maintain the unit activity across sessions and cue conditions. All data recorded on the same day were saved to one data acquisition file or merged offline (PlexUtil, Plexon Inc. Dallas, TX) to optimize offline unit isolation across sessions and cue conditions.
The microdrive was connected to a 31-channel wireless headstage (Triangle BioSystems Inc., Durham, NC) with a 2X gain headstage that passed the signal to a high-gain amplifier (total = 10,000X; Plexon, Inc., Dallas, TX). Recordings were band-passed filtered between 0.8 Hz–6 kHz for single units and between 3.3 Hz– 89 Hz for local field potentials (LFP). The signal was then processed by a Multichannel Acquisition Processor (Plexon, Inc., Dallas, TX), which allowed for real-time thresholding and waveform discrimination. Spike waveforms above a threshold set by the experimenter were time-stamped and digitized at 40kHz for 1ms.
Two LEDs, approximately 2 cm apart located on top of the head stage were used for reflective color marker tracking with CinePlex (Plexon, Inc., Dallas, TX). This tracking mode optimized LED tracking in the presence of an illuminated background. Timestamped x and y coordinates of the rat’s position were provided in real time to a Multichannel Acquisition Processor (MAP, Plexon Inc., Dallas TX). The third computer controlled all behavioral equipment with a DIG-716P2 Smart Control Output Interface (Med Associates Inc, St. Albans, VT) including food pellet delivery, auditory white noise and the display of floor images with custom written software in Med PC. SortClient (Plexon, Inc., Dallas, TX) collected rat head position, single unit data and timestamps in real time for later offline analysis.
Analysis
Single units were isolated offline based on the relative amplitudes of signals recorded simultaneously by the four wires of each tetrode (Offline Sorter, Plexon, Inc., Dallas, TX). Final sorted files were converted to NEX files and individual session files containing position and unit timestamps were exported from the final sorted file based on event start and stop timestamps (Nex Technologies, Littleton, MA). Custom written Matlab programs (Mathworks Inc., Natick, MA) were used to read NEX files (readNexFile.m; author Benjamin Kraus), fix bad position coordinates, and filter data for speed (>3 cm/s) (adapted from FixMyPOS.m. author R. Jonathan Robitsek). In general, position coordinates were obtained by averaging the coordinates of the two LEDs to obtain one position coordinate for each timestamp. In the event that tracking of one LED was missing or out of range, the position coordinates for the remaining LED were used instead.
Custom Matlab programs were used to calculate spatial information content, construct speed filtered spike position, firing rate, and threshold firing rate maps as well as Gaussian smoothed (3x3pixels) firing rate maps. Correlation coefficients were also calculated for spatial analysis. Place fields were identified using threshold firing rate maps that filtered the firing rate maps for pixels in which the firing rate of the cell was 3x greater than the grand average firing rate of that cell. At least five adjacent and contiguous pixels were required for the identification of a place field (Burwell and Hafeman, 2003). Spatial information content was calculated for all hippocampal cells in all three sessions per condition using unsmoothed data and the formula:
Where i is the spatial bin number, Pi is the probability for occupancy of bin i, Ri is the mean firing rate for bin i and R is the mean firing rate (Skaggs et al., 1993). Cells were included in the analysis if spike counts were at least 50 and spatial information content scores were at least 0.25 in the rotated 10-min session and either the first or second standard 10-min session.
In order to compare spatial firing patterns across sessions in each condition, firing rate maps for the rotated and second standard session were rotated clockwise in 90 degree increments and correlation coefficients were calculated in comparison to the first standard session. In the event that a cell did not start firing until the rotated session, the second standard session was compared to the rotated session for correlations. Place field responses within a condition were classified by visually inspecting the place fields between the standard and cue-rotated sessions as rotation, remap, or no change and confirmed with a correlation approach. Place fields were classified as having predictably rotated if the field rotated at least once in concert with the rotation of the relevant cue. Correlation coefficients for rotation responses were highest for the location concordant with the rotated cue and exceeded all other correlations by a minimum of 0.15. Place fields were classified as having remapped if the field appeared or disappeared, if the highest correlation coefficient was for a location discordant with the rotated cue, or if the place field shifted to an unpredictable location with the rotation of the relevant cue and the correlation coefficient was similar across all 90 degree increments. Finally, place fields were classified as having not changed if the place field remained in the same location following the rotation of the relevant cue and correlation coefficients were highest for the 0 degree rotation increment.
RESULTS
A total of 249 hippocampal CA1 cells were recorded from eleven rats. This data set included 172 cells recorded from eight animals that experienced multiple conditions, and 77 cells that were recorded from three animals that experienced only the Object Floor condition. Of the cells recorded in the multiple-condition subjects, 104 cells were recorded in at least two of the three cue conditions on the same day. On these days, the order of the cue conditions was randomized. To avoid duplicate recordings of the same cell in the same condition, electrodes were advanced in increments of at least 26 μm between recording days until new cells were evident. A total of 106 cells were recorded in the Landmark Wall condition, 106 cells in the Landmark Floor condition, and 105 cells in the Object Floor condition. Of these, 67, 62, and 57 cells exhibited spatial firing patterns and met analysis criterion in the Landmark Wall, Landmark Floor, and Object Floor conditions, respectively. Of the 77 hippocampal CA1 cells recorded from three rats subjected only to the Object Floor condition, 30 cells exhibited spatial firing patterns and met analysis criterion. The responses of these cells are discussed separately below. Figure 2 shows the location of electrode tips in CA1.
Figure 2.
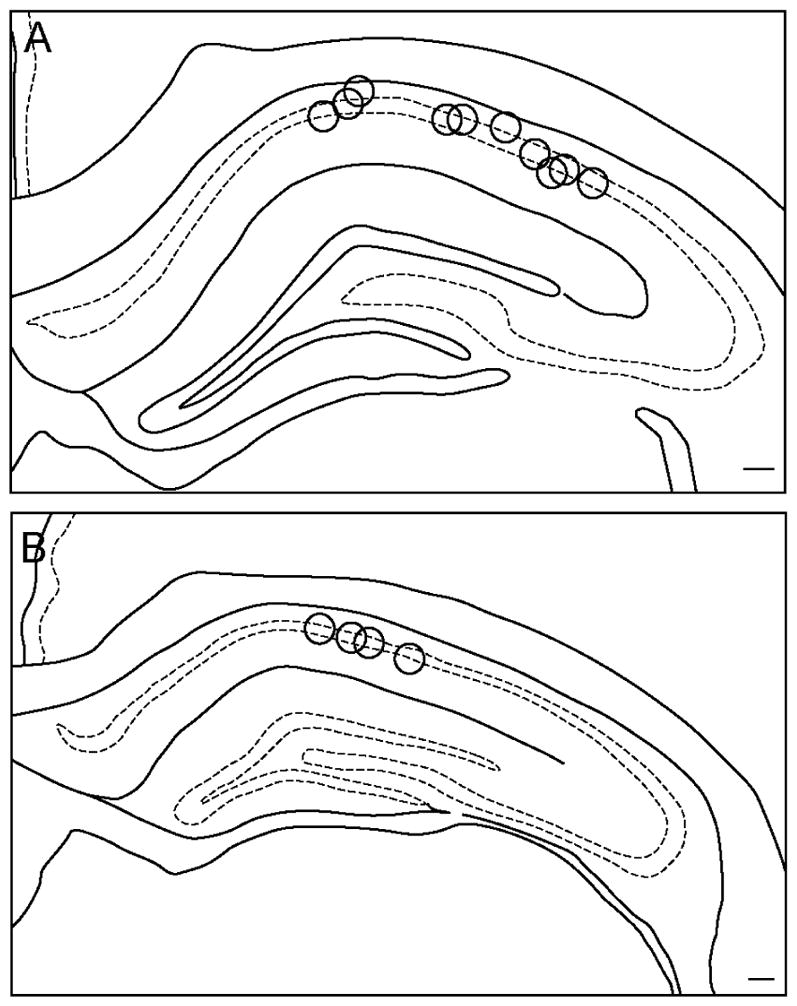
Location of electrode recording sites. Shown are locations of tetrode recording surfaces in coronal sections of dHIP between −3.30 to 3.60 mm relative to bregma (A) and −3.80 to −4.16 mm relative to bregma (B). Scale bars = 250 um.
Place fields rotate following Landmark Floor and Landmark Wall manipulations, but remap following Object Floor manipulations
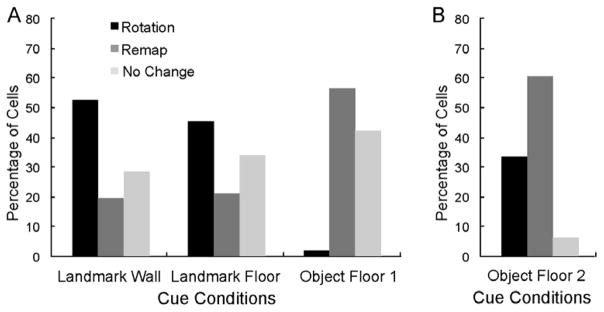
In the Landmark Wall and Floor conditions, the predominant response to 90 degree cue rotation was a place field rotation in concordance with the visual cue (Figure 3A). Of the 67 cells in the Landmark Wall condition, responses of 52.24% were classified as rotation, 22.40% as remap, and 25.37% as no change. The results were similar for the 62 cells in the Landmark Floor condition; responses of 43.55% of cells were classified as rotation, 22.58% as remap, and 33.87% as no change. A Chi Square Analysis revealed that responses in the Landmark Wall and Floor conditions were not significantly different (X2 (2) = 1.296, p=0.523). In contrast, in the Object Floor condition, the predominant response to 90 degree cue rotations was a place field remap. Of the 57 cells in the Object Floor condition, only 1.75% of responses (one cell) were classified as rotation, whereas 57.90% and 40.35% were classified as remap and no change, respectively. A Chi Square Analysis revealed that responses in the Object Floor condition were significantly different from responses in the Landmark Wall condition (X2(2) = 39.210, p<0.0001) and the Landmark Floor condition (X2(2) = 31.761, p<0.0001). A one-way analysis of variance revealed the average speed of animals did not significantly differ across conditions (mean±SE was 23± 0.80 for Landmark Floor, 22±0.84 for Landmark Wall, and 21 ± 0.85 for Object Floor, (F(2,163)=1.333, p=0.267)), and thus cannot account for the differences in responses to cues.
Figure 3.
Distribution of place field responses in three visual cue conditions. A. Cells in the Landmark Wall (n = 67) and Landmark Floor (n = 62) conditions responded similarly to the visual cue manipulations; the largest proportion of recorded place fields rotated with Landmark cues. Cells in the Object Floor condition (n = 57) responded differently; the largest proportion of place fields remapped in response to rotations, and only one cell rotated concordantly with rotation of the objects. B. Cells in the Object Floor condition (n = 30) recorded from animals that experienced only that cue condition. Again, the largest proportion of cells remapped in response to rotations of the object cues.
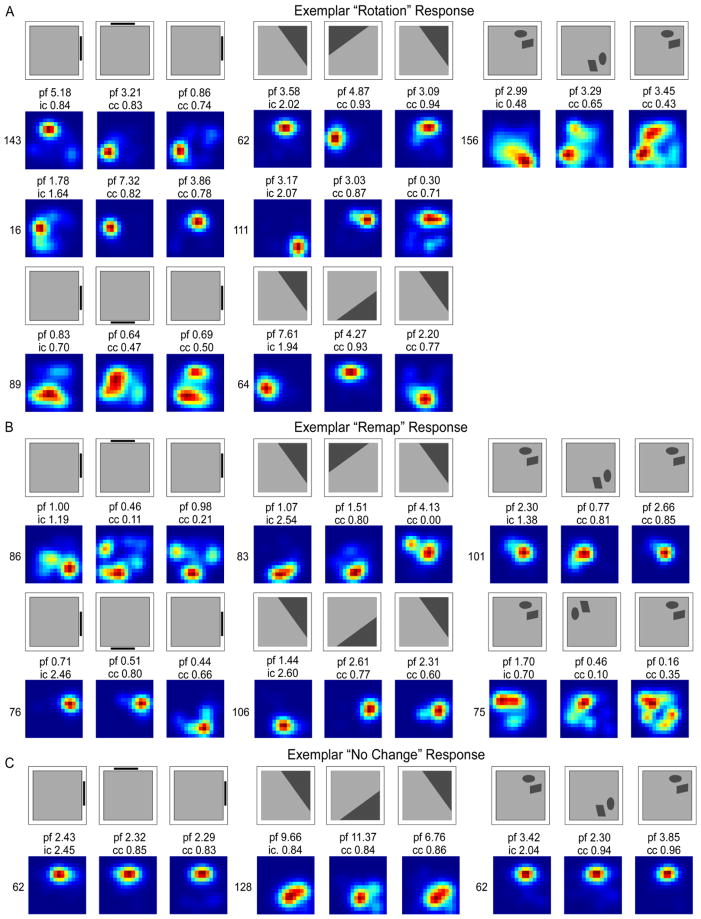
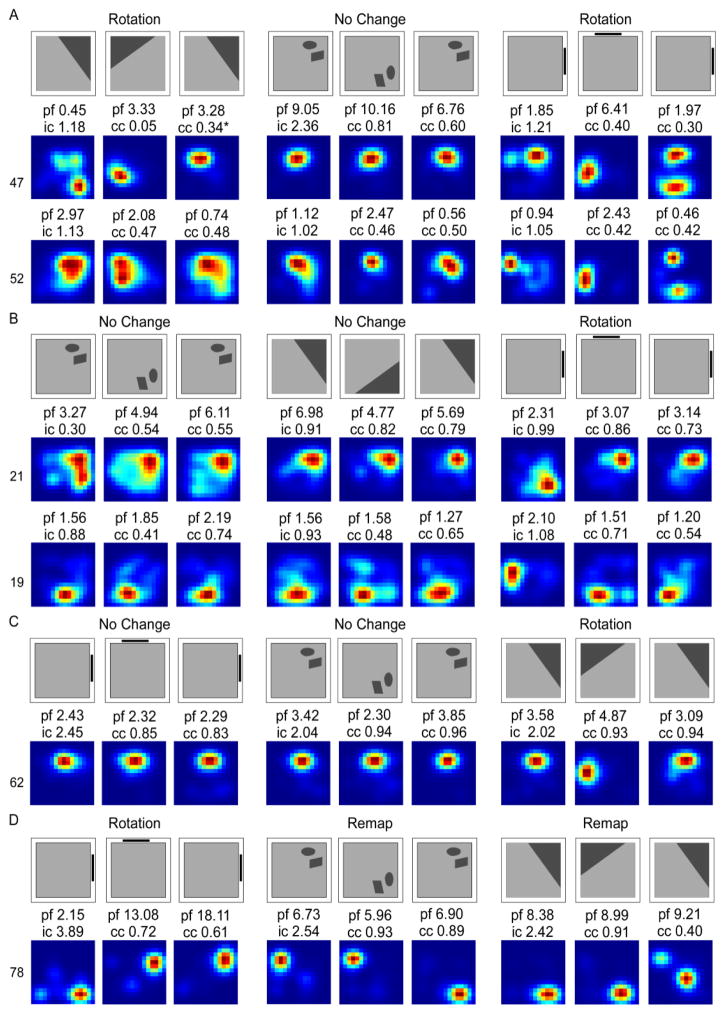
Figure 4 illustrates exemplar responses in each cue condition. Place cell responses were defined as a rotation response if the place field rotated in concordance with the cue between the first standard and rotation sessions, between the rotation session and the second standard, or both. In the Landmark Wall condition, rotation responses included a rotation in the manipulated session, followed by a return to the original firing field location (Figure 4A; Cell 89), a rotation in the manipulated session followed by a no change in the second standard configuration (Figure 4A; Cell 143), and a no change in the manipulated session followed by a rotation with the cue in the second standard configuration (Figure 4A; Cell 16).
Figure 4.
Smoothed rate maps showing exemplar place field responses. Rotation (A), remap (B), and no change (C) responses are shown for the Landmark Wall (left), Landmark Floor (middle), and Object Floor (right) conditions. Above each rate map series is a schematic showing the direction of cue rotation. Abbreviations: peak firing field rate (pf), information content score for the first standard session (ic), the correlation coefficient (cc) for the rotation and second standard sessions.
Similar responses were observed in the Landmark Floor condition. Rotation responses included a rotate and return response (Figure 4A; Cell 62), a rotation in the manipulated session followed by a no change in the second standard configuration (Figure 4A; Cell 111) as well as a rotation in the manipulated session followed by an unpredictable rotation in the second standard configuration (Figure 4A; Cell 64).
The sole rotation response in the Object Floor condition is also illustrated (Figure 4A; Cell 156). This place field rotated in the manipulated session and then did not change its firing position in the second standard configuration. Remap and no change responses are also illustrated in Figure 4B and C, respectively. Remap responses included unpredictable rotations (Figure 4B; Cells 76, 83, 106, and 101) and unpredictable shifts in firing field locations accompanied by low correlation coefficients (Figure 4B; Cells 86, and 75).
Remapping responses in the object condition did not differ qualitatively from those seen in the other two conditions. Approximately half of the remapping classifications were unpredictable rotations in the object and landmark conditions. In the object condition, it is unlikely that partial rotations underlie the remap response. 55% of cells classified as remap were unpredictable rotations; these rotations were often in the opposite direction of the cue rotation. In 10% of remap classifications, place fields either appeared or disappeared, and in the remaining 35%, correlation coefficients were low across all rotation increments making it unlikely that remapped fields are due to partial rotations. If the field had partially rotated, the correlation coefficients of the rotated session should have at least been higher than the remaining rotation increments.
Finally, a one-way analysis of variance revealed that correlation coefficients calculated for the comparison of standard sessions before and after the rotation session were not significantly different across visual cue conditions [F(2, 80)= 2.619, p=0.079]. Responses included in this analysis were rotation, remap and no change.
Experience influences place cell responses to Landmark and Object cue manipulations differently
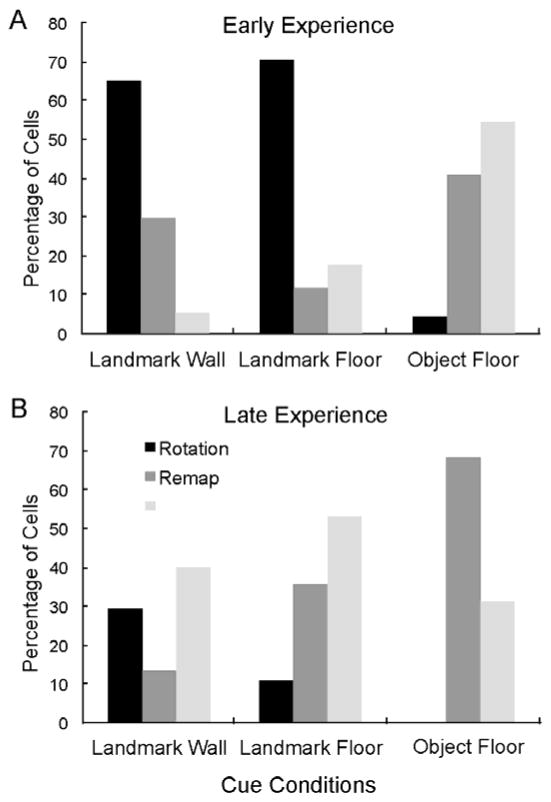
We also found an effect of experience on place cell response to cue manipulations that differed for the Landmark versus the Object Floor conditions. Experience was defined as the number of days the animal was exposed to the exploratory maze, regardless of the cue condition, and included days in which the animal was exposed to the maze, but no recordings were obtained, e.g. habituation. Visual inspection of the data indicates that there was often a transition in responses around the 8th to 9th day. Thus, data were grouped into Early Experience (cells recorded up to day 8) and Late Experience (cells recorded on days 9–16). In both of the Landmark cue conditions, the percentage of rotation responses decreased and the percentage of no change responses increased with experience (Figure 5). In the Object Floor condition, however, the percentage of remap responses increased and no change responses decreased with experience (Figure 5). Frequency analysis indicated that occurrences of rotation, remapping, and no-change cells were significantly different across Early and Late experience for each of the landmark conditions (p<0.0001), and there was a trend toward a difference for the Object Floor condition (p<0.058, Table 1). Within the Early experience phase, both landmark conditions were different from the Object Floor, but not from each other (Table 1). All paired comparisons were significantly different in the Late experience phase. These data suggest that rats became less reliant on the Landmark cues for orientation and navigation with experience and that the Object cues were more likely to influence hippocampal representations with increasing experience.
Figure 5.
Effect of experience on place field responses differs between Landmark and Object conditions. Early experience (A) was the first eight days in the apparatus and late experience (B) was the last 8 days in the apparatus. In both the Landmark Wall and Floor rotations decreased with experience, and no change responses increased. In the Object Floor condition, however, place field remapping increased with experience, whereas no change responses decreased.
Table 1.
Effects of experience on responses to stimulus conditions
| Within Cue Condition | LW | LF | OF | |
|---|---|---|---|---|
| Early vs Late | X2(2)=26.79 | X2(2)=22.39 | X2(1)=3.59 | |
| (p<0.0001) | (p<0.0000) | (p<0.058) | ||
|
| ||||
| Across Cue Conditions | LW vs LF | LW vs OF | LF vs OF | |
|
| ||||
| Early | X2(3)=33.41 | X2(2)=5.15 | X2(2)=26.40 | X2(2)=23.60 |
| (p<0.0000) | (p<0.076) | (p<0.0000) | (p<0.0000) | |
| Late | X2(3)=29.33 | X2(2)=7.08 | X2(2)=25.69 | X2(2)=8.71 |
| (p<0.0000) | (p<0.029) | (p<0.0000) | (p<0.01) | |
Chi-square analysis of frequencies of cells showing rotation, remapping, or no change in response to cue manipulations. Degrees of freedom are in parentheses. For the Early vs Late analysis for the Object Floor Condiition, rotations were not included as there was only one occurance. Abbreviations: LF, Landmark Floor; LW, Landmark Wall; OF, Object Floor.
For the Early Experience, we were also interested in whether place fields that rotated with the cue between the first and second sessions also rotated back concordantly in the final session. For the Landmark Wall and Landmark Floor conditions, the majority of rotation fields returned to the original location (71% and 63%, respectively). In the Object Floor condition, the one field that showed a rotation response did not return to the same position. With experience, fields were less likely to rotate or return to the original configuration.
A number of cells were recorded in multiple conditions on the same day, and we were interested in how the same cell responded across different conditions (Figure 6). Overall, there was more correspondence across the two Landmark conditions (50%) followed by the two Floor conditions (33%). Responses of cells across the Landmark Wall and the Object floor showed the least correspondence (12.5%).
Figure 6.
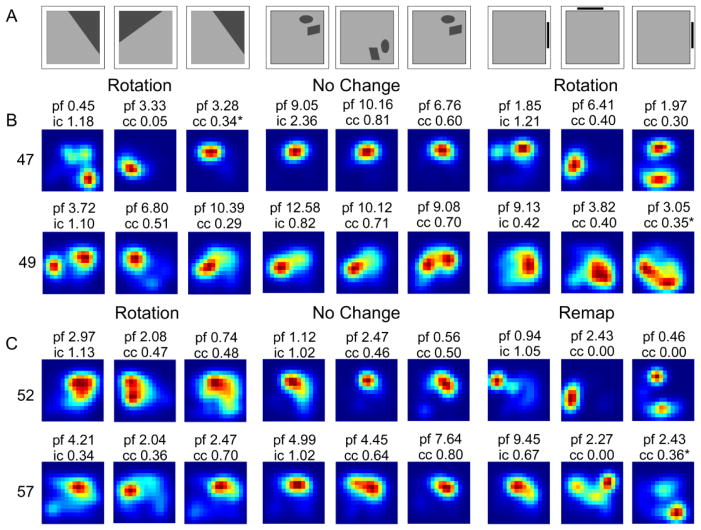
Cells responded similarly across Landmark conditions and differently across Landmark and Object conditions. Shown are smoothed rate maps showing exemplar responses of cells recorded during early (D3–8) experience in all visual cue conditions. The peak firing field rate (pf), Information content score for the first standard session (ic) and the Correlation Coefficient (cc) for the rotated session and second standard are listed above each map. A. Schematic of condition order and rotation direction. B. Cell 47 and 49 rotated with cues in the Landmark conditions, but did not change in response to rotations of cues in the Object condition. C. Cells 52 and 57 rotated with cues in the Landmark Floor condition, remapped in the Landmark Wall condition, and did not change in response to rotations of cues in the Object Floor Condition.
Eight cells were recorded on the same day in both the Landmark Wall and Landmark Floor conditions. Of these, half of the cells responded similarly. Three cells (47, 49, and 58) exhibited rotation responses in both conditions and one cell (41) exhibited no change in both conditions. The remaining four cells (50, 52, 54, and 57) all remapped in the Landmark Wall condition and rotated in the Landmark Floor condition. Nine cells were recorded in the Landmark Floor and Object Floor conditions. Of these, one third of the cells responded similarly. These cells (41, 43, and 44) exhibited no change in both conditions. The remaining six cells had different response in the Landmark and Object Floor conditions. The place fields of five of these cells (47, 49, 50, 52, and 57) rotated in the Landmark Floor condition, but did not change in the Object Floor condition. The remaining cell (58) rotated in the Landmark Floor condition, but remapped in the Object Floor condition. Finally, a total of eight cells were recorded in the Landmark Wall and Object Floor condition. Of these, one cell had similar responses in both conditions. This cell (41) did not change its place field location in either condition. Of the remaining seven cells, three cells (47, 49, and 51) rotated in the Landmark Wall, but did not change their response in the Object Floor condition. One cell (58) rotated in the Landmark Wall and remapped in the Object Floor condition, and three cells (50, 52, and 57) remapped in the Landmark Wall condition and did not change in the Object Floor condition.
The order in which multiple cue conditions were presented to animals on the same day did not affect the place field response of the same cell. That is to say, cells recorded in the same animal on the same day that rotated, remap or did not change in one condition were equally likely to rotate, remap or not change in subsequent conditions. Animals presented with the Object Floor condition before the Landmark Floor or Wall condition, had cells that rotated in the Landmark conditions and those same cells remapped or did not change in the Object Floor condition (Figure 7).
Figure 7.
Order of conditions does not impact place field response. Shown are smoothed rate maps for cells recorded in all cue conditions. Rate maps and schematics are organized in the order the animal was run in each condition. The peak firing field rate (pf), Information content score for the first standard session (ic) and the Correlation Coefficient (cc) for the rotated session and second standard are listed. Firing rate maps were smoothed for illustrative purposes. Cells rotated (A, B, C) or remapped (D) fields in the last, but not previous, conditions, indicating that order of conditions did not impact place field responses.
To determine if exposure to Landmark conditions influenced whether the Object Floor cues were used for orientation, three naïve animals were implanted and experienced only the Object Floor condition. Similar to animals that experienced all three cue conditions, the predominant response to 90 degree Object cue rotation was a place field remap (Figure 3B). Rotation responses, however, were also evident. These animals were tested in a different space in which extraneous cues were better controlled. This is consistent with the observation of the low number of “no change” responses. It is also possible that because the animal’s experience in the recording room was limited to one condition as opposed to three, the reduced daily experience impeded their ability to notice uncontrolled extraneous cues that the other animals exposed to all three conditions were using. Of the 30 cells recorded, responses of 60% were classified as remap, 33.3% as rotation and 6.7% as no change. When these cells were added to the Object Floor cells in Figure 3A, the distribution of cells showing Rotation, Remapping, and No Change responses still differed significantly from distributions in the Landmark Wall condition (X2(2) = 42.659, p<0.0001) and the Landmark Floor condition (X2(2) = 34.928, p<0.0001).
These data suggest that rats are more likely to use Object cues for orientation if they have not experienced peripherally located cues (i.e. Landmark cues). Given that coherent rotations were not the dominant response, Object cues seem to afford qualitatively different stimulus control over place fields than do Landmark cues.
DISCUSSION
This study explored the properties of visual cues used by the hippocampus for orientation and navigation. Ours is the first study to employ cues restricted to the visual domain to examine the influence of stimulus properties on the use of cues for spatial orientation. We provide evidence for the hypothesis that hippocampal place cells are biased to employ large, distally located cues for this purpose. Moreover, such cues can be located on the wall or the floor in the same way that either a tall rock or a pond viewed at a distance can be used for orienting and navigating. We manipulated three types of 2D visual cues and examined responses of hippocampal place cells. Rotation of large, peripherally located, 2D cues, whether placed on a wall or on the floor adjacent to walls, were more likely to result in concordant rotations of hippocampal place fields, suggesting that both types of cues are used for orientation. We also found that place fields responded to manipulations of smaller 2D objects on the floor, but not in a way that suggested these stimuli were normally used for orienting. Even if the rats experienced only 2D objects, and not larger peripherally located cues, manipulation of the 2D object cues was more likely to result in place field remapping and less likely to result in concordant rotation. Our data suggest that the hippocampus processes visual cues for at least two purposes. Large, distal cues are more likely to be processed as stable landmarks and to be used for orientation and navigation. In contrast, smaller, more proximal cues are more likely to be processed as non-stationary objects or features in the environment.
The control of hippocampal place cell activity by polarizing 2D cues on the wall of an apparatus is well documented (O’Keefe and Conway, 1978; Muller and Kubie, 1987; Fenton et al., 2000a, b), and other studies have used the color of the entire floor as a context indicator (Jeffery and Anderson, 2003; Anderson et al., 2006; Ji and Wilson, 2007). To our knowledge, this is the first demonstration that large, polarizing 2D visual cues on the floor of an arena and on the wall of an arena are equally effective in controlling hippocampal place fields. In addition, although a few studies have examined the effects of manipulation of 3D objects on hippocampal place fields (Cressant et al., 1997, 1999; Burke et al., 2011), ours is the first study to assess the effects of 2D floor-based cues on hippocampal place fields. Finally, we provide the first evidence that hippocampal responses to visual cues on the floor are modulated differentially consistent with whether or not cues might be beneficial for navigation.
Why do large floor-based cues adjacent to the perimeter of an arena and smaller cues away from the perimeter control hippocampal place fields in such different ways? A key feature of a visual cue that results in its use as a landmark for orientation is saliency, and size tends to correlate with saliency. Cue size alone, however, does not determine the degree of stimulus control over place field location (Muller and Kubie, 1987). Caduff and Timpf (2008) argued that saliency is not an inherent property, but a combined evaluation of the actual feature or cue, the environment, and the observer’s point of view. Other work suggests that relationship between the position of objects and the location of the rat impact the perception of object stability (Cressant et al., 1997, 1999). In our study, the 2D objects were differently shaped and were located away from walls such that the appearance would change based on the rat’s vantage point. For example, the rat was able to walk between the objects and the wall. In contrast, the two larger cues located on the wall or on the floor adjacent to the wall would remain relatively unchanged as the rat moves about the maze. Thus, our data are consistent with other findings that an interaction of environmental space, characteristics of cues, including size, and vantage point influence the nature of stimulus control over place field responses.
Remapping was predominant in the object cue condition demonstrating that these cues were able to control spatial firing patterns. It may be, however, that in absence of a more salient landmark, our object cues were in competition with uncontrolled extraneous cues. To address this possibility we recorded cells from animals that were exposed only to the Object Floor condition (Fig 3B). In that experiment we carefully controlled the possibility of modulation by extraneous stable cues. Judging by the low number of cells that exhibited no change responses, we think we were successful in this regard. A relatively larger proportion of cells showed rotation responses, but the predominant response of place fields to rotation of object floor cues was to remap.
A number of studies have addressed the influence of experience on landmark stability. In one study, place cell responses to cue manipulations were examined in a double rotation task in which sets of distal visual cues and local multimodal sensory cues were counter rotated by 90 degrees resulting in cue conflict between local and distal cues (Shapiro et al., 1997). After repeated trials, the percentage of place field rotations concordant with distal cues decreased and the percentage of remap responses increased. The authors suggested that rats learned to encode distinct representations for the organization of stimuli in the double rotation condition and the standard environment. A more consistent finding, however, is that when external visual cues are non-stationary or unavailable, place cells rely on idiothetic cues to update spatial representations (Knierim et al., 1995; Sharp et al., 1995; Jeffery et al., 1997; Jeffery, 1998). Consistent with this idea, our data show that an experience-dependent decrease in rotations that are concordant to landmark cues is accompanied by an increase in no change responses. Because the animals were disoriented prior to placement in the arena, idiothetic cues would not have been useful. It is more likely that the animals learned over time that the landmark cues were not reliable for updating spatial position and began to rely on extraneous uncontrolled, but stable, environmental cues. Thus with experience, extraneous uncontrolled cues in the environment may have gained stimulus control over place fields as the landmark cues were losing stimulus control.
The effects of the 2D object cues on place fields also changed with experience, but in a qualitatively different way. As the animal gained more experience in the maze, manipulation of the object cues on the floor resulted in increased place field remapping. Although somewhat speculative, one interpretation is that as increased experience led to a heightened awareness for stationary extramaze cues, it may have become increasingly apparent to the animals that the objects were moving. This observation may have increased the attention of the animals to those cues, thus increasing the associability of the cues. Similarly, if the animals viewed the objects as portable, this may have negatively impacted the use of the objects for orientation. The object cues were rarely used for orientation, but the increased remap response with experience suggests that the object cues were increasingly available for associative learning and for incorporation into the contextual representation. Data from rat (Biegler and Morris, 1993) and human (Burgess et al., 2004) also suggest that geometric stability is a prerequisite for a cue to be included into an animal’s representation of allocentric space, but that the lack of geometric stability does not prevent a cue from acquiring associative strength. Even though the pair of object cues in the current study was located toward the walls, the physical space between the objects and between the objects and walls may have decreased the geometric stability of the cue and its likelihood to be used for orientation.
Two lines of evidence in the present study suggest that the hippocampus represents different classes of cues that could be functionally described as landmarks and objects. First, manipulation of the large distal cues tended to produce concordant rotations, whereas manipulation of the pair of object cues tended to result in place field remapping. Indeed, the single concordant rotation in response to rotation of the object cues may have been an unpredictable rotation that happened to line up with the cue rotation. Second, the influence of experience on place cell responses to cue manipulations was qualitatively different for the large distally located cues as compared with the pair of object cues located away from the wall. For the large distal cues, numbers of rotations concordant with landmark manipulation decreased with experience and the number of no change responses increased. In contrast, for the pair of object cues, responses of no change decreased with experience and remap responses increased. Taken together, the evidence suggests that the hippocampus processes at least two classes of cues for two purposes: landmarks are processed for navigational relevance and objects are processed for associative properties.
Whether cues are classified as landmarks or objects in the hippocampus or whether the hippocampus relies on other structures for this process is an open question. Hippocampal lesions do not disrupt the activity of head direction cells, in the postsubiculum and anterior thalamus or their stability across multiple days in both novel and familiar environments (Golob and Taube, 1997). That animals can still orient in both without hippocampal processing of landmark or objects suggests that classification of these cues occurs elsewhere. One possibility is that the later and medial entorhinal areas (LEA and MEA respectively) determine the navigational relevance of object and landmark cues. In fact, many studies have shown that the MEA is involved in processing landmarks for spatial navigation or orientation (Hafting et al., 2005; Hargreaves et al., 2007; Savelli et al., 2008; but see Clark and Taube, 2011). Recent lesion, recording, and immediate early gene studies suggest that the LEA is involved in processing object and object place information and is therefore a reasonable candidate for object processing (Deshmukh and Knierim, 2011; Van Cauter et al., 2013; Wilson et al., 2013). Additional research is required to further elucidate the contributions of the medial and lateral entorhinal cortices, as well as landmark and object processing.
To conclude, here we have shown that cues restricted to the visual modality can impact the behavior of hippocampal place cells. Consistent with studies in other brain regions, our data suggest that whether such cues are used for orientation depends on size, proximity, and portability. Large, distally located objects are more likely to be used as landmarks for orientation and navigation. Smaller, more proximal, portable objects are less likely to be used for navigation, but appear to be available for contextual and associative learning.
Acknowledgments
This research was supported, in part, by NSF Awards IOB-0522220, EFRI-0937848, and IOB-1146334 to RDB and 1T32NS062443 to KMS.
References
- Dreher B, Sefton AJ, Ni SY, Nisbett G. The morphology, number, distribution and central projections of Class I retinal ganglion cells in albino and hooded rats. Brain, behavior and evolution. 1985;26(1):10–48. doi: 10.1159/000118764. [DOI] [PubMed] [Google Scholar]
- Fukuda Y. A three-group classification of rat retinal ganglion cells: histological and physiological studies. Brain research. 1977;119(2):327–34. doi: 10.1016/0006-8993(77)90314-6. [DOI] [PubMed] [Google Scholar]
- Salinas-Navarro M, Mayor-Torroglosa S, Jimenez-Lopez M, Aviles-Trigueros M, Holmes TM, Lund RD, Villegas-Perez MP, Vidal-Sanz M. A computerized analysis of the entire retinal ganglion cell population and its spatial distribution in adult rats. Vision research. 2009;49(1):115–26. doi: 10.1016/j.visres.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Anderson MI, Killing S, Morris C, O’Donoghue A, Onyiagha D, Stevenson R, Verriotis M, Jeffery KJ. Behavioral correlates of the distributed coding of spatial context. Hippocampus. 2006;16:730–742. doi: 10.1002/hipo.20206. [DOI] [PubMed] [Google Scholar]
- Auger SD, Mullally SL, Maguire EA. Retrosplenial cortex codes for permanent landmarks. PloS one. 2012;7:e43620. doi: 10.1371/journal.pone.0043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegler R, Morris RG. Landmark stability is a prerequisite for spatial but not discrimination learning. Nature. 1993;361:631–633. doi: 10.1038/361631a0. [DOI] [PubMed] [Google Scholar]
- Burgess N, Spiers HJ, Paleologou E. Orientational manoeuvres in the dark: dissociating allocentric and egocentric influences on spatial memory. Cognition. 2004;94:149–166. doi: 10.1016/j.cognition.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Nematollahi S, Uprety AR, Wallace JL, Barnes CA. The influence of objects on place field expression and size in distal hippocampal CA1. Hippocampus. 2011;21:783–801. doi: 10.1002/hipo.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Hafeman DM. Positional firing properties of postrhinal cortex neurons. Neuroscience. 2003;119:577–588. doi: 10.1016/s0306-4522(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Caduff D, Timpf S. On the assessment of landmark salience for human navigation. Cognitive processing. 2008;9:249–267. doi: 10.1007/s10339-007-0199-2. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Taube JS. Intact landmark control and angular path integration by head direction cells in the anterodorsal thalamus after lesions of the medial entorhinal cortex. Hippocampus. 2011;21:767–782. doi: 10.1002/hipo.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressant A, Muller RU, Poucet B. Failure of centrally placed objects to control the firing fields of hippocampal place cells. J Neurosci. 1997;17:2531–2542. doi: 10.1523/JNEUROSCI.17-07-02531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressant A, Muller RU, Poucet B. Further study of the control of place cell firing by intra-apparatus objects. Hippocampus. 1999;9:423–431. doi: 10.1002/(SICI)1098-1063(1999)9:4<423::AID-HIPO8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Frontiers in behavioral neuroscience. 2011;5:69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Csizmadia G, Muller RU. Conjoint control of hippocampal place cell firing by two visual stimuli. Ii. A vector-field theory that predicts modifications of the representation of the environment. J Gen Physiol. 2000a;116:211–221. doi: 10.1085/jgp.116.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Csizmadia G, Muller RU. Conjoint control of hippocampal place cell firing by two visual stimuli. I. The effects of moving the stimuli on firing field positions. J Gen Physiol. 2000b;116:191–209. doi: 10.1085/jgp.116.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Ranck JB., Jr Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Exp Brain Res. 1981;41:399–410. doi: 10.1007/BF00238898. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Cho CE, Kerr KM, Barredo JL, Alleyne JE, Patterson YR, Burwell RD. The Floor Projection Maze: A novel behavioral apparatus for presenting visual stimuli to rats. J Neurosci Methods. 2009;181:82–88. doi: 10.1016/j.jneumeth.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob EJ, Taube JS. Head direction cells and episodic spatial information in rats without a hippocampus. Proc Natl Acad Sci U S A. 1997;94:7645–7650. doi: 10.1073/pnas.94.14.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Yoganarasimha D, Knierim JJ. Cohesiveness of spatial and directional representations recorded from neural ensembles in the anterior thalamus, parasubiculum, medial entorhinal cortex, and hippocampus. Hippocampus. 2007;17:826–841. doi: 10.1002/hipo.20316. [DOI] [PubMed] [Google Scholar]
- Jacobson TK, Ho JW, Kent BW, Yang F-C, Burwell RD. Automated visual cognitive tasks for recording neural activity using a Floor Projection Maze. Journal of Visualized Experiments Behavior. doi: 10.3791/51316. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ. Learning of landmark stability and instability by hippocampal place cells. Neuropharmacology. 1998;37:677–687. doi: 10.1016/s0028-3908(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Anderson MI. Dissociation of the geometric and contextual influences on place cells. Hippocampus. 2003;13:868–872. doi: 10.1002/hipo.10162. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Donnett JG, Burgess N, O’Keefe JM. Directional control of hippocampal place fields. Exp Brain Res. 1997;117:131–142. doi: 10.1007/s002210050206. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Knierim JJ. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci. 2002;22:6254–6264. doi: 10.1523/JNEUROSCI.22-14-06254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley KS. The mechanism of vision: XV. Preliminary studies of the rat’s capacity for detail vision. Journal of General Psychology. 1938;18:123–193. [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser MB, Moser EI. Progressive transformation of hippocampal neuronal representations in “morphed” environments. Neuron. 2005;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minini L, Jeffery KJ. Do rats use shape to solve “shape discriminations”? Learn Mem. 2006;13:287–297. doi: 10.1101/lm.84406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. J Neurosci. 2011;31:7441–7449. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. Exploring the role of space-defining objects in constructing and maintaining imagined scenes. Brain and cognition. 2013;82:100–107. doi: 10.1016/j.bandc.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Clarendon; Oxford: 1978. [Google Scholar]
- Savelli F, Yoganarasimha D, Knierim JJ. Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus. 2008;18:1270–1282. doi: 10.1002/hipo.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Tanila H, Eichenbaum H. Cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Blair HT, Etkin D, Tzanetos DB. Influences of vestibular and visual motion information on the spatial firing patterns of hippocampal place cells. J Neurosci. 1995;15:173–189. doi: 10.1523/JNEUROSCI.15-01-00173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. Citeseer. 1993. An information-theoretic approach to deciphering the hippocampal code. [Google Scholar]
- Tanila H, Shapiro ML, Eichenbaum H. Discordance of spatial representation in ensembles of hippocampal place cells. Hippocampus. 1997;7:613–623. doi: 10.1002/(SICI)1098-1063(1997)7:6<613::AID-HIPO4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Van Cauter T, Camon J, Alvernhe A, Elduayen C, Sargolini F, Save E. Distinct roles of medial and lateral entorhinal cortex in spatial cognition. Cereb Cortex. 2013;23:451–459. doi: 10.1093/cercor/bhs033. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JN. Rats maintain an overhead binocular field at the expense of constant fusion. Nature. 2013;498:65–69. doi: 10.1038/nature12153. [DOI] [PubMed] [Google Scholar]
- Wilson DI, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA. Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus. 2013 doi: 10.1002/hipo.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]