Abstract
In order to determine if highly negative stigma is a more salient cue than other negative emotional, non-stigmatized cues, participants underwent EEG while passively viewing or actively regulating their emotional response to images of highly negative stigmatized (e.g., homeless individuals, substance abusers), or highly negative non-stigmatized (e.g., a man holding a gun, an injured person) individuals. ERP analyses focused on the N2 (associated with detecting novelty), the early positive potential (associated with processing emotion), and a sustained late positive potential (associated with modulating regulatory goals). A salience effect for highly negative stigma was revealed in the early positive potential, with higher magnitude ERP responses to images of highly negative stigmatized as compared to highly negative non-stigmatized individuals 355 ms post-stimulus onset. Moreover, the amplitude of this effect was predicted by individual differences in implicit bias. Our results also demonstrated that the late positivity response was not modulated by regulatory goals (passively view versus to reappraise) for images of highly negative stigmatized individuals, but was for images of highly negative, non-stigmatized individuals (replicating previous findings). Our findings suggest that the neural response to highly negative stigma is salient and rigid.
Keywords: emotion regulation, social stigma, ERP
Stigmas are powerful and have a deleterious impact on how those who possess them are treated (e.g., Crocker, Major, & Steele, 1998; Langlois et al., 2000). Emerging research in the field of social neuroscience has demonstrated that perceivers engage neural activity in both affective and cognitive regions when they evaluate individuals who are socially stigmatized (e.g., Cikara, Farnsworth, Harris, & Fiske, 2010; Cikara & Fiske, 2011; Harris & Fiske, 2006; Krendl, Macrae, Kelley, Fugelsang, & Heatherton, 2006; Krendl, Moran, & Ambady, 2012; Krendl, Heatherton, & Kensinger, 2009), particularly when the stigmas are highly negative (e.g., Harris & Fiske, 2006; Krendl, Macrae, Kelley, Fugelsang, & Heatherton; Krendl, Kensinger, & Ambady, 2012). Moreover, recent research by Krendl and colleagues (2012) suggests that the cognitive responses to highly negative social stigmas are obligatory. Specifically, using fMRI and an emotion regulation paradigm, the authors found that participants had heightened activation in neural regions associated with cognitive control during their initial (e.g., during the first two seconds) evaluation of images of highly negative stigmatized individuals (e.g., homeless individuals and substance abusers) as compared to non-stigmatized individuals regardless of their explicit regulatory goals.
This finding suggested that the cognitive response to highly negative social stigmas may be obligatory, but two important questions remain. First, are negative socially stigmatized individuals registered at earlier stages of processing than negative, non-stigmatized individuals; and is the obligatory response effective in modulating the automatic affective response that the highly negative stigmatized groups elicit? Understanding both when stigma is dissociated from negative, non-stigma and why the cognitive responses to stigma are engaged are important questions because despite extensive research demonstrating that individual differences in motivation to overcome prejudice can reduce bias, bias persists (e.g., Amodio, Devine, Harmon-Jones, 2008; Devine, 1989; Dunton & Fazio, 1997).
Neurophysiological research has demonstrated that race elicits heightened activation in early perceptual processes (e.g., the N1 or N2; for review, see Ito & Bartholow, 2009), which often automatically elicits the need for cognitive control (e.g., ERN; Amodio et al., 2004; Amodio, Devine, & Harmon-Jones, 2008; Bartholow, Dickter, & Sestir, 2006). However, the timecourse of detecting non-race stigma remains unknown. It has been widely shown that highly negative socially stigmatized individuals such as homeless individuals and substance abusers produce a powerful negative emotional response (e.g., Fiske, Cuddy, Glick, & Xu, 2002). This finding suggests that socially stigmatized individuals elicit heightened attention from perceivers. Importantly, negative, non-stigmatized stimuli also elicit heightened attention from perceivers (e.g., Hajcak, MacNamara, & Olvet, 2010). However, it remains unknown whether social stigma is salient because it is negative (in which case the neurophysiological response to stigmatized individuals should be the same as it is to negative, non-stigma individuals), or because it is stigmatized (in which case the neurophysiological response to stigmatized individuals should be different from the response to negative, non-stigma individuals). An additional consideration is that although neuroimaging work has demonstrated that stigmatized individuals also elicit a powerful cognitive response (Krendl et al., 2006; Krendl, Kensinger, & Ambady, 2012), it remains unknown whether that cognitive response is effective in regulating perceivers’ negative affective response to stigmatized individuals. The current investigation will examine both of these questions.
Because detecting social stigma requires decoding more complex cues (e.g., Brewer, 1988; Fiske & Neuberg, 1990; see also, Ito & Urland, 2003, 2005) than detecting race stigma (for which perceptual cues from faces are sufficient), it is unlikely that perceptual processes would be engaged to the same extent in response to identifying non-race stigma. Simply put, accurately identifying someone as being homeless from simply seeing a photograph of that individual requires perceivers to decode contextual information (e.g., the types of clothing the individual is wearing, the individual’s surrounding environment). Thus, we hypothesized that the ERP components involved in processing negative emotion that were also sensitive to cognitive goals – specifically the N2, the early P3, and the late positivity potential (LPP). – would be engaged in detecting these highly negative socially stigmatized targets.
During emotion perception, the N2 occurs in parallel to the early P3 response during an early and relatively unconscious stage of processing (Liddell, Williams, Rathjen, Shevrin, & Gordon, 2004). The frontocentral N2 response – occurring 200–300 ms post-stimulus onset – is involved in detecting novelty, and may signal need for inhibition and cognitive control (for review, see Folstein & Van Pettern, 2008), whereas the early P3 – generally starting around 250 ms post-stimulus onset – directs attention toward emotional information (e.g., Dolcos & Cabeza, 2002; Eimer & Holmes, 2002). The LPP, which is distributed along the posterior midline, peaks around 500 and 2000 ms post-stimulus onset and can persist for the entire duration of the presentation of the emotional stimulus (for review, see Hajcak, MacNamara, & Olvet, 2010). Interestingly, the LPP appears to be modulated both by emotional intensity (e.g., Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Dolcos & Cabeza, 2002; Schupp, Cuthbert, Bradley, Cacioppo, Ito, & Lang, 2000), as well as the explicit regulatory goals of the perceiver (e.g., Cuthbert et al., 2000; Hajcak, Moser, & Simons, 2006; Schupp et al., 2000). For instance, the LPP peak is higher when participants are instructed to maintain their negative emotions to highly negative images as compared to when they are instructed to reappraise their emotional response to those images (e.g., reinterpret the image in such a way that it no longer elicits a negative response; Hajcak & Nieuwenhuis, 2006).
Because the LPP plays an important role in processing emotional information, we used the magnitude of this ERP response as an operational measure of whether or not regulatory processes were successfully engaged when evaluating highly negative stigmatized individuals. Simply put, if the regulatory response elicited when individuals passively viewed images of highly negative stigmatized individuals was effective in reducing negative affect toward those individuals, then the sustained late positivity response on those trials should be reduced as compared to when individuals passively viewed images of negative, non-stigmatized individuals (which does not elicit an obligatory regulatory response).
Moreover, because the sustained positivity response is modulated by cognitive control, we anticipated that its amplitude would be modulated by individual differences in bias (e.g., Experiment 2; Amodio, Devine, & Harmon-Jones, 2008). This prediction stems from previous fMRI research that has demonstrated that the magnitude of activity in neural regions underlying cognitive control (e.g., the dorsolateral prefrontal cortex and the anterior cingulate cortex) is positively correlated with individual differences in implicit bias toward stigmatized individuals (Knutson Mah, Manly, & Grafman, 2007; Krendl, Kensinger & Ambady, 2012; Richeson et al., 2003).
There are two key questions in the current study: to determine first, whether negative socially stigmatized individuals are registered at somewhat early stages of cognitive and affective processing than negative, non-stigmatized individuals, and second, how effective is perceivers’ cognitive response to highly negative socially stigmatized individuals in regulating their negative affective response to those individuals? In order to answer these questions, we conducted an emotion regulation task with stigma and negative, non-stigma images using electroencephalography (EEG).
Method & Materials
Participants
A total of 23 right-handed adults (Mage = 21.4 years, SD = 2.0 years, 10 female) from the greater Boston area provided written informed consent to participate in this study. This sample size was selected in order to parallel the sample size used in the fMRI study by Krendl, Kensinger, and Ambady (2012) on which this study was based. Given the sensitivity of EEG to movement, we oversampled in order to ensure we would have at least the same number of participants as were in the fMRI study (N = 16). Of the 23 participants who enrolled in the study, two participants were excluded because they had excessive movement during the task (thereby rendering fewer than 50 percent of their trials valid), and one additional participant was excluded after indicating less than an overall 75 percent success rate (58%) in regulating his emotional response to the images. This left 20 participants (Mage = 21.3 years, SD = 2.1 years, 8 female). They participated for monetary compensation. The study was approved by the Institutional Review Board at Boston College.
Materials
For the current study, we compiled 50 images of stigmatized targets (substance abusers and homeless individuals), 50 negative images portraying negative social, but non-stigmatized, targets (e.g., a man holding a gun, a couple at a cemetery) from the International Affective Picture Set (IAPS; Lang, Bradley, & Cuthbert, 2005)1, and 25 images of neutral, control targets (images of people with no visible stigmas). The images in the stigmatized condition depicted homeless individuals, alcoholics, or drug addicts. Their stigmas were clearly identifiable in the images, and have been used in previous neuroimaging research by the first authors (e.g., Krendl, Heatherton, & Kensinger, 2009; Krendl, Moran, & Ambady, 2012; Krendl, Kensinger, & Ambady, 2012). These target groups were selected as the stigmatized group for several reasons: first, homeless individuals, drug addicts, and alcoholics are widely stigmatized (e.g., Goffman, 1963; Fiske, Cuddy, Glick, & Xu, 2002); second, their stigmatized condition is clearly visible from a photograph; finally, previous research suggests that homeless individuals and substance abusers elicit a powerful negative emotional response (e.g., Fiske, Cuddy, Glick, & Xu, 2002). The IAPS images, however, were selected based on the fact that they were highly negative, social (e.g., a person was the central object of each image), and non-stigmatized. For instance, all of the people featured in these images were White, and none conveyed any attributes that are commonly stigmatized (e.g., drug use). The control images were selected to match the stigmatized and non-stigmatized images in dimensions such as the race, age, and gender of the individuals featured in the stigmatized and negative, non-stigmatized conditions.
All images were pre-rated for valence and arousal by 23 participants in order to ensure the final set of IAPS images and images of stigmatized targets did not differ in valence or arousal. These participants were different than those who participated in the EEG study, but were all undergraduates who were recruited from the same undergraduate institution where the EEG study was conducted. The participants’ ratings were highly reliable (valence: Cronbach’s α = .97; arousal: Cronbach’s α = .90). These participants rated the pilot images on a scale of 1 to 7, where lower numbers indicated low valence (e.g., negative) or arousal, and high numbers indicated high valence (e.g., positive) and arousal (IAPS: Mvalence = 2.29, SD = .93; Marousal = 5.56, SD = .78; Stigma: Mvalence = 2.07, SD = .48; Marousal = 5.31, SD = .55; Neutral images: Mvalence = 4.80, SD = .80; Marousal = 3.96, SD = .53).
Behavioral Tasks
Procedure
Participants completed a practice session consisting of 15 trials prior to beginning the task. Participants were instructed during this session that the goal of the decrease condition was for them “to try to change your emotional response to a series of images.” The experimenter suggested several possible strategies for doing this, including reimagining an individual with an injury as someone who was injured while defending someone else, or someone who is ill as going on to make a full recovery. Participants were reminded that the same strategy would not be effective for all the images, so they should use whatever strategy was most effective for them for a given image. Participants were told to engage these strategies for the entire time that the image was on the screen. Importantly, during the practice sessions, participants practiced reappraising their emotional response out loud so that two experimenters could monitor the level of detail and likelihood of success for the strategies. At the end of the practice session, participants were asked to report the percentage of trials during which they were able to successfully regulate their negative emotional response.
Regulation task during the EEG recording session
During the EEG session, participants were instructed to passively view or decrease their negative emotional response to the images described above. Prior to seeing each image, a prompt was presented on the screen for 2 seconds instructing participants to passively view the image (“attend”), or actively decrease their negative emotional response to the image (“decrease”). The image then appeared on the screen for 8 seconds, during which time participants either passively viewed the image (e.g., did not try to change their natural emotional response to the image), or “decreased” their emotional response. After the 8-second presentation interval elapsed, participants rated the relative strength of their negative emotions to the image they had just seen (1 = very weak negative emotion, 7 = very strong negative emotion; Figure 1). The images were presented in pseudorandom order such that any given negative image (stigmatized or non-stigmatized) was paired with the passive viewing instructions for approximately half the subjects, and decrease instructions for the other half. The neutral, control images were only paired with passive viewing instructions.
Figure 1.
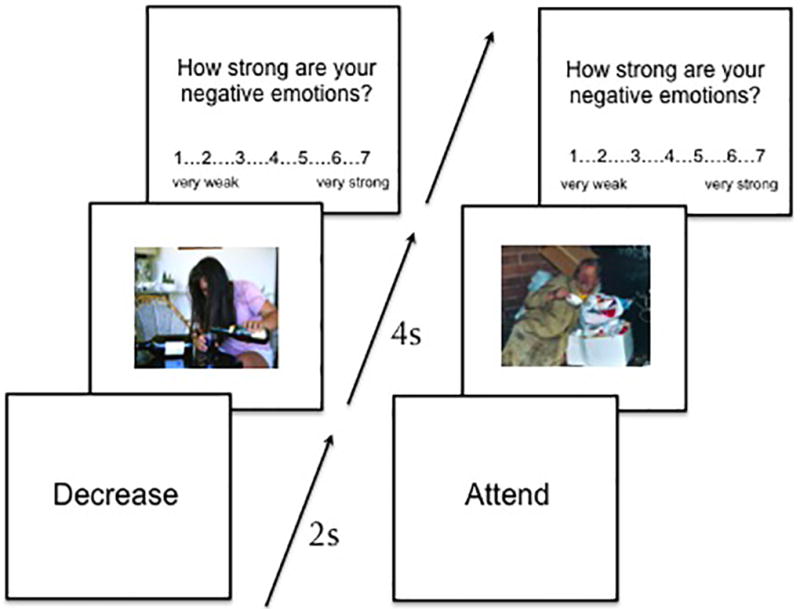
Graphical representation of task. In the left panel, participants saw a prompt for 2s prior to the onset of the image (of an alcoholic) instructing them to decrease their negative emotional response to a subsequent image. The image then appeared, remaining on the screen for 8s. Finally, they rated their negative emotional response to the image. The panel on the right depicts a passive viewing trial with an image of a homeless individual.
The experimental task was divided into four separate runs, each lasting approximately 8 minutes. Each run consisted of an equal number of stigmatized and negative, non-stigmatized images in the passive viewing and the decrease condition. Participants also completed 6 to 7 trials per run of the passive viewing condition for the neutral, control images. Within each run, the images were presented in a pseudorandomized order, and the order in which the runs were presented was counterbalanced across participants. Participants were given a short break between each run, during which they reported their overall success in decreasing their negative affect on the previous run.
At the conclusion of the task, participants completed an Implicit Association Task (IAT; Greenwald, Schwartz & McGhee, 1998) to measure their implicit attitudes toward homeless individuals. Here, participants viewed and categorized 12 images (e.g., of either homeless individuals or controls) and 12 words (that were either pleasant or unpleasant) in a stereotypically congruent (e.g., “unpleasant” and “homeless” paired on the same side of the screen) or stereotypically incongruent (e.g., “pleasant” and “homeless” presented on the same side of the screen) manner. The congruent and incongruent blocks were presented in a pseudorandom manner.
EEG data acquisition and ERP analysis
Subjects were seated directly in front of a computer monitor at a distance of approximately 60 cm, and fitted with an Active Two electrode cap (Behavioral Brain Sciences Center, Birmingham, UK). On-screen images were 360 pixels in height with a resolution of 72 pixels/inch, subtending approximately 2° of visual angle horizontally and vertically. A full array of 128 Ag-AgCl BioSemi (Amsterdam, the Netherlands) active electrodes was connected to the cap in equidistant concentric circles from the 10–20 position, Cz. Two mini-biopotential electrodes were placed bilaterally on each mastoid process. The electrooculogram (EOG) generated from blinks and eye movement was recorded from one electrode approximately 1 cm below the left eye (to record vertical eye movement), and one electrode approximately 1 cm from the canthus of the right eye (to assess horizontal eye movement). Electrode gel (Signagel, Parker Laboratories, Inc.) was used as the conducting medium for all electrodes. The EEG, which was recorded continuously through the task, was digitized using ActiView software (BioSemi, Amsterdam, the Netherlands) at a sampling rate of 512 Hz/second.
EEG recordings were referenced according to BioSemi's design, which uses two electrodes mounted in the cap on either side of the electrode between CPz and Pz as the ground electrodes (see Figure 2 for electrode configuration). Offline analyses were performed using the EMSE Software Suite (Source Signal Imaging, San Diego, CA, USA). Data were referenced to a common average reference with low and high cutoffs of 0.03 and 30 Hz, respectively. The EEG was segmented for each trial beginning 200 ms prior to the onset of the image (which served as the baseline) and continued for 2,000 ms post-onset. Trials were also corrected for excessive EOG activity using the EMSE manual ocular artifact tool. The investigators first manually identified examples of EOG artifact data and EOG artifact-free data. The EMSE software then produced a logarithmic ratio of artifact data to clean data using a covariance technique that simultaneously models the two types of EEG data. Finally, ocular artifact was removed from the recording where it was detected by the correction tool. We also discarded any trials from averaging if they contained movement greater than 90 µV. An average of 21.8 trials (SD = 3.13 trials) per condition were valid for each subject (range 13 to 25 valid trials per condition). There was no significant difference in the number of valid trials per condition (F < 1). Individual bad channels were also corrected using the EMSE spatial interpolation filter. For any given participant, an average of 1.67 electrodes (SD = 1.59) were interpolated (range 0 to 6 interpolated electrodes), but none of those were electrodes that we used for the reported analyses. ERPs were averaged as a function of each condition in each subject, and were corrected relative to the baseline. We then created a group average ERP as a function of condition.
Figure 2.
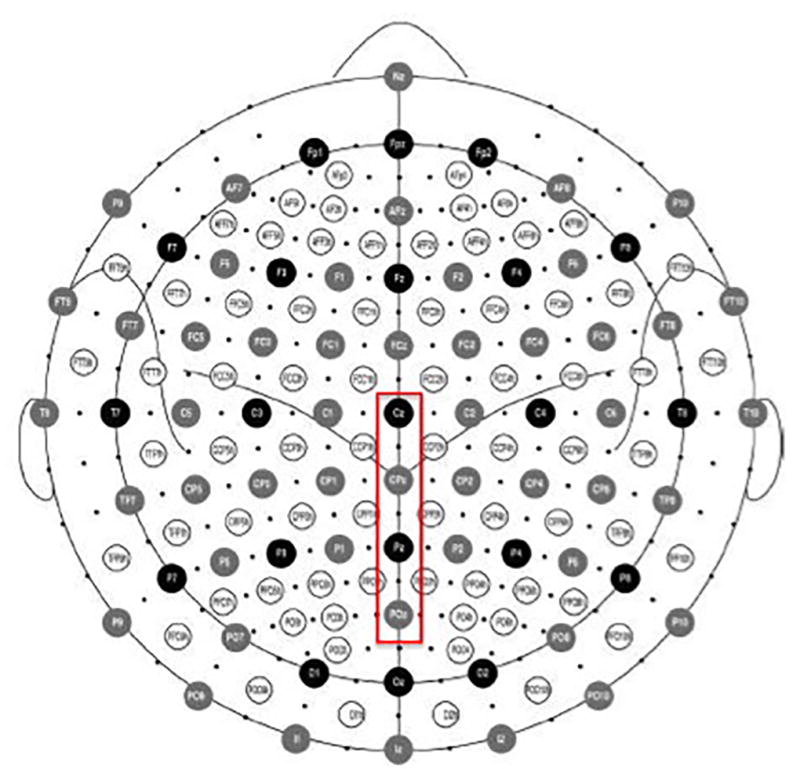
Position of 128 electrodes on the BioSemi headcap with the midline centro-parietal electrodes (from Cz-POz) that were used for our analyses outlined in red.
Although each picture was presented for a total of 8 seconds, we were primarily interested in ERP activity during the first 2 seconds immediately following picture onset because prior fMRI research by Krendl and colleagues (2012) suggested that stigmatized individuals are first dissociated from negative, non-stigmatized individuals in this time frame. Moreover, numerous studies have demonstrated that regulatory goals modulate the neural response as soon as 300 ms post-stimulus onset (for review, see Hajcak, MacNamara, & Olvet, 2010), suggesting that the time frame was appropriate. The average activity in the 200 ms period prior to the picture onset served as the pre-stimulus baseline, resulting in each ERP average consisting of a time window lasting 2200 ms. Average waveforms used in the ANOVAs reported below were analyzed 200 ms prior to stimulus onset (baseline) to 2000 ms after stimulus onset in 100-ms time bins for each participant and each condition. In order to evaluate the late positivity response and the sustained late positivity responses, we averaged the signal value extending through the midline (centro-parietally) from Cz posteriorly through POz, which have been previously identified in emotion processing (e.g., Deveney & Pizzagalli, 2008; Dolcos & Cabeza, 2002; Eimer & Holmes, 2002; Hajcak, Dunning, & Foti, 2009; see Figure 2 for electrode locations). Consistent with previous research, we defined the late positivity associated with identifying emotional information as a positive, posterior peak occurring between 300 and 500 ms post-stimulus onset (for review, see Hajcak, Olvet, & MacNamara, 2010). We defined the sustained late positivity as a sustained positive peak around these midline electrodes occurring between 500 ms – 2000 ms post-stimulus onset (for review, see Hajcak, Olvet, & MacNamara, 2010).
Results
Behavioral results
Regulation task and IAT
In order to determine whether participants successfully decreased their negative emotional response during the reappraisal conditions, we first entered the behavioral ratings of negative affect toward the stigmatized images and the negative non-stigmatized images into a 2 (instruction: decrease or passive viewing) × 2 (image type: stigma v. negative non-stigmatized) ANOVA. Results revealed a main effect of instruction (F(1,19) = 57.79, p < .001, η2partial = .75), an effect of image type (F(1,19) = 4.67, p = .044, η2partial = .20), and an image type × instruction interaction (F(1,19) = 13.08, p = .002, η2partial = .41).
Subsequent analyses demonstrated that the effect of instruction emerged because participants expressed stronger negative affect towards both the stigmatized images and the negative non-stigmatized images in the passive viewing condition than they did in the decrease condition (t(19) = 6.14, p < .001; 95% CI [.83,1.68]); t(19) = 8.83, p < .001; 95% CI [1.21,1.96]), respectively; Table 1). The effect of image type emerged because participants rated the negative non-stigmatized images as more negative than the images of the stigmatized targets in the passive viewing condition (t(19) = −3.24, p = .004; 95% CI [−.57,−.12]). However, there was no difference in the mean affect ratings between the negative non-stigmatized and stigmatized images in the decrease condition (t < 1; 95% CI [−.16,.18]).
Table 1.
Mean ratings of negative emotion (1 = very weak negative emotion, 7 = very strong negative emotion) in the passive viewing and decrease conditions for images of stigmatized and negative, non-stigmatized individuals. SD ().
| Passive Viewing | Decrease | |
|---|---|---|
| Stigma | 4.03 (.98) | 2.79 (.82) |
| Negative, non-stigma | 4.38 (.93) | 2.78 (.72) |
We next evaluated participants’ implicit attitudes toward homeless individuals through their IAT scores. Paired t-tests between the congruent and incongruent blocks revealed that participants demonstrated the predicted IAT bias against homeless individuals, with reaction times for the stereotypically incongruent blocks (homeless: MRT = 1135.61 ms, SD = 314.86 ms) being significantly longer than reaction times for the stereotypically congruent blocks (homeless: MRT = 867.66, SD = 220.27 ms, t(19) = 4.96, p < .001; 95% CI [154.92,380.98]).
ERP results
Effect of image type on ERP response: Passive viewing condition
Our first goal in the ERP analyses was to determine whether negative socially stigmatized individuals are registered at somewhat early stages of cognitive and affective processing than negative, non-stigmatized individuals and control images. We therefore first examined the N2 and early P3 responses in the passive viewing condition for the stigmatized, negative, non-stigmatized, and control images using a repeated measures ANOVA. Results for both components are discussed in turn below.
N2
We entered the N2 response at Fz during the 200 to 300 ms post-stimulus onset time window for each participant into a repeated measures ANOVA with image type (stigmatized; negative, non-stigmatized; and neutral, control) as a within-subjects factor. Results revealed a main effect of image type (F(2,38) = 5.84, p = .006, η2partial = .39), which was driven by the fact that the N2 response was greater to both stigmatized, and negative, non-stigmatized images as compared to control images (t(19) = 2.67, p = .015; 95% CI [.43, 3.55]; t(19) = 3.34, p = .003; 95% CI [.69, 3.00], respectively). However, the N2 response did not differ between the stigmatized and negative, non-stigmatized images (t < 1). See Figure 3.
Figure 3.
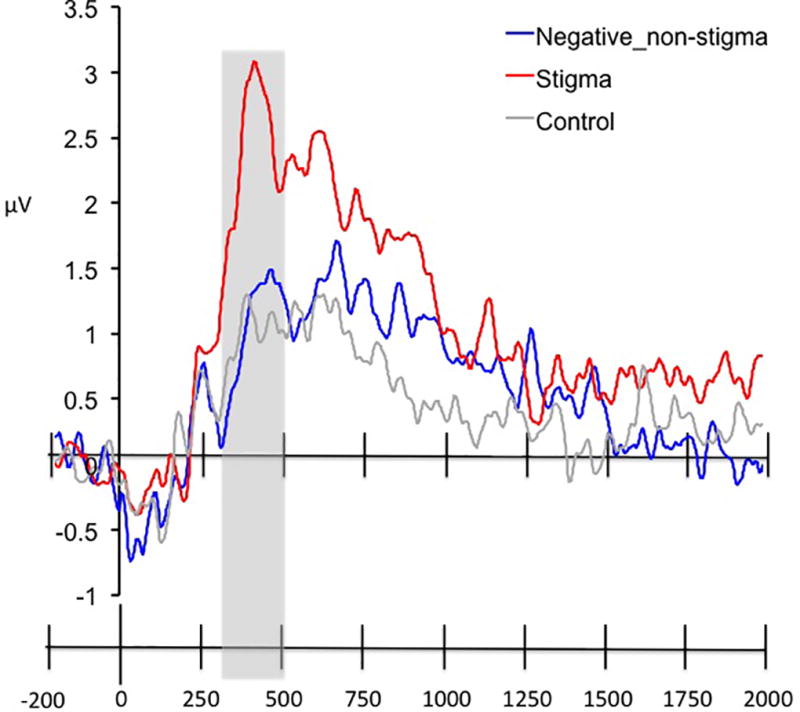
ERP waveforms for the N2 response at Fz for passively viewing stigmatized, negative, non-stigmatized, and neutral, control images. The time window of interest (200 to 300 msec post-stimulus onset) is highlighted in grey.
Early P3
We next examined the P3 response by averaging across the midline centroparietal electrodes (from Cz posteriorly through POz; Figure 2) waveforms in the passive viewing condition between 300 to 500 ms post-stimulus onset. Figure 4 presents the average ERPs elicited by images of stigmatized and negative, non-stigmatized individuals in the passive viewing condition from 200 ms pre-stimulus onset to 2000 ms post-stimulus onset. Visual inspection of these results revealed a positivity response peaking at 355 ms post-stimulus onset for both images of stigmatized individuals (M = 3.09 µV) and negative, non-stigmatized images (M = 1.71 µV; see Figure 4). The ANOVA revealed a significant main effect of image type (F(2,38) = 9.67, p < .001, η2partial = .34), which was driven by the fact that the late positivity was greater in response to images of stigmatized individuals as compared to both negative, non-stigmatized individuals, and neutral, control images (t(19) = 4.85, p < .001; 95% CI [.74,1.87]; t(19) = 3.62, p = .002; 95% CI [.55,2.07], respectively). See Table 2 for mean voltages by condition.
Figure 4.
ERP waveforms for the late positivity response (averaged across the electrodes from Cz to POz) for passively viewing stigmatized, negative, non-stigmatized, and neutral, control images. The time window of interest (300 to 500 msec post-stimulus onset) is highlighted in grey.
Table 2.
Mean voltages (in µV) when the peak amplitude for the late positivity responses occurring between 300 and 500 msec post-stimulus onset in response to passively viewing images of stigmatized individuals, negative, non-stigmatized individuals, and neutral, control images. SD ().
| 300–500 ms post- onset |
|
|---|---|
| Stigmatized | 2.23 (.2.49) |
| Negative, non-stigmatized | .93 (2.49) |
| Neutral, control | .92 (2.82) |
Effect of image type and regulatory goals on ERP response
Our second goal of the current study was to determine how effective perceivers’ obligatory cognitive and affective response to highly negative socially stigmatized individuals is in regulating their negative affective response to those individual. Here, we hypothesized that the instructed regulatory goal would modulate the LPP response, but not the N2 or early P3 response, to images of stigmatized as compared to images of negative, non-stigmatized individuals. For each component, we conducted a 2 (image type: stigma or negative non-stigmatized) × 2 (regulatory goal: decrease or passive viewing) repeated measures ANOVA using the same electrodes and time windows as were used when evaluating the passive viewing condition alone.
N2
First, we examined how, if at all, the instructed regulatory goal modulated the N2 response to images of stigmatized as compared to images of negative, non-stigmatized individuals. The ANOVA revealed no main effects or interactions (all Fs < 1.02, ps > .32, η2partials ≤ .05).
Early P3
Next, we examined how, if at all, the instructed regulatory goal modulated the early P3 response to images of stigmatized as compared to images of negative, non-stigmatized individuals. The ANOVA revealed a main effect of image type (F(1,19) = 38.33, p < .001, η2partial = .67), but no main effect of instruction or interactions (both Fs < 1, ps > .40, η2partials < .04).
Late positivity potential
Finally, we examined how, if at all, the instructed regulatory goal modulated the late positivity response to images of stigmatized as compared to images of negative, non-stigmatized individuals. Because regulatory goals could affect the late positivity in different time windows for stigmatized as compared to negative, non-stigmatized images, we also included time as an additional variable in the analysis in order to identify when, if at all, regulatory goals interacted with the type of image being evaluated.
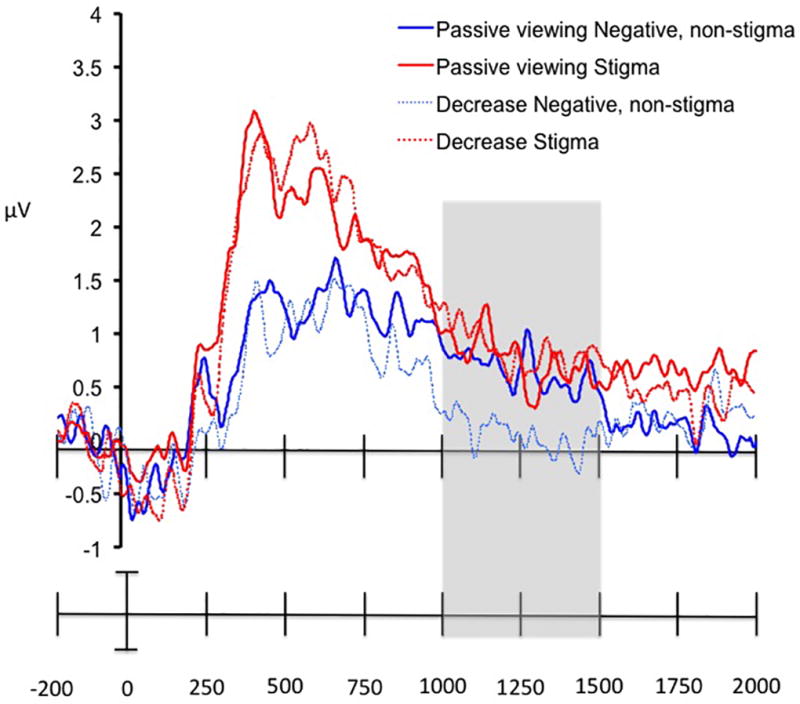
Visual inspection of the ERP waveform in the centroparietal electrodes from Cz posteriorly through POz) revealed a sustained late positivity response peaking 625 post-stimulus onset for images of stigmatized individuals (M = 2.55 µV), and at 676 ms post-stimulus onset for images of negative, non-stigmatized individuals (M = 1.71 µV). Once it peaked, the late positivity was sustained until 1200 ms post-stimulus onset (see Figure 5). In order to examine the effect of image type, regulatory goal, and time, we divided the 600 – 2000 ms time window into three sub-blocks: 600–1000 ms, 1000–1500 ms, and 1500 – 2000 ms based on visual inspection of the data (Figure 5). This approach resulted in our conducting a 2 (image type: stigma or negative non-stigmatized) × 2 (regulatory goal: decrease or passive viewing) × 3 (time block: 600–1000 ms, 1000–1500 ms, and 1500 – 2000 ms) repeated measures ANOVA. Results revealed a significant main effect of image type (F(1,19) = 13.87, p = .001, η2partial = .42), and time block (F(2,38) = 16.69, p < .001, η2partial = .47), a trend toward an effect of image type × time block (F(2,38) = 2.86, p = .07, η2partial = .13), and an image type × regulatory goal × time block interaction (F(2,38) = 5.29, p = .009, η2partial = .22). There were no other main effects or interactions (all Fs < 1.5, ps > .30, η2partials < .06).
Figure 5.
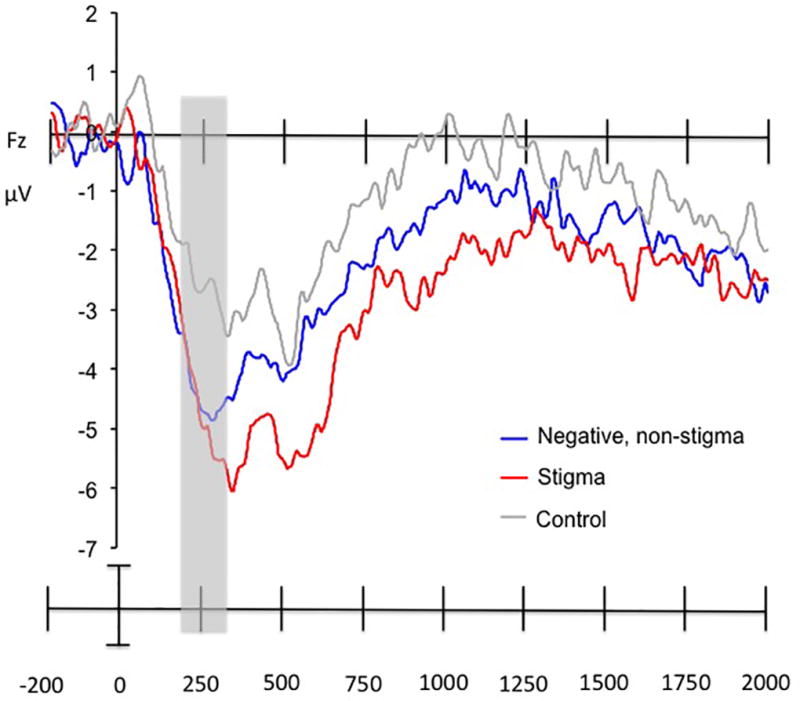
Average ERP waveforms for the sustained late positivity response (averaged across the electrodes from Cz to POz) in the passive viewing and decrease conditions for stigmatized, negative non-stigmatized, and neutral, control images. The time window in which the only effect of instruction was observed (1000 to 1500 msec post-stimulus onset) is highlighted in grey. The effect of instruction was significant for the negative, non-stigmatized images, but not the stigmatized images.
The effect of image type emerged because, irrespective of regulatory goal, the images of stigmatized individuals elicited a higher overall late positivity ERP response than did images of negative, non-stigmatized individuals (t(19) = 3.75, p = .015; 95% CI [.40,1.40]). The effect of time block emerged because the overall magnitude of the late positivity declined between 600 to 2000 ms post-onset for both images of stigmatized and negative, non-stigmatized individuals. This interpretation was confirmed by a significant linear contrast (F(1,19) = 15.51, p = .001).
In order to unpack the three-way interaction, we conducted two separate 2 (regulatory goal: decrease or passive viewing) × 3 (time block: 600–1000 ms, 1000–1500 ms, and 1500 – 2000 ms) repeated measures ANOVAs for stigmatized images and negative, non-stigmatized images, respectively. For the stigmatized images, results revealed a main effect of time block (F(2,38) = 17.48, p < .001, η2partial = .48), but no other main effects or interactions (all Fs < 1, ps > .20, η2partials < .04). For the negative, non-stigmatized images, results revealed a main effect of time block (F(2,38) = 11.21, p < .001, η2partial = .37), no main effect of regulatory goal (F(1,19) = 1.70, p = .21, η2partial = .08), but a regulatory goal × time block interaction (F(2,38) = 3.68, p = .035, η2partial = .16).
We then compared the overall amplitude of the late positivity response to negative, non-stigmatized images in the decrease as compared to the passive viewing condition using a Bonferroni correction to adjust the significance threshold to p < .017. Results revealed that the late positivity to negative, non-stigmatized images was significantly reduced during the decrease as compared to passive viewing condition between 1000–1500 ms (t(19) = 3.78, p = .001; 95% CI [.53,1.82]), but not during 500 – 1000 ms or 1500 – 2000 ms post-stimulus onset (p > .36 for both; 95% CIs [−.35,.92; −.37,.25, respectively]). See Table 3 for mean voltages by image type and regulatory goal.
Table 3.
Mean voltages (in µV) when the peak amplitude for the sustained late positivity responses occurring between 500 – 1000, 1000 – 1500, and 1500 – 2000 msec post-stimulus onset as a function of regulatory goals in response to viewing images of stigmatized and negative, non-stigmatized individuals. SD ().
| 500 – 1000 ms post- onset |
1000–1500 ms post- onset |
1500–2000 ms post- onset |
||||
|---|---|---|---|---|---|---|
| Passive viewing |
Decrease | Passive viewing |
Decrease | Passive viewing |
Decrease | |
| Stigmatized | 1.95 (2.07) | 2.10 (2.46) | .77 (1.38) | .87 (1.50) | .66 (.91) | .46 (.1.00) |
| Negative, non-stigmatized | 1.23 (1.94) | 1.02 (2.15) | 1.23 (1.94) | .05 (1.18) | .12 (1.08) | .21 (.88) |
The late positivity response and behavior
In order to more closely examine why regulatory goals did not affect the magnitude of the late positivity response in the stigma condition, we correlated the amplitude of each individuals’ late positivity response when they were attempting to decrease their negative emotional response to stigmatized individuals with a measure of their implicit bias (IAT score) and explicit bias (self-reported negative emotional response to the stigmatized individuals in the passive viewing condition) toward the stigmatized targets using a Pearson’s bivariate correlation (because both implicit and explicit bias were normally distributed, as assessed by Kolmogorov-Smirnov tests; both ps = .20). We chose to examine the effect of bias on amplitude because explicit negative attitudes have been shown to predict changes in the ERP response (e.g., Hajcak & Nieuwenhuis, 2006). Moreover, previous fMRI research has demonstrated that the magnitude of activity in neural regions underlying cognitive control (e.g., the dorsolateral prefrontal cortex and the anterior cingulate cortex) is positively correlated with individual differences in implicit bias toward stigmatized individuals (Knutson Mah, Manly, & Grafman, 2007; Krendl, Kensinger & Ambady, 2012; Richeson et al., 2003). For both the implicit and explicit data, higher scores indicate a higher magnitude of bias.
Results revealed that the peak amplitude of the late positivity response in the decrease condition for stigmatized images was correlated with implicit, but not explicit, attitudes toward stigmatized individuals (r(20) = .44, p = .05; r(20) = −.37, p = .11, respectively). However, these results should be taken with caution given that the overall power is rather low, thereby increasing our risk of a Type II error.
Affect ratings
One possibility that emerged from the ERP results is that the stigma images may have elicited more varied negative affective responses as compared to the negative, non-stigmatized images. Alternatively, the stigma images may have been perceived to be more unusual relative to the negative, non-stigmatized images and control images, thereby resulting in heightened attention (which would be reflected in a heightened late positivity response). In order to examine these possibilities, we presented 21 undergraduates at Indiana University with all of images from the ERP task (presented in the same as during the ERP task). Each image was presented for 2 seconds in the absence of regulation prompts (passive viewing or decrease), and participants were then asked to rate: 1) how surprised they were to see that image (1 = not at all, 7 = very much); and 2) how strong their negative emotions were to the image (1 = very weak, 7 = very strong). Participants were asked to indicate (yes or no) whether the image elicited anger, contempt, disgust, distress, fear, guilty, pity, indifference, and unconcern. They were also given an option at the end of indicate whether the image elicited any other negative emotions not listed.
Ratings were then entered into an ANOVA with image type (control, negative, non-stigma, or stigma images) as the within-subject factor. First, we verified that participants in our sample viewed the negative, non-stigma images as being more negative as compared to the stigma images (which would be consistent with the ratings by the participants in the ERP study) by finding a significant effect of image type for the overall affect ratings: F(2,40) = 97.87, p < .001, η2partial = .83, which was driven by the fact that the negative, non-stigma images (Mrating = 4.00, SD = 1.10) were viewed as being more negative than the stigma images (Mrating = 3.70, SD = 1.34; t(20) = 2.63, p = .016; 95% CI [.06,.55]). The control images were rated as being less negative than both (Mrating = 1.44, SD = .34; both ps < .001).
Next, we examined whether the stigma images were perceived as being more unexpected than the other images (based on the question asking how surprising participants found each image to be). Here, the ANOVA revealed a main effect of image type: F(2,40) = 39.16, p < .001, η2partial = .66, which was driven by the fact that the negative, non-stigma images (Mrating = 3.92, SD = 1.16) were viewed as being more unexpected than the stigma images (Mrating = 2.97, SD = 1.12; t(20) = 7.38, p < .001; 95% CI [.69,1.23]). The control images (Mrating = 2.15, SD = 1.17) were rated as being less negative than both stigma (t(20) = 3.41, p = .003; 95% CI [.32,1.32]) and negative, non-stigma images (t(20) = 7.38, p < .001; 95% CI [.69,1.23]).
Finally, we examined whether there were more varied emotions elicited for stigma as compared to negative, non-stigma images. Here, we used a proportion score (number of times participants indicated they experienced a specific emotion for an image in a specific condition divided by the total number of images per condition) as the dependent variable. We found a main effect of image type for all emotions except for contempt (all Fs > 14.92, η2partials > .46, and ps < .001). Subsequent t-tests demonstrated that, as compared to the stigma images, the negative non-stigma images elicited more anger (t(17) = 3.06, p = .007; 95% CI [.04,.23]), distress (t(17) = 3.58, p = .002; 95% CI [.08,.30]), and fear (t(17) = 7.23, p < .001; 95% CI [.21,.38]), whereas stigma images elicited more disgust and pity as compared to negative, non-stigma (t(17) = 3.17, p = .006; 95% CI [.04,.20]; t(17) = 3.91, p = .001; 95% CI [.07,.24], respectively). The neutral, control images elicited more indifference and lack of concern as compared to any other image type (all ps < .001). Importantly, the negative, non-stigma images also elicited more self-reported other negative feelings as compared to the stigma images (t(17) = 22.66, p = .017; 95% CI [.01,.11]); see Table 4 for all means).
Table 4.
Mean proportion of images by condition (control, negative, non stigma, and stigma) that participants reported elicited the following emotions. SD ().
| Control | Negative, non stigma |
Stigma | |
|---|---|---|---|
| Anger** | .03 (.03)b | .31 (.18)a | .17 (.16) |
| Contempt | .10 (.16) | .18 (.17) | .20 (.21) |
| Disgust** | .05 (.05)b | .37 (.14)a | .48 (.21) |
| Distressed** | .06 (.05)b | .56 (.19)a | .39 (.26) |
| Fear** | .04 (.05)b | .47 (.21)a | .19 (.23) |
| Guilt** | .02 (.02)b | .18 (.12) | .21 (.19) |
| Pity** | .06 (.06)b | .45 (.16)a | .63 (.26) |
| Indifferent** | .63 (.32)b | .28 (.22) | .28 (.27) |
| Unconcerned** | .71 (.31)b | .24 (.23) | .24 (.31) |
| Other** | .03 (.04)b | .15 (.19)a | .10 (.13) |
Main effects noted next to emotion type with ** (for all main effects, p < .001)
For between image comparison, all significance is p < .017 (Bonferroni correction) with a denoting that negative, non-stigma significantly differs from stigma, and b denoting that control significantly differs from negative, non stigma and stigma.
Discussion
The results from this study demonstrate two key findings: First, the earlier of the two positive components investigated here dissociated images of non-race stigmatized individuals 355 ms post-stimulus onset; second, instructed regulatory goals (to passively view versus to reappraise) did not affect the ERP response to images of highly negative stigmatized individuals, although there was evidence of a diminished late positivity to negative, non-stigmatized images when regulation was instructed. Together, our findings suggest that highly negative non-race stigma may be detected at somewhat early stages of affective processing than negative, non-stigma, and has a powerful effect on neural processing.
This study is the first to report that highly negative social stigma is distinguished from other emotional stimuli by 355 ms after onset. This finding is consistent with previous neurophysiological research demonstrating that race stigmas are detected rapidly, often within 250 msec of presentation (for review, see Ito & Urland, 2009), but extends this previous research to more perceptually complex negative social groups. The fact that highly negative social stigma is distinguished from other emotional stimuli in 355 ms may shed light on the powerful negative affective response that it elicits (Crocker, Major, & Steele, 1998; Fiske, Cuddy, Glick, & Xu, 2002; Goffman, 1963). That is, the rapid detection of social stigma is likely a marker of its salience, which may cause it to elicit an even stronger affective response than other negative, non-stigmatized images.
Our findings suggest that attention and emotion play an important role in detecting social stigma. Specifically, we found that the frontocentral N2 response – which has been implicated in detecting novelty and need for response inhibition (for review, see Folstein & Van Pettern, 2008) – dissociated stigmatized and negative, non-stigmatized individuals from control images, but not from each other. However, the early P3 – which has been implicated in directing attention toward emotional information (e.g., Dolcos & Cabeza, 2002; Eimer & Holmes, 2002) – distinguished the stigmatized individuals from the negative, non-stigmatized individuals. Together, these findings suggest that in early processing, the N2 may “flag” both stigmatized and negative, non-stigmatized individuals. Socially stigmatized individuals are then dissociated from negative, non-stigma by 355 ms post-stimulus onset by the early positivity component. This finding is consistent with prior work demonstrating an additive effect of emotion on attention, which is initiated in the parietal cortex (Schupp, Stockburger, Codispoti, Junghofer, Weike, et al., 2007). Moreover, this process seems to occur automatically, given that the regulatory goal did not interact with either the N2 or the P3 response. It is important to note that the images of highly negative, non-stigmatized individuals were rated as eliciting more negative affect during the task, and more varied negative affect as compared to the images of the highly negative stigmatized individuals. Thus, these results suggest that highly negative social stigma is not salient simply because it is more negative or arousing than negative, non-stigmatized images.
However, when considered in tandem with our second finding – that regulatory goals were not effective in reducing the sustained emotional response to images of stigmatized individuals – one possibility is that the conflicting emotions elicited by stigma as compared to negative, non-stigma (pity and disgust) may have been more difficult to resolve than the self-reported negative emotions (fear, anger, and distress) that were elicited more robustly by negative, non-stigma images. Indeed, individuals who are stigmatized have widely been shown to elicit complex negative affective responses from perceivers (e.g., Crocker, Major, & Steele, 1998; Fiske et al., 2002; Harris & Fiske, 2006; Krendl, Heatherton, & Kensinger, 2009; Krendl, Kensinger, & Ambady, 2012). Simply put, it is possible that developing strategies that effectively decrease conflicting emotions (pity and disgust) may be more difficult to do than developing a strategy that simply reduces negative emotions (anger, fear, and distress). Although this assertion is merely speculative, it could explain (at least in part) why regulatory goals were less likely to affect the ERP response to stigmatized images as compared to negative, non-stigmatized images.
The fact that the sustained late positivity response to highly negative stigmatized individuals was not reduced at any time during the decrease condition may suggest that, even if individuals are engaging in regulatory processes when they evaluate stigmatized individuals (as shown in previous fMRI research; e.g., Krendl et al., 2006; Krendl, Heatherton, & Kensinger, 2009; Krendl, Kensinger, & Ambady, 2012), those processes are not successful in reducing all markers of affective response. Moreover, we found that individual differences in bias affected the amplitude of the late positivity response in the decrease condition. Specifically, we found that individuals with greater implicit bias toward highly negative stigmatized individuals had a higher amplitude late positivity response when they were instructed to decrease their negative emotional response to those images. Together, these finding suggests that this marker of the affective response to highly negative stigma may be too rigid to be effectively reduced, although it is worth noting that we did observe a significant reduction in the magnitude of this potential in the decrease as compared to passive viewing condition for the negative, non-stigmatized image.
Although these ERP results, constrained to the initial 2-second evaluative period, suggest a rigidity to the affective response to highly negative stigma, the behavioral ratings made by participants suggest some malleability of that response. It is important to note that the behavioral response we collected was made following a full 8-second regulatory block, whereas the ERP results focus on the first 2 seconds. Thus, it may be the case that participants were able to regulate their affective response over this longer time period despite an initial rigidity in the affective response. Because the extant fMRI research on the neural correlates engaged when individuals perceive stigma has focused primarily on evaluations made in less than 4 seconds (e.g., Cikara, Farnsworth, Harris, & Fiske, 2010; Cikara & Fiske, 2011; Harris & Fiske, 2006 (Experiment 2); Krendl, Macrae, Kelley, Fugelsang, & Heatherton, 2006; Krendl, Heatherton, & Kensinger, 2009), gaining a better understanding of the neural processes engaged during this initial evaluative period is central to understanding how stigma is perceived.
Together, the results of the current study contribute to our understanding of why negative bias toward highly negative stigmatized individuals might persist, despite widespread efforts to reduce and overcome that bias. Specifically, the fact that highly negative socially stigmatized individuals are dissociated from negative non-stigmatized individuals as early as 355 ms post-stimulus onset suggests that highly negative stigma is a salient cue. Moreover, its salience appears to be connected to the rigidity of the response that individuals have toward highly negative stigma, even in the presence of explicit regulatory goals to overcome that bias. Together, these findings contribute to the growing social neuroscience literature on person perception. These findings may lay the foundation for future work to developing effective interventions to overcome the pervasive and pernicious negative bias toward stigma.
Acknowledgments
Funding support for this study was provided by NRSA training grant 1F32AG034039 (awarded to ACK) and NIMH grant MH080833 (awarded to EAK). This research was conducted while EAK was a Searle Scholar.
Footnotes
IAPS images used: 2100, 2110, 2120, 2130, 2205, 2220, 2221, 2410, 2490, 2661, 2690, 2691, 2700, 2810, 2900, 3180, 3220, 3230, 3280, 3530, 4621, 4631, 6211, 6212, 6243, 6244, 6510, 6530, 6540, 6560, 6561, 6570, 6830, 8230, 8480, 9041, 9042, 9160, 9220, 9230, 9270, 9402, 9415, 9417, 9421, 9430, 9452, 9520, 9530, 9700.
References
- Amodio DM, Harmon-Jones E, Devine PG, Curtin JJ, Sigan, Covert AE. Neural signals for the detection of unintentional race bias. Psychological Science. 2004;15(2):88–93. doi: 10.1111/j.0963-7214.2004.01502003.x. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Devine PG, Harmon-Jones E. Individual differences in the regulation of intergroup bias: the role of conflict monitoring and neural signals for control. Journal of personality and social psychology. 2008;94(1):60. doi: 10.1037/0022-3514.94.1.60. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Dickter CL, Sestir MA. Stereotype activation and control of race bias: Cognitive control and inhibition and its impairment by alcohol. Journal of Personality and Social Psychology. 2006;90(2):272–287. doi: 10.1037/0022-3514.90.2.272. [DOI] [PubMed] [Google Scholar]
- Brewer MB. A dual process model of impression formation. In: Srull TK, Wyer RS, editors. A dual process model of impression formation. Advances in social cognition. Vol. 1. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc.; 1988. pp. 1–36. (1988) [Google Scholar]
- Cikara M, Farnsworth RA, Harris LT, Fiske ST. On the wrong side of the trolley track: Neural correlates of relative social valuation. Social cognitive and affective neuroscience. 2010;5(4):404–413. doi: 10.1093/scan/nsq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikara M, Fiske ST. Bounded empathy: Neural responses to outgroup targets' (mis)fortunes. Journal of Cognitive Neuroscience. 2011;23:3791–3803. doi: 10.1162/jocn_a_00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J, Major B, Steele C. Social stigma. 4. Vol. 2. New York, NY, US: McGraw-Hill; 1998. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Deveney CM, Pizzagalli DA. The cognitive consequences of emotion regulation: an ERP investigation. Psychophysiology. 2008;45(3):435–444. doi: 10.1111/j.1469-8986.2007.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine PG. Stereotypes and prejudice: their automatic and controlled components. Journal of personality and social psychology. 1989;56(1):5. [Google Scholar]
- Dolcos F, Cabeza R. Event-related potential of emotional memory: encoding pleasant, unpleasant, and neutral pictures. Cogntiive, Affective, and Bheavioral Neuroscience. 2002;2(3):252–263. doi: 10.3758/cabn.2.3.252. [DOI] [PubMed] [Google Scholar]
- Dunton BC, Fazio RH. An individual difference measure of motivation to control prejudiced reactions. Personality and Social Psychology Bulletin. 1997;23(3):316–326. [Google Scholar]
- Eimer M, Holmes A. An ERP study on the time course of emotional face processing. Neuroreport. 2002;13(4):427–431. doi: 10.1097/00001756-200203250-00013. [DOI] [PubMed] [Google Scholar]
- Fiske ST, Cuddy AJC, Glick P, Xu J. A model of (often mixed) stereotype content: Competence and warmth respectively follow from perceived status and competition. Journal of Personality and Social Psychology. 2002;82(6):878–902. [PubMed] [Google Scholar]
- Fiske ST, Neuberg SL. A continuum of impression formation, from category-based to individuating processes: Influences of information and motivation on attention and interpretation. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 23. New York: Academic Press; 1990. pp. 1–74. [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffman E. Stigma: Notes on the management of spoiled identity. New York, NY: Simon & Schuster; 1963. [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the implicit association test. Journal of personality and social psychology. 1998;74(6):1464. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology. 2010;35(2):129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology. 2009;120(3):505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective, & Behavioral Neuroscience. 2006;6(4):291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: Appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6(3):517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Dehumanizing the lowest of the low: Neuroimaging responses to extreme out-groups. Psychological Science. 2006;17(10):847–853. doi: 10.1111/j.1467-9280.2006.01793.x. [DOI] [PubMed] [Google Scholar]
- Ito TA, Bartholow BD. The neural correlates of race. Trends in cognitive sciences. 2009;13(12):524–531. doi: 10.1016/j.tics.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TA, Urland GR. Race and gender on the brain: Electrocortical measures or attention to the race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology. 2003;85(4):616–626. doi: 10.1037/0022-3514.85.4.616. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland GR. The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Human brain mapping. 2007;28(10):915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl AC, Kensinger EA, Ambady N. How does the brain regulate negative bias to stigma? Social Cognitive & Affective Neuroscience. 2012;7(6):715–726. doi: 10.1093/scan/nsr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl AC, Moran JM, Ambady N. Does context matter in evaluations of stigmatized individuals? An fMRI study. Social Cognitive & Affective Neuroscience. 2012 doi: 10.1093/scan/nss037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl AC, Heatherton TF, Kensinger EA. Aging minds and twisting attitudes: An fMRI investigation of age differences in inhibiting prejudice. Psychology & Aging. 2009;24(3):530–541. doi: 10.1037/a0016065. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Macrae CN, Kelley WM, Fugelsang JF, Heatherton TF. The good, the bad, and the ugly: An fMRI investigation of the functional anatomic correlates of stigma. Social Neuroscience. 2006;1(1):5–15. doi: 10.1080/17470910600670579. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Manual and Affective Ratings. Technical Report A-6. Gainesville, FL: Univeristy of Florida; 2005. [Google Scholar]
- Langlois JH, Kalakanis L, Rubenstein AJ, Larson A, Hallam M, Smoot M. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychological Bulletin. 2000;126(3):390–423. doi: 10.1037/0033-2909.126.3.390. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Williams LM, Rathjen J, Shevrin H, Gordon E. A temporal dissociation of subliminal versus supraliminal fear perception: an event-related potential study. Journal of cognitive neuroscience. 2004;16(3):479–486. doi: 10.1162/089892904322926809. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, Trawalter S, Shelton JN. An fMRI investigation of the impact of interracial contact on executive function. Nature neuroscience. 2003;6(12):1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Schupp, Cuthbert, Bradley, Cacioppo, Ito, Lang Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, Hamm AO. Selective visual attention to emotion. The Journal of Neuroscience. 2007;27(5):1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


