Abstract
Different regimens of silver diamine fluoride (SDF) have been used to manage early childhood caries. So far, there is limited information regarding the concentrations and frequency of applications for effective caries control in primary teeth. This study aimed to compare the efficacy of 2 commercially available SDF solutions at preprepared concentrations of 38% and 12% when applied annually or biannually over 18 mo in arresting dentine caries in primary teeth. This randomized double-blinded clinical trial recruited kindergarten children aged 3 to 4 y who had at least 1 tooth with dentine caries. The children were randomly allocated to receive 4 treatment protocols: group 1, annual application of 12% SDF; group 2, biannual application of 12% SDF; group 3, annual application of 38% SDF; and group 4, biannual application of 38% SDF. Clinical examinations at 6-mo intervals were conducted to assess whether active carious lesions became arrested. Information on the children’s background and oral hygiene habits was collected through a parental questionnaire at baseline and follow-up examinations. A total of 888 children with 4,220 dentine carious tooth surfaces received treatment at baseline. After 18 mo, 831 children (94%) were examined. The caries arrest rates were 50%, 55%, 64%, and 74% for groups 1, 2, 3, and 4, respectively (P < 0.001). Lesions treated with SDF biannual application had a higher chance of becoming arrested compared with those receiving SDF annual application (odds ratio, 1.33; 95% confidence interval, 1.04–1.71; P = 0.025). The interaction between concentration and lesion site was statistically significant (P < 0.001). Compared with 12% SDF, the use of 38% SDF increased a chance of becoming arrested (P < 0.05), except lesions on occlusal surfaces. Based on the 18-mo results, SDF is more effective in arresting dentin caries in the primary teeth of preschool children at 38% concentration than 12% concentration and when applied biannually rather than annually.
Knowledge Transfer Statement: The results of this study can be used by clinicians and dental public health professionals when deciding which concentrations and frequency of application of silver diamine fluoride solution should be adopted for arresting dentine caries. With consideration of caries arrest treatment with silver diamine fluoride, which is painless, simple, and low cost, this information could lead to more appropriate therapeutic decisions for caries control in young children or those who lack access to affordable conventional dental care.
Keywords: randomized controlled trial, children, primary teeth, dental caries, remineralization, early childhood caries
Introduction
Early childhood caries (ECC) is highly prevalent, especially in poor and disadvantaged children (Chu et al. 1999; Tinanoff and Reisine 2010). Epidemiological studies reported that ECC was mostly left untreated (Chu et al. 2012; Schwendicke et al. 2015). The conventional restorative approach requires sophisticated dental equipment and well-trained health personnel, especially in apprehensive young children (Chu and Lo 2007). Effective and feasible caries treatment protocols are required to address this major dental public health problem. Clinical studies have shown that the use of 38% silver diamine fluoride (SDF) is effective in preventing and also arresting caries (Chu et al. 2002; Llodra et al. 2005). A clinical trial demonstrated that SDF treatment was more effective (relative risk, 67%) than interim restorative treatment using a glass ionomer cement (relative risk, 39%) in arresting caries of primary teeth (dos Santos et al. 2012).
Different concentrations of SDF solutions are commercially available. An in vitro study using a 40% aqueous solution of silver fluoride in Australia suggested that such a high concentration of fluoride may allow a substantial amount of fluoride to enter the systemic circulation and potentially cause dental fluorosis in young children (Gotjamanos 1997). Although this suggestion of the risk of dental fluorosis was refuted by the Health Department of Western Australia (Neesham 1997), some researchers have recommended the use of a lower concentration of SDF to minimize this risk (Yee et al. 2009). However, a one-off application of a low concentration (12%) of SDF was not effective in arresting dental caries (Yee et al. 2009). In contrast, a 30-mo clinical study found that 3 applications of 10% SDF over 3 consecutive weeks was effective in arresting caries in children (Braga et al. 2009). There is no study so far to investigate the effectiveness of regular applications of a low concentration of SDF in arresting caries of young children.
Rosenblatt et al. (2009) performed a review on SDF and concluded that it is a safe, effective, efficient, and “equitable” caries control agent for preventing and arresting dental caries. Milgrom and Chi (2011) advocated that SDF therapy is an important prevention-centered caries management strategy during critical periods in early childhood. However, more randomized, controlled clinical trials on SDF are thus needed before recommending the widespread use of this potentially useful interventional agent. At present, no study has shown whether low and high SDF concentrations are equally effective or if 6- or 12-mo applications differ in the treatment outcome. The objective of this study was to compare the efficacy of 2 commercially available SDF solutions at preprepared concentrations of 38% and 12% when applied annually or biannually over 18 mo in arresting dentine caries in primary teeth. The null hypothesis tested was that there was no difference in the effectiveness of SDF in caries arrest in the primary teeth of children at 1) concentrations of 12% or 38% and 2) an application frequency of every 6 or 12 mo.
Methods
The study was approved by the Institutional Review Board (IRB) of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB reference number: UW 09-302). The trial was registered in the Registry of Clinical Trials run by the United States National Library of Medicine (ClinicalTrials.gov identifier: NCT02385474). Healthy preschool children aged 3 to 4 y who had at least 1 tooth with untreated active dentine caries attending the first year in 37 kindergartens were invited to join this study. Informed consent was sought from parents of each participating child. Teeth with exposed pulp or nonvital teeth were excluded in this study.
A trained dentist conducted the field examination in the kindergarten through a careful visual inspection with the aid of a Community Periodontal Index (CPI) periodontal probe and a dental mirror with light-emitting diode intraoral illumination. The oral hygiene status was measured using the visible plaque index (VPI). The buccal and lingual surfaces of 6 index teeth (55, 51, 63, 71, 75, and 83) were examined for recording the VPI scores. The tooth status (decayed, missing, filled surfaces [dmfs] score), tooth discoloration, and hypermobility were recorded. The caries was diagnosed at the cavitation level. A lesion was recorded as active if softness was detected upon gentle probing or if the decayed tooth was extracted or restored at the follow-up examination. If the dentine surface was hard to probing, it was classified as arrested caries (Chu et al. 2002; Llodra et al. 2005; Yee et al. 2009; Zhi et al. 2012). For caries arrest assessment, all surfaces (buccal, lingual, mesial, distal, and occlusal for posterior teeth) of each tooth were assessed. The follow-up oral examinations were conducted by the same examiner at 6, 12, and 18 mo. Intraexaminer agreement on the plaque and caries assessment was conducted in 10% of the children at all examinations. A parental questionnaire was administered at baseline and 18-mo follow-up visits regarding their children’s oral hygiene habits, frequency of toothbrushing, use of fluoride toothpaste, dental visit behavior, snacking habits, main caretaker, parental condition, parental educational level, and family total income.
A 2-factor factorial design (concentration: 12% vs. 38% and frequency: annually vs. biannually) was adopted. Participating children with dental caries were categorized as having a higher caries rate (more than 3 caries tooth surfaces) or a lower caries rate (3 or fewer caries tooth surfaces). A dental assistant who held the random allocation list prepared the materials according to the child’s assigned group. The children were then allocated by a stratified randomization method (block size of 8) using a personal computer into 1 of 4 groups as follows:
Group 1: Topical application of 12% SDF solution every 12 mo
Group 2: Topical application of 12% SDF solution every 6 mo
Group 3: Topical application of 38% SDF solution every 12 mo
Group 4: Topical application of 38% SDF solution every 6 mo
In this study, the 12% SDF solution was Cariostop 12% (Biodinâmica Química e Farmacêutica LTDA), while the 38% SDF solution was Saforide (Toyo Seiyaku Kasei Co. Ltd.). SDF was applied after the examination by an independent operator blinded to the group allocation. A normal saline solution was applied to the carious tooth surfaces of the group 1 and 3 children during the half-yearly follow-up visits to blind the children. The examiner was blinded to the treatment group allocation of the children and the identity of the solutions throughout the study.
The results of previous clinical trials showed that around 70% of the active dentin caries became arrested after 24 mo (Chu et al. 2002). An absolute difference of 10% in the caries arrest rates between treatment groups was considered clinically significant. The estimated sample size was based on the expected proportion of arrested caries, with the power of the study set at 80% (β = 0.2) and with α = 0.05 as the statistical significance level. The sample size per study group, calculated by using the software Sample Power 2.0 (SPSS, Inc.), was 353 active carious tooth surfaces. Based on the results of epidemiological surveys (Lo et al. 2009; Chu et al. 2012), we estimated that the mean baseline active carious surfaces would be 3. The intraclass correlation coefficient (ICC) for dental caries data at the surface level within the individual would be approximately 0.3 (Masood et al. 2015). Following the equation for the required sample size in a multilevel study (Twisk 2006), the estimated sample size would be at least 565 active carious surfaces and with at least 188 children being recruited for each group at baseline. The anticipated dropout rate was approximately 15% (Duangthip et al. 2016); thus, 221 children in each group or 884 children in total needed to be recruited at baseline.
Statistical Analysis
An intention-to-treat analysis was undertaken. All data were analyzed using the software SPSS 23.0 for Windows (SPSS, Inc.). Cohen’s κ statistics were used to assess the intraexaminer reliability in caries diagnosis, assessment of VPI at baseline, and follow-up examinations. The χ2 test was used to assess the categorical data of children’s demographic information (age, sex, place of birth, parental condition, main caretaker, father’s and mother’s education level, and monthly family income level), oral health–related habits (bottle feeding before bed, stop bottle feeding age, start toothbrushing age, daily toothbrushing frequency, use of fluoride toothpaste), dental visits, caries condition (baseline ECC status, tooth position and lesion site), and adverse effects among the 4 treatment groups. Analysis of variance (ANOVA) was performed to assess the comparability between the treatment groups according to the baseline conditions of the children such as frequency of snack time; decayed, missing, filled teeth (dmft)/dmfs score; number of nonvital teeth; number of included teeth/surfaces; and VPI score. The McNemar test was used to compare the changes in oral health–related habits at baseline and the follow-up examinations.
Since more than 1 caries lesion could be chosen from 1 child, the generalized estimating equations (GEE) approach was used to adjust for the clustering effect. The first level was the tooth surface while the second level was the child (subject). This analysis accounted for the correlation (clustering) between observations of multiple surfaces from the same child. Therefore, a multilevel logistic regression analysis was performed to analyze the effects of independent variables on the caries arrest rates at the 18-mo examinations. Treatment group was replaced with SDF concentration (12% or 38%), frequency (annually or biannually), and interaction between concentration and frequency to evaluate the effects of these factors in the presence of other significant independent variables adjusted in the model. Based on prior knowledge, tooth position, lesion site, lesion size, plaque on lesion, and overall VPI score are significant factors affecting caries arrest (Duangthip 2015). Therefore, besides the concentration and frequency of application, the above-mentioned factors and their significant interaction with the assigned treatment were included as variables in the base model. Other potential variables (namely, the child’s demographic characteristics collected at baseline, oral health–related behaviors and dental visits collected at 18 mo, and clinical characteristics at 18 mo) with P < 0.1 in the univariate analysis were selected and added to the base model. All possible subset models approach was used, and these models were compared. The goodness of fit of the models was estimated by the corrected quasi-likelihood information criterion (QICC). QICC is a reverse value for goodness of fit so that the smaller the value, the better the model fit. The model with all variables being significant and showing the lowest QICC was selected as the best-fit logistic regression model. The level of statistical significance for all tests was set at P < 0.05.
Results
A total of 4,251 kindergarten children were screened and 888 eligible children were randomly allocated into 4 treatment groups with 222 children in each group. Among them, 419 children (48%) were regarded as having high caries rates. The mean (SD) age of the children was 3.8 (0.6) years, and 519 (58.4%) were boys. Background information and clinical characteristics of children in the 4 study groups at baseline are shown in Table 1. There was no statistically significant difference in mean age, sex, place of birth, parental condition, main caretaker, father’s and mother’s education level, monthly family income level, and oral health–related behaviors among the 4 groups. Regarding the clinical characteristics, the mean (SD) dmft and dmfs at baseline were 3.84 (2.79) and 5.15 (4.75), respectively. The mean (SD) VPI score was 0.69 (0.20). There was no statistically significant difference in the baseline of the mean dmft and dmfs scores, number of teeth and surfaces included for treatment, number of nonvital teeth, and mean VPI scores between the 4 groups. According to the tooth position, 2,421 (57%) surfaces were in the upper anterior arch, 561 surfaces (13%) were in the upper posterior arch, 115 (3%) surfaces were in the lower anterior arch, and 1,123 (27%) surfaces were in the lower posterior arch. There was no statistically significant difference in the distribution of caries surfaces according to tooth position or lesion site.
Table 1.
Baseline Demographic Information, Oral Health–Related Habits, and Clinical Characteristics of Children in the 4 Study Groups (N = 888).
| Group 1 (12%, Annual) (n = 222) | Group 2 (12%, Biannual) (n = 222) | Group 3 (38%, Annual) (n = 222) | Group 4 (38%, Biannual) (n = 222) | |
|---|---|---|---|---|
| Demographic backgrounda | ||||
| Sex | ||||
| Male | 134 (60) | 130 (59) | 132 (60) | 123 (55) |
| Main caretaker | ||||
| Father or mother | 151 (69) | 145 (65) | 150 (68) | 145 (65) |
| Grandparents | 44 (19) | 44 (20) | 40 (18) | 49 (22) |
| Maid or other people | 27 (12) | 33 (15) | 32 (14) | 28 (13) |
| Father’s education level | ||||
| Primary education | 35 (17) | 37 (17) | 32 (15) | 36 (17) |
| Secondary education | 145 (68) | 140 (66) | 143 (67) | 135 (64) |
| Postsecondary education | 32 (15) | 36 (17) | 38 (18) | 41 (19) |
| Mother’s education level | ||||
| Primary education | 37 (17) | 34 (16) | 38 (17) | 42 (19) |
| Secondary education | 161 (73) | 152 (70) | 149 (68) | 147 (67) |
| Postsecondary education | 22 (10) | 30 (14) | 31 (14 ) | 30 (14) |
| Monthly family income | ||||
| Below HK$10,000 | 80 (37) | 80 (37) | 80 (38) | 84 (41) |
| HK$10,001–20,000 | 78 (37) | 66 (31) | 74 (35) | 65 (31) |
| Above HK$20,000 | 56 (26) | 69 (32) | 57 (27) | 59 (28) |
| Oral health–related habitsa | ||||
| Toothbrushing frequency | ||||
| Once or less daily | 132 (59) | 123 (55) | 129 (58) | 122 (55) |
| Twice or more daily | 90 (41) | 99 (45) | 93 (42) | 100 (45) |
| Use of fluoride toothpaste | 119 (54) | 119 (54) | 115 (52) | 120 (54) |
| Use of bottle feeding before bed | 117 (53) | 113 (51) | 127 (57) | 111 (50) |
| Mean (SD) daily snacking frequency | 2.37 (1.61) | 2.36 (1.31) | 2.24 (1.26) | 2.42 (1.39) |
| Clinical characteristics, mean (SD)a | ||||
| Baseline dmft | 3.82 (2.72) | 3.81 (2.83) | 3.92 (2.91) | 3.83 (2.72) |
| Baseline dmfs | 5.00 (4.49) | 5.20 (4.86) | 5.41 (5.16) | 5.00 (4.47) |
| No. of teeth included | 3.65 (2.67) | 3.76 (2.64) | 3.71 (2.86) | 3.62 (2.48) |
| No. of surfaces included | 4.73 (4.11) | 4.83 (4.13) | 4.83 (4.47) | 4.61 (3.71) |
| No. of nonvital teeth | 0.05 (0.29) | 0.07 (0.40) | 0.11 (0.47) | 0.05 (0.34) |
| VPI score at baseline | 0.68 (0.21) | 0.68 (0.19) | 0.70 (0.20) | 0.69 (0.20) |
Values are presented as n (%) unless otherwise indicated.
dmfs, decayed, missing, filled surfaces; dmft, decayed, missing, filled teeth; SD, standard deviation; VPI, visible plaque index.
No statistically significant difference regarding children’s demographic background, oral health–related habits, and clinical characteristics among 4 groups.
Follow-up Findings
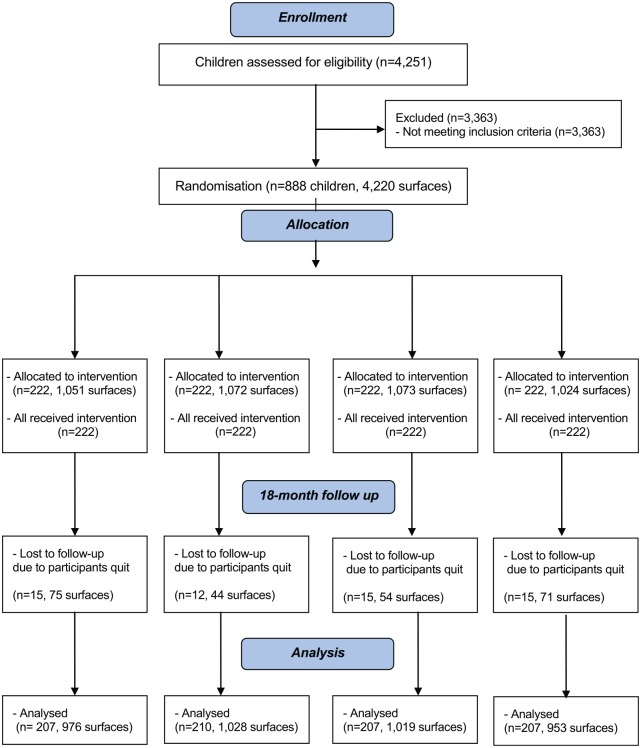
The flow of children is summarized in the CONSORT flowchart (Fig.). The subject and surface dropout rate were 6.4% and 5.8%, respectively. There was no statistically significant difference in subject or surface dropout rates among the 4 groups (χ2 test, P > 0.05). The main reason for participants leaving this study was due to changing kindergartens. All the 831 remaining children returned the parental questionnaires after the 18-mo examination. There were no statistically significant differences among the 4 groups of children on sex, bottle-feeding habit, oral health–related behaviors, dental visits, or mean daily snacking frequency at the 18-mo examination. At 18 mo, 25% of the study children were still using nursing bottles for feeding. Regarding the oral health–related behaviors, 64% brushed their teeth twice daily or more but 2% of them did not brush their teeth. Regarding the age of starting brushing, 14% started brushing at age 12 mo or younger, 23% at 13 to 18 mo, 22% at 19 to 24 mo, and 39% after 24 mo. After receiving SDF treatment, 5% of the children visited a dentist for dental checkup. No statistically significant difference was found in baseline caries experience, number of caries teeth and surfaces included, and number of nonvital teeth of children who dropped out and remained in the 18-mo follow-up (P > 0.05). Intraexaminer reliability of VPI scores and caries arrest assessment, as measured by Cohen’s κ statistics, were at least 0.91 at baseline and all follow-up examinations.
Figure.
Flow diagram of participants’ progress over 18 mo.
The oral hygiene status in mean VPI score at baseline and 6-, 12-, and 18-mo examination was 0.69, 0.43, 0.45, and 0.36, respectively, and no significant differences were found in VPI scores among the 4 groups (P > 0.05). Compared with baseline examination, there was significant improvement in all 4 groups from baseline to 6-mo follow-up but not at 12- or 18-mo examinations. At the 18-mo follow-up examination, almost all of the soft lesions (99%–100%) had visible plaque, while only 72% to 81% of the arrested lesions had visible plaque. However, no significant interaction between plaque on lesion and the assigned treatment was found. There were significantly more soft caries surfaces than arrested caries with visible plaque (P < 0.001). No significant difference in caries experience (dmft and dmfs) was found among the 4 treatment groups at all examinations.
Effectiveness of SDF Treatment
At baseline, the respective total numbers of active cavitated dentin lesions in groups 1 to 4 were 1,051, 1,072, 1,073, and 1,024. Before adjustment of covariates, at the 18-mo follow-up, the treatment effectiveness among 4,220 included caries surfaces was 50%, 55%, 64%, and 74% for groups 1 to 4, respectively (Table 2). Statistically significant differences were found in caries arrest rates between the 4 groups at all follow-up examinations (χ2 test, P < 0.001). The proportions of arrested surfaces of upper anterior teeth, upper posterior teeth, lower anterior teeth, and lower posterior teeth are shown in Table 2. Besides the known black staining on carious lesions after SDF treatment, no major adverse event occurred during the 18-mo study.
Table 2.
Caries Arrest Rates at 6-, 12-, and 18-mo Follow-up Examinations.
| Group 1 (12%, Annual) | Group 2 (12%, Biannual) | Group 3 (38%, Annual) | Group 4 (38%, Biannual) | P Value | |
|---|---|---|---|---|---|
| All surfaces, n (%) | |||||
| Baseline | (n = 1,051) | (n = 1,072) | (n = 1,073) | (n = 1,024) | |
| 6 mo | 337/1,051 (32.1) | 346/1,072 (32.3) | 471/1,073 (43.9) | 449/1,024 (43.8) | <0.001 |
| 12 mo | 409/1,007 (40.6) | 502/1,046 (48.0) | 540/1,041 (51.9) | 618/987 (62.6) | <0.001 |
| 18 mo | 487/976 (49.9) | 566/1,028 (55.1) | 649/1,019 (63.7) | 701/953 (73.6) | <0.001 |
| Upper anterior teeth, n (%) | |||||
| Baseline | (n = 605) | (n = 612) | (n = 619) | (n = 585) | |
| 6 mo | 213/605 (35.2) | 208/612 (34.0) | 314/619 (50.7) | 284/585 (48.5) | <0.001 |
| 12 mo | 276/587 (47.0) | 331/596 (55.5) | 367/610 (60.2) | 403/565 (71.3) | <0.001 |
| 18 mo | 335/572 (58.6) | 382/582 (65.6) | 450/599 (75.1) | 447/543 (82.3) | <0.001 |
| Upper posterior teeth, n (%) | |||||
| Baseline | (n = 140) | (n = 140) | (n = 143) | (n = 138) | |
| 6 mo | 29/140 (20.7) | 33/140 (23.6) | 41/143 (28.7) | 56/138 (40.6) | 0.004 |
| 12 mo | 30/132 (22.7) | 44/138 (31.9) | 41/134 (30.6) | 67/133 (50.4) | <0.001 |
| 18 mo | 33/126 (26.2) | 55/138 (39.9) | 57/131 (43.5) | 74/128 (57.8) | <0.001 |
| Lower anterior teeth, n (%) | |||||
| Baseline | (n = 33) | (n = 26) | (n = 29) | (n = 27) | |
| 6 mo | 24/33 (72.7) | 17/26 (65.4) | 22/29 (75.9) | 16/27 (59.3) | 0.533 |
| 12 mo | 23/32 (71.9) | 19/26 (73.1) | 22/29 (75.9) | 23/27 (85.2) | 0.208 |
| 18 mo | 29/32 (90.6) | 21/26 (80.8) | 28/29 (96.6) | 25/26 (96.2) | 0.108 |
| Lower posterior teeth, n (%) | |||||
| Baseline | (n = 273) | (n = 294) | (n = 282) | (n = 274) | |
| 6 mo | 71/273 (26.0) | 88/294 (29.9) | 94/282 (33.3) | 93/274 (33.9) | 0.005 |
| 12 mo | 80/256 (31.3) | 108/286 (37.8) | 110/268 (41.0) | 125/262 (47.7) | 0.001 |
| 18 mo | 90/246 (36.6) | 108/282 (38.3) | 114/260 (43.8) | 155/256 (60.5) | <0.001 |
Logistic Regression Model at 18-mo Follow-up
For the base model, both high concentration (38% SDF) and high frequency (biannually) were associated with an increased chance to arrest caries lesions (P < 0.05). All other variables in the base model (tooth position, lesion site, lesion size, plaque on lesion, and overall VPI score) were also significantly associated with effectiveness of caries arrest (P < 0.05). No statistically significant interaction between SDF concentration and frequency of application was found. However, the interaction between concentration and lesion site was statistically significant. This interaction was included in the base model together with the assigned treatment (concentration and frequency of application) and other variables mentioned above.
Six variables (mother’s education, main caretaker, toothbrushing frequency, use of bottle feeding before bed, start toothbrushing age, dental visit) with P < 0.1 in the univariate analysis were selected as the additional independent variables. As a result, 1 base model plus 63 possible subset models were generated. Comparing these 64 subset logistic regression models, the best-fit model with all additional significant variables was selected (Table 3). It had a lower QICC (3,233) than the base model (3,515). The ICC was 0.13.
Table 3.
Adjusted Logistic Regression with the Best Goodness of Fit Estimated by the Corrected Quasi-Likelihood Information Criterion.
| Explanatory Variable | Predicted Probabilitya | Odds Ratio | 95% Confidence Interval | P Value | Pairwise Comparison |
|---|---|---|---|---|---|
| Base model | |||||
| Frequency | 0.025 | ||||
| (1) Annuallyb | 0.53 | ||||
| (2) Biannually | 0.61 | 1.33 | 1.04–1.71 | ||
| SDF concentration | <0.001 | ||||
| (1) 12%b | |||||
| (2) 38% | 2.73 | 1.86–3.99 | |||
| Lesion site | <0.001 | ||||
| (1) Mesialb | |||||
| (2) Buccal | 2.50 | 1.69–3.71 | |||
| (3) Lingual | 1.03 | 0.67–1.57 | |||
| (4) Distal | 1.53 | 1.14–2.06 | |||
| (5) Occlusal | 1.31 | 0.85–2.01 | |||
| Lesion site × SDF concentration | 0.001 | ||||
| (1.1) Mesial × 12% | 0.53c | ||||
| (1.2) Mesial × 38% | 0.76 | (1.2) > (1.1) | |||
| (2.1) Buccal × 12% | 0.74 | ||||
| (2.2) Buccal × 38% | 0.90 | 1.12 | 0.63–1.98 | (2.2) > (2.1) | |
| (3.1) Lingual × 12% | 0.54 | ||||
| (3.2) Lingual × 38% | 0.75 | 0.92 | 0.91–0.49 | (3.2) > (3.1) | |
| (4.1) Distal × 12% | 0.64 | ||||
| (4.2) Distal × 38% | 0.75 | 0.64 | 0.46–1.01 | (4.2) > (4.1) | |
| (5.1) Occlusal × 12% | 0.60 | ||||
| (5.2) Occlusal × 38% | 0.63 | 0.41 | 0.25–0.68 | ||
| Tooth position | <0.001 | ||||
| (1) Upper anteriorb | 0.53 | (1) > (2), (4) | |||
| (2) Upper posterior | 0.22 | 0.25 | 0.17–0.36 | (3) > (2), (4) | |
| (3) Lower anterior | 0.77 | 2.88 | 1.06–7.83 | ||
| (4) Lower posterior | 0.24 | 0.27 | 0.19–0.40 | ||
| Size of lesion | <0.001 | ||||
| (1) Smallb | 0.53 | ||||
| (2) Large | 0.25 | 0.29 | 0.24–0.37 | ||
| Lesion with visible plaque | <0.001 | ||||
| (1) Yesb | 0.53 | ||||
| (2) No | 0.99 | 82.58 | 35.70–191.00 | ||
| High VPI score | 0.19 | 0.10–0.37 | <0.001 | ||
| Mean VPI = 0.40 | 0.30 | ||||
| Additional significant variables | |||||
| Age of start brushing | 0.001 | ||||
| (1) No brushingb | 0.53 | (2), (3) > (1) | |||
| (2) 12 mo or younger | 0.85 | 4.89 | 1.81–13.22 | (3) > (5) | |
| (3) 13–18 mo | 0.85 | 4.81 | 1.89–12.22 | ||
| (4) 19–24 mo | 0.78 | 3.06 | 1.20–7.85 | ||
| Over 24 mo | 0.78 | 3.02 | 1.20–7.55 | ||
| Mother’s education | 0.024 | ||||
| (1) Primary educationb | 0.53 | (1) > (2) | |||
| (2) Secondary education | 0.42 | 0.62 | 0.44–0.88 | ||
| (3) Postsecondary education | 0.46 | 0.73 | 0.45–1.18 | ||
| Main caretaker | 0.030 | ||||
| (1) Parentsb | 0.53 | NS | |||
| (2) Grandparents | 0.46 | 0.74 | 0.53–1.03 | ||
| (3) Maid or others | 0.43 | 0.66 | 0.46–0.94 |
NS, no significant multiple comparisons were identified; SDF, silver diamine fluoride; VPI, visible plaque index.
Predicted probability of arrested caries with the mean VPI score of 0.40 (after excluding missing data) and other variables as reference category.
Reference category.
Predicted probability for lesion site × SDF concentration was calculated using estimates for both main and interaction effects.
At 18 mo, lesions of the children receiving biannual SDF application had a higher chance of becoming arrested compared with those receiving annual SDF application (odds ratio [OR], 1.33; 95% confidence interval [CI], 1.04–1.71; P = 0.025). The interaction between SDF concentration and lesion site was statistically significant (P < 0.001). Lesions on mesial, distal, buccal, and lingual surfaces that received SDF concentration at 38% had a higher chance of becoming arrested than those receiving a SDF concentration at 12% (P < 0.05). However, there is no statistically significant difference in arresting caries on occlusal surfaces by 38% SDF and 12% SDF. Lesions in the posterior teeth (P < 0.001), large lesions (P < 0.001), and lesions in a child with a higher VPI score (P < 0.001) had a lower chance of becoming arrested. Similarly, lesions with visible plaque had a lower chance of becoming arrested compared with those without visible plaque (P < 0.001). Children who started brushing at age 18 mo and younger (P = 0.001), whose mother completed primary education (P = 0.024), or whose caretakers were their own parents (P = 0.030) had a higher chance of having arrested caries.
Discussion
This study used a 2 × 2 factorial design, and this allowed simultaneous study of 2 factors at 2 levels together with their potential interaction effect (Stamm 2004; Bria et al. 2006). Stratification was used to reduce baseline differences in disease severity and therefore reduce the potential bias to the treatment outcomes (Meier 1981; Kingman 1984). Block randomization was used to ensure that the number of participants in each group was almost the same (Matts and Lachin 1988). In this study, the allocation ratio of 1:1:1:1 among the 4 treatment groups was achieved.
According to the sample size calculation, the obtained ICC in the present study (0.13) was smaller than the estimated one (0.3). In addition, the completeness of follow-up is considered very satisfactory as the subject dropout rate was approximately 6% over 18 mo, which was lower than the anticipated rate in the planning study. Therefore, the efficiency or power in the multilevel analysis could be maintained. Since staining on the treated tooth surfaces was commonly found among the 4 SDF treatment groups, detection bias may be less likely to occur during the examination. Randomization was performed at the child (subject) level. It could not be done at the tooth surface level due to the contamination of the intervention in each child. Therefore, besides the treatment effects, information on potential confounding factors, especially at the tooth surface level, should be collected and analyzed. Predicting the model of treatment effectiveness at the subject level would not be precise because the assessment at the subject level could not allow the predicting variables that were related to the lesion site to be investigated.
The clinical diagnosis of caries activity was based on the visual-tactile assessment of the caries lesions (Nyvad and Fejerskov 1997). A blunt CPI probe with light force was used to assess the surface condition to avoid damaging the caries surface and avoid missing certain parts of the surface that were not arrested. Despite the fact that a gold-standard tool for caries activity assessment has not been available, the visual-tactile clinical assessment is the only validated method for assessing caries lesions in a single session (Ekstrand et al. 2009). After SDF application, the black staining layer over the arrested dentin was identified to be a hard and impermeable layer of silver phosphate (Yamaga et al. 1972), and the collagens were protected from being exposed in the arrested cavitated dentinal lesion (Mei et al. 2014). Arrested dentin caries surfaces had a significantly higher microhardness value than the soft active caries surfaces (Chu and Lo 2008). Although caries activity could also be assessed by the depth of lesions obtained from the longitudinal radiographic examinations (Lunder and von der Fehr 1996), such facilities could not be available in the epidemiological or field studies like the current study. However, the reliability of clinical assessment of dentin caries in field settings could be improved through trainings and calibrations (Chu et al. 2012). Cohen’s κ statistics in lesion activity and oral hygiene assessments could be maintained to be over 0.9 in this study. The negative control group was not used in this study due to ethical issues. In this study, group 3 (annual application of 38% SDF) was used as a positive control for comparison with other treatment groups because this treatment protocol has been proven to be effective in arresting dentine caries in a previous study (Chu et al. 2002). In the present study, the differences in treatment effectiveness by varying the concentration and frequency were studied.
The first null hypothesis that there was no difference in effectiveness between 12% and 38% SDF in arresting dentine caries in primary teeth was not supported in this study. The treatment effectiveness of 38% SDF was more effective than 12% SDF in arresting caries. Since the interaction effect between the concentration and lesion site was found to be significant, the combination of lesion site and SDF concentration should be taken into consideration when applying SDF solution for caries arrest. The second null hypothesis that there was no difference in treatment effectiveness when SDF was applied annually or biannually was also not supported by the present findings. The results of this study agreed with the findings reported by Zhi and coworkers (2012) that biannual application could significantly increase the proportion of surfaces that became arrested after 18 mo at the surface level. Nevertheless, this study was the first to evaluate the interaction effect between concentration and frequency on the treatment effectiveness of SDF. The interaction between the concentration of the SDF solutions and application frequency on treatment effectiveness was disproven in this study. This indicated that the treatment effectiveness of SDF was not significantly modified by the presence of both factors.
Based on the results of the present study, 38% SDF with biannual application is the most effective therapeutic regime for arresting dentin caries in preschool children. Besides SDF concentration and frequency of application, other factors such as lesion site, tooth position, VPI score, plaque on lesion, lesion size, the age of starting toothbrushing, mother’s education, and main caretaker were significantly associated with treatment effectiveness. It should be highlighted that the disturbance of dental plaque is an effective measure contributing to the control of caries progression and affecting the success rate of SDF. Once cavities have been developed, the trapping of food over these lesions made the natural cleansing and remineralization actions of saliva almost impossible. Even a protective layer could be formed after SDF treatment (Mei et al. 2014); it might soon be dissolved and, therefore, the lesions cannot be arrested. Therefore, further oral health education on toothbrushing should be emphasized in the kindergarten oral health programs.
SDF at 38% contains approximately 254,000 ppm silver ions (Mei et al. 2013). Approximately 18% of orally administered silver are absorbed (Hadrup and Lam 2014). Vasquez et al. (2012) measured the serum concentrations of silver and fluoride after oral SDF application and reported occasional SDF application should pose little toxicity risk when used in adults. Although no severe adverse effect was found in the present study during 18 mo, the possibility of having toxicity in children due to silver ingestion cannot be excluded. Thus, one needs to pay attention to the safety aspect when applying high-concentration silver agents to young children.
This study reported the 18-mo results of SDF therapy on young children. As the application frequencies were only completed once biannually or annually, a longer period of evaluation is more desirable. In addition, it should be noted that the results of the present study were based on dentine caries lesions that became arrested. Thus, these findings may not be transferable to different types of carious lesions such as enamel carious lesions.
Due to the simplicity and noninvasive approach of SDF treatment as well as teachers’ support during the examination, the caries arrest treatment could be easily carried out in most of the study children. These findings could provide evidence-based support for the further development of using SDF in dental public health programs. However, other issues related to feasibility, cost, sustainability, and patients’ acceptability should also be considered when translating research to practice.
Conclusion
Based on the 18-mo results of this randomized clinical trial, it can be concluded that SDF is more effective in arresting dentin caries in the primary teeth of preschool children at 38% concentration rather than 12% concentration and when applied biannually rather than annually.
Author Contributions
M.H.T. Fung, contributed to design and data acquisition, drafted and critically revised the manuscript; D. Duangthip, contributed to design and data interpretation, drafted and critically revised the manuscript; M.C.M. Wong, contributed to design, data analysis, and interpretation, drafted and critically revised the manuscript; E.C.M. Lo, C.H. Chu, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The administering institution, the University of Hong Kong, provided administrative and technical supports to our work. We are grateful to the funding bodies, administrating institution, all participating kindergartens, headmistresses, teachers, child participants, and their parents for their support and cooperation in this research.
Footnotes
This study was financially supported by the Food and Health Bureau Health and Health Service Research Fund (09101101) and Research Grant Council General Research Fund (765213M).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Braga MM, Mendes FM, De Benedetto MS, Imparato JC. 2009. Effect of silver diamine fluoride on incipient caries lesions in erupting permanent first molars: a pilot study. J Dent Child (Chic). 76(1):28–33. [PubMed] [Google Scholar]
- Bria E, Di Maio M, Nistico C, Cuppone F, Terzoli E, Cognetti F, Giannarelli D. 2006. Factorial design for randomized clinical trials. Ann Oncol. 17(10):1607–1608. [DOI] [PubMed] [Google Scholar]
- Chu CH, Fung DS, Lo EC. 1999. Dental caries status of preschool children in Hong Kong. Br Dent J. 187(11):616–620. [DOI] [PubMed] [Google Scholar]
- Chu CH, Ho PL, Lo EC. 2012. Oral health status and behaviours of preschool children in Hong Kong. BMC Public Health. 12:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CH, Lo EC. 2007. Dental caries prevention and treatment for preschool children in China. Chin J Dent Res. 10(Suppl):54–60. [Google Scholar]
- Chu CH, Lo EC. 2008. Microhardness of dentine in primary teeth after topical fluoride applications. J Dent. 36(6):387–391. [DOI] [PubMed] [Google Scholar]
- Chu CH, Lo EC, Lin HC. 2002. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentine caries in Chinese pre-school children. J Dent Res. 81(11):767–770. [DOI] [PubMed] [Google Scholar]
- dos Santos VE, Jr, de Vasconcelos FM, Ribeiro AG, Rosenblatt A. 2012. Paradigm shift in the effective treatment of caries in schoolchildren at risk. Int Dent J. 62(1):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangthip D, Chu CH, Lo EC. 2016. A randomized clinical trial on arresting dentine caries in preschool children by topical fluorides—18 month results. J Dent. 44:57–63. [DOI] [PubMed] [Google Scholar]
- Duangthip D. 2015. A randomized clinical trial on arresting dentin caries in preschool children by topical fluorides [dissertation]. Hong Kong SAR: University of Hong Kong. [DOI] [PubMed] [Google Scholar]
- Ekstrand KR, Zero DT, Martignon S, Pitts NB. 2009. Lesion activity assessment. Monogr Oral Sci. 21:63–90. [DOI] [PubMed] [Google Scholar]
- Gotjamanos T. 1997. Safety issues related to the use of silver fluoride in paediatric dentistry. Aust Dent J. 42(3):166–168. [DOI] [PubMed] [Google Scholar]
- Hadrup N, Lam HR. 2014. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—a review. Regul Toxicol Pharmacol. 68(1):1–7. [DOI] [PubMed] [Google Scholar]
- Kingman A. 1984. Stratification methods in caries clinical trials. J Dent Res. 63(Spec No):773–777. [PubMed] [Google Scholar]
- Llodra JC, Rodriguez A, Ferrer B, Menardia V, Ramos T, Morato M. 2005. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 84(8):721–724. [DOI] [PubMed] [Google Scholar]
- Lo EC, Loo EK, Lee CK. 2009. Dental health status of Hong Kong preschool children. Hong Kong Dent J. 6(1):6–12. [Google Scholar]
- Lunder N, von der Fehr FR. 1996. Approximal cavitation related to bite-wing image and caries activity in adolescents. Caries Res. 30(2):143–147. [DOI] [PubMed] [Google Scholar]
- Masood M, Masood Y, Newton JT. 2015. The clustering effects of surfaces within the tooth and teeth within individuals. J Dent Res. 94(2):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts JP, Lachin JM. 1988. Properties of permuted-block randomization in clinical trials. Control Clin Trials. 9(4):327–344. [DOI] [PubMed] [Google Scholar]
- Mei ML, Chu CH, Lo EC, Samaranayake LP. 2013. Fluoride and silver concentrations of silver diamine fluoride solutions for dental use. Int J Paediatr Dent. 23(4):279–285. [DOI] [PubMed] [Google Scholar]
- Mei ML, Ito L, Cao Y, Lo EC, Li QL, Chu CH. 2014. An ex vivo study of arrested primary teeth caries with silver diamine fluoride therapy. J Dent. 42(4):395–402. [DOI] [PubMed] [Google Scholar]
- Meier P. 1981. Stratification in the design of a clinical trial. Control Clin Trials. 1(4):355–361. [DOI] [PubMed] [Google Scholar]
- Milgrom P, Chi DL. 2011. Prevention-centered caries management strategies during critical periods in early childhood. J Calif Dent Assoc. 39(10):735–741. [PubMed] [Google Scholar]
- Neesham DC. 1997. Fluoride concentration in AgF and dental fluorosis. Aust Dent J. 42(4):268–269. [PubMed] [Google Scholar]
- Nyvad B, Fejerskov O. 1997. Assessing the stage of caries lesion activity on the basis of clinical and microbiological examination. Community Dent Oral Epidemiol. 25(1):69–75. [DOI] [PubMed] [Google Scholar]
- Rosenblatt A, Stamford TC, Niederman R. 2009. Silver diamine fluoride: a caries “silver-fluoride bullet.” J Dent Res. 88(2):116–125. [DOI] [PubMed] [Google Scholar]
- Schwendicke F, Dörfer CE, Schlattmann P, Foster Page L, Thomson WM, Paris S. 2015. Socioeconomic inequality and caries: a systematic review and meta-analysis. J Dent Res. 94(1):10–18. [DOI] [PubMed] [Google Scholar]
- Stamm JW. 2004. The classic caries clinical trial: constraints and opportunities. J Dent Res. 83(Spec No):C6–14. [DOI] [PubMed] [Google Scholar]
- Tinanoff N, Reisine S. 2010. Update on early childhood caries since the Surgeon General’s Report. Acad Pediatr. 9(6):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twisk JW. 2006. Applied multilevel analysis: practical guides to biostatistics and epidemiology. Cambridge (UK): Cambridge University Press. [Google Scholar]
- Vasquez E, Zegarra G, Chirinos E, Castillo JL, Taves DR, Watson GE, Dills R, Mancl LL, Milgrom P. 2012. Short term serum pharmacokinetics of diammine silver fluoride after oral application. BMC Oral Health. 12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaga R, Nishino M, Yoshida S, Yokomizo I. 1972. Diammine silver fluoride and its clinical application. J Osaka Univ Dent Sch. 12:1–20. [PubMed] [Google Scholar]
- Yee R, Holmgren C, Mulder J, Lama D, Walker D, van Palenstein Helderman W. 2009. Efficacy of silver diamine fluoride for arresting caries treatment. J Dent Res. 88(7):644–647. [DOI] [PubMed] [Google Scholar]
- Zhi QH, Lo EC, Lin HC. 2012. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent. 40(11):962–967. [DOI] [PubMed] [Google Scholar]