Abstract
In the present study, effects of salbutamol on the inflammatory parameters, angiogenesis, interleukin-1 beta (IL-1β) and vascular endothelial growth factor (VEGF) levels were investigated in an air pouch model of inflammation. Inflammation was induced by intrapouch administration of 1% solution of sterile carrageenan in male Wistar rats. Salbutamol (125, 250 and 500 µg/rat) and salbutamol (500 µg/rat) plus propranolol (100 μg/rat) were injected intrapouch. After 6 and 72 h, fluid inside the pouches was collected to measure volume of exudates, leukocytes number and IL-1β levels. To determine angiogenesis, the granulation tissues were dissected out and weighed 3 days after carrageenan injection, then hemoglobin concentration was assessed using a hemoglobin assay kit. In addition, amount of VEGF in the exudates was measured 72 h after induction of inflammation. Leukocyte accumulation and the volume of exudates were significantly inhibited by salbutamol administration. In addition, salbutamol decreased the production of VEGF and IL-1β. Moreover, all used doses of salbutamol significantly inhibited angiogenesis. Interestingly, effects of salbutamol on the attenuation of angiogenesis and inflammatory parameters was similar to diclofenac sodium. Co-administration of propranolol with salbutamol clearly reversed anti-inflammatory effects of salbutamol. Salbutamol can decrease acute and chronic inflammation by β2-adrenergic receptors activation. The observed IL-1β and VEGF inhibitory properties of salbutamol may be responsible for anti-inflammatory and anti-angiogenic effect of the agent.
Keywords: Salbutamol, Inflammation, Angiogenesis, Air pouch, Carrageenan, VEGF, IL-1β
INTRODUCTION
Numerous studies have indicated the existence of strong bidirectional communication between the immune system and the nervous system. Evidence confirms that catecholamines and their metabolites can regulate the immune response (1). Salbutamol is a well-known β2-adrenergic receptor agonist in the treatment of asthma (2). It selectively binds to and activates β2-receptors on the surface of many cells. Inhibitory effect of salbutamol on inflammatory processes is seen for CD4 cells, monocytes and macrophages. In addition, anti-inflammatory effects of β2-receptors on pulmonary inflammation models (3) support the role of receptors in inflammatory conditions (4).
Rheumatoid arthritis (RA) is a chronic autoimmune disease (5) that usually involves multiple joints and causes functional disability with serious pain (6). An animal model for RA, namely air pouch model has been extensively used to investigate on various types of inflammation (7), granulomatous inflammation, angiogenesis (8,9) and evaluation of anti-rheumatic and anti-inflammatory agents (10). Angiogenesis (formation of new blood vessels) is a normal and common physiologic process in the body (11,12). Despite its role in normal function of embryogenesis and menstrual cycle, angiogenesis also contributes in the pathogenesis of tumor growth, neovascular glaucoma and RA (12,13).
In the pathogenesis of RA, angiogenesis leads to leukocyte recruitment and inflammation in the synovium followed by endothelial proliferation and increase in the synovial blood vessels (13,14,15). Since angiogenesis is a key element in the development of cancer and inflammatory diseases, anti-angiogenic drugs may be important clues to control angiogenesis dependent diseases such as RA (16,17). With regard to the potential modulatory role of β2-adrenoceptors in inflammatory conditions (18,19) we hypothesized that β2-agonists (such as salbutamol) may produce anti-inflammatory and anti-angiogenic effects. Therefore, the aims of the current study were to investigate the anti-inflammatory and anti-angiogenic effects of salbutamol on the rat air pouch model of inflammation.
Production of interleukin-1 beta (IL-1β), as the main regulator of other inflammatory cytokines was determined. Its neutralization has proven to be a potent treatment for RA (20,21,22). In addition, vascular endothelial growth factor (VEGF) plays an important role for regulating angiogenesis. It seems that inhibition of VEGF activity is a logic strategy to suppress angiogenesis related diseases (23,24). So that, to validate angiogenesis results, VEGF levels were measured in this study as well.
MATERIALS AND METHODS
Chemicals
Following chemicals were obtained: salbutamol powder (Exir Pharmaceutical Company, Iran), propranolol (Hakim Pharmaceutical Company, Iran), carrageenan (Sigma Company, Germany), Ethylene-diaminetetraacetic acid (EDTA, Merck Company, Germany), hemoglobin kit and Drabkin reagent (ZistChem Diagnostics, Iran), VEGF kit (Biospes Company, China) and IL-1β kit (Glory Science Company, USA). All other chemicals were of highest grade commercially available.
Animals
Male Wistar rats (200-250 g, Pasture Institute, Iran) were housed in standard cages under a 12L:12D schedule with free access to food and water. All animal experiments were approved by the animal research Ethics Committee of Tabriz University of Medical Science (document ID: 8523) and performed in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals (revised 1985).
In the first set of experiments for assessment of acute inflammation, the rats were divided into 6 groups of 6 rats each including saline, vehicle-treated (control), salbutamol-treated by doses of 125, 250 and 500 µg/rat, and propranolol (100 µg/rat) plus salbutamol (500 µg/rat) treatment with intra-pouch administration.
After 6 h of carrageenan injection, the levels of inflammatory parameters were assayed.
In other set of experiments for evaluation of chronic inflammation, the rats were allocated into 9 groups of 6 rats each including saline, vehicle-treated (control), diclofenac sodium (1 mg/rat as positive control), salbutamol-treated by doses of 25, 125, 250 and 500 µg/rat, propranolol-treated (100 µg/rat), and propranolol (100 µg/rat) plus salbutamol (500 µg/rat) treatment with intra-pouch injection. After 72 h of carrageenan injection, the inflammatory markers were measured.
Except saline groups, all the animals received intrapouch administration of a phlogistic agent (1 mL of carrageenan 1% solution).
Stock solutions were diluted with saline and then 1 mL of the diluted solution was administered into the pouches of all rats just before carrageenan injection and then for 2 consecutive days.
Creation of air pouch in rats
The rats were anesthetized with isoflurane, shaved their dorsal cervical thoracic region, and then sterile air (20 and 10 mL) was subcutaneously injected (on day 1 and day 3, respectively).
On day 6, inflammation was induced by intrapouch injection of 1 mL of 1% solution of sterile carrageenan (25,26). Six and 72 h after carrageenan administration, the inflammatory parameters were measured.
Quantification of cell migration and exudation
Six and 72 h after carrageenan injection, the animals were sacrificed. The pouches were washed with 3 mL of phosphate-buffered saline (PBS), then opened and the exudates volumes were measured. In addition, samples from the exudate were collected to determine total leukocyte counts (26).
Determination of IL-1β and VEGF concentration
Six hours and 3 days after inflammation induction, the rats were sacrificed by isoflurane and their harvested exudates were centrifuged at 10,000 g for 10 min, then IL-1β and VEGF levels were assessed in supernatants using commercial ELISA kits according to the manufacturer's instructions.
Measurement of granulation tissue weight and angiogenesis
Inflammation stimulates cell proliferation, fibroblast and macrophage accumulation and collagen formation in the inner membrane of the pouch cavity named granulation tissue (8). The granulation tissue was dissected out and weighed 6 and 72 h after carrageenan injection. Determination of angiogenesis in granulation tissue was performed based on the methods described previously by Ghosh, et al. (27). Briefly, 3 days after carrageenan injection, the dissected granulation tissue was washed in PBS (pH = 7.4), homogenized in 3 mL Drabkin reagent on an ice bed by a homogenizer (HO4 AP-Edmund Bϋhler, B. Braun, Germany). The homogenate was centrifuged at 10,000 g for 30 min. Finally, concentration of hemoglobin in the supernatant was measured spectrophotometrically at 540 nm and expressed as mg hemoglobin/100 g wet tissue.
Statistical analysis
Data was expressed as mean ± SEM and analyzed by SPSS software (Ver. 17) for any statistical significance (P < 0.05) between groups using one way analysis of variance (ANOVA) with LSD post-test. Statistical analysis between saline and vehicle-treated (control) groups was performed by independent samples T-test.
RESULTS
Effects of salbutamol on inflammatory parameters 6 h after carrageenan injection
Table 1 shows the effects of intrapouch administration of carrageenan on inflammatory parameters. Carrageenan recovered the exudates after 6 h, also resulted in a significant increases in accumulation of leukocytes compared to that observed with saline alone (P < 0.001). In addition, carrageenan significantly increased granulation tissue formation compared to the control group (Table 1).
Table 1.
Effects of carrageenan on the inflammatory parameters 6 h after injection.
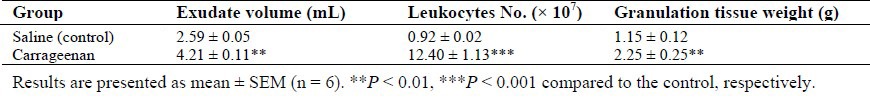
As shown in Fig. 1, intra-pouch administration of salbutamol (125 μg/rat) did not change white blood cell number (Fig. 1A), quantity of recovered exudates from the air-pouches (Fig. 1B), and the granulation tissue weight (Fig. 1C) from that of the control carrageenan group. However, salbutamol by doses of 250 and 500 μg/rat led to significant reductions in the number of leukocytes (87.16 × 106 and 42.44 × 106, respectively) compared to control value (124.08 × 106) (P < 0.01 and P < 0.001, respectively). Also, the quantity of recovered exudates and granulation tissue weight were decreased from that of the control carrageenan group by some doses of salbutamol. Moreover, IL-1β level was significantly reduced in salbutamol-treated groups (Fig. 1D). At the same time, anti-inflammatory effects of 500 μg/rat salbutamol (reduction of leukocytes number, exudates volume and granulation tissue weight) were significantly inhibited by co-administration of propranolol (P < 0.01).
Fig. 1.
Effects of salbutamol on (A) total number of leukocytes in the pouch fluid, (B) pouch fluid volume, (C) granulation tissue weight, and (D) IL-1β level 6 h after carrageenan injection. Results are presented as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control, respectively. Sal, salbutamol (μg/rat); Prop, propranolol (100 μg/rat).
Effects of salbutamol on inflammatory parameters 72 h after carrageenan injection
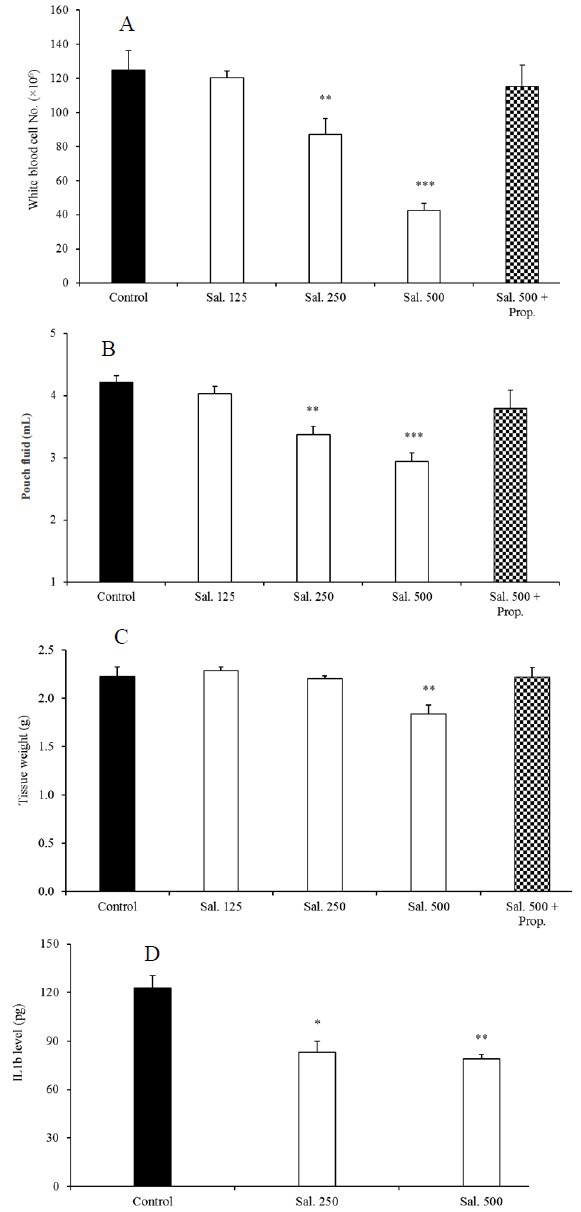
Table 2 shows significantly changes of inflammatory parameters between carrageenan and saline control groups 72 h after carrageenan injection. In addition, comparison between the effects of carrageenan on the inflammatory parameters 6 h and 72 h after its administration was summarized in Table 3. As shown in Fig. 2A, salbutamol lowered the total number of leukocytes from 377.3 × 106 in control to 110.4 × 106, 107.7 × 106, and 90.19 × 106 in groups treated with 125, 250 and 500 μg/rat of salbutamol, respectively. Volume of intrapouch exudate (Fig. 2B) was decreased by all doses of salbutamol (P < 0.001). Compared to the control group, granulation tissue weight was significantly reduced only by 500 µg/rat of salbutamol (Fig. 2C). Salbutamol (250 and 500 µg/rat) significantly decreased IL-1β level in the pouch fluid 72 h after inflammation induction (Fig. 2D).
Table 2.
Effects of carrageenan on the inflammatory parameters 72 h after injection.

Table 3.
Comparison between the effects of carrageenan on the inflammatory parameters 6 h and 72 h after injection.

Fig. 2.
Effects of salbutamol on (A) total number of leukocytes in the pouch fluid, (B) pouch fluid volume, (C) granulation tissue weight, and (D) IL-1β level 72 h after carrageenan injection. Results are presented as mean ± SEM (n = 6). **P < 0.01, ***P < 0.001 compared to the control, respectively. Sal, salbutamol (μg/rat); Prop, propranolol (100 μg/rat); Diclo, diclofenac sodium (1 mg/rat).
Interestingly, effects of salbutamol in attenuating of inflammatory parameters were similar to diclofenac sodium (Fig. 2). Administration of propranolol alone did not produce significant changes in inflammatory parameters in comparison with the control group. However, its administration with salbutamol (125, 250 and 500 µg/rat) clearly reversed anti-inflammatory effects of salbutamol in this study (P < 0.001).
Effects of salbutamol on VEGF level and angiogenesis
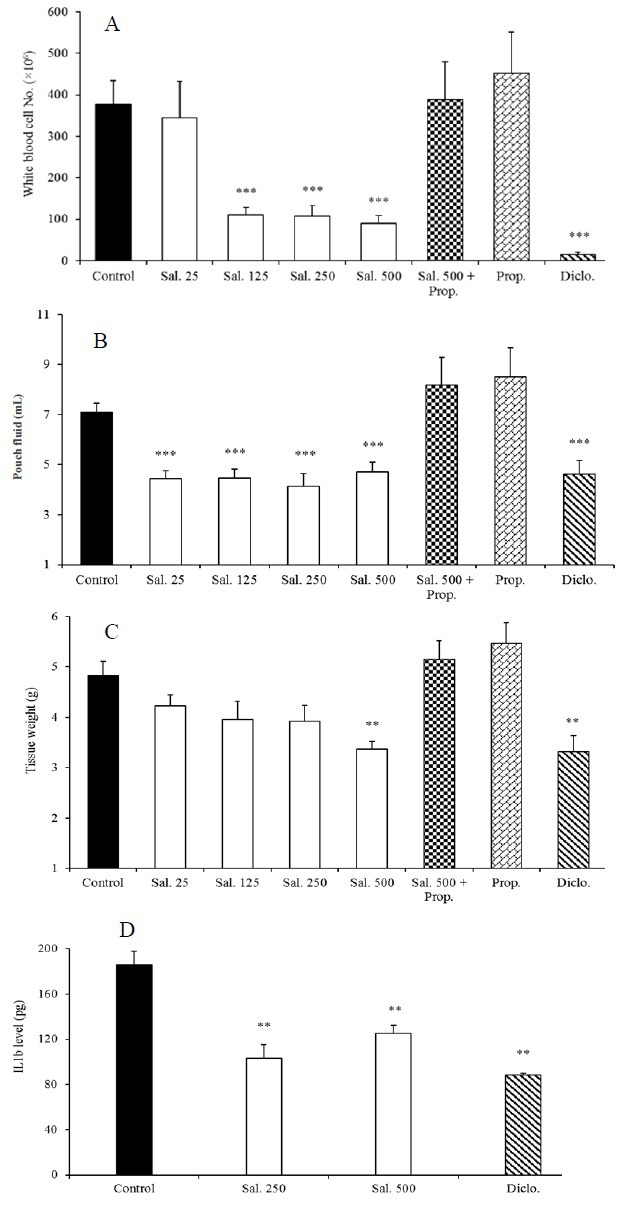
Determination of angiogenesis in granulation tissue was performed by measuring concentration of hemoglobin. In comparison with the control rats, a significant reduction (P < 0.001) in hemoglobin content was shown in salbutamol-treated groups (Fig. 3). In agreement with the above findings, a significant decrease in VEGF concentrations in response to salbutamol was also observed.
Fig. 3.
Effects of salbutamol on angiogenesis (based on the hemoglobin content) and VEGF level 3 days after carrageenan injection. Results are presented as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control, respectively. # Indicates significant change from the control; #P < 0.05 and ## P < 0.01, respectively. Sal, salbutamol (μg/rat); Prop, propranolol (100 μg/rat).
DISCUSSION
Neurotransmitters of sympathetic nervous system (mainly norepinephrine and epinephrine) communicate with many physiologic systems, including the immune system via adrenergic receptors to modulate production of regulatory cytokines (18,19). It seems that manipulation of the system may help to a better understanding its role in the immune response (28).
In the current study, salbutamol was used as a pharmacologic manipulator of adrenergic system and selective activator of β2-receptors and its effects against carrageenan-induced acute and chronic inflammation were investigated in air pouch model. Recruitment of leukocytes from the vessel to an area of inflammation is the host's initial defense system (29). Our results showed efficacy of salbutamol as an inhibitor of leukocyte accumulation during inflammation. Results of some previous works revealed that immune cells such as monocytes and lymphocytes expressed adrenergic receptors and their manipulations may exhibit anti-inflammatory effects (18,19). Catecholamines may act as regulators of lymphocyte trafficking. Maybe, this effect is related to catecholamine-induced changes in expression of adhesion molecules by immune cells or the endothelium (18,19). Although, β2-adrenergic receptor may have important functions in inflammatory conditions, however, the exact mechanisms by which the receptor might mediate leukocyte infiltration and accumulation is little known (30). Besides inhibitory effects of salbutamol on the accumulation of leukocytes, volume of exudate was affected by the agent in our study. It has become clear that salbutamol produces significant suppression in carrageenan-induced paw edema in rats (4). Consistent with these results, salbutamol both in acute and chronic inflammation decreased pouch fluid dose dependently.
In addition, for the first time it was found that salbutamol suppresses angiogenesis in the inflammatory granulation tissue. In this condition, IL-1β (an important angiogenic agent in inflammation) and VEGF might have pivotal role in neovascularization modulation by salbutamol. IL-1β modulates expression of proangiogenic and inflammatory molecules and produces a lot of cellular responses such as activation of leukocytes and endothelial cells (21,31). However, biological activity of IL-1β during angiogenesis especially in air-pouch model is not well known. Johnson, et al. suggested that production of IL-1β might be triggered by mediation of β-adrenergic receptor (32). The data obtained in this study showed that salbutamol decreased level of IL-1β in the inflammatory exudates. This result is in agreement with the results reported by Hallsworth, et al. in human airway smooth muscle cells (33). Also, in agreement with our results, Walker, et al. reported that IL-1β in Müller cells cultured was lowered by β-adrenoceptors stimulation with isoproterenol (2).
The process of angiogenesis (either physiologic or pathologic) is very complex and involves the interactions of several cell types and mediators (3,34). In synovial inflammation, angiogenesis and endothelial proliferation occur and control of neovascularization attenuates synovitis in RA (15,35). Similar to our results, Uzkeser, et al. reported that salbutamol dose dependently reduced the weight of granulomatous tissue in a chronic inflammation model (4). Our data revealed that salbutamol can reduce angiogenesis in the granulomatous tissue. Some researchers indicated that isoproterenol (a β-adrenoceptors agonist) could not decrease aortic microvessel sprouting, while propranolol caused increase in VEGF-mediated microvessel sprouting (36). In response to pro-inflammatory cytokines, VEGF is produced within the synovium and induces neovascularization, cell proliferation, vascular permeability and stimulates leukocyte extravasation (35).
Here, we also introduced a new function for salbutamol as a potent inhibitor of VEGF-mediated angiogenesis. Reduction in VEGF concentration was correlated with anti-angiogenic activity of salbutamol. These data are consistent with the results of Lu, et al. showing that inhibition of VEGF results in decreasing of disease activity in RA animal models (37).
Although salbutamol and diclofenac sodium belong to different pharmacological groups, interestingly anti-inflammatory effects of salbutamol were similar to diclofenac sodium on the main inflammatory parameters. At the same time, co-administration of a β-adrenoceptor antagonist (propranolol) reversed anti-inflammatory effects of salbutamol indicating that β2-receptors may play pivotal role in this condition.
CONCLUSION
Regarding the results of this work, it may be concluded that salbutamol by stimulating β2-adreoceptors suppresses production of pro-inflammatory mediators, and then produces potent anti-inflammatory activity in both acute and chronic inflammation. Salbutamol effectively reduces the production of VEGF and IL-1β corresponds to a reduction in the angiogenesis, so that it may be considered as an angiogenesis regulator with potential therapeutic uses in inflammatory diseases. Co-administration of propranolol with salbutamol clearly blocked these effects indicating the important role of β-receptor activation in anti-inflammatory and anti-angiogenesis effect of salbutamol. Further studies need to understand the precise mechanism of action of salbutamol in such conditions.
ACKNOWLEDGMENTS
This study was supported by Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran. The authors would like to thank Drug Applied Research Center, Tabriz University of Medical Sciences for providing laboratory facilities and supporting this work. Salbutamol was gifted by Exir Pharmaceutical Company, Iran.
REFERENCES
- 1.Serafeim A, Gordon J. The immune system gets nervous. Curr Opin Pharmacol. 2001;1(4):398–403. doi: 10.1016/s1471-4892(01)00069-8. [DOI] [PubMed] [Google Scholar]
- 2.Prenner BM. Role of long-acting beta2-adrenergic agonists in asthma management based on updated asthma guidelines. Curr Opin Pulm Med. 2008;14(1):57–63. doi: 10.1097/MCP.0b013e3282f27121. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Fievez L, Cheu E, Bureau F, Rong W, Zhang F, et al. Anti-inflammatory effects of formoterol and ipratropium bromide against acute cadmium-induced pulmonary inflammation in rats. Eur J Pharmacol. 2010;628(1-3):171–178. doi: 10.1016/j.ejphar.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Uzkeser H, Cadirci E, Halici Z, Odabasoglu F, Polat B, Yuksel TN, et al. Anti-inflammatory and antinociceptive effects of salbutamol on acute and chronic models of inflammation in rats: involvement of an antioxidant mechanism. Mediators Inflamm. 2012;2012:438912. doi: 10.1155/2012/438912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22(1):1–12. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- 6.Dalmarco EM, Astolfi G, de Liz R, de Córdova CM, Fröde TS. Modulatory effect of mycophenolate mofetil on carrageenan-induced inflammation in the mouse air pouch model. Int Immunopharmacol. 2012;13(4):476–482. doi: 10.1016/j.intimp.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Martin SW, Stevens AJ, Brennan BS, Davies D, Rowland M, Houston JB. The six-day-old rat air pouch model of inflammation: Characterization of the inflammatory response to carrageenan. J Pharmacol Toxicol Methods. 1994;32(3):139–147. doi: 10.1016/1056-8719(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 8.Edwards JC, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981;134(2):147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- 9.Sato K, Komatsu N, Higashi N, Imai Y, Irimura T. Granulation tissue formation by nonspecific inflammatory agent occurs independently of macrophage galactose-type C-type lectin-1. Clin Immunol. 2005;115:47–50. doi: 10.1016/j.clim.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Colville-Nash P, Lawrence T. Air-pouch models of inflammation and modifications for the study of granuloma-mediated cartilage degradation. Methods Mol Biol. 2003;225:181–189. doi: 10.1385/1-59259-374-7:181. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 12.Colville-Nash PR, Scott DL. Angiogenesis and rheumatoid arthritis: pathogenic and therapeutic implications. Ann Rheum Dis. 1992;51(7):919–925. doi: 10.1136/ard.51.7.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paleolog EM. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002;4:S81–S90. doi: 10.1186/ar575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clavel G, Bessis N, Boissier MC. Recent data on the role for angiogenesis in rheumatoid arthritis. Joint Bone Spine. 2003;70(5):321–326. doi: 10.1016/s1297-319x(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 15.Clavel G, Valvason C, Yamaoka K, Lemeiter D, Laroche L, Boissier MC, et al. Relationship between angiogenesis and inflammation in experimental arthritis. Eur Cytokine Netw. 2006;17(3):202–210. [PubMed] [Google Scholar]
- 16.Makhni E. Angiogenesis: An Examination of both Tumorigenic and Rehabilitative Properties. MIT Undergrad Res J. 2003;8:23–26. [Google Scholar]
- 17.Kubota Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J Med. 2012;61(2):47–56. doi: 10.2302/kjm.61.47. [DOI] [PubMed] [Google Scholar]
- 18.Kavelaars A. Regulated expression of alpha-1 adrenergic receptors in the immune system. Brain Behav Immun. 2002;16(6):799–807. doi: 10.1016/s0889-1591(02)00033-8. [DOI] [PubMed] [Google Scholar]
- 19.Lorton D, Lubahn C, Bellinger DL. Potential use of drugs that target neural-immune pathways in the treatment of rheumatoid arthritis and other autoimmune diseases. Curr Drug Targets Inflamm Allergy. 2003;2(1):1–30. doi: 10.2174/1568010033344499. [DOI] [PubMed] [Google Scholar]
- 20.Lane T, Lachmann HJ. The emerging role of interleukin-1beta in autoinflammatory diseases. Curr Allergy Asthma Rep. 2011;11(5):361–368. doi: 10.1007/s11882-011-0207-6. [DOI] [PubMed] [Google Scholar]
- 21.Dinarello CA. Interleukin-1β and the Autoinflammatory Diseases. New Engl J Med. 2009;360(23):2467–2470. doi: 10.1056/NEJMe0811014. [DOI] [PubMed] [Google Scholar]
- 22.Jesus AA, Goldbach-Mansky R. IL-1 blockade in autoinflammatory syndromes. Annu Rev Med. 2014;65:223–244. doi: 10.1146/annurev-med-061512-150641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appleton I, Brown NJ, Willis D, Colville-Nash PR, Alam C, Brown JR, et al. The role of vascular endothelial growth factor in a murine chronic granulomatous tissue air pouch model of angiogenesis. J Pathol. 1996;180(1):90–94. doi: 10.1002/(SICI)1096-9896(199609)180:1<90::AID-PATH615>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106(4):148–156. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- 25.Sedgwick AD, Sin YM, Edwards JC, Willoughby DA. Increased inflammatory reactivity in newly formed lining tissue. J Pathol. 1983;141(4):483–495. doi: 10.1002/path.1711410406. [DOI] [PubMed] [Google Scholar]
- 26.Duarte DB, Vasko MR, Fehrenbacher JC. Models of inflammation: carrageenan air pouch. Curr Protoc Pharmacol. 2012 doi: 10.1002/0471141755.ph0506s56. Chapter 5: Unit.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ajoy Kumar Ghosh A, Hirasawa N, Ohtsu H, Watanabe T, Ohuchi K. Defective angiogenesis in the inflammatory granulation tissue in histidine decarboxylase-deficient mice but not in mast cell-deficient mice. J Exp Med. 2002;195(8):973–982. doi: 10.1084/jem.20011782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Rey A, Besedovsky HO. Sympathetic nervous system-immune interactions in autoimmune lymphoproliferative diseases. Neuroimmuno-modulation. 2008;15(1):29–36. doi: 10.1159/000135621. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin. 2012;33(3):342–350. doi: 10.1038/aps.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voronov E, Carmi Y, Apte RN. Role of IL-1-mediated inflammation in tumor angiogenesis. Adv Exp Med Biol. 2007;601:265–270. doi: 10.1007/978-0-387-72005-0_28. [DOI] [PubMed] [Google Scholar]
- 32.Johnson JD, Cortez V, Kennedy SL, Foley TE, Hanson H, Fleshner M. Role of central beta-adrenergic receptors in regulating proinflammatory cytokine responses to a peripheral bacterial challenge. Brain Behav Immun. 2008;22(7):1078–1086. doi: 10.1016/j.bbi.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallsworth MP, Twort CH, Lee TH, Hirst SJ. Beta(2)-adrenoceptor agonists inhibit release of eosinophil-activating cytokines from human airway smooth muscle cells. Br J Pharmacol. 2001;132(3):729–741. doi: 10.1038/sj.bjp.0703866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005;4(1):3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- 35.Szekanecz Z, Besenyei T, Paragh G, Koch AE. New insights in synovial angiogenesis. Joint Bone Spine. 2010;77(1):13–19. doi: 10.1016/j.jbspin.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stati T, Musumeci M, Maccari S, Massimi A, Corritore E, Strimpakos G, et al. beta-blockers promote angiogenesis in the mouse aortic ring assay. J Cardiovasc Pharmacol. 2014;64(1):21–27. doi: 10.1097/FJC.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Kasama T, Kobayashi K, Yoda Y, Shiozawa F, Hanyuda M, et al. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol. 2000;164(11):5922–5927. doi: 10.4049/jimmunol.164.11.5922. [DOI] [PubMed] [Google Scholar]


