Abstract
In recent years, the use of the antioxidant in reducing heavy metal toxicities has increased worldwide. Curcumin has been reported to have a strong antioxidant activity. In this study, we investigated the protective effects of curcumin on lead acetate-induced testicular damage in rats. The sample used 40 male rats divided into 5 groups: negative control (rats were given daily with corn oil); positive control (rats were given daily with lead acetate 50 mg/kg BW orally once in a day for 35 days); and the treatment group (rats were given the curcumin 100 mg, 200 mg, and 400 mg/kg BW orally once in a day for 40 days, and on the 5th day, were given lead acetate 50 mg/kg BW one h after the curcumin administration). After 40 days, levels of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in testicular tissue, and sperm count, motility and viability in the epididymis were measured in rats. Testis samples were also collected for histopathological studies. Results showed that lead acetate administration significantly decreased the SOD, GPx, and increased MDA levels. Lead acetate also decreased the sperm count, motility, viability, and altered histopathological testis (testicular damage, necrosis of seminiferous tubules and loss of spermatid) compared to the negative control. However, administration of curcumin significantly improved the histopathological in testis, increased the sperm count, motility, viability, and also significantly increased the SOD, GPx, and decreased MDA in testis of lead acetate-treated rats. From the results of this study we concluded that the curcumin could be a potent natural product provide a promising protective effect against lead acetate induced testicular toxicity in rats.
Keywords: Curcumin, Antioxidant, Lead acetate, Testis
INTRODUCTION
Lead acetate is a biotoxic environmental and industrial pollutant, which accumulates in almost all the body tissues such as the liver, lung, bones, kidneys, reproductive organs and immune system (1). It has been reported the physiological, biochemical, and behavioral effects of this toxic lead in animal, including disorders of central and peripheral nervous systems (2), cardiovascular system (3), kidney (4), liver (5) and reproductive system (6).
The mechanism of lead-induced testicular toxicity is the oxidative stress and it develops when there is an imbalance between the free radicals and the scavenging capacity of antioxidants in the testis (7). A recent study confirmed that lead-induced testicular toxicity, the possible involvement of reactive oxygen species (ROS) or free radicals such as superoxide ion (O2-), hydroxyl radical (OH-) and nitric oxide (NO) (8). It was reported that lead increased the level of generation of ROS, lipid peroxidation, and inhibited the activity of antioxidants such as glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (4,6). Malondialdehyde (MDA) is secondary products of lipid peroxidation, may be used as an indicator of cell membrane injury. The increase in MDA levels in testicular toxicity suggests enhanced lipid peroxidation leading to tissue damage and decreased the activity of antioxidant defense mechanisms to prevent the formation of excessive free radicals (7,9).
The increasing concentration of MDA is an evidence tissue damage of lead toxicity processes caused by increased free radicals (10).
It has been shown that antioxidants can prevent or reduce the oxidation of other molecules by ROS in a tissue or cell organisms, scavenge free radicals and attenuate their deleterious effects (11,12). Antioxidant activity or inhibition of generation of free radicals plays a crucial role in protection against such testicular toxicity. So, it has been claimed that protective agents against free radicals, such as antioxidants, may be a useful therapeutic for heavy metal toxicity in testis.
It has been reported that the natural products or medicinal plants have antioxidant properties in reducing free radical-induced tissue damage. Medicinal plants have advantages over the conventionally used drugs that are much expensive and known to have harmful side effects for the treatment of various disease (13). Many authors tried active compound of medicinal plant like quercetine (14), cathecin (15), allicin (16) against lead toxicity.
Recently, it has been focused on the protective effect of plant products or medicinal plants having antioxidant properties such as curcumin for therapeutic in reducing free radical-induced tissue damage (13,17). It is a constituent of rhizomes of the plant Curcuma which is a member of the family Zingiberaceae. Curcumin has pharmacological effects such as antibacterial, antiviral, antifungal (18), anticancer (19), anti-inflammatory (20) and antioxidant (13). The present study aimed to investigate the protective effect of curcumin against lead acetate induced testicular damage in male albino rats.
MATERIALS AND METHODS
Chemicals
Lead acetate and curcumin were purchased from Sigma-Aldrich (USA;Cat. No 6080-56-4 and 458-37-7, respectively). MDA in tissues was obtained from NWLSS (USA, Cat. No. NWK-MDA01). Determination of tissue levels of SOD and GPx activities was performed using assay kits from Cayman Chemicals (USA, Cat. No. 706002 and 703102, respectively).
Experimental animals
Male rat (Wistar rat) weighing approximately 200-250 g (2.5-3 months) were obtained from Gadjah Mada University, Yogyakarta, Indonesia for experimental purpose. They were housed in plastic cages in an air-conditioned room with a temperature maintained at 26 ± 2°C and 12 h alternates light and dark cycles. The rats were given ad libitum with tap water and fed with standard commercial rat chow. This study was reviewed by the Ethical Clearance Committee for preclinical research, Institute of Tropical Disease, Airlangga University and obtained ethical clearance under No.183/ITD/12/2015.
Experimental design
Forty male Wistar rats were treated once daily by oral gavage for 40 days and divided randomly into five groups as the following: negative control group (rats were given daily with corn oil); positive control group (rats were given daily with corn oil and lead acetate 50 mg/kg BW (7,21) orally once in a day for 35 days) and the treatment group (rats were given the curcumin dissolved in corn oil in dose of 100 mg, 200 mg, and 400 mg/kg BW orally by gastric gavages (17,22) once in a day for 40 days and lead acetate 50 mg/kg BW were given on 5th day, one hour after the curcumin administration for 35 days). On day 40, all of the rats were sacrificed after anesthetization by diethyl ether inhalation, then their testis and epididymis were excised. The sperm count, motility, viability was assessed from cauda epididymis. Tissues of testis were homogenized in ice-cold 50 mM sodium phosphate buffer (pH 7.4) containing 0.1 mM ethylene diamine tetraacetic acid (EDTA). The supernatant was separated by centrifugation at 1000 g for 20 min at 4°C. The supernatant was used for the analyzes of MDA and antioxidant enzymes (SOD and GPx). The testis was also fixed in a 10% neutral buffered formalin solution for histopathological examination
Sperm count
The new improved Neuber's counting chamber (hemocytometer) was used in counting the total number of spermatozoa. About 10 mL of the diluted sperm suspension was transferred to each counting chamber of the hemocytometer and was allowed to stand for 5 min, and thereafter observed under a binocular light microscope (23).
Sperm motility
Ten mL of sperm suspension was assessed by counting all progressive motile, non-progressive motile and immotile spermatozoa. The motility was determined by visual estimation (using microscope at a magnification of 400×) of the proportion of spermatozoa moving forward (motile) and those that didn't move were considered nonmotile. The percentage of motile spermatozoa were thereafter determined (23).
Sperm viability
Sperm viability was investigated using the eosin stain. The staining was conducted with one drop of freshly collected semen and two drops of eosin solution. Quantitative viability expressed as a percentage was determined by counting viable and nonviable spermatozoa per chamber. Viable spermatozoa cannot absorb eosin stain while nonviable spermatozoa can absorb the stain. The dye exclusion was evaluated in 100 spermatozoa. Sperm viability was defined as the percentage of dead sperm cells (23).
Measurement of MDA level
MDA was determined in the supernatant of homogenate testicular tissue by the thiobarbituric acid (TBA) method which estimates the MDA formation. The concentration of MDA was measured at 532 nm and calculated by the absorbance coefficient of MDA-TBA complex (24). MDA is expressed as nanomoles MDA/g tissue.
Measurement of antioxidant enzymes
Tissue preparation for enzyme assay of rat testes were rapidly thawed from -70°C at room temperature for 5 min and manually homogenised in cold phosphate buffer (pH 7.4) and debris removed by centrifugation at 3500 g for 10 min (Centrifuge 5415 R, Eppendorf AG, Hamburg, Germany). Supernatants were recovered and used for enzyme activity and protein assays.
The activity of SOD was measured with SOD detection kit according to the manufacturer's instructions. The SOD activity is then evaluated by the degree of inhibition of this reaction. The level of SOD was measured at 505 nm and through a standard curve and expressed as U/mg protein (25).
The activity of GPx was measured with GPx detection kit according to the manufacturer's instructions. The GPx was evaluated spectrophotometrically against blank at 340 nm. GPx 1 unit was l mol of oxidized NADPH per min per mg of tissue protein. The GPx activity was expressed as U/mg protein (26).
Histopathological examination
The tissue of testis was fixed in a 10% neutral buffered formalin solution, embedded in paraffin and used for histopathological examination with hematoxylin and eosin (H&E) stain (27).
Statistical analysis
Data were presented as means ± standard deviation. One-way ANOVA has carried post hoc test, and the statistical comparisons among the groups were performed with an LSD test using a statistical package program (SPSS V. 17.0).
RESULTS
Effects of curcumin on lead acetate-induced changes in semen analysis
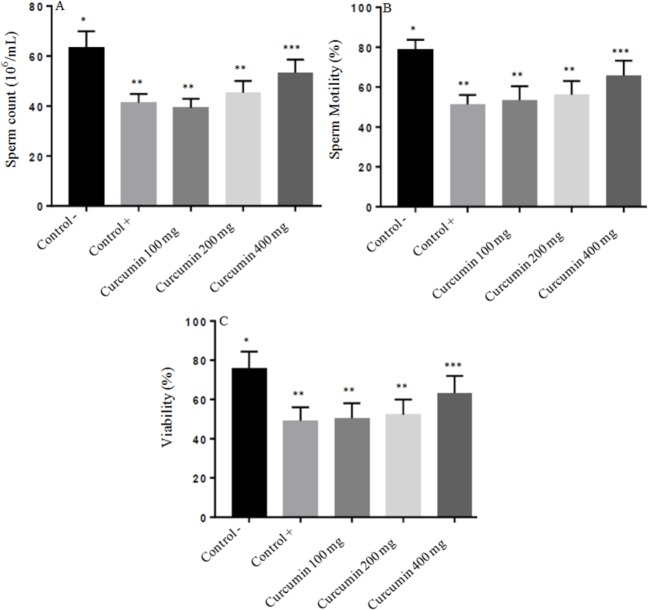
Fig. 1A showed the results of epididymal spermatozoa count in all groups. The epididymal spermatozoa count were 63.44 ± 5.27, 41.13 ± 4.69, 39.20 ± 4.85, 45.23 ± 5.89, 53.62 ± 5.61 × 106/mL in negative control, positive control, treatment curcumin at dose 100 mg/Kg BW, 200 mg/Kg BW and dose 400 mg/Kg BW, respectively. Lead acetate exposure of male rats induced a significant decrease of epididymal spermatozoa count (P < 0.05) when compared with the negative control. However, male rats treated with curcumin only at dose 400 mg/kg BW but not at dose 100 mg/Kg and 200 mg/kg BW showed a significant increase (P < 0.05) in epididymal spermatozoa count as compared to the positive control rats.
Fig. 1.
The protective effect of curcumin on lead acetate-induced testicular toxicity in: (A) sperm Count; (B) sperm motility; and (C) sperm viability. Asterisk (*) within a bar graph indicates significant difference between the means (P < 0.05). Each point represents the mean of eight experiments.
The results of sperm motility in this experiment were 78.53 ± 6.75%, 51.24 ± 5.63%, 53.30 ± 6.31%, 56.20 ± 5.15%, 65.80 ± 6.23% in negative control, positive control, treatment curcumin at dose 100 mg/Kg BW, 200 mg/Kg BW, and 400 mg/Kg BW, respectively. In the positive control (lead acetate treatment) group, the sperm motility was significantly decreased when compared with negative control group (P < 0.05). Treatment with curcumin at dose 400 mg/Kg BW but not at dose 100 mg/Kg and 200 mg/kg BW markedly enhanced sperm motility in lead acetate treatment which was significantly different from the positive control (P < 0.05) (Fig. 1B).
Fig. 1C and Fig. 2 show the results of sperm viability in all groups. The epididymal spermatozoa count was 75.91 ± 5.66%, 49.04 ± 4.97%, 50.25 ± 6.10%, 52.94 ± 5.92%, 63.02 ± 6.82% in negative control, positive control, treatment curcumin at dose 100 mg/Kg BW, 200 mg/Kg BW, and 400 mg/Kg BW, respectively. Lead acetate exposure of male rats induced a significant increase of sperm viability (P < 0.05) when compared with the negative control. However, male rats treated with curcumin only at dose 400 mg/kg BW but not at dose 100 mg/Kg and 200 mg/kg BW showed a significant increase (P < 0.05) in sperm viability as compared to positive control rats.
Fig. 2.
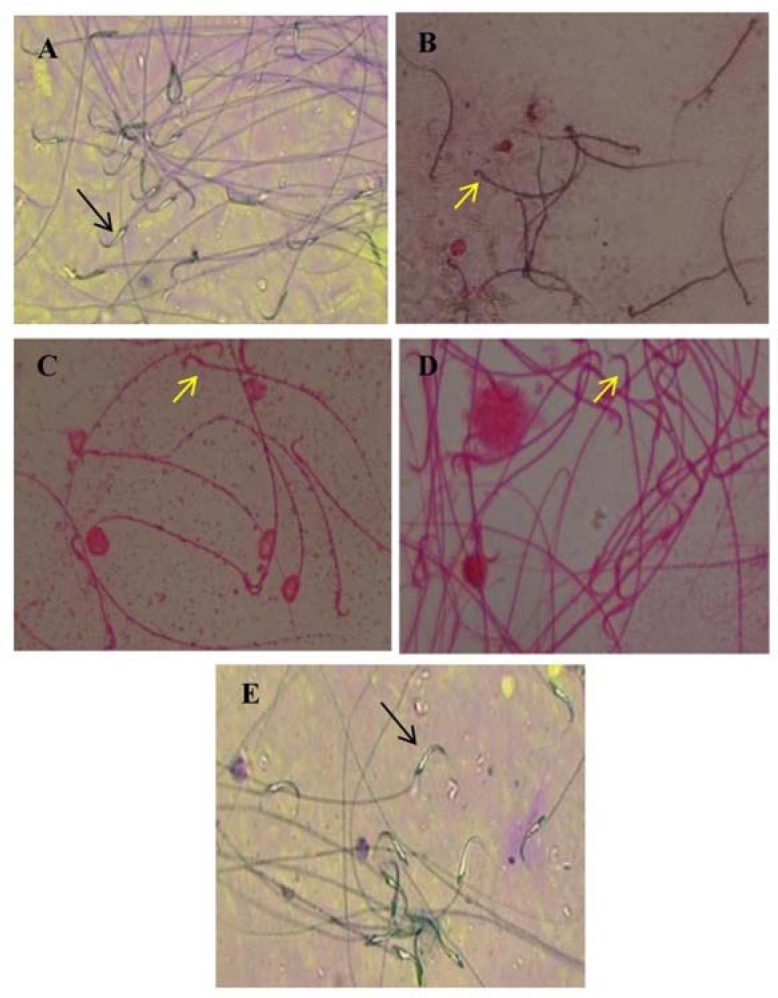
The protective effect of curcumin on lead acetate-induced testicular toxicity in sperm viability. Sperm viability (black arrow) or nonviability (yellow arrow).
Effects of curcumin on lead acetate-induced changes in MDA, SOD, and GPx in testicular tissue
The levels of MDA in the tissue homogenates of testes was 21.83 ± 2.86, 32.50 ± 1.87, 33.50 ± 2.89, 26.83 ± 3.31, 25.50 ± 3.08 nmol/mg protein in negative control, positive control, treatment curcumin at dose 100 mg/Kg BW, 200 mg/Kg BW, and dose 400 mg/Kg BW, respectively. In the positive control (lead acetate treatment) group, the level of MDA was significantly increased compared to the negative control group (P < 0.05).
Treatment with curcumin at dose 400 mg/Kg BW but not at dose 100 mg/Kg and 200 mg/kg BW markedly reduced testis MDA in lead acetate treatment which was significantly different from the positive control (P < 0.05) (Fig. 3A).
Fig. 3.
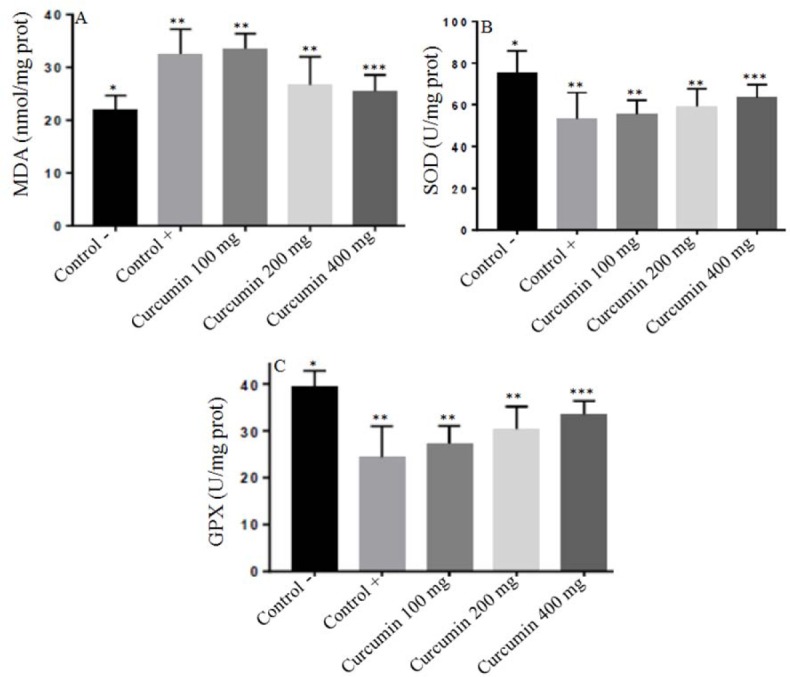
The protective effect of curcumin on lead acetate-induced testicular toxicity in: (A) MDA levels; (B) SOD levels; and (C) GPx levels. Asterisk (*) within a bar graph indicates significant difference between the means (P < 0.05). Each point represents the mean of eight experiments.
Fig. 3B showed the results of the level of SOD changes in all groups. The level of SOD in the tissues of the testes was 75.53 ± 7.75, 53.24 ± 8.63, 55.30 ± 6.31, 59.20 ± 7.15, 63.80 ± 5.15 U/mg protein in negative control, positive control, treatment curcumin at dose 100 mg/Kg BW, 200 mg/Kg BW and dose 400 mg/Kg BW, respectively. The administration of lead acetate significantly decreased testis SOD levels (P < 0.05) in rats compared with the negative control. Treatment with curcumin at dose 400 mg/Kg BW but not at dose 100 mg/Kg and 200 mg/kg BW markedly enhanced testis SOD in lead acetate treatment which was significantly different from the positive control (P < 0.05).
The levels of GPx in the tissue homogenates of testes was 39.97 ± 4.65, 24.04 ± 4.97, 27.74 ± 5.10, 30.94 ± 3.92, 33.02 ± 3.81 U/mg protein in negative control, positive control, treatment curcumin at dose 100 mg/Kg BW, 200 mg/Kg BW, and 400 mg/Kg BW, respectively. In the positive control (lead acetate treatment) group, the level of GPx was significantly decreased compared to the negative control group (P < 0.05). Treatment with curcumin at dose 400 mg/Kg BW but not at dose 100 mg/Kg and 200 mg/kg BW markedly enhanced testis GPx in lead acetate treatment which was significantly different from the positive control (P < 0.05) (Fig. 3C).
Effects of curcumin on lead acetate induce testicular damage
Histological observations on the negative control showed that in each seminiferous tubule, spermatids, spermatocytes, and spermatozoa are observable and they appear normal. In the positive control, rats were administered only with lead acetate showed testicular damage, necrosis of seminiferous tubules and loss of spermatid. Lead acetate thus had a deleterious effect on testicular tissues. In the rats, treated with curcumin, the number and morphological integrity of seminiferous tubule are being preserved. Observations indicate that the testicular toxic effects lead acetate was reduced by curcumin (Fig. 4).
Fig. 4.
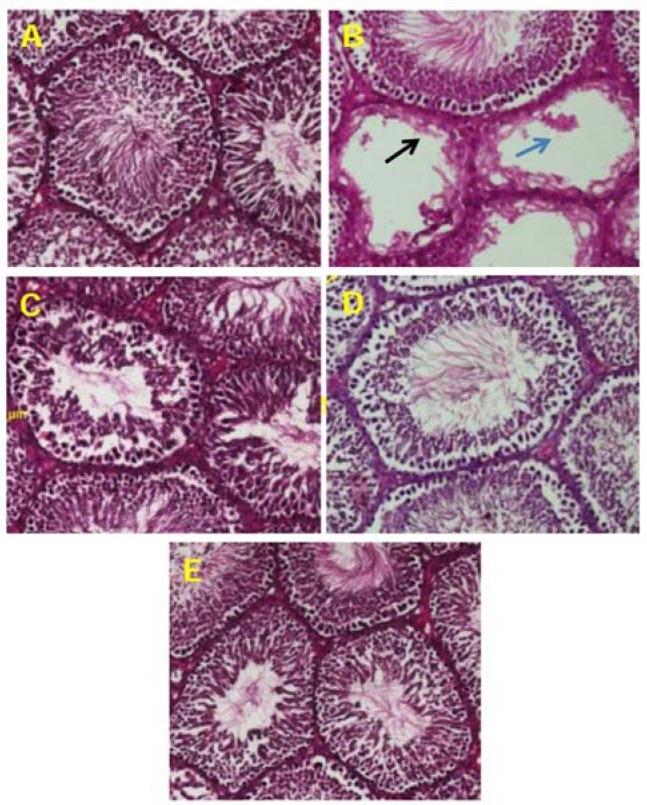
(A) normal testis sections in negative control group; (B) histopathological view of testis sections in lead acetate treated group shown seminiferous tubules such as the endothelial cells appear destroyed and or dispersed, and loss of spermatid (indicated by arrows) as compared to the negative control group; (C and D) rats treated with curcumin 100 mg/kg BW and 200 mg/kg BW showed like a positive control; (E) rats treated with curcumin 400 mg/kg showed regeneration in testicular cells, using haematoxyline and eosin stain technique (×400).
DISCUSSION
Lead acetate may induce oxidative stress leading to the generation of free radicals and alteration in oxygen free radical scavenging enzyme system or antioxidant and damage membrane structure (28). In the current study, we evaluated the protective role of curcumin against the oxidative stress changes in the testicular tissue resulting from the administration of lead acetate in rats. The biochemical mechanisms involved in the testis toxicity of lead acetate were studied by measuring the levels of MDA and by screening the activities of primary antioxidant enzymes such as SOD and GPx. It also testicle tissue samples was investigated for histopathological studies.
These results showed that lead acetate administration significantly decreased the SOD, GPx and increased MDA levels. Lead acetate also decreased the sperm count, motility, viability, and altered histopathological testis (testicular damage, necrosis of seminiferous tubules and loss of spermatid) compared to the negative control group. Lead-induced testicular damages have been attributed, at least in part, to toxicant-induced oxidative stress. It results suggest that lead stimulates the formation of ROS, thus causing oxidative damage to various tissues resulting in loss of membrane functions. Long-term exposure to lead increases MDA or lipid peroxidation and causes inhibition of SOD activity inducing oxidative damage in testes (27,29). The various toxic effects induced by lead in biological systems have been linked to increased MDA or lipid peroxidation, as an early and sensitive consequences of lead exposure. Lead acetate toxicity leads to the generation of free radical damage by two separate pathways, including hydroperoxides, singlet oxygen, and hydrogen peroxides, evaluated by MDA levels as the final products of lipid peroxidation, and the direct depletion of antioxidant reserves (1). The present investigation resulted in significantly increased of MDA levels in the testis of lead acetate-treated mice in comparison to the negative control. This means that it increased the oxidative stress in the lead acetate-treated mice. Therefore, the significantly lower levels of MDA in the tissues of curcumin treated groups as compared with the lead acetate group indicate attenuation of lipid peroxidation. It is known that lead acetate-induced oxidative stress and tissue damage could be caused by two mechanisms including increased generation of ROS and by causing direct depletion of antioxidant reserves (30). Intense lipid peroxidation caused by lead exposure may affect the mitochondrial and cytoplasmic membranes, causing more severe oxidative damage in the tissues and consequently releasing lipid hydroperoxides into circulation which reflects the induction of oxidative stress (31). The curcumin, which behaves as a powerful antioxidant and free radical scavenger, can decrease the MDA level perturbed by lead acetate in rats testis, as observed in this study. Treatment of rats with curcumin at a dose of 400 mg/kg BW prevented the levels of MDA to rise when the rats were challenged with lead acetate. This means that curcumin minimized the toxic effect of lead acetate via its antioxidant activity. The antioxidant protective mechanism decreases the oxidative stress and scavenges the free radical responsible for the testis damage and thus inhibit the lipid peroxidation as measured by MDAlevels. The findings of this study suggest that curcumin could attenuate oxidative stress by decreasing the lipid peroxidation (MDA level) in the lead-treated testis.
A similar result has shown that vitamin C and vitamin E enhanced the antioxidant status and inhibited lipid peroxidation in rats with lead acetate induced testis injury. These findings indicate that the antioxidant activity of vitamin C and vitamin E are targeted primarily towards the lipid component of cells. Antioxidants such as vitamin C and vitamin E have been reported to inhibit free radical formation and are effective in minimizing lipid peroxidation in several different biological systems (32,33).
SOD and GPx are important antioxidant enzymes. They constitute a mutually supportive defense mechanism against free radical. SOD decomposes superoxide radicals (O2-) to produce H2O2. GPx is a selenoenzyme which has played a major role in the decrease of H2O2 and hydroperoxide to produce nontoxic products. Therefore, the activities of SOD and GPx have been used to assess oxidative stress in cells (4,14,31). Many studies have shown that lead acetate has a high affinity for SH groups in several enzymes such as SOD and GPx, thus it can alter antioxidant activities by inhibiting functional SH groups in these enzymes (1). In the present study, the activity of SOD and GPx in mouse testis was decreased by lead acetate treatment. This decreased SOD and GPx activities with lead acetate treatment is in agreement with previous studies. This suggested that lead acetate exposure induced oxidative stress by inhibiting the activity of this antioxidant enzyme. Interestingly, the administration of curcumin increased the activities of SOD and GPx in the testis of lead-treated rats, which might be due to the ability of curcumin to reduce the accumulation of free radicals. Curcumin acts as a scavenger for the oxygen-derived free radicals, thus protecting from testis damage (34).
The decrease in lipid peroxidation due to curcumin has been attributed to alterations in the antioxidant defense system which includes enzymes such as glutathione-S-transferase, catalase (CAT), SOD, GPx, and nonenzymatic molecule such as glutathione, which normally protect against free radical toxicity. The primary mechanism of action of curcumin may involve the scavenging of free radicals which can inhibit free radical formation (12,17). It has been found a decrease MDA levels and an increase in the antioxidant enzyme parameters including SOD, CAT, and GPx in the plasma and tissue such as liver, kidney, and brain of animals that were administered curcumin in association with heavy metal, in comparison to the group that was administered heavy metal alone (4,35).
Histopathological results demonstrating structural changes in testis tissue of heavy metal toxicity such as lead acetate were reported by some researchers. In the present study, histopathological view of testis sections in the lead acetate treated group showed the testicular damage, necrosis of seminiferous tubules, and loss of spermatid, as compared to the negative control group. The testicular damage, necrosis of seminiferous tubules and loss of spermatid were considerably mild in the groups treated with curcumin 400 mg/kg.
In summary, our data indicate that lead acetate-induced testicular toxicity might be related to oxidative damage. Co-administration of curcumin lessened the effects of lead acetate-induced testicular toxicity possibly by inhibiting free radical-mediated process. Further investigation of these promising protective effects of curcumin against lead acetate-induced testicular damage may have a considerable impact on developing clinically feasible strategies to treat patients with lead acetate-induced testicular failure.
The therapeutic potential of curcumin has been evaluated against several environmental toxicants. Singh, et al. (34) studied the protective effect of curcumin against the reproductive toxicity of cadmium chloride. They found that the presence of curcumin with cadmium chloride showed protective effects against its reproductive toxicity and this may be due to the activity of curcumin as antioxidant. Chandra, et al. (30) reported that the use of curcumin attenuated the damaged effects of chromium on the reproduction of male rats, improved its spermatogenic damage, decreased sperm count, testosterone level, and induced antioxidant defense.
CONCLUSION
The present results showed that lead acetate significantly decreased the sperm count, motility, and viability. Treatment with curcumin significantly enhanced sperm count, motility, and viability. The lead acetate treatment also significantly increased the MDA and decreased the antioxidant enzymes (SOD and GPx) in testis. The inhibition of antioxidant enzymes will increase free radicals in testicular tissues and might affect the rat reproductive fertility. The presence of curcumin with lead acetate showed protective effects as attenuating lead acetate against its testicular toxicity and this may be due to the activity of curcumin as antioxidant. The antioxidant enzymes (SOD and GPx) were increased and MDA was decreased after curcumin administration, and the enzymatic activities (SOD and GPx) and MDA in rats can be used as biomarkers of heavy metal toxicity such as lead acetate. Moreover, histological results of lead acetate showed damage of the seminiferous tubules and inhibition of spermatogenesis. Using diet rich in curcumin could be used to overcome the testicular toxicity of lead acetate.
ACKNOWLEDGEMENTS
This study was supported by the Indonesian Ministry of Health for Research in Study Program of Environmental Health, Polytechnic of Health Surabaya.
REFERENCES
- 1.Flora G, Gupta D, Tiwari A. Toxicity of lead: A review with recent updates. Interdiscip Toxicol. 2012;5(2):47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ani M, Moshtaghi AA, Aghadavood M. Protective effects of selenium and zinc on the brain acetyl cholinesterase activity in lead intoxified rat. Res Pharm Sci. 2006;2:80–84. [Google Scholar]
- 3.Vaziri ND, Gonick HC. Cardiovascular effects of lead exposure. Indian J Med Res. 2008;128(4):426–435. [PubMed] [Google Scholar]
- 4.Sudjarwo SA, Koerniasari Protective effects of ethanol extract of mangosteen (Garcinia mangostana L.) pericarp against lead acetate-induced nephrotoxicity in mice. Global J Pharmacol. 2015;9(4):385–391. [Google Scholar]
- 5.Koerniasari, Setiawan, Ngadino, Rustanti IEW, Sudjarwo SA. Protective effect of ethanol extract of mangosteen (Garcinia mangostana L.) pericarp against lead acetate induced hepatotoxicity in mice. Int J Curr Res. 2015;7(2):12518–12522. [Google Scholar]
- 6.Adhikari N, Sinha N, Narayan R, Saxena DK. Lead-induced cell death in testes of young rats. J Appl Toxicol. 2001;21(4):275–277. doi: 10.1002/jat.754. [DOI] [PubMed] [Google Scholar]
- 7.Owolabi JO, Ghazal OK, Williams FE, Ayodele EO. Effect of Moringa oleifera (Drumstick) leaf extracts on lead induced testicular toxicity in adult Wistar rat (Rattus novergicus) Int J Biotech Biomed Res. 2012;2(12):4003–4009. [Google Scholar]
- 8.Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 2006;11(2):114–127. [PubMed] [Google Scholar]
- 9.Asadpour R, Shahbazfar AA, Kianifard D, Azari M, Zaboli N. Comparison of the protective effects of garlic (Allium sativum L) extract, vitamin E and N acetyl cystein on testis structure and sperm quality in rats treated with lead acetate. Revue Med Vet. 2013;164(1):27–41. [Google Scholar]
- 10.Ayinde OC, Ogunnowo S, Ogedegbe RA. Influence of Vitamin C and Vitamin E on testicular zinc content and testicular toxicity in lead exposed albino rats. BMC Pharmacol Toxicol. 2012;13:17–25. doi: 10.1186/2050-6511-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adibmoradi M, Morovvati H, Moradi HR, Sheybani MT, Amoli JS, Mazaheri Nezhad Fard R, et al. Protective effects of wheat sprout on testicular toxicity in male rats exposed to lead. Reprod Syst Sex Disord. 2015;4(4):1–9. [Google Scholar]
- 12.Agarwal R, Goel SK, Behari JR. Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury. J Appl Toxicol. 2010;30(5):457–468. doi: 10.1002/jat.1517. [DOI] [PubMed] [Google Scholar]
- 13.Elsayed ASI. The curcumin as antioxidant natural herb, with emphasize on its effects against some disease. Inter J Appl Biol Pharm Tech. 2016;7(1):26–40. [Google Scholar]
- 14.Abd El-Latief HM. Protective effect of quercetin and or zinc against lead toxicity on rat testes. Global J Pharmacol. 2015;9(4):366–376. [Google Scholar]
- 15.Chen L, Yang X, Jiao H, Zhao B. Tea catechins protect against lead-induced cytotoxicity, lipid peroxidation, and membrane fluidity in HepG2 Cells. Toxicol Sci. 2002;69(1):149–156. doi: 10.1093/toxsci/69.1.149. [DOI] [PubMed] [Google Scholar]
- 16.Aslani MR, Najarnezhad V, Mohri M, Azad M. The effect of allicin on blood and tissue lead content in mice. Comp Clin Pathol. 2011;20(2):121–125. [Google Scholar]
- 17.Sudjarwo SA, Sudjarwo KE, Sudjarwo GW, Koerniasari Mechanisms of endothelial cell protection by curcumin in hypercholesterolemia. J Appl Pharm Sci. 2011;1(10):32–35. [Google Scholar]
- 18.Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res Int. 2014 doi: 10.1155/2014/186864. Article ID 186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrone D, Ardito F, Giannatempo G, Dioguardi M, Troiano G, Lo Russo L, et al. Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther Med. 2015;10(5):1615–1623. doi: 10.3892/etm.2015.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 21.Batra N, Nehru B, Bansal MP. The effect of zinc supplementation on the effects of lead on the rat testis. Reprod Toxicol. 1998;12(5):535–540. doi: 10.1016/s0890-6238(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 22.Tarasub N, Tarasub C, Na Ayutthaya WD. Protective role of curcumin on cadmium-induced nephrotoxicity in rats. J Environ Chem Ecotoxicol. 2011;3(2):17–24. [Google Scholar]
- 23.Raji Y, Salman TM, Akinsomisoye OS. Reproductive functions in male rats treated with methanolic extract of Alstonia boonei stem bark. African J Biomed Res. 2005;8:105–111. [Google Scholar]
- 24.Kheradmand A, Alirezaei M, Asadian P, Rafiei Alavi E, Joorabi S. Antioxidant enzyme activity and MDA level in the rat testis following chronic administration of ghrelin. Andrologia. 2009;41(6):335–340. doi: 10.1111/j.1439-0272.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- 25.Rasyidah TI, Suhana S, Nur-Hidayah H, Kaswandi MA, Noah RM. Evaluation of antioxidant activity of Zingiber Officinale (Ginger) on formalin-induced testicular toxicity in rats. J Med Bioeng. 2014;3(3):149–153. [Google Scholar]
- 26.Unlucerci Y, Bekpinar S, Kocak H. Testis glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase activities in aminoguanidine-treated diabetic rats. Arch Biochem Biophys. 2000;379(2):217–220. doi: 10.1006/abbi.2000.1876. [DOI] [PubMed] [Google Scholar]
- 27.Sakr SA, Mahran HA, El-Deeb MM. Ameliorative effect of curcumin on fluoxetine-induced reproductive toxicity and oxidative stress in male albino rats. Oxid Antioxid Med Sci. 2013;2(1):29–35. [Google Scholar]
- 28.Dorostghoal M, Seyyednejad SM, Jabari A. Protective effects of Fumaria parviflora L. on lead-induced testicular toxicity in male rats. Andrologia. 2014;46(6):437–446. doi: 10.1111/and.12100. [DOI] [PubMed] [Google Scholar]
- 29.Shan G, Tang T, Zhang X. The protective effect of ascorbic acid and thiamine supplementation against damage caused by lead in the testes of mice. J Huazhong Univ Sci Technolog Med Sci. 2009;29(1):68–72. doi: 10.1007/s11596-009-0114-4. [DOI] [PubMed] [Google Scholar]
- 30.Chandra AK, Chatterjee A, Ghosh R, Sarkar M. Effect of curcumin on chromium-induced oxidative damage in male reproductive system. Environ Toxicol Pharmacol. 2007;24(2):160–166. doi: 10.1016/j.etap.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 2006;11(2):114–127. [PubMed] [Google Scholar]
- 32.Ayinde OC, Ogunnowo S, Ogedegbe RA. Influence of vitamin C and vitamin E on testicular zinc content and testicular toxicity in lead exposed albino rats. BMC Pharmacol Toxicol. 2012;13:17–25. doi: 10.1186/2050-6511-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tariq SA. Role of ascorbic acid in scavenging free radicals and lead toxicity from biosystems. Mol Biotechnol. 2007;37(1):62–65. doi: 10.1007/s12033-007-0045-x. [DOI] [PubMed] [Google Scholar]
- 34.Singh P, Deora k, Sankhla v, Mogra p. Curcumin rendered protection against cadmium chloride induced testicular damage in Swiss albino mice. J Cell Mol Biol. 2012;10(2):31–38. [Google Scholar]
- 35.Soliman MM, Baiomy AA, Yassin MH. Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatoxicity and nephrototoxicity in Wistar rats. Biol Trace Elem Res. 2015;167(1):91–102. doi: 10.1007/s12011-015-0280-0. [DOI] [PubMed] [Google Scholar]