Abstract
Sodium benzoate (SB) is one of the food additives and preservatives that prevent the growth of fungi and bacteria. SB has been shown to improve the symptoms of neurodegenerative disease such as Alzheimer's disease. The aim of this study was to evaluate the effect of SB on the cell survival and cellular antioxidant indices after exposure to aluminum maltolate (Almal) in PC12 cell line as a model of neurotoxicity. The cells exposed to different concentrations of SB (0.125 to 3 mg/mL) in the presence of Almal (500 µM) and cell viability, the level of reactive oxygen species (ROS), glutathione content and catalase activity were measured. The results showed that low concentrations of SB caused an increase in the cell survival, but cell viability was reduced in high concentrations. SB could neither prevent the level of ROS production nor change glutathione content. SB (0.5 mg/mL) significantly increased the catalase enzyme activity as compared to the Almal. This study suggested that SB did not completely protect the cell to aluminum-induced free radicals toxicity. Possibly SB improves the symptoms of neurodegenerative disease by other mechanisms.
Keywords: Sodium benzoate, Aluminum, Neurotoxicity, PC12, Oxidative stress, Glutathione
INTRODUCTION
Neurodegenerative diseases are multi-factorial disorders generally under the influence of various environmental and genetic factors. The prevalence of this disease is rising in the elderly population. One of the main reasons for the prevalence of the disease in old age is oxidative stress which causes brain damage by the imbalance between the production of reactive oxygen species (ROS) and antioxidant defense system. In addition, environmental factors such as smoking, diet, the presence of toxins such as pesticides and herbicides, heavy metals and infection have a role in the prevalence of these diseases. Aluminum is one of the metals which has been implicated in the etiology of neuro-degenerative disorders such as Alzheimer's disease (1,2). Various applications of aluminum in the medical, packaging and agriculture industry make inevitable exposure of human to it. So aluminum can enter the body by food, drinking water, beverages and medications containing aluminum (3,4). Evidence has shown that aluminum contributes to neuronal death and neuro-fibrillary tangle formation in Alzheimer's Disease (5,6,7).
Sodium benzoate (SB) is one of the food additives and preservatives commonly used in a variety of products, including beverages, fruit juices, jams, sauces, and pickles (8,9).
SB prevents the growth of fungi and bacteria under acidic conditions, and it is known as a safe food additive, but according to the U.S. Food and Drug Administration the permitted concentrations of 0.1% by weight can be added to the food (8,9,10); SB is even used as an FDA-approved drug, a component of Ucephan, in the treatment of urea cycle disorder in children (11). Some studies have suggested neuropharmacological effects of SB in vivo and in vitro. SB, as a metabolite of cinnamon, increased the levels of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) in the mouse CNS as well as primary human neurons and astrocytes (12). SB also protected memory and learning in an animal model of Alzheimer's disease (13). Yadave, et al. reported immunosuppressive potential of SB by the mechanism of modulating the expression of various stimulatory molecules and regulatory cytokines in splenocytes (14). In addition, the results of a double-blind clinical study showed that SB can improve cognitive impairment in the early phase of Alzheimer's disease (15).
In the light of studies that showed protective effects of SB on the nervous system, the aim of this study was to evaluate the effect of SB on the cell survival and cellular antioxidant indices, which have not been previously reported. These include ROS and gluthation (GSH) in PC12 cell line after exposure to aluminum maltolate (Almal) as a model of neurotoxicity.
MATERIALS AND METHODS
Materials
PC12 rat pheochromocytoma cells were obtained from Pasteur Institute (Tehran, Iran). 3-hydroxy-2-methyl-4-pyron (maltol), aluminum chloride (AlCl3.6H2O), and sodium benzoate (SB) were purchased from the Merck Company (Germany). Glutathione (reduced form, GSH), 5,5'-dithiobis (2-nitrobenzoic acid) (DTNB) and 2',7'-dichlorodihydro-fluorescein diacetate (DCFH-DA) were from Sigma (St. Louise, MO, U.S.A.). Also, fetal bovine serum, Dulbecco's modified Eagle's medium (DMEM), horse serum, trypsin, penicillin, and streptomycin were obtained from Gibco BRL Life Technologies (Grand Island, NY, USA).
Preparation of aluminum maltolate complex
Almal was prepared according to the procedure previously described by Bertholf, et al. (16). A stock solution (25 mM) of Almal was prepared and sterilized using a 0.22 μM filter.
Cell culture
PC12 cells were cultured in DMEM, containing 10% heat-inactivated horse serum, 5% fetal bovine serum, 100 U/mL penicillin and streptomycin, incubated at a temperature of 37°C in a humidified atmosphere of 95% air and 5% CO2, and passaged when it reached 80% confluency.
Cell treatment
For the experiments, PC12 cells were seeded overnight and then treated with Almal (250, 500, 750, and 1000 μM) or various concentrations of SB in phosphate buffered saline (PBS) (0.125, 0.25, 0.5, 1, 1.5, 2, 2.5, and 3 mg/mL) incubated at 37°C for 48 h. After analysis of the result of Almal cytotoxicity by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium (MTT) assay, Almal 500 μM was selected for co-treatment with SB (0.125, 0.25, 0.5 and 1 mg/mL) for 48 h incubation. Also, for MTT assay, the cells were seeded in 96-well micro plates at a density of 5000 cells/well or 2 × 105 cells/well in a 24-well plate for ROS detection and 3 × 106 cells/75 cm2 flask precultured for determining glutathione content and also measuring the amount of catalase enzyme activity.
Cell viability
After 48 h treatment, the cell viability was determined by MTT, a standard colorimetric assay (17). Briefly, after 48 h of treatment, the medium was removed and 20 μL of MTT solution (5 mg/mL in PBS) was added for 4 h at 37°C; then, to dissolve the blue formazan product, 200 μL of dimethylsulfoxide (DMSO) was added per well. The absorption was measured at 570 nm (630 nm as a reference) by the automated plate reader (BioTek, Absorbance Reader). Results were reported as a percentage of cell viability relative to the untreated cells (control group).
Measurement of intracellular reactive oxygene species
The production of intracellular ROS was quantified using a DCFH-DA assay. After treatment, the PC12 cells were once washed with prewarmed PBS and then incubated with 10 µM DCFH-DA at 37°C for 30 min in the dark place. They were rewashed and resuspended in PBS for analysis by fluorometer (BMG Labtech, Germany) with excitation and emission wavelengths of 485 and 530 nm, respectively. H2O2 (250 μM) was used as a positive control.
Measurement of glutathione
To determine the GSH level, PC12 were treated with different concentrations of SB and Almal; then, the cell lysate was prepared. To prevent the interference of testing with proteins, the cell lysate solution was mixed with sulfosalicylic acid 5% solution at a ratio of 1:1. The homogenate was centrifuged at 10,000 rpm for 10 min at 4°C. The supernatants were collected for the assay. The content of GSH was assessed according to the method previously described (18). In this method, reduced GSH reacts with the DTNB reagent in an alkaline environment, producing a yellow complex. The absorption was read at 412 nm using a spectrophotometer (Unico, S-2150, China), employing GSH as the standard at 0-300 μM.
Measurement of catalase activity
To determine the activity of catalase, after treatments, PC12 cells were washed twice with ice-cold PBS and homogenized. The homogenate was centrifuged at 13,000 rpm for 20 min at 4°C.
The supernatants were collected for the assay. Catalase activity was measured based on the decomposition of H2O2 at 240 nm. Enzyme activity units were equal to the amount of enzyme that consumes 1 µM H2O2 within a minute under the experimental conditions. Enzyme activity was calculated as follows (19) and the special enzyme activity was reported as U/mg protein.
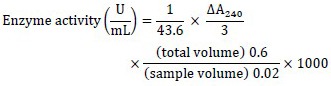
Determination of protein concentration
Protein was determined using Bradford method (20). Bovine serum albumin (BSA) was used as the standard.
Data analysis
All data obtained from at least three independent experiments are expressed as mean ± SEM. The significance of the difference was analyzed by a one-way ANOVA followed by Tukey as post-test using SPSS version 22. P < 0.05 was considered statistically significant.
RESULTS
Effect of sodium benzoate on cell viability
Various concentrations of SB in the present study were used according to the study conducted by Yadav, et al. (14). Results indicated that exposure of the PC12 cells to different concentrations of SB for 48 h significantly decreased the viability at concentrations of 1.5, 2, 2.5, and 3 mg/mL (P < 0.05) compared with the control group (Fig. 1). Similar to the results of the mentioned study, the highest non-cytotoxic concentration of SB after 48 h of treatment was near 1 mg/mL. Thus, up to 0.5 mg/mL SB was used for studying the effect of SB on aluminium toxicity.
Fig. 1.
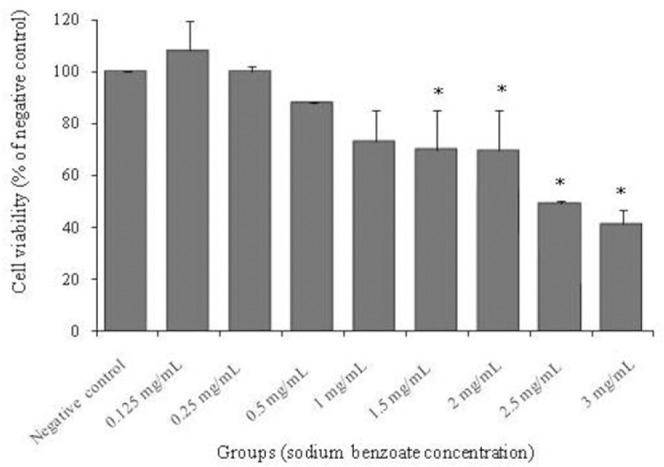
The effect of 48 h treatment with different concentrations of sodium benzoate (0.125, 0.25, 0.5, 1, 1.5, 2, 2.5, and 3 mg/mL) on viability of PC12 cells. Cell viability was determined using MTT assay. Values are expressed as mean ± S.E.M of at least three independent experiments. *Significant difference (P < 0.05) compared to the negative control group.
Effect of aluminum maltolate on cell viability
To evaluate the effect of Almal on cell viability, PC12 cells were exposed to different concentrations of Almal (250, 500,750, and1000 µM) for 48 h; then, the MTT assay was performed. Almal induced cytotoxicity in a concentration-dependent manner, at concentrations 250, 500, 750 and 1000 µM where19%, 37%, 58% and 65% cytotoxicity were observed, respectively compared to the negative control group. At all concentrations, the decrease in cell viability was significantly different (P < 0.05) compared to the control group (Fig. 2).
Fig. 2.
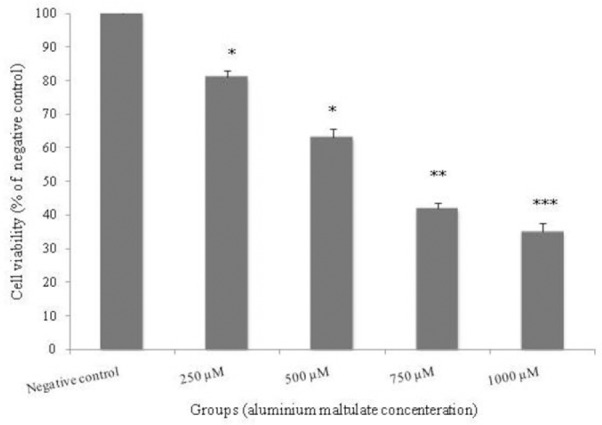
The effect of 48 h treatment with different concentrations of aluminum maltolate (250, 500, 750, and 1000 μM) on viability of PC12 cells. Cell viability was determined using MTT assay. Values are expressed as mean ± S.E.M of at least three independent experiments. *Significant difference (*P < 0.05, ** P < 0.01, ***P < 0.001) compared to the negative control group.
In this study, the concentration of 500 µM Almal, the low concentration near the LC50, was selected for further cytotoxic study.
Effect of combination of aluminum maltolate and sodium benzoate on cell viability
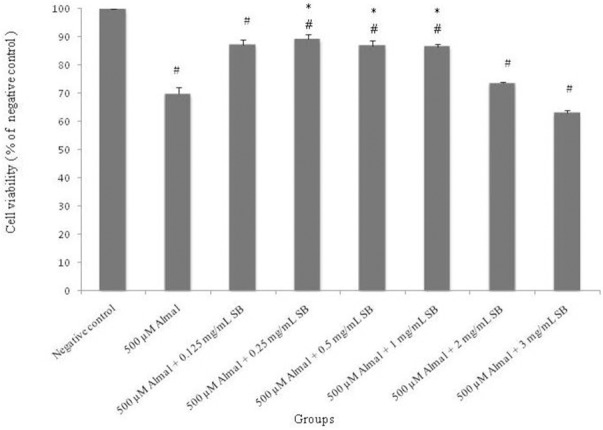
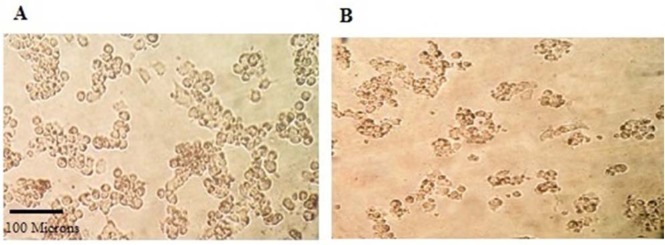
Co-treatment of Almal (500 μM) with SB (0.125, 0.25, 0.5, 1, 2 and 3 mg/mL) for 48 h in PC12 cells revealed that SB at low concentrations (0.125, 0.25, 0.5 and 1 mg/mL) protects the cells significantly (P < 0.05) compared with Almal group, but not completely because cell viability was decreased compared to the negative control group. Also, SB is cytotoxic at high concentrations (2 and 3 mg/mL) (Fig. 3). In addition, effect of Almal (500 μM) on morphology of PC12 cells treated for 48 h, showed that Almal decreased live cells, changed the cell morphology and reduced the cell size as compared with the negative control (Fig. 4).
Fig. 3.
The effect of 48 h treatment with different concentrations of sodium benzoate (0.125, 0.25, 0.5,1, 2 and 3 mg/mL) against aluminum maltolate (500 μM) cytotoxicity. Cell viability was determined using MTT assay. The values were expressed as mean ± S.E.M of at least three independent experiments. Almal; aluminum maltolate, SB; sodium benzoate. *Significant difference P < 0.05 (#compared to the negative control group, *compared to the 500 μM aluminum maltolate group).
Fig. 4.
Effect of treatment on morphology of PC12 cells. The effect of (A) vehicle-phosphate buffered saline (negative control); (B) 500 μM aluminum maltolate on morphology of PC12 cells treated for 48 h. Cells were examined under light microscope at 10 × magnification.
Effect of sodium benzoate on aluminum maltolate-induced ROS production
The protective effect of SB on aluminum-induced intracellular production of ROS was monitored by fluorescence probe DCFH-DA in PC12 cells.
ROS production was measured following the exposure of PC12 cells to Almal (500 μM) and different concentrations of SB (0.125, 0.25 and 0.5 mg/mL) for 48 h. Also, H2O2 (250 μM) was used as a positive control group. A significant increase in the ROS was observed in all groups compared to the negative control (P < 0.05). Whereas SB at 0.125 and 0.25 mg/mL significantly decreased the fluorescence resulting from ROS (P < 0.05) compared to the Almal group, it did not completely show the protective effect to reduce ROS similar to the negative control group (Fig. 5).
Fig. 5.
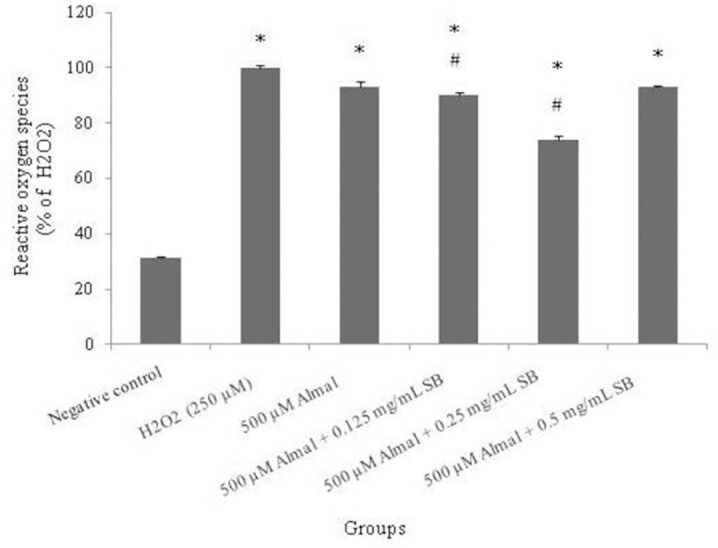
The effect of 48 h treatment with different concentrations of sodium benzoate (0.125, 0.25 and 0.5 mg/mL) on reactive oxygene speices production of aluminum maltolate (500 μM) in PC12 Cells. The reactive oxygene speices production was assessed according to changes in the fluorescence intensity the oxidation product of 2',7’-dichlorodihydrofluorescein diacetate (DCFH-DA). The values are expressed as mean ± S.E.M of at least three independent experiments. Almal, aluminum maltolate; SB, sodium benzoate. *Significant difference P < 0.05 (*compared to the negative control group, #compared to the aluminum maltolate group).
Effect of sodium benzoate and aluminum maltolate on glutathione content
To investigate the effect of Almal and SB on oxidative stress indices, intracellular GSH concentration was measured in all groups. Results showed neither Almal nor SB (in all concentration) had a considerable effect on GSH levels (Fig. 6).
Fig. 6.
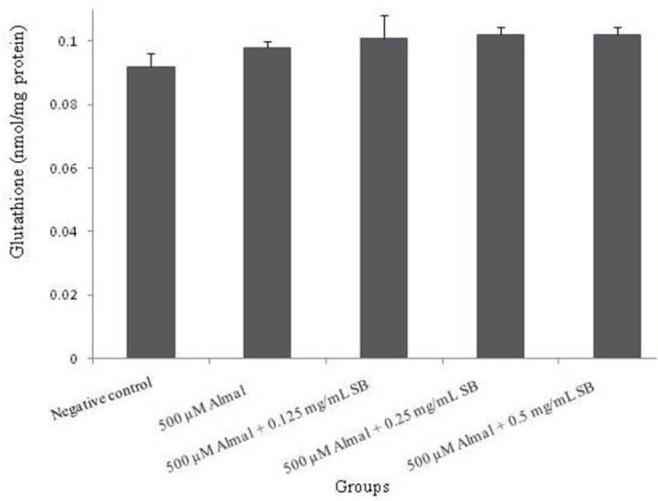
The effect of 48 h treatment with different concentrations of sodium benzoate (0.125, 0.25 and 0.5 mg/mL) and Almal (500 μM) on intracellular glutathione content in PC12 cells. The values are expressed as mean ± S.E.M of at least three independent experiments. Almal, aluminum maltolate; SB, sodium benzoate.
Effect of sodium benzoate and aluminum maltolate on catalase enzyme activity
Catalase activity was significantly decreased (77.5%) in PC12 cells (P < 0.05) when exposed to Almal for 48 h compared to the untreated cells. Also, it was found that co-treatment with different concentrations of SB (0.125, 0.25 and 0.5 mg/mL) changed the catalase activity. Exposure of the cells to 0.5 mg/mL SB significantly (P < 0.05) increased the catalase enzyme activity as compared to the Almal group (Fig. 7).
Fig. 7.
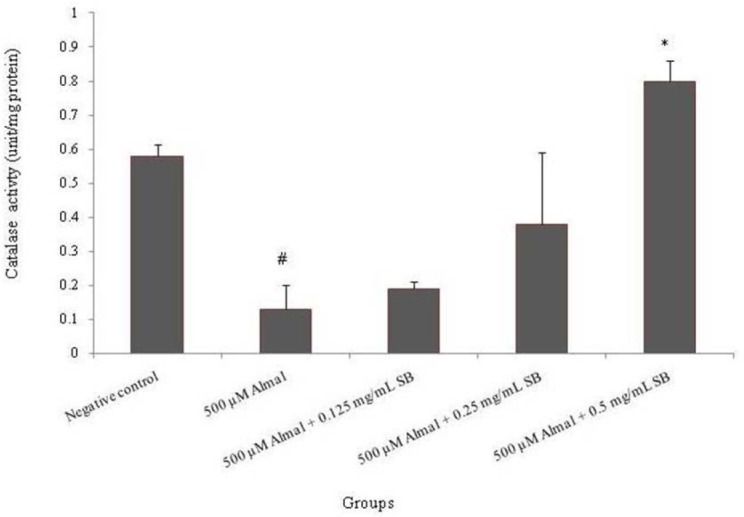
The effect of 48 h treatment with different concentrations of sodium benzoate (0.125, 0.25 and 0.5 mg/mL) and aluminum maltolate (500 μM) on catalase enzyme activity in PC12 Cells. The values are expressed as mean ± S.E.M of at least three independent experiments. Almal; aluminum maltolate, SB; sodium benzoate. *Significant difference P < 0.05 (#compared to the negative control group, *compared to the 500 μM aluminum maltolate group).
DISCUSSION
In previous studies, two forms of aluminum chloride and Almal were used (21,22,23). Almal is a lipophilic complex and permeable to the cell membrane (24). Maltolate is a typical component of the human diet and it is one of the byproducts of the breaking of sucrose. Maltolate has a strong tendency to combine with aluminum, causing the formation of Almal complex in the gastrointestinal tract. Thus, the study of the combined toxicity of Almal contributes to human health (25). Some studies have shown the beneficial effects of SB on improving neurodegenerative diseases such as Alzheimer's diseases, Parkinson's diseases, multiple sclerosis and other diseases (14).
In the present study, the results of MTT assay showed the exposure of PC12 cells to Almal caused cell death in a dose dependent manner (Fig. 2). Similar effects were also observed by the treatment of cells with various concentrations of SB in the absence of Almal. High concentrations of SB (2 and 3 mg/mL) reduced the percentage of living cells in the MTT assay (Fig. 1). This shows that SB at high concentrations is cytotoxic. However, treatment of the cells in different concentrations of SB (up to 1 mg/mL) inhibited cell death induced by Almal (Fig. 3). Similar to a previous study, we also observed that the highest non-cytotoxic dose of SB was 1 mg/mL in vitro (14).
The results of fluorescence measurement of DCFH-DA-labeled cells showed that 48 h exposure to Almal increased the fluorescent intensity of the dye, suggesting that ROS production is associated with aluminum toxicity on cells, as previously reported (26). However, previous research suggested that SB has the hydroxyl radical-scavenging property, and it can protect the cells against free radicals (27,28). In the current study, we showed that SB cannot reduce the ROS production as a result of Almal toxicity on PC12 cells. There are two possible mechanisms which may be associated with the lack of preventive impact of SB on the Aluminium toxicity. First of all, SB generates benzene in combination with free radicals of oxygen and hydrogen (29). Therefore, it is likely that SB produced benzene in contact with the Almal-induced ROS, which caused damage to the cells. Also, possibly hydroxyl radicals are not involved in Al-induced oxidative stress, the same as a previous report which indicated that pre-treatment of cells with a hydroxyl radical scavenger (SB) significantly alleviated oxidative stress induced by copper but was not effective in the case of zinc in Scenedesmus sp. While several types of ROS, including hydroxyl radicals involved in lipid peroxidation, this study concluded that the hydroxyl radical is not involved in zinc-induced lipid peroxidation (28).
In the present study, we found that GSH content, as one of the antioxidant defense mechanisms, did not change in all treated cells. There were no significant differences between Almal group or different concentrations of SB groups compared with the negative control (Fig. 6). Despite these results, other studies have shown that aluminum can reduce the GSH content in the cells (26,30). It is likely that the amount of glutathione has increased as a compensatory response of the cell toward the reduction of oxidative stress in the face of aluminum for 48 h; also it is probable that another important antioxidant system such as thioredoxin (which are similar to the role of glutathione in the cells) is involved in the reduction of ROS production (31), thus preventing the change in the content of glutathione by aluminum toxicity. Furthermore, a previous study suggested that lowering the intracellular GSH concentration did not directly trigger the cell death, but it promoted the onset of Almal decrease in the cell viability (26).
Superoxide dismutase, catalase, and glutathione reductase are major enzymes in cellular defense against ROS (32). In this study, catalase activity as one of the cell antioxidant defense mechanisms, was evaluated. The results showed that Almal reduces the activity of catalase and SB can prevent this effect. Some studies suggested that the inhibitory effect of aluminum on the activity of catalase may be due to the effect of the direct reaction with enzymes, or even due to the effect of aluminum on the post-transcriptional changes or post-translational steps in the synthesis of the enzymes. Anane, et al. have also suggested that the response of superoxide dismutase and catalase to aluminum depends on the characteristics of the tissue (33,34).
In this study, Almal induced severe ROS generation in PC12 cells (Fig. 4) and decreased catlase activity, which may be the result of inactivation and damages of the catalase molecules by ROS. Moreover, under oxidative stress, increasing the activities of antioxidant enzymes are usually connected with their activation of preexisting inactive molecules or de-novo synthesis (35). Accordingly, results showed that co-treatment of the cells with Almal and SB modified the elevated levels of ROS and improved catalase activity to compensate the oxidative damages.
Even though SB has been known as a radical scavenger (28), our results showed that the protective effect of SB is not related to this property. Totally, SB does not have a great impact, at least, on the antioxidant defense system. It is possible that only the other mechanism of SB, as a D-amino acid oxidase inhibitor, is responsible for neurodegenerative disease in clinical studies (15). Recent data indicated that D-amino acids that are the neurotransmitters for the co-agonist site of the N-methyl-D-aspartate receptor (NMDAR), have neuroprotective effect. Therefore, NMDA-enhancing agents have potential for improving cognitive function in patients with CNS disorders (36,37).
Clinical studies have also suggested that SB, a D-amino acid oxidase inhibitor, is beneficial for neurocognitive function in patients with early-phase Alzheimer's disease and schizophrenia (15,37). However, it is important to note that the SB toxic dose is not very different from the protective doses. As we have seen, doubling the effective dose is toxic and decreases the cell viability. Also, Noorafshan, et al. reported oral administration of SB for four weeks induced anxiety and motor impairment in rats (38). Furthermore, human studies in the context of the effects of SB suggested that high intake of SB may be associated with attention deficit hyperactivity disorder (ADHD) and hyperactivity in children, but this needs to be further investigated (39,40,41).
CONCLUSION
This study suggests that SB did not completely protect the cell to aluminum-induced free radicals toxicity. Although previous studies indicated that SB is a radical scavenger and also, has beneficial effects on neurocognitive disease, but our results show SB does not have a significant effects on the antioxidant defense system. Possibly SB improves the symptoms of neurodegenerative disease by other mechanisms.
To investigate the effects of SB on other parameters related to oxidative stress, further studies are suggested on superoxide dismutase activity and also lipid peroxidation.
ACKNOWLEDGMENTS
The present article was extracted from the thesis written by Rozhin Abbasi Borojeni. The instrumental facilities provided by Diagnostic Laboratory Sciences and Technology Research Center of Shiraz University of Medical Sciences is greatly acknowledged. This investigation was financially supported by the Vice Chancellor of Research Affairs of Shiraz University of Medical Sciences (94-01-103-10812).
REFERENCES
- 1.Kumar V, Gill KD. Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol. 2009;83:965–978. doi: 10.1007/s00204-009-0455-6. [DOI] [PubMed] [Google Scholar]
- 2.Amjad S, Umesalma S. Protective effect of Centella asiatica against aluminium-induced neurotoxicity in cerebral cortex, striatum, hypothalamus and hippocampus of rat brain-histopathological, and biochemical approach. J Mol Biomark Diagn. 2015;2015:212. [Google Scholar]
- 3.Savory J, Herman MM, Ghribi O. Mechanisms of aluminum-induced neurodegeneration in animals: Implications for Alzheimer's disease. J Alzheimers Dis. 2006;10:135–144. doi: 10.3233/jad-2006-102-302. [DOI] [PubMed] [Google Scholar]
- 4.Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 2007;10:1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci. 2011;124(2):225–250. doi: 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng K-W, Fu H, Liu G-X, Wang X-M. Aluminum maltolate induces primary rat astrocyte apoptosis via overactivation of the class III PI3K/Beclin 1-dependent autophagy signal. Toxicol In Vitro. 2012;26:215–220. doi: 10.1016/j.tiv.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Walton J. Evidence for participation of aluminum in neurofibrillary tangle formation and growth in Alzheimer's disease. J Alzheimers Dis. 2010;22:65–72. doi: 10.3233/JAD-2010-100486. [DOI] [PubMed] [Google Scholar]
- 8.Nair B. Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int J Toxicol. 2001;20:23–50. doi: 10.1080/10915810152630729. [DOI] [PubMed] [Google Scholar]
- 9.Reddy MV, Aruna G, Parameswari SA, Banu BH, Reddy PJ. Estimated daily intake and exposure of sodium benzoate and potassium sorbate through food products in school children of tirupati, india. Int J Pharm Pharm Sci. 2015;7:129–133. [Google Scholar]
- 10.Heydaryinia A, Veissi M, Sadadi A. A comparative study of the effects of the two preservatives, sodium benzoate and potassium sorbate on Aspergillus niger and Penicillium notatum. Jundishapur J Microbiol. 2011:4. [Google Scholar]
- 11.Gropman A, Summar M, Leonard J. Neurological implications of urea cycle disorders. J Inherit Metab Dis. 2007;30:865–879. doi: 10.1007/s10545-007-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K. Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J Neuroimmune Pharmacol. 2013;8:739–755. doi: 10.1007/s11481-013-9447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modi KK, Roy A, Brahmachari S, Rangasamy SB, Pahan K. Cinnamon and its metabolite sodium benzoate attenuate the activation of p21 rac and protect memory and learning in an animal model of Alzheimer's disease. PloS one. 2015;10:e0130398. doi: 10.1371/journal.pone.0130398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav A, Kumar A, Das M, Tripathi A. Sodium benzoate, a food preservative, affects the functional and activation status of splenocytes at non cytotoxic dose. Food Chem Toxicol. 2016;88:40–47. doi: 10.1016/j.fct.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Lin C-H, Chen P-K, Chang Y-C, Chuo L-J, Chen Y- S, Tsai GE, et al. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2014;75:678–685. doi: 10.1016/j.biopsych.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Bertholf RL, Herman MM, Savory J, Carpenter RM, Sturgill BC, Katsetos CD, et al. A long-term intravenous model of aluminum maltol toxicity in rabbits: tissue distribution, hepatic, renal, and neuronal cytoskeletal changes associated with systemic exposure. Toxicol Appl Pharmacol. 1989;98:58–74. doi: 10.1016/0041-008x(89)90134-8. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 19.Zal F, Mostafavi Pour Z, Vessal M. Comparison of the effects of vitamin e and/or quercetin in attenuating chronic cyclosporine a induced nephrotoxicity in male rats. Clin Expe Pharmacol Physiol. 2007;34:720–724. doi: 10.1111/j.1440-1681.2007.04623.x. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Satoh E, Okada M, Takadera T, Ohyashiki T. Glutathione depletion promotes aluminum-mediated cell death of PC12 cells. Biol Pharm Bull. 2005;28:941–946. doi: 10.1248/bpb.28.941. [DOI] [PubMed] [Google Scholar]
- 22.Saberzadeh J, Arabsolghar R, Takhshid MA. Alpha synuclein protein is involved in Aluminum-induced cell death and oxidative stress in PC12 cells. Brain Res. 2016;1635:153–160. doi: 10.1016/j.brainres.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Ohyashiki T, Satoh E, Okada M, Takadera T, Sahara M. Nerve growth factor protects against aluminum-mediated cell death. Toxicology. 2002;176:195–207. doi: 10.1016/s0300-483x(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 24.Johnson VJ, Kim SH, Sharma RP. Aluminum-maltolate induces apoptosis and necrosis in neuro-2a cells: potential role for p53 signaling. Toxicol Sci. 2005;83:329–339. doi: 10.1093/toxsci/kfi028. [DOI] [PubMed] [Google Scholar]
- 25.Griffioen KJ, Ghribi O, Fox N, Savory J, DeWitt DA. Aluminum maltolate-induced toxicity in NT2 cells occurs through apoptosis and includes cytochrome c release. Neurotoxicology. 2004;25:859–867. doi: 10.1016/j.neuro.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Satoh E, Okada M, Takadera T, Ohyashiki T. Glutathione depletion promotes aluminum-mediated cell death of PC12 cells. Biol Pharm Bull. 2005;28:941–946. doi: 10.1248/bpb.28.941. [DOI] [PubMed] [Google Scholar]
- 27.Cervantes A, Pinedo HM, Lankelma J, Schuurhuis GJ. The role of oxygen-derived free radicals in the cytotoxicity of doxorubicin in multidrug resistant and sensitive human ovarian cancer cells. Cancer Lett. 1988;41(2):169–177. doi: 10.1016/0304-3835(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi BN, Gaur JP. Relationship between copper- and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta. 2004;219:397–404. doi: 10.1007/s00425-004-1237-2. [DOI] [PubMed] [Google Scholar]
- 29.Hu M, Wang J, Cai J, Wu Y, Wang X. [Analysis of sodium benzoate biotoxicity by atomic force microscope] Sheng Wu Gong Cheng Xue Bao. 2008;24:1428–1432. doi: 10.1016/s1872-2075(08)60064-3. [DOI] [PubMed] [Google Scholar]
- 30.Murakami K, Yoshino M. Aluminum decreases the glutathione regeneration by the inhibition of NADP-isocitrate dehydrogenase in mitochondria. J Cell Biochem. 2004;93:1267–1271. doi: 10.1002/jcb.20261. [DOI] [PubMed] [Google Scholar]
- 31.Silva-Adaya D, Gonsebatt ME, Guevara J. Thioredoxin system regulation in the central nervous system: experimental models and clinical evidence. Oxid Med Cell Longev. 2014;2014:590808. doi: 10.1155/2014/590808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammadirad A, Abdollahi M. A systematic review on oxidant/antioxidant imbalance in aluminium toxicity. Int J Pharmacol. 2011;7:12–21. [Google Scholar]
- 33.Anane R, Creppy EE. Lipid peroxidation as pathway of aluminium cytotoxicity in human skin fibroblast cultures: prevention by superoxide dismutase+catalase and vitamins E and C. Hum Exp Toxicol. 2001;20:477–481. doi: 10.1191/096032701682693053. [DOI] [PubMed] [Google Scholar]
- 34.Swain C, Chainy GB. Aluminum effect on lipid peroxidation and on the activities of superoxide dismutase and catalase in the cerebral hemisphere and liver of young chicks. J Trace Elem Med Biol. 1997;11:77–82. doi: 10.1016/s0946-672x(97)80030-2. [DOI] [PubMed] [Google Scholar]
- 35.Lushchak VI. Classification of oxidative stress based on its intensity. EXCLI J. 2014;13:922. [PMC free article] [PubMed] [Google Scholar]
- 36.Esposito S, Pristera A, Maresca G, Cavallaro S, Felsani A, Florenzano F, et al. Contribution of serine racemase/D-serine pathway to neuronal apoptosis. Aging Cell. 2012;11:588–598. doi: 10.1111/j.1474-9726.2012.00822.x. [DOI] [PubMed] [Google Scholar]
- 37.Lane H-Y, Lin C-H, Green MF, Hellemann G, Huang C-C, Chen P-W, et al. Add-on treatment of benzoate for schizophrenia: a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA Psychiatry. 2013;70:1267–1275. doi: 10.1001/jamapsychiatry.2013.2159. [DOI] [PubMed] [Google Scholar]
- 38.Noorafshan A, Erfanizadeh M, Karbalay-Doust S. Sodium benzoate, a food preservative, induces anxiety and motor impairment in rats. Neurosciences (Riyadh) 2014;19:24–28. [PubMed] [Google Scholar]
- 39.Kilic N, Balkan E, Akgoz S, Sen N, Dogruyol H. Comparison of the effectiveness and side-effects of tolterodine and oxybutynin in children with detrusor instability. Int J Urol. 2006;13:105–108. doi: 10.1111/j.1442-2042.2006.01240.x. [DOI] [PubMed] [Google Scholar]
- 40.Arnold LE, Lofthouse N, Hurt E. Artificial food colors and attention-deficit/hyperactivity symptoms: conclusions to dye for. Neurotherapeutics. 2012;9:599–609. doi: 10.1007/s13311-012-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beezhold BL, Johnston CS, Nochta KA. Sodium benzoate–rich beverage consumption is associated with increased reporting of ADHD symptoms in college students a pilot investigation. J Atten Disord. 2014;18:236–241. doi: 10.1177/1087054712443156. [DOI] [PubMed] [Google Scholar]