Abstract
Allopurinol, an inhibitor of xanthine oxidase, reduces both plasma uric acid and oxidative stress and shows useful effects on some complications of diabetes. However, it is not defined which of the above mentioned properties are involved. Moreover, to the best of our knowledge no study has been done on the effects of allopurinol on diabetic retinopathy. In the present study, the effect of allopurinol on experimental diabetic retinopathy and its possible mechanism has been investigated. Thirty two rats were divided into four groups of eight rats each; (1) normal, (2) diabetic control, (3) diabetic + allopurinol (50 mg/kg.day), (4) diabetic + benzbromarone (10 mg/kg.day). Drugs were administered daily and orally from the day after diabetes induction for eight weeks. Thereafter retinal function and structure were evaluated by electroretinography and microscopic studies. Uric acid and oxidative stress biomarkers were measured biochemically. Diabetes significantly increased plasma uric acid and oxidative stress markers and reduced body weight and amplitude of electroretinogram (ERG) b-wave and oscillatory potentials. Treatment of diabetic rats with allopurinol caused a significant increase in the amplitude of ERG b-wave (87%) and decrease in blood sugar (20%), uric acid (49%), and 8-iso-prostaglandin F2a (56%), but had no effect on the number of retinal ganglionic cells and oscillatory potentials. Benzbromarone showed no significant effects on the considered parameters except the reduction of uric acid. Allopurinol improved the b-wave amplitude of diabetic rats. It seems that this beneficial effect is due to the reduction of oxidative stress rather than its effect on plasma uric acid.
Keywords: Diabetic retinopathy, Allopurinol, Benzbromarone, Electroretinography
INTRODUCTION
Diabetes mellitus (DM) is a widespread metabolic disorder characterized by hyperglycemia. The number of suffering patients is on the rise globally. Though DM patients take antidiabetic drugs, the hyperglycemia cannot be controlled adequately and it causes undesirable abnormalities in different organs of the body. Diabetic retinopathy (DR) is considered as the most prevalent complication of DM (1), and a leading cause of visual impairment and acquired blindness (2). In diabetic patients, the high incidence of retinopathy has become a great concern (3). In comparison with all other complications, retinopathy may be initiated as a direct manifestation of glucose toxicity (4). In the initial stages of DR, there are neuronal dysfunctions and apoptosis of retinal neurons, which occurs before vascular lesions. Neuronal dysfunction can be seen through changes occurring in the electroretinogram and retinal histology (5).
A major goal of DM control is to prevent chronic complications. Since glycemic control does not entirely prevent DM complications (6), there is a growing interest in developing alternative agents to prevent them.
Allopurinol, an inhibitor of xanthine oxidase (XO) that simultaneously reduces plasma uric acid (UA) and oxidative stress, may have therapeutic effects on neuronal and vascular dysfunction in DM. To this point, few studies have been carried out to investigate the effects of allopurinol in DM complications and also the obtained results were inconclusive.
In studies that showed a positive outcome, it was not defined which property of allopurinol, i.e. reduction of oxidative stress or uric acid was involved (7,8,9,10,11,12,13). However, to the best of our knowledge no study has been done on the effects of allopurinol on DR. XO is the main enzyme in purine metabolism and catalyzes formation of UA from xanthine and hypoxanthine (7,14). Some studies have suggested that hyperuricemia is an important risk factor for diabetic complications (such as DR) and UA lowering may have a protective effect in diabetes (7,8,9,15,16,17).
XO also acts as a major source of reactive oxygen species (ROS) in diabetes (7,17,18). Superoxide anion radicals (O2 -) and hydrogen peroxide (H2O2) are generated as byproducts of purine metabolism at the same time when UA is forming by XO action (7,17). XO level and activity in diabetes increase (10). Some studies have implied that targeting XO with allopurinol can reduce oxidative injury in DM and free radical reduction is responsible mechanism for allopurinol in diabetes (10,11,12,13).
In the present study, the effect of allopurinol on the early functional and pathological changes of DR in streptozotocin-treated diabetic rats has been investigated. Benzbromarone, a uricosuric agent, were also used to define the probable useful effect of allopurinol that is due to its UA lowering effect or reduction of oxidative stress.
MATERIALS AND METHODS
Animals
In this experimental study thirty two healthy adult male Sprague–Dawley rats weighing 180-220 g were taken from the Center of Comparative and Experimental Medicine, Shiraz University of Medical Sciences (Shiraz, Iran). Animals were fed a standard rodent diet and water, and kept at 12:12 h light/dark cycle. The study protocol was approved by the Bioethics Committee of Shiraz University of Medical Sciences (Registration No. 2016-284) and all animals were treated according to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of diabetes
After seven days of quarantine period, animals were induced with diabetes through a single intraperitoneal (i.p.) injection of 60 mg/kg of streptozotocin (Sigma-Aldrich, Germany) after 10 h fasting. Streptozotocin was dissolved in 0.1 M citrate buffer solution (pH 4.5) and immediately injected. Normal control rats received the same amount of sodium citrate buffer. Three days after streptozotocin injection, a blood sample was drawn from the tail vein and plasma glucose concentration was checked using the Accu-Chek blood glucose test meter (Roche Diagnostic, Germany). Rats with blood sugar higher than 300 mg/dL (more than 90% of injected animals) were considered diabetics (DM type I). No exogenous insulin treatment was given (19).
Experimental design
Experimental animals were randomly divided into four groups: normal rats, which received vehicle; diabetic controls, which received vehicle; diabetic rats, which received 50 mg/kg.day of allopurinol (Sigma-Aldrich, Germany); and diabetic rats, which received 10 mg/kg.day of benzbromarone (Fluka, Germany).
Allopurinol and benzbromarone were dissolved in sodium carboxymethyl cellulose (Fluka, Switzerland). Drugs were administered daily and orally from the day after diabetes induction for eight weeks. In each group, eight rats were assigned to evaluate the electrophysiology, biochemistry and histopathology of the retina (20).
Biochemical assays
Rats were anesthetized by an i.p. injection of ketamine/xylazine (80 and 10 mg/kg, respectively) and blood from the heart was collected and its plasma was separated not later than 2 h after sampling. Butylated hydroxytoluene (Sigma-Aldrich, Germany) 0.005% was added to avoid ex vivo peroxidation of lipids. 8-iso-prostaglandin F2a (8-Iso-F2α) levels in plasma and malondialdehyde (MDA) levels in extracts from retinal homogenates as two important indices of lipid peroxidation were measured.
The 8-Iso-F2α concentrations in plasma samples were assayed by an ELISA Kit (Enzo Life Sciences Inc., USA) according to the manufacturer's protocol following the extraction procedure. Final optical densities were read at 405 nm to determine 8-Iso-F2α levels (21).
After plasma separation, rats were sacrificed and their eyes were immediately enucleated. Right eyes were dissected quickly and the retina was carefully removed from the eyeballs. Then, MDA levels were assessed based on the reaction of MDA with thiobarbituric acid (Sigma-Aldrich, Germany) which forms a pink chromogen compound. MDA concentration was determined by comparison to a standard curve of 1, 1', 3, 3’-tertaethoxy propane (Sigma-Aldrich, Germany). The absorbance was read spectrophotometrically at 532 nm. Results are shown as nmol/g retina wet weight using the linear regression equation (22).
To confirm the effects of studied drugs, plasma UA levels were also measured by uricase method, using a commercial kit obtained from Pars Azmun (Iran), and by a colorimeter at 546 nm.
Retinal assessment
To evaluate the retinal dysfunction, full-field electroretinography (ERG) recordings and histology were taken at four and eight weeks after diabetes induction, respectively.
Electroretinography
Before ERG recordings, animals were dark-adapted for at least 6 h before the experiment and further procedures were conducted under dim red illumination and at a constant temperature of 25°C. Stimuli consisted of white flashes (light-emitting diode flash) delivered by the ganzfeld bowl. Scotopic ERG was recorded according to the International Society for Clinical Electrophysiology of Vision (ISCEV) guidelines. ERG responses were recorded by using a RetiScan RetiPort electrophysiology apparatus (RETI-port/scan21; Roland Consult, Germany). Each ERG record represents an average of 3–6 responses and signals were low-pass filtered at 50 Hz cutoff frequency.
ERG analysis consisted of the amplitudes of a-waves, b-waves and oscillatory potentials (23).
Histology
Changes in cell density in the ganglion cell layer (GCL) were evaluated by cross-sectional slides under light microscopy. After tissue processing, eyes were cut sagittally into five-micrometer sections. Sections were subsequently stained using hematoxylin and eosin (H&E), and ganglion cell counts were performed using 400× total magnification (24).
Statistical analysis
The ERG responses were analyzed only in the left eye. All data in figures and tables are presented as mean ± S.E.M.
The intergroup variation was measured by one-way analysis of variance (ANOVA) followed by Tukey post hoc test to assess their significance. P < 0.05 was considered statistically significant. The statistical analysis was done using the SPSS Statistical Software version 16.
RESULTS
Diabetes induction
In the three diabetic groups, 3 days after streptozotocin administration, plasma glucose levels were increased nearly by four times higher than the normal levels. This severe hyperglycemia was persistent during the experimental period. There was a small but significant reduction in plasma glucose level of allopurinol-treated group compared to diabetic group (P = 0.044). Decrease in plasma glucose level of benzbromarone-treated group was non-significant (Table 1).
Table 1.
Rat blood glucose levels and body weights.
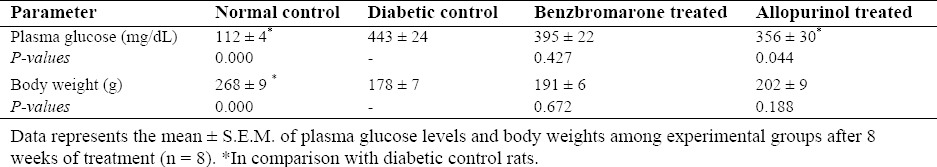
Total mortality rate of diabetic animals was about 18%. Mean body weights of streptozotocin-treated rats from all diabetic groups were significantly lower than those of control group (33% reduction). Although benzbromarone- and allopurinol-treated groups showed weight gain during the investigations and increase in their mean body weight (7% and 13% increase, respectively), but these differences did not reach a significant value (Table 1).
Oxidative stress
Streptozotocin injection induced a significant increase in plasma 8-Iso-F2α levels (159%) and retina MDA contents (237%) compared to normal control group. After eight weeks of treatments, the mean plasma 8-Iso-F2α levels of the allopurinol-received rats decreased significantly (P = 0.023). Although, allopurinol reduced retinal MDA, it did not reach a statistical significance (P = 0.089). Benzbromarone had no significant effect on these parameters (Table 2).
Table 2.
Biochemical obtained values in studied groups.
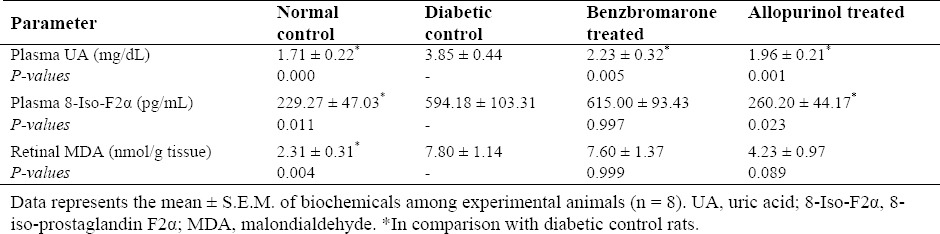
Plasma uric acid
At the end of the experiment, considerable hyperuricemia was observed in diabetic control group. Evaluation of mean plasma UA levels revealed that UA in the DM type I was 125% higher than the normals, while lower levels were seen in the treatment groups; 49% decrease in allopurinol-treated group and 42% decrease in benzbromarone-treated group were observed. There was no significant difference of the UA values in benzbromarone- and allopurinol-treated groups (Table 2).
After evaluation of the ERG parameters, amplitudes of b-wave and oscillatory potentials showed a significant decrease in DM type I compared to normal control group. Obtained results in the treatment groups revealed that allopurinol could only improve b-wave amplitudes and return it to normal range, but benzbromarone showed no significant effect (Fig. 1).
Fig. 1.
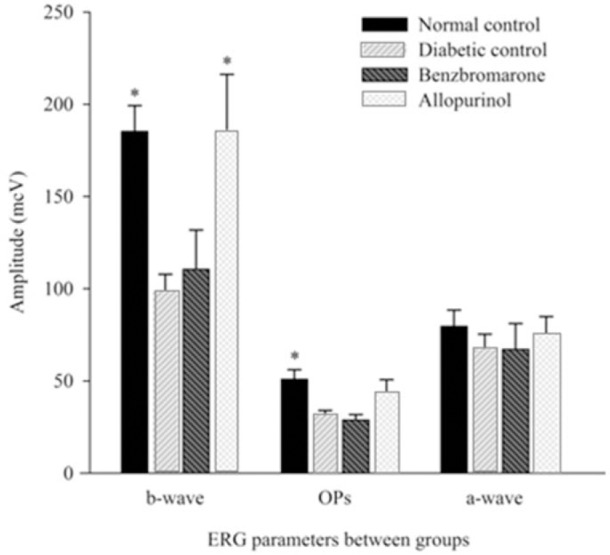
Amplitudes of the rats electroretinography waves. Each bar graph represents the mean value of different electroretinogram waves between experimental animals (n = 8). Standard errors represented by vertical bars. *Mean value was significantly different compared with diabetic control group, by One-Way ANOVA and Tukey post hoc test (P < 0.05). OPs, oscillatory potentials; ERG, electroretinogram.
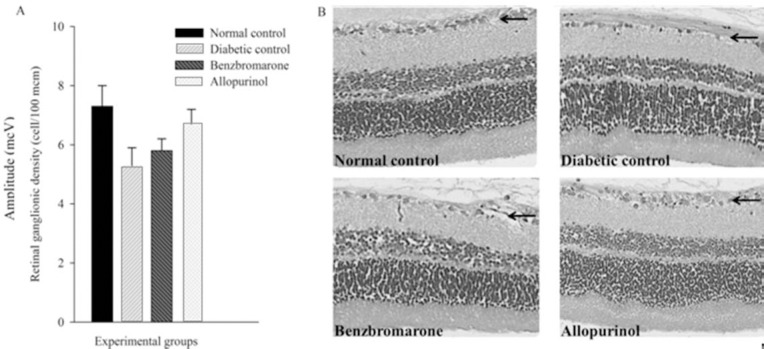
Analysis of retinal cross sections exhibited approximately 27% reduction in retinal ganglion cells (RGCs) of DM I rats compared with normal ones, then again it did not have statistical significance (P = 0.094). No evident changes in retinal morphology were observed among drug-received groups (Fig. 2).
Fig. 2.
Density of retinal ganglion cells in research groups. (A) Each bar graph represents the mean value of cell numbers in retinal ganglion cell layer (GCL) between experimental animals (n = 8). Standard errors represented by vertical bars. (B) Representative micrographs from rat retina. The GCL is indicated by arrows.*Mean value was significantly different compared with diabetic control group, by One-Way ANOVA and Tukey post hoc test (P <0.05). Diabetes reduced the number of ganglion cells of retina, but the change was not significant (P = 0.094).
DISCUSSION
In the present study, the effect of allopurinol on experimental DR and its possible mechanism has been investigated.
Diabetes was induced by a single injection of streptozotocin, a well-known agent that destroys β-cells and produces an experimental model of type I diabetes (25). After treatment of diabetic rats with allopurinol for 8 weeks, a small but significant reduction in blood sugar was observed. This could be due to its antioxidant properties (see below). Benzbromarone had no meaningful effect on blood glucose.
As expected, diabetic control animals showed 3-4 times increase in biomarkers of oxidative stress, i. e. plasma 8-Iso-F2α and retinal MDA (Table 2). The F2-isoprostanes are produced by the enzyme-independent free radical oxidation of arachidonic acid and are generated in conditions of increased oxidative stress. It seems that it is the best and the most reliable marker of oxidative stress in vivo (26,27). Allopurinol attenuated oxidative stress in diabetic rats, an effect which can be attributed to its inhibitory effect on XO. It should be noted that the little hypoglycemic effect of allopurinol may have made some contribution to the observed results. No significant relationship found between benzbromarone administration and oxidative stress biomarkers.
Similar to previous studies, diabetes caused hyperuricemia (15,16,17). Both drugs in their given doses, resulted in considerable reduction in UA, and in this respect were the same.
Allopurinol decreases UA production through inhibition of XO (which is an enzyme involved in oxidative stress), but benzbromarone acts through inhibition of renal reabsorption of UA without any relation to XO. Some studies have linked ameliorative effects of allopurinol on diabetes to its ability in reducing UA production (7,8,9), though other studies have stated that there is no link between UA lowering property of allopurinol and diabetes recovery (10).
Diabetes caused a decline in amplitude of the ERG b-wave and oscillatory potentials and an obvious but non-significant reduction of RGCs. The ERG b-wave is generated by neurons of inner nuclear layer (INL) and is considered to represent the activity of bipolar cells and functional integrity of the INL. Therefore b-wave reduction could be due to bipolar cells loss or dysfunction (28). The oscillatory potentials are three to four low-amplitude, high-frequency wavelets superimposed on the ascending limb of the ERG b-wave. The oscillatory potentials are considered to be the result of amacrine cells activity, such as; feedback from amacrine and bipolar cells, and/or amacrine to ganglion cell communication. Therefore, the oscillatory potentials attenuation reflects amacrine cells loss or dysfunction (29,30).
To support the result, we also assessed alteration in cell number of the GCL. Retinal ganglion cells (RGCs) play an important role in transmitting visual signals to the visual cortex and their loss in experimental diabetes may represent neurodegeneration of GCL (31). Since some studies have stated that histological changes are detected after a longer period of time from the onset of diabetes (32), it is suggested that lengthier experiments may be more suitable for histological assessments. However, keeping diabetic animals alive for more than 2 months is very difficult and it was not done in our study.
Several factors are involved in the pathophysiology of DR. Oxidative stress is one of the major mechanisms which is involved in retinal neurodegeneration. Other potential mechanisms of cell loss in retinal neurons during diabetes include; glutamate excitotoxicity, ischemic insult and reduced growth factor signaling (32,33).
To best of our knowledge, this is the first study that has investigated allopurinol effects on DR. Allopurinol therapy only resulted in correction of b-wave amplitude and a non-significant increase in RGCs. Meanwhile, benzbromarone had a similar effect on UA but showed no effect on functional and histological changes in DR, which implies that the beneficial effects of allopurinol were due to its antioxidant property.
It should be noted that allopurinol had a small, but significant effect on blood sugar and this effect may contribute to the ophthalmic protective effect of this compound. However, we have no explanation for the ineffectiveness of allopurinol on the oscillatory potentials changes in diabetes. This may imply that mechanisms other than oxidative stress are involved in the amacrine cells dysfunction in diabetes and allopurinol cannot restore their function alone.
In the present study, diabetes had no effect on the amplitude of a-wave. This observation may imply that DM had no effect on the photoreceptors function, but there are controversial reports in the a-wave changes in DM (34,35). It is claimed that the duration of diabetes plays an important role in producing changes in the amplitude of a-wave, and at least 10-12 weeks after the onset of diabetes is necessary to observe any change in this wave (30,36,37).
Although the hypoglycemic effect of allopurinol might have some contribution in the obtained beneficial results in diabetic retinopathy, it must be re-emphasized that blood glucose lowering effect of allopurinol was very small.
In summary, experimental type I diabetes resulted in cellular dysfunction in INL and GCL cells of rat retina. Allopurinol therapy had protective effects on INL neurons (especially bipolar cells) but had no positive effect on amacrine cells and ganglionic layer. Benzbromarone did not show to have any beneficial properties on the retina. Overall, the data suggest that it is unlikely the effects of allopurinol were due to a decrease in uric acid production. The presented evidences support the hypothesis that a reduction in oxidative stress contributed to the beneficial effects of allopurinol.
CONCLUSION
Allopurinol improves the ERG b-wave amplitude of diabetic rats. It seems that this beneficial effect is due to the reduction of oxidative stress rather than its effect on plasma uric acid. It has no significant effects on the number of retinal ganglion cells, and amplitude of ERG a-wave and oscillatory potentials.
ACKNOWLEDGEMENTS
The present article was extracted from the dissertation written by Mohsen Goharinia and was financially supported by the Research Grant from Shiraz University of Medical Sciences, Iran (Grant number: 7262). The authors would like to thank Maryam Mojahed and Marjan Tavakoli for their technical support. The authors would also like to thank the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for their invaluable assistance in editing this article.
REFERENCES
- 1.Liu X, Zhu B, Zou H, Hu D, Gu Q, Liu K, et al. Carbamylated erythropoietin mediates retinal neuroprotection in streptozotocin-induced early-stage diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2015;253:1263–1272. doi: 10.1007/s00417-015-2969-3. [DOI] [PubMed] [Google Scholar]
- 2.Cai X, McGinnis JF. Diabetic retinopathy: animal models, therapies, and perspectives. J Diabetes Res 2016. 2016 doi: 10.1155/2016/3789217. 3789217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2016;61:187–196. doi: 10.1016/j.survophthal.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Lorenzi K, Oates PJ. The polyol pathway and diabetic retinopathy. Diabetic Retinopathy: 2007. 2007 610385. [Google Scholar]
- 5.Kur J, Burian MA, Newman EA. Light adaptation does not prevent early retinal abnormalities in diabetic rats. Scientific reports. 2016:6. doi: 10.1038/srep21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care. 2011;34:968–74. doi: 10.2337/dc10-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afshari M, Larijani B, Rezaie A, Mojtahedi A, Zamani MJ, Astanehi-Asghari F, et al. Ineffectiveness of allopurinol in reduction of oxidative stress in diabetic patients; a randomized, double-blind placebo-controlled clinical trial. Biomed Pharmacother. 2004;58:546–50. doi: 10.1016/j.biopha.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Kosugi T, Nakayama T, Heinig M, Zhang L, Yuzawa Y, Sanchez-Lozada LG, et al. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol. 2009;297:F481–F488. doi: 10.1152/ajprenal.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4:128–132. [PubMed] [Google Scholar]
- 10.Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51(4):1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 11.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 12.Inkster ME, Cotter MA, Cameron NE. Treatment with the xanthine oxidase inhibitor, allopurinol, improves nerve and vascular function in diabetic rats. Eur J Pharmacol. 2007;561:63–71. doi: 10.1016/j.ejphar.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto S, Koshiishi I, Inoguchi T, Nawata H, Utsumi H. Confirmation of superoxide generation via xanthine oxidase in streptozotocin-induced diabetic mice. Free Radic Res. 2003;37:767–772. doi: 10.1080/1071576031000107344. [DOI] [PubMed] [Google Scholar]
- 14.Kushiyama A, Tanaka K, Hara S, Kawazu S. Linking uric acid metabolism to diabetic complications. World J Diabetes. 2014;5:787–795. doi: 10.4239/wjd.v5.i6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JJ, Yang IH, Kuo HK, Chung MS, Chen YJ, Chen CH, et al. Serum uric acid concentration is associated with worsening in severity of diabetic retinopathy among type 2 diabetic patients in Taiwan--a 3-year prospective study. Diabetes Res Clin Pract. 2014;106:366–372. doi: 10.1016/j.diabres.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Mohora M, Virgolici B, Coman A, Muscurel C, Gaman L, Gruia V, et al. Diabetic foot patients with and without retinopathy and plasma oxidative stress. Rom J Intern Med. 2007;45:51–57. [PubMed] [Google Scholar]
- 17.Xia J, Wang Z, Zhang F. Association between related purine metabolites and diabetic retinopathy in type 2 diabetic patients. Int J Endocrinol 2014. 2014 doi: 10.1155/2014/651050. 651050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munteanu M, Sturza A, Schiller A, Timar R. Endothelial dysfunction in diabetes–clasic sources of vascular oxidative stress (nadph oxidases, enos uncoupling and xanthine oxidase) Rom J Diabetes Nut Metabol Dis. 2013;20:149–155. [Google Scholar]
- 19.Minaiyan M, Ghannadi A, Movahedian A, Hakim-Elahi I. Effect of Hordeum vulgare L. (Barley) on blood glucose levels of normal and STZ-induced diabetic rats. Res Pharm Sci. 2014;9:173–178. [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CC, Hsu YJ, Lee TM. Impact of elevated uric acid on ventricular remodeling in infarcted rats with experimental hyperuricemia. Am J Physiol Heart Circ Physiol. 2011;301:H1107–H1117. doi: 10.1152/ajpheart.01071.2010. [DOI] [PubMed] [Google Scholar]
- 21.Firuzi O, Shakibazad N, Amoozgar H, Borzoee M, Abtahi S, Ajami G, et al. Effects of omega-3 polyunsaturated fatty acids on heart function and oxidative stress biomarkers in pediatric patients with dilated cardiomyopathy. Int Cardiovasc Res J. 2013;7:8–14. [PMC free article] [PubMed] [Google Scholar]
- 22.Dilsiz N, Sahaboglu A, Yildiz MZ, Reichenbach A. Protective effects of various antioxidants during ischemia-reperfusion in the rat retina. Graefes Arch Clin Exp Ophthalmol. 2006;244:627–633. doi: 10.1007/s00417-005-0084-6. [DOI] [PubMed] [Google Scholar]
- 23.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, et al. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 24.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai AK, Lo AC. Animal models of diabetic retinopathy: summary and comparison. J Diabetes Res 2013. 2013 doi: 10.1155/2013/106594. 106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, et al. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal. 2015;23:1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson MJ, Gopaul N, Davies IR, Hughes DA, Carrier MJ. Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care. 2002;25:537–541. doi: 10.2337/diacare.25.3.537. [DOI] [PubMed] [Google Scholar]
- 28.Kusari J, Zhou S, Padillo E, Clarke KG, Gil DW. Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2007;48:5152–5159. doi: 10.1167/iovs.07-0427. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des. 2007;13(26):2699–2712. doi: 10.2174/138161207781662920. [DOI] [PubMed] [Google Scholar]
- 30.Liu YJ, Lian ZY, Liu G, Zhou HY, Yang HJ. RNA sequencing reveals retinal transcriptome changes in STZinduced diabetic rats. Mol Med Rep. 2016;13:2101–2109. doi: 10.3892/mmr.2016.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xin H, Zhou F, Liu T, Li GY, Liu J, Gao ZZ, et al. Icariin ameliorates streptozotocin-induced diabetic retinopathy in vitro and in vivo. Int J Mol Sci. 2012;13:866–878. doi: 10.3390/ijms13010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:1156–1163. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung KI, Kim JH, Park HY, Park CK. Neuroprotective effects of cilostazol on retinal ganglion cell damage in diabetic rats. J Pharmacol Exp Ther. 2013;345:457–463. doi: 10.1124/jpet.113.203067. [DOI] [PubMed] [Google Scholar]
- 34.Kohzaki K, Vingrys AJ, Bui BV. Early inner retinal dysfunction in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2008;49:3595–3604. doi: 10.1167/iovs.08-1679. [DOI] [PubMed] [Google Scholar]
- 35.Nasralah Z, Robinson W, Jackson GR, Barber AJ. Measuring visual function in diabetic retinopathy: progress in basic and clinical research. J Clin Exp Ophthalmol 2013. 2013 [Google Scholar]
- 36.Hou SZ, Liang CY, Liu HZ, Zhu DM, Wu YY, Liang J, et al. Dendrobium officinale prevents early complications in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2016;2016:6385850. doi: 10.1155/2016/6385850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: electro-retinographical and morphological observations. Exp Eye Res. 2002;74:615–625. doi: 10.1006/exer.2002.1170. [DOI] [PubMed] [Google Scholar]