Abstract
Introduction:
Biomarkers predictive of response to mechanistic target of rapamycin (mTOR) inhibitor, everolimus, in endocrine receptor (ER)-positive metastatic breast cancer (MBC) are a work in progress. We evaluated the feasibility of directly measuring mTOR activity and phosphatase and tensin homolog (PTEN) expression and correlating their expression with response and survival.
Materials and Methods:
MBC patients who received everolimus with endocrine therapy (ET) after progression on an aromatase inhibitor and had adequate tissue preservation for estimation of mTOR activity and PTEN expression were selected for analysis from a prospectively maintained database. Progression-free survival (PFS) and overall survival (OS) were estimated by Kaplan–Meier method, and correlation between mTOR activity and PTEN expression with survival was done by log-rank test.
Results:
Thirteen ER-positive MBC patients were available for analysis. PTEN expression was lost in 11/13 (84.6%) patients and retained in 2/13 patients (15.4%). mTOR activity was absent in four patients (30.7%), weak in six patients (46.1%), and moderate in 3 patients (23.2%). Median PFS for the entire population was 2.5 months while median OS was not reached. Patients with an absent mTOR activity showed a longer PFS (5 vs. 1.5 vs. 2 months) than those with weak and moderate activity, respectively (P = 0.043). There was no correlation between loss of PTEN expression and PFS.
Conclusions:
Measurement of direct mTOR activity in patients with MBC receiving everolimus/ET combination appears feasible. Absent mTOR activity may predict for longer PFS with everolimus-ET combination and requires further study.
Keywords: Everolimus, mechanistic target of rapamycin, metastatic breast cancer, phosphatase and tensin homolog
Introduction
PI3K/Akt/mechanistic target of rapamycin (mTOR) is a canonical intracellular signaling pathway with a well-established role in tumor cell growth and proliferation.[1] Akt-induced activation of the endocrine receptor (ER) pathway, irrespective of estrogen availability, is a known mechanism of resistance to endocrine therapy (ET), and evidence has shown that a combination of mTOR inhibitors and ET can overcome this resistance in metastatic breast cancers (MBCs).[2]
ER-positive tumors constitute approximately 75% of patients with MBC, with ET recommended as the standard first line of care in this subgroup of patients.[3,4] Postprogression on first-line ET, everolimus combined with ET has gained ground as the recommended second-line option based primarily on the Phase 3 BOLERO-2 trial, which showed a meaningful progression-free survival (PFS) benefit over ET alone.[5,6]
While the histological subtyping of breast cancers and gene expression profiling-based classification of breast cancers has helped direct therapy, validated biomarkers suggestive of response or resistance to mTOR inhibitors are a work in progress. Markers that have generated interest include p70 S6 kinase (S6K1), 4E-BP1, and phosphatase and tensin homolog (PTEN) loss/PIK3CA mutations.[7,8] There is also some early data on measuring mTOR activity itself or mTOR mutations though data regarding its correlation with response to mTOR inhibitors are lacking.[9,10]
With a primary aim of evaluating the feasibility of examining potential biomarkers in patients with ER-positive MBC receiving everolimus with ET, an exploratory retrospective study was conducted to quantify mTOR activity and PTEN deleted on chromosome 10 expression and correlate this expression with response and survival.
Materials and Methods
A prospectively maintained database of MBC patients having received everolimus from March 2012 to June 2014 was audited, and patients who had adequate tissue preservation for estimation of mTOR activity and PTEN expression were selected for analysis. Inclusion criteria were –
Patients should have received everolimus with ET after progression on an aromatase inhibitor (AI)
Adequate tissue specimen available for evaluation mTOR activity and PTEN expression.
Protocol for measurement of mechanistic target of rapamycin activity and phosphatase and tensin homolog expression
Deparaffinization
The histopathology specimen was deparaffinized by incubating the sections in 3 washes of xylene for 5 min each, 2 washes of 100% ethanol for 10 min each, and 2 washes of 90% ethanol for 10 min each followed by 2 washes in distilled H20 (dH20) for 2 min each.
Antigen unmasking
For antigen unmasking of mTOR, citrate (at pH 6) was used with the section being incubated for 40 min in water bath at 98°C. This was followed by cooling the slides on bench top for 30 min.
For antigen unmasking of PTEN, TE-Tris ethylenediamine tetraacetic acid (at pH 9) was used with incubation in pressure cooker for 2 whistles. Slides were then cooled on bench top for 30 min.
Staining
The sections are washed once in TBS (Triss- Buffered Saline) for 5 min with 0.1% Tween-20. They are then incubated in 3% H2O2 in the dark for 10 min followed by 2 repeat washes in TBS for 5 min with 0.1% Tween-20.
Blocking
The section is blocked by 100–400 ml followed by draining and blotting without washing. 100-400 μl primary antibody diluted in recommended antibody diluent is added to each section (kit recommendation) (dilution for mTOR: 1:100, and for PTEN is 1:100, Dako).
After dilution, mTOR slide is incubated overnight at 4°C while PTEN slide is incubated for 90 min in room temperature. Postincubation, antibody solution is removed and sections washed in 1X TBS for 5 min each with 0.1% Tween-20 twice. The amplifier is added and section incubated for 5–10 min at room temperature (provided by kit, Thermo scientific). Amplifier is then removed and section washed in 1X TBS for 5 min each with 0.1% Tween-20 twice. Secondary antibody plus enzyme (polymer) provided by kit (Thermofisher) is added and incubated in room temperature for 10 min in the dark. Excess solution is removed and sections washed in 1X TBS for 5 min once followed by addition of 100–400 μl DAB substrate to each section. Once the sections develop, slides are immersed in dH20 for 5 min followed by counterstaining with hematoxylin-eosin and repeat washing in distilled H20 for 5 min.
Dehydration of sections
The procedure is as follows:
Incubate sections in 90% ethanol two times for 5 min each
Repeat in 100% ethanol, incubating sections two times for 5 min each
Repeat in xylene, incubating sections 3 times for 5 min each.
This was followed by evaluation of activity under microscope.
Positive and negative controls are also run along with test slides according to kit instructions.
Clinical variables
Response rates – complete response, partial response, and stable disease were calculated. Response rates were measured by RECIST criteria or by clinical examination. Toxicity was recorded by National Cancer Institute Common Toxicology Criteria for Adverse Events 4 (NCI CTCAE 4.0).
Statistical analysis
Standard summary statistics were used for continuous and discrete variables. Association between mTOR activity and PTEN expression with PFS and overall survival (OS) was analyzed by the log-rank test. PFS and OS were calculated by the Kaplan–Meier product-limit method. PFS was calculated from date of the first dose of everolimus to date of documented progression or last date of follow-up. OS was calculated from date of the first dose of everolimus to date of death.
Results
Thirteen patients with ER-positive MBC, pretreated with at least one prior line of AI, and treated with everolimus with ET, had adequate biopsy samples for biomarker evaluation. Their baseline characteristics, responses to therapy, and survival are shown in Table 1. Four patients did not have a response assessment because the everolimus-ET combination was stopped prematurely before a response assessment was possible.
Table 1.
Baseline patient characteristics and survival
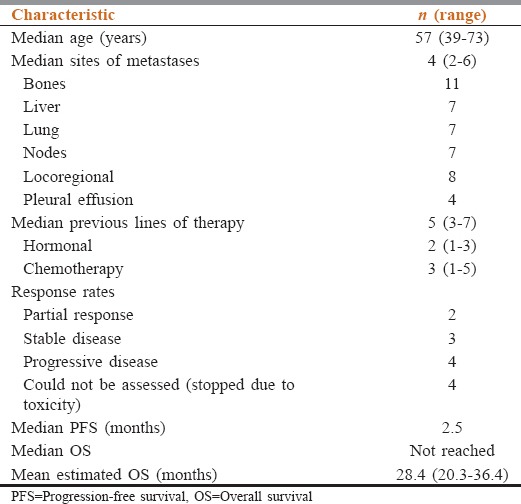
PTEN activity was lost in 11/13 patients (84.6%) and retained in the remaining (15.4%). mTOR activity was absent in 4 patients (30.7%), weak in 6 patients (46.1%), and moderate in 3 patients (23.2%).
Median PFS for the entire population was 2.5 months (range 1.5–10.5 months) while median OS was not reached. Loss of PTEN expression showed no correlation with PFS (P = 0.462). Patients with an absent mTOR activity showed a longer PFS compared to those with weak/moderate activity (5 vs. 1.5 vs. 2 months). This difference was statistically significant (P = 0.043) [Figure 1]. There was no correlation between retention of PTEN expression and OS (P = 0.584). There was also no correlation between patients with absent mTOR activity versus patients with weak and moderate activity with respect to OS (P = 0.241).
Figure 1.
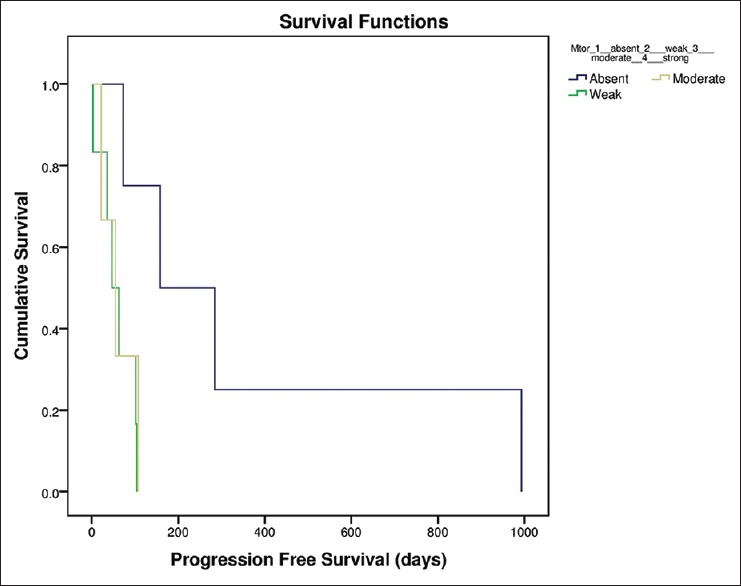
Mechanistic target of rapamycin progression-free survival
Discussion
mTOR is actually a combination of two separate multiprotein complexes: mTOR complex 1 (mTORC1) and mTOR complex 2. The activity of the rapamycin analogs is purportedly due to their inhibition of mTORC1, which itself is a component of the intracellular growth-signaling pathway consisting receptor tyrosine kinase, PI3K, PIP3, and Akt. While phosphorylated Akt actively inhibits tumor suppressor protein and activates mTORC1, PTEN actively inhibits this pathway and thus loss of PTEN activity ensures a constantly activated mTORC1. Downstream targets of mTORC1 include 4E-BP1 and S6 kinase 1, which functions in the G1 phase of the cell cycle and encodes ribosomal proteins, facilitating translation.[11]
A majority of the components of this intracellular pathway have been under evaluation as potential biomarkers of activity or resistance with mTOR inhibitors. Our study focused on mTOR activity and PTEN expression. While a number of studies have used S6K1 activity and its subsequent reduction as a biomarker of response to mTOR inhibitors,[12,13] we used direct measurement of mTOR antigen activity. mTOR activity was absent or weak in 76.8% (10/13 patients), and there was some suggestion that patients with absent activity had better PFS compared to patients with weak/moderate activity and this reached statistical significance (P = 0.043). The small numbers in this study imply that this particular aspect needs further study. While this method of mTOR activity estimation needs much larger studies and validation, it potentially circumvents some of the problems associated with using S6K1 activity as a surrogate for mTOR inhibition.[14] S6K activity is elevated in many cancer cells, but whether it truly is tumorigenic is questionable, as its elevation may simply be an endpoint of alterations occurring prior in the pathway. There is also evidence to suggest that cells “sensitive” as well as “insensitive” to rapamycin analogs may have decrease in S6K1 activity, thereby decreasing its sensitivity.[15] Concurrent or prior chemotherapy used may also alter S6K1 levels, further complicating its analysis as a specific marker of mTOR inhibition.[16,17] The results of our study may also appear contrary to studies which have shown that high levels of S6K1 and potentially, pAKT, as markers of activation of the mTORC pathway may be predictive of response to mTOR inhibitors; however, while downstream signals have been better characterized as biomarkers, upstream markers such as the mTOR activity itself or PIK3CA status correlate less well as biomarkers[14,18]
PTEN is a well-characterized tumor suppressor, coded by the PTEN gene on chromosome 10q23, with loss of its function being a well-known determinant for the development of cancers.[19] Ideally, PTEN status determination by DNA sequencing and protein quantification are complementary and both are required for a correct estimation of activity. In our study, we directly measured PTEN activity, with a dichotomous distribution into “lost” (11/13) and “retained” (2/13). The small numbers in this analysis precluded any meaningful correlation with outcomes, i.e., there was no statistically significant difference in OS in patients with either “lost” or “retained” activity. Animal models have shown that mTOR inhibitors probably act downstream to PTEN and may reduce neoplastic proliferation and tumor size;[20] conversely, loss of PTEN may increase resistance to endocrine therapies.[21] However, preclinical and clinical data are not conclusive on whether mTOR inhibitors are effective in patients with PTEN loss with studies showing contrary results.[22,23]
Translational components of the BOLERO-2 (using next generation sequencing) and TAMRAD (using IHC, Sanger sequencing and NGS) evaluated a number of potential biomarkers such as PIK3CA, PTEN, pAkt, 4E-BP1, FGFR1, and S6RP amongst others for response to everolimus.[10,24] While the TAMRAD analysis showed that patients with high p4E-BP1, low 4E-BP1, low LKB1, low pAkt, and low PI3K were likely to attain more benefit with tamoxifen/everolimus combination, the BOLERO-2 subgroup analysis observed quantitative differences in response to everolimus-ET combination for PIK3CA exon-specific mutations (exon 20 vs. exon 9).
Our study is a pilot study which attempted to examine the feasibility of direct estimation of mTOR activity (instead of using surrogates) and PTEN expression in a small series of patients. It is a hypothesis generating study, which calls for further analysis concerning the direct measurement of mTOR activity as a biomarker of response to mTOR inhibitors. Whether absent direct mTOR activity (as opposed to weak/moderate activity) predicts for longer PFS with everolimus/ET, as seen in this small cohort, also requires further analysis. Assessment of PTEN activity was also carried out and appears feasible for larger application in prospective studies. Further studies, possibly with the addition of PIK3CA and other markers may help in identifying a combination of viable biomarkers predicting for response or resistance to mTOR inhibitors.
Conclusions
Measurement of direct mTOR activity in patients with MBC receiving everolimus/ET combination is feasible and requires validation in larger studies as an accurate method of biomarker analysis. Absent mTOR activity may predict for longer PFS with everolimus-ET combination and requires further study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 2.Paplomata E, O’Regan R. New and emerging treatments for Estrogen receptor-positive breast cancer: Focus on everolimus. Ther Clin Risk Manag. 2013;9:27–36. doi: 10.2147/TCRM.S30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–9. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 4.Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: Meta-analysis. J Natl Cancer Inst. 2006;98:1285–91. doi: 10.1093/jnci/djj357. [DOI] [PubMed] [Google Scholar]
- 5.Yardley DA, Noguchi S, Pritchard KI, Burris HA, 3rd, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–84. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Overall survival results from BOLERO-2†. Ann Oncol. 2014;25:2357–62. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–23. doi: 10.1158/1078-0432.ccr-03-0043. [DOI] [PubMed] [Google Scholar]
- 8.Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do KA, et al. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin Cancer Res. 2012;18:5816–28. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dekter HE, Romijn FP, Temmink WP, van Pelt J, de Fijter JW, Smit NP, et al. A spectrophotometric assay for routine measurement of mammalian target of rapamycin activity in cell lysates. Anal Biochem. 2010;403:79–87. doi: 10.1016/j.ab.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Hortobagyi GN, Chen D, Piccart M, Rugo HS, Burris HA, 3rd, Pritchard KI, et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Results from BOLERO-2. J Clin Oncol. 2016;34:419–26. doi: 10.1200/JCO.2014.60.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21:656–64. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulay A, Zumstein-Mecker S, Stephan C, Beuvink I, Zilbermann F, Haller R, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–61. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka C, O’Reilly T, Kovarik JM, Shand N, Hazell K, Judson I, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26:1596–602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 14.O’Reilly T, McSheehy PM. Biomarker development for the clinical activity of the mTOR inhibitor everolimus (RAD001): Processes, limitations, and further proposals. Transl Oncol. 2010;3:65–79. doi: 10.1593/tlo.09277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane HA, Wood JM, McSheehy PM, Allegrini PR, Boulay A, Brueggen J, et al. MTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009;15:1612–22. doi: 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 16.Jeon YJ, Kim IK, Hong SH, Nan H, Kim HJ, Lee HJ, et al. Ribosomal protein S6 is a selective mediator of TRAIL-apoptotic signaling. Oncogene. 2008;27:4344–52. doi: 10.1038/onc.2008.73. [DOI] [PubMed] [Google Scholar]
- 17.Tee AR, Proud CG. DNA-damaging agents cause inactivation of translational regulators linked to mTOR signalling. Oncogene. 2000;19:3021–31. doi: 10.1038/sj.onc.1203622. [DOI] [PubMed] [Google Scholar]
- 18.Slamon DJ, Hurvitz SA, Chen D, Andre F, Tseng LM, Jerusalem GH, et al. Predictive biomarkers of everolimus efficacy in HER2+ advanced breast cancer: Combined exploratory analysis from BOLERO-1 and BOLERO-3. ASCO Meet Abstr. 2015;33(15 Suppl):512. [Google Scholar]
- 19.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 20.Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice. Proc Natl Acad Sci U S A. 2001;98:10320–5. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillon LM, Miller TW. Therapeutic targeting of cancers with loss of PTEN function. Curr Drug Targets. 2014;15:65–79. doi: 10.2174/1389450114666140106100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O’Bryant CL, et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–82. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 23.Weigelt B, Warne PH, Downward J. PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene. 2011;30:3222–33. doi: 10.1038/onc.2011.42. [DOI] [PubMed] [Google Scholar]
- 24.Treilleux I, Arnedos M, Cropet C, Wang Q, Ferrero JM, Abadie-Lacourtoisie S, et al. Translational studies within the TAMRAD randomized GINECO trial: Evidence for mTORC1 activation marker as a predictive factor for everolimus efficacy in advanced breast cancer. Ann Oncol. 2015;26:120–5. doi: 10.1093/annonc/mdu497. [DOI] [PubMed] [Google Scholar]


