Abstract
Human papillomavirus (HPV) associated head and neck squamous cell cancers (HNSCC) have become increasingly common in the West, but the same cannot be said about India. These cancers have a different biology and confer a better prognosis, however, its current role in the management of patients in India is not clearly defined. At the 35th Indian Cooperative Oncology Network conference held in September 2016, a panel of radiation, surgical and medical oncologists, pathologists, and basic scientists from across the country having experience in clinical research with respect to HPV in HNSCC reviewed the available literature from India. All the ideas and facts were thereafter collated in this report. Various topics of controversy in dealing with the diagnosis and management of HPV-associated HNSCC have been highlighted in this report in context to the Indian scenario. Furthermore, the prevalence of the same and its association with tobacco and high-risk sexual behavior has been touched on. Conclusively, a set of recommendations has been proposed by the panel to guide the practicing oncologists of the country while dealing with HPV-associated HNSCC.
Keywords: Consensus recommendations, head and neck squamous cell cancers, human papillomavirus, India
Introduction
Human papillomavirus (HPV) associated head and neck squamous cell cancers (HNSCC) have generated significant research interest in recent times. With the changing paradigm of risk factors in the West,[1] there has been a frenzy of research in the past decade.
This article is an attempt to present the work done in India, and establish the current status of the role of HPV in HNSCC in India. Furthermore, the issues relevant to the Indian practice are highlighted with particular emphasis on its prevalence, interaction with tobacco and standardization of detection methods. This will hopefully be a practical guide for the oncologist and serve as a benchmark for the future work in India.
Methods
In September 2016, the ICON: Indian Cooperative Oncology Network held its 35th conference in Mumbai, India, wherein it invited a panel of radiation, surgical and medical oncologists, pathologists, and basic scientists from across the country having experience in clinical research with respect to HPV in HNSCC to identify its current status and role in India. Four weeks before the meeting subtopics were decided to be debated on. These subtopics were distributed among the participants for reviewing the available literature. A draft was then made comprising the important points needed to be highlighted at the meeting.
After detailed discussion on these topics at the meeting and follow-up of resolutions and queries/issues, all the ideas and facts were collated in this report to provide an insight to the practicing oncologists in the country regarding the current status of HPV in HNSCC in India. While data from the West has been cited at places, an attempt has been made to base this report on the work done in India in the Indian context.
Discussion
Tobacco burden and head and neck squamous cell cancers
Tobacco and alcohol consumption are well-established primary risk factors for HNSCC worldwide. The risk of HNSCC in a smoker is up to 10 times as compared to a never smoker depending on the duration and quantity of smoking.[2] In India, 60% of tobacco users use smokeless tobacco, which increases the relative risk of oral cancers to as high as 15.0 as compared to never users.[3] In the Global Adult Tobacco Survey conducted in 2009–2010, 34.6% of the Indian adult population used tobacco. Of these, 25.1% were only smokers, 59.5% were only smokeless tobacco users, and the remaining 15.4% were both smokers and smokeless tobacco users.[4]
A decrease in the incidence of HNSCC has been noted in the West paralleling the decrease in smoking;[5] however, there has been an unusual increase in the incidence of oropharyngeal cancers from 16.3% during the 1980s to 72.7% during the 2000s in the USA,[1] and other developed countries.[6] This led to the exploration of alternate causes of carcinogenesis in these cases. Many epidemiological and laboratory studies have attributed HPV infection of the oral cavity and oropharynx in the causation of cancer of the upper aerodigestive tract, especially the oropharynx.
In India and other developing nations, consumption of tobacco has been relatively stable over the years. Most studies from India report a history of tobacco use in nearly 80% patients[7,8,9,10,11,12] Mishra et al. reported the prevalence of cigarette and bidi smoking in India to have decreased by about 5% among males from 1998 to 2015. However, this modest decrease in tobacco consumption has been offset by the rising population, increasing the absolute numbers of smokers between the age groups of 15–69 years from 79 million in 1998 to 108 million in 2015.[13] A report analyzing the Mumbai Cancer Registry found that the rates of oral cancer have been steadily rising over 15 years, from 1995 to 2009, at the rate of 2.7% annually.[14] A study from South India reported the overall incidence of HNSCC to have been decreasing over 10 years from 1986 to 98, however, a trend toward rising incidence of oral tongue cancers was noted.[15]
Human papillomavirus in the Indian community and high-risk sexual behavior
There is sparse Indian literature describing HPV infection of the oral cavity in the general population and the two existing studies are highly discordant. In one, the saliva rinse of 396 normal individuals from various parts of North Karnataka was collected and polymerase chain reaction (PCR) based high-risk (HR) HPV genotyping was carried out. It reported 2.75% were positive for HPV16 and 22% positive for HPV18.[16] In another study, oral mucosal smears were prepared from 60 healthy individuals and PCR-based HR-HPV genotyping was carried out. It reported 65% of individuals to be positive for HPV16/18.[17] Western literature reports a low prevalence of HPV infection (<10%) in normal oral mucosa[18,19,20,21,22] There is not enough evidence to clearly establish the role of latent HPV infection of the oral cavity in normal individuals in the development of cancer. Western literature indicated an association between HR sexual behavior (oral sex and number of sexual partners) and HNSCC.[23] Indian data are sparse in this respect as eliciting detailed sexual history is usually uncomfortable for the patients as well as the clinicians. Bahl et al. reported HR sexual behavior in 58% of patients with oropharyngeal carcinoma who tested positive for HPV DNA, which was significantly higher than the HPV DNA negative cohort.[11]
Interaction of tobacco and human papillomavirus
Indian data show discordant results for the interaction between tobacco and HPV correlation. Bahl et al. showed that in the North Indian population with oropharyngeal cancer, the incidence of HPV positivity did not significantly correlate with tobacco or alcohol consumption.[11] On the other hand, a recent study conducted in Northeast India showed significant association between HR-HPV DNA positivity and tobacco chewing and alcohol consumption among patients with HNSCC, however, no significant association was shown with tobacco smoking.[10] The lack of clarity in the Indian studies regarding the association may be due to (a) very small proportion of nontobacco users compared to users in HNSCC and (b) low incidence of HPV positivity (see below) in HNSCC.
Prevalence of human papillomavirus-related head and neck squamous cell cancers in India
Oral cavity
HPV prevalence in squamous cell carcinomas (SCC) of the oral cavity in India has been reported to be 33.6% in the Eastern region,[24] 48% in South India,[25] 15% in West India,[26] 27.5% in Central India,[27] and 28% in Northeast India.[10] Koppikar et al. detected HR-HPV subtypes in only 6% of patients with oral SCC.[28] This shows a great discrepancy in the HPV positivity rates among patients with Oral SCC, probably attributable to the different techniques of HPV detection and reporting. A majority of HPV-positive oral SCC in these studies were oral tongue cancers. A couple of studies from South India have shown the incidence of HPV positivity in oral tongue cancers being as high as 51.2%[29] and 48%.[25]
In the developed nations, the incidence of HPV positivity in oral SCC is also reported to be highly variable ranging from as low as 10% to as high as 80%.[30]
The clinical significance of the presence of HPV in oral cancers is currently unknown.
Oropharynx
There is substantial Western literature on the prevalence of HPV in oropharyngeal SCC reported to be between 28% to 68%.[31,32,33,34] A recent systematic review and meta-analysis showed a prevalence of 47.7% HPV-positive oropharyngeal cancers.[35] Ironically, while the prognostic impact of HPV positivity is clearly proven in oropharyngeal cancer, the Indian data are sparse. Bahl et al. reported 22.8% HPV positivity in 105 oropharyngeal cancer patients.[11] A similar incidence of 20% was reported by Murthy et al.[12] and 15% by Sannigrahi et al. in patients with oropharyngeal cancer.[9]
Larynx and hypopharynx
Due to the lack of prognostic impact, the prevalence of HPV has not been extensively studied in laryngeal and hypopharyngeal cancers. The reported prevalence of HPV in laryngeal cancer and hypopharyngeal cancer varies widely ranging from 3% to 85%[36,37,38] and 4.8% to 34%,[39,40] respectively. However, the meta-analysis by Mehanna et al., the HPV prevalence in nonoropharyngeal sites was 21.8% with a declining trend with time.[35] Indian data show a prevalence of HPV-related laryngeal and hypopharyngeal cancers ranging from 5% to 20%[9,10,12]
Human papillomavirus virology and methods of detection
HPV is a nonenveloped, double-stranded DNA virus belonging to Papillomaviridaefamily which infects the epithelial cells of mucosa and skin. Nearly 120 types of HPV have been completely sequenced which are broadly divided into “low-risk” and “HR” types, based on their oncogenic potential. The viral DNA contains “early” genes encoding 6 proteins, namely, E1, E2, E4-E7, and “late” genes encoding 2 proteins, namely, L1 and L2. E6 and E7 are mainly responsible for the oncogenic activity of the virus. E6 degrades p53, thereby limiting its activity of suppressing the cell cycle progression and inducing apoptosis. E7 destabilizes Rb protein thereby activating the cyclin-dependent kinases at G1-S phase checkpoint of the cell cycle, thus promoting cellular proliferation. As a feedback mechanism, there occurs an increase in p16 expression, in an attempt to curb the cell cycle progression. Disruption of these major regulatory pathways ultimately leads to cellular transformation and immortalization.
Methods of detection of human papillomavirus
As HPV-related HNSCC have a significantly different clinical behavior and prognosis (as discussed later), it is critical that these tumors be identified in an accurate and cost effective manner. A number of methods are used in the diagnosis of HPV-associated HNSCC. The information about different methodologies, their principles, advantages, and disadvantages is essential to understand and choose the most appropriate method for the diagnosis of HPV-related HNSCC [Table 1].
Table 1.
Comparison of various human papillomavirus testing methods
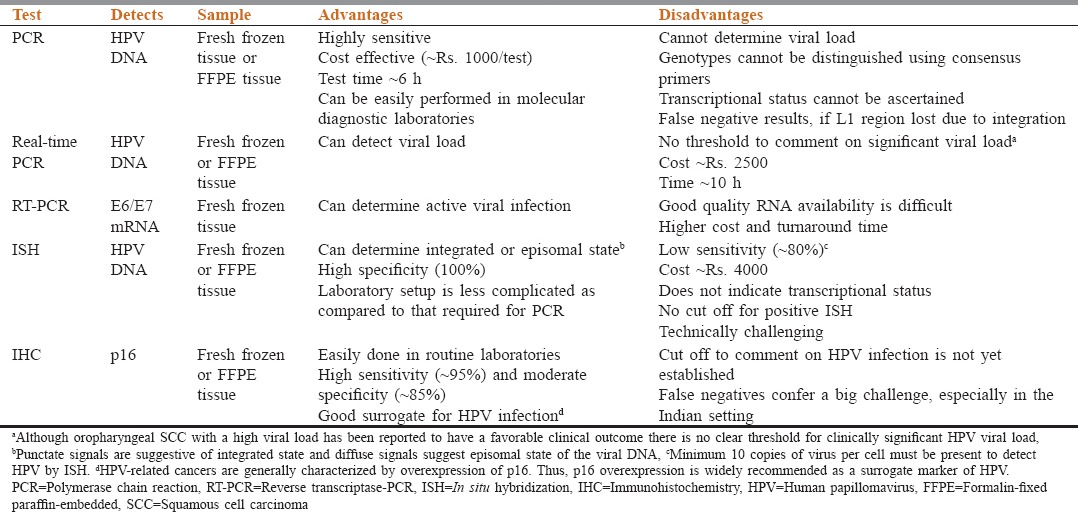
Although oropharyngeal SCC with a high viral load has been reported to have a favorable clinical outcome,[41] there is no clear threshold for clinically significant HPV viral load
Punctate signals are suggestive of integrated state and diffuse signals suggest episomal state of the viral DNA[42]
Minimum 10 copies of virus per cell must be present to detect HPV by in situ hybridization (ISH)[43]
HPV-related cancers are generally characterized by overexpression of p16. Thus, p16 overexpression is widely recommended as a surrogate marker of HPV.[44,45]
Immunohistochemistry for p16
There are several technical and practical questions which are still unaddressed and it is therefore important for the clinicians and pathologists to consider the following, before the intended use of immunohistochemistry (IHC) for p16 in patients with HNSCC as a routine test.
Choice of p16 antibody used for immunohistochemistry staining
Several p16 antibodies are available commercially, but their performance is yet to be compared.[46] E6H4™ clone of p16 antibody used as a component of CINtec p16 Histology Kit (Roche) is the most widely used clone and it has been validated in several studies. Therefore, its use in p16 IHC is strongly recommended, due to excellent interobserver concordance.[47,48]
Need for a clear scoring system for p16 immunohistochemistry staining
Various studies have chosen different cutoff values for a positive p16 staining pattern, widely ranging from >1% to >70%[49,50,51,52,53,54]
A recent systematic review by Grønhøj Larsen et al.[55] concluded that reliability of p16 staining to predict HPV is much better when cutoff for staining is set at >70% of diffused cytoplasmic and nuclear staining. Chen et al.[56] demonstrated that cytoplasmic staining <30% of positively stained tumor cells were HPV negative whereas diffuse nuclear and cytoplasmic staining in the range 50%–90% were HPV positive.
The American Society for Clinical Pathology and College of American Pathologists are of the opinion that diffuse cytoplasmic and nuclear staining in >70% tumor cells must be a criteria for p16 staining to be regarded as positive.[57]
Thus, it may be most appropriate to set the cutoff to be at least >50% (preferably ≥70%) diffuse nuclear and cytoplasmic staining labeling as p16 positive [Figure 1].
Figure 1.
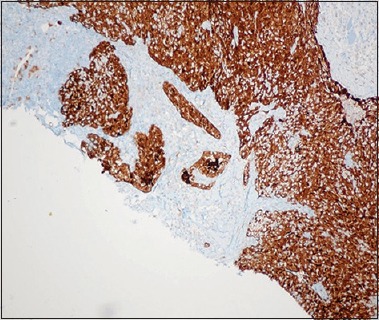
p16 IHC showing >70% staining
Is p16 staining alone sufficient for high-risk human papillomavirus infection?
None of the methods described can be labeled as an ideal test for HPV, offering optimal sensitivity, specificity, and cost effectiveness. It has therefore been suggested to combine different HPV tests (particularly p16 IHC and PCR) to minimize their individual limitation and yet optimally utilize their combined strength.
Thavaraj et al.[58] proposed use of p16 as a triage test, followed by DNA ISH. The discrepant cases p16(+)/ISH(−) were subjected to PCR assay. Using this approach, the authors could classify 98% of cases as either HPV (+) or HPV (−) and discrepant cases reduced to a minor subset of 2%.
Although the accuracy of the result increases steeply on combining multiple tests, performing multiple steps to achieve the results is cumbersome and drastically increases the test cost, limiting its practical use. This may, however, be the best approach in treatment de-escalation trials.
Is there other underlying mechanism in discordant cases?
Several studies have reported discordance between HPV DNA and p16 tests. A wide range of cases with HPV-positive oropharyngeal squamous cell carcinoma (OPSCC) that is p16 negative has been reported.[9,8,12,58,59,60,61] It has been postulated that the absence p16 overexpression may be a result of epigenetic mechanism, namely, p16 methylation, driven by prolonged tobacco exposure.[62] The significant discordance in the p16 and PCR positivity, particularly in India, with a high tobacco burden, may be attributed to p16 hypermethylation.
Clinicopathological features of human papillomavirus-related head and neck squamous cell cancers
HPV-related HNSCC, usually in the oropharynx, presents with an early stage primary tumor and an advanced stage nodal disease (N2/N3).[63] Association of smoking and HPV-related HNSCC is not clear as discussed previously. HR sexual behavior is considered a potential risk factor for HPV infection.[64] Murthy et al.[12] showed a relatively higher incidence of HPV-related HNSCC in females. Prototypic HNSCC are generally moderately differentiated, whereas HPV-induced SCC are predominantly nonkeratinizing SCC and may be often described as poorly differentiated SCC or basaloid carcinomas exhibiting lobular growth of cells with hyperchromatic nuclei, scanty cytoplasm, and mitotic figures.[65,66,67]
Prognosis and survival in human papillomavirus-related head and neck squamous cell cancers
A review and meta-analysis of multiple retrospective series showed that HPV-related HNSCC have a significantly better overall survival (OS) and disease-free survival (DFS).[68] A site-specific analysis revealed that for nonoropharyngeal HNSCC OS and DFS were similar for both HPV-positive and HPV-negative patients.[68] Another report from a prospective clinical trial showed similar benefit in survival for HPV-positive HNSCC.[69] Tobacco use increases the risk of death by 1% with each additional pack year irrespective of HPV positivity[70] thus negating any survival benefit gained with the presence of active HPV infection. The prognostic implications of HPV have led to the proposal of a new staging system for HPV-positive oropharyngeal SCC by the International Collaboration for Oropharyngeal cancer Network for Staging which has been recommended for incorporation into the 8th edition of the AJCC.[63] From India, Elango et al. have compared the outcomes of patients with HPV-positive and negative oral tongue SCC. They found no significant difference in OS between the two groups, however, a significantly lower rate of disease recurrence at 2 years was seen in the HPV-positive group (7% vs. 32%; P = 0.014).[25] Murthy et al. have reported the 5y OS to be nonsignificant between HPV-positive and HPV-negative oropharyngeal carcinomas (56% vs. 54%).[12] They attributed tobacco use in the reduction of the OS benefit gained with HPV positivity and showed a large and clinically significant improvement in OS and cancer-specific survival among p16+ve tobacco nonusers.
Treatment de-escalation strategies in human papillomavirus-related head and neck squamous cell cancers
Considering the better outcomes seen with HPV-positive OPSCC, an attempt is being made to reduce the toxicity of treatment without affecting the disease outcomes. Various strategies are being tried under Phase 2 or 3 clinical trial settings to achieve this objective, including change of concurrent chemotherapy or use of biological therapy or reduction in RT doses/volumes after response to induction chemotherapy or addition of surgery as the primary treatment modality. With the advent of robotic technology, use of transoral robotic surgery is also evolving. It must be emphasized that all these strategies are currently under investigation and must not be practiced routinely outside a clinical trial.
Panel recommendations and guidance
Based on the panel's discussions, the recommendations while dealing with the diagnosis and management of HPV-related HNSCC in clinical practice is summarized in Box 1. Until more data are available, these should be used as the guiding principles.
Box 1.
Panel recommendations for human papillomavirus diagnosis and management in head and neck squamous cell cancers
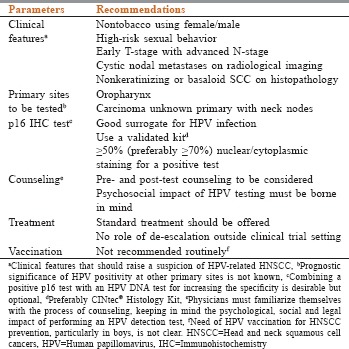
Clinical features that should raise a suspicion of HPV-related HNSCC
Prognostic significance of HPV positivity at other primary sites is not known
Combining a positive p16 test with an HPV DNA test for increasing the specificity is desirable but optional
Preferably CINtec® Histology Kit
Physicians must familiarize themselves with the process of counseling, keeping in mind the psychological and social and legal impact of performing an HPV detection test
Need of HPV vaccination for HNSCC prevention, particularly in boys, is not clear.
Future directions
To establish a definitive role of HPV in the prognosis and treatment of HNSCC in India, a robust effort is required by the practicing oncologists and researchers in the country. The panel recommends the following areas which require the focus and attention of the institutions dealing with these cancers.
Collaboration to estimate the true prevalence of HPV-related oropharynx in the country as a whole. An effort in its direction is currently being made by the head and neck cooperative oncology group of the Foundation for Head and Neck Oncology
Standardization of testing and reporting of p16 IHC for HPV detection
Research to establish definite risk factors for HPV-related HNSCC including documentation of information regarding sexual practices of the patients
Accurate documentation of use of alcohol/tobacco, their form, frequency, and duration in routine practice
Exploring the role of de-escalating treatment severity in HPV-positive HNSCC to reduce treatment-associated toxicity in well-conducted clinical trials
Although HPV vaccination has shown reduced rates of HPV infection whether this translates into reduction in the incidence of HPV-related HNSCC is not yet known. A futuristic cost effective strategy for the use of HPV vaccine in males would depend on the availability of such data.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: An emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–35. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 3.Datta S, Chaturvedi P, Mishra A, Pawar P. A review of Indian literature for association of smokeless tobacco with malignant and premalignant diseases of head and neck region. Indian J Cancer. 2014;51:200–8. doi: 10.4103/0019-509X.146713. [DOI] [PubMed] [Google Scholar]
- 4.International Institute for Population Sciences. Ministry of Health and Family Welfare. Government of India, Global Adult Tobacco Survey India 2009-2010. 2010 [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults – United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:1157–61. [PubMed] [Google Scholar]
- 6.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh V, Husain N, Akhtar N, Kumar V, Tewari S, Mishra S, et al. Do human papilloma viruses play any role in oral squamous cell carcinoma in North Indians? Asian Pac J Cancer Prev. 2015;16:7077–84. doi: 10.7314/apjcp.2015.16.16.7077. [DOI] [PubMed] [Google Scholar]
- 8.Jitani AK, Raphael V, Mishra J, Shunyu NB, Khonglah Y, Medhi J. Analysis of human papilloma virus 16/18 DNA and its correlation with p16 expression in oral cavity squamous cell carcinoma in North-Eastern India: A chromogenic in-situ hybridization based study. J Clin Diagn Res. 2015;9:EC04–7. doi: 10.7860/JCDR/2015/13022.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sannigrahi MK, Singh V, Sharma R, Panda NK, Radotra BD, Khullar M. Detection of active human papilloma virus-16 in head and neck cancers of Asian North Indian patients. Oral Dis. 2016;22:62–8. doi: 10.1111/odi.12382. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Rai AK, Das D, Das R, Kumar RS, Sarma A, et al. Alcohol and tobacco increases risk of high risk HPV infection in head and neck cancer patients: Study from North-East Region of India. PLoS One. 2015;10:e0140700. doi: 10.1371/journal.pone.0140700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahl A, Kumar P, Dar L, Mohanti BK, Sharma A, Thakar A, et al. Prevalence and trends of human papillomavirus in oropharyngeal cancer in a predominantly North Indian population. Head Neck. 2014;36:505–10. doi: 10.1002/hed.23317. [DOI] [PubMed] [Google Scholar]
- 12.Murthy V, Swain M, Teni T, Pawar S, Kalkar P, Patil A, et al. Human papillomavirus/p16 positive head and neck cancer in India: Prevalence, clinical impact, and influence of tobacco use. Indian J Cancer. 2016;53:387–93. doi: 10.4103/0019-509X.200668. [DOI] [PubMed] [Google Scholar]
- 13.Mishra S, Joseph RA, Gupta PC, Pezzack B, Ram F, Sinha DN, et al. Trends in bidi and cigarette smoking in India from 1998 to 2015, by age, gender and education. BMJ Glob Health. 2016;1:e000005. doi: 10.1136/bmjgh-2015-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shridhar K, Rajaraman P, Koyande S, Parikh PM, Chaturvedi P, Dhillon PK, et al. Trends in mouth cancer incidence in Mumbai, India (1995-2009): An age-period-cohort analysis. Cancer Epidemiol. 2016;42:66–71. doi: 10.1016/j.canep.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elango JK, Gangadharan P, Sumithra S, Kuriakose MA. Trends of head and neck cancers in urban and rural India. Asian Pac J Cancer Prev. 2006;7:108–12. [PubMed] [Google Scholar]
- 16.Kulkarni SS, Kulkarni SS, Vastrad PP, Kulkarni BB, Markande AR, Kadakol GS, et al. Prevalence and distribution of high risk human papillomavirus (HPV) Types 16 and 18 in Carcinoma of cervix, saliva of patients with oral squamous cell carcinoma and in the general population in Karnataka, India. Asian Pac J Cancer Prev. 2011;12:645–8. [PubMed] [Google Scholar]
- 17.Pattanshetty S, Kotrashetti VS, Nayak R, Bhat K, Somannavar P, Babji D. PCR based detection of HPV 16 and 18 genotypes in normal oral mucosa of tobacco users and non-users. Biotech Histochem. 2014;89:433–9. doi: 10.3109/10520295.2014.887143. [DOI] [PubMed] [Google Scholar]
- 18.Dodson TB. The frequency of human papilloma virus (HPV) is higher in premalignant and malignant oral mucosal lesions than normal mucosa. J Evid Based Dent Pract. 2010;10:174–5. doi: 10.1016/j.jebdp.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Migaldi M, Pecorari M, Forbicini G, Nanni N, Grottola A, Grandi T, et al. Low prevalence of human papillomavirus infection in the healthy oral mucosa of a Northern Italian population. J Oral Pathol Med. 2012;41:16–20. doi: 10.1111/j.1600-0714.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 20.Kristoffersen AK, Enersen M, Kverndokk E, Sunde PT, Landin M, Solheim T, et al. Human papillomavirus subtypes in oral lesions compared to healthy oral mucosa. J Clin Virol. 2012;53:364–6. doi: 10.1016/j.jcv.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 21.González-Losa Mdel R, Manzano-Cabrera L, Rueda-Gordillo F, Hernández-Solís SE, Puerto-Solís L. Low prevalence of high risk human papillomavirus in normal oral mucosa by hybrid capture 2. Braz J Microbiol. 2008;39:32–4. doi: 10.1590/S1517-83822008000100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farsi NJ, El-Zein M, Gaied H, Lee YC, Hashibe M, Nicolau B, et al. Sexual behaviours and head and neck cancer: A systematic review and meta-analysis. Cancer Epidemiol. 2015;39:1036–46. doi: 10.1016/j.canep.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Nagpal JK, Patnaik S, Das BR. Prevalence of high-risk human papilloma virus types and its association with P53 codon 72 polymorphism in tobacco addicted oral squamous cell carcinoma (OSCC) patients of Eastern India. Int J Cancer. 2002;97:649–53. doi: 10.1002/ijc.10112. [DOI] [PubMed] [Google Scholar]
- 25.Elango KJ, Suresh A, Erode EM, Subhadradevi L, Ravindran HK, Iyer SK, et al. Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac J Cancer Prev. 2011;12:889–96. [PubMed] [Google Scholar]
- 26.D’Costa J, Saranath D, Dedhia P, Sanghvi V, Mehta AR. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998;34:413–20. doi: 10.1016/s1368-8375(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 27.Gheit T, Vaccarella S, Schmitt M, Pawlita M, Franceschi S, Sankaranarayanan R, et al. Prevalence of human papillomavirus types in cervical and oral cancers in central India. Vaccine. 2009;27:636–9. doi: 10.1016/j.vaccine.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 28.Koppikar P, deVilliers EM, Mulherkar R. Identification of human papillomaviruses in tumors of the oral cavity in an Indian community. Int J Cancer. 2005;113:946–50. doi: 10.1002/ijc.20664. [DOI] [PubMed] [Google Scholar]
- 29.Ramshankar V, Soundara VT, Shyamsundar V, Ramani P, Krishnamurthy A. Risk stratification of early stage oral tongue cancers based on HPV status and p16 immunoexpression. Asian Pac J Cancer Prev. 2014;15:8351–9. doi: 10.7314/apjcp.2014.15.19.8351. [DOI] [PubMed] [Google Scholar]
- 30.Kumaraswamy KL, Vidhya M. Human papilloma virus and oral infections: An update. J Cancer Res Ther. 2011;7:120–7. doi: 10.4103/0973-1482.82915. [DOI] [PubMed] [Google Scholar]
- 31.Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–3. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 32.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 33.Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122:2656–64. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 34.Reimers N, Kasper HU, Weissenborn SJ, Stützer H, Preuss SF, Hoffmann TK, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–8. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 35.Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer – Systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–55. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 36.Halec G, Holzinger D, Schmitt M, Flechtenmacher C, Dyckhoff G, Lloveras B, et al. Biological evidence for a causal role of HPV16 in a small fraction of laryngeal squamous cell carcinoma. Br J Cancer. 2013;109:172–83. doi: 10.1038/bjc.2013.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young RJ, Urban D, Angel C, Corry J, Lyons B, Vallance N, et al. Frequency and prognostic significance of p16(INK4A) protein overexpression and transcriptionally active human papillomavirus infection in laryngeal squamous cell carcinoma. Br J Cancer. 2015;112:1098–104. doi: 10.1038/bjc.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Zhang Z, Sun R, Lin H, Gong L, Fang M, et al. HPV infection and anemia status stratify the survival of early t2 laryngeal squamous cell carcinoma. J Voice. 2015;29:356–62. doi: 10.1016/j.jvoice.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigo JP, Hermsen MA, Fresno MF, Brakenhoff RH, García-Velasco F, Snijders PJ, et al. Prevalence of human papillomavirus in laryngeal and hypopharyngeal squamous cell carcinomas in northern Spain. Cancer Epidemiol. 2015;39:37–41. doi: 10.1016/j.canep.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Shaughnessy JN, Farghaly H, Wilson L, Redman R, Potts K, Bumpous J, et al. HPV: A factor in organ preservation for locally advanced larynx and hypopharynx cancer? Am J Otolaryngol. 2014;35:19–24. doi: 10.1016/j.amjoto.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Cohen MA, Basha SR, Reichenbach DK, Robertson E, Sewell DA. Increased viral load correlates with improved survival in HPV-16-associated tonsil carcinoma patients. Acta Otolaryngol. 2008;128:583–9. doi: 10.1080/00016480701558880. [DOI] [PubMed] [Google Scholar]
- 42.Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–72. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 43.Badaracco G, Venuti A, Morello R, Muller A, Marcante ML. Human papillomavirus in head and neck carcinomas: Prevalence, physical status and relationship with clinical/pathological parameters. Anticancer Res. 2000;20:1301–5. [PubMed] [Google Scholar]
- 44.Klussmann JP, Gültekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–53. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragin CC, Taioli E, Weissfeld JL, White JS, Rossie KM, Modugno F, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95:1432–8. doi: 10.1038/sj.bjc.6603394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson M, Schache A, Sloan P, Thavaraj S. HPV specific testing: A requirement for oropharyngeal squamous cell carcinoma patients. Head Neck Pathol. 2012;6 Suppl 1:S83–90. doi: 10.1007/s12105-012-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westra WH. Detection of human papillomavirus (HPV) in clinical samples: Evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol. 2014;50:771–9. doi: 10.1016/j.oraloncology.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis JS., Jr p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(Suppl 1):S75–82. doi: 10.1007/s12105-012-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis JS, Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–96. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer CA, Zlobec I, Green E, Probst S, Storck C, Lugli A, et al. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126:1256–62. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 51.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–8. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 52.Bishop JA, Ma XJ, Wang H, Luo Y, Illei PB, Begum S, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–82. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–73. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 54.Jordan RC, Lingen MW, Perez-Ordonez B, He X, Pickard R, Koluder M, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–54. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grønhøj Larsen C, Gyldenløve M, Jensen DH, Therkildsen MH, Kiss K, Norrild B, et al. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: A systematic review. Br J Cancer. 2014;110:1587–94. doi: 10.1038/bjc.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen ZW, Weinreb I, Kamel-Reid S, Perez-Ordoñez B. Equivocal p16 immunostaining in squamous cell carcinoma of the head and neck: Staining patterns are suggestive of HPV status. Head Neck Pathol. 2012;6:422–9. doi: 10.1007/s12105-012-0382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ChuteDJ, Aramouni GT, Brainard JA, Hoschar AP, Kroeger A, Yen-Lieberman B. Hybrid capture 2 human papilloma virus testing for head and neck cytology specimens. J Am Soc Cytopathol. 2014;3:173–82. doi: 10.1016/j.jasc.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Thavaraj S, Stokes A, Guerra E, Bible J, Halligan E, Long A, et al. Evaluation of human papillomavirus testing for squamous cell carcinoma of the tonsil in clinical practice. J Clin Pathol. 2011;64:308–12. doi: 10.1136/jcp.2010.088450. [DOI] [PubMed] [Google Scholar]
- 59.Evans MF, Matthews A, Kandil D, Adamson CS, Trotman WE, Cooper K. Discrimination of ‘driver’ and ‘passenger’ HPV in tonsillar carcinomas by the polymerase chain reaction, chromogenic in situ hybridization, and p16(INK4a) immunohistochemistry. Head Neck Pathol. 2011;5:344–8. doi: 10.1007/s12105-011-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuo KT, Hsiao CH, Lin CH, Kuo LT, Huang SH, Lin MC. The biomarkers of human papillomavirus infection in tonsillar squamous cell carcinoma-molecular basis and predicting favorable outcome. Mod Pathol. 2008;21:376–86. doi: 10.1038/modpathol.3800979. [DOI] [PubMed] [Google Scholar]
- 61.Wittekindt C, Gültekin E, Weissenborn SJ, Dienes HP, Pfister HJ, Klussmann JP. Expression of p16 protein is associated with human papillomavirus status in tonsillar carcinomas and has implications on survival. Adv Otorhinolaryngol. 2005;62:72–80. doi: 10.1159/000082474. [DOI] [PubMed] [Google Scholar]
- 62.Kulkarni V, Saranath D. Concurrent hypermethylation of multiple regulatory genes in chewing tobacco associated oral squamous cell carcinomas and adjacent normal tissues. Oral Oncol. 2004;40:145–53. doi: 10.1016/s1368-8375(03)00143-x. [DOI] [PubMed] [Google Scholar]
- 63.O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging: A multicentre cohort study. Lancet Oncol. 2016;17:440–51. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 64.Rettig E, Kiess AP, Fakhry C. The role of sexual behavior in head and neck cancer: Implications for prevention and therapy. Expert Rev Anticancer Ther. 2015;15:35–49. doi: 10.1586/14737140.2015.957189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westra WH. The morphologic profile of HPV-related head and neck squamous carcinoma: Implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6(Suppl 1):S48–54. doi: 10.1007/s12105-012-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thariat J, Badoual C, Faure C, Butori C, Marcy PY, Righini CA. Basaloid squamous cell carcinoma of the head and neck: Role of HPV and implication in treatment and prognosis. J Clin Pathol. 2010;63:857–66. doi: 10.1136/jcp.2010.078154. [DOI] [PubMed] [Google Scholar]
- 67.Chernock RD. Morphologic features of conventional squamous cell carcinoma of the oropharynx: ‘keratinizing’ and ‘nonkeratinizing’ histologic types as the basis for a consistent classification system. Head Neck Pathol. 2012;6(Suppl 1):S41–7. doi: 10.1007/s12105-012-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int J Cancer. 2007;121:1813–20. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 69.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 70.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]


