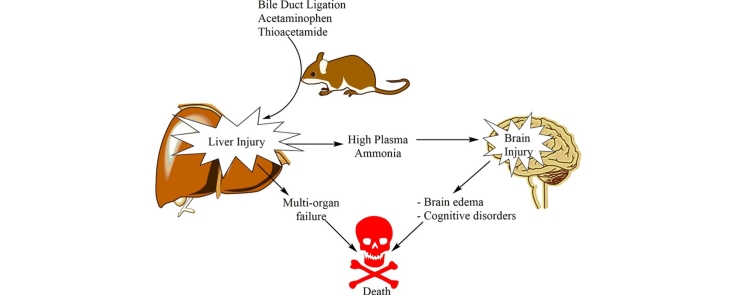
Graphical abstract
Chemical compounds studied in this article: 4-Nitrophenol (PubChemCID: 980); Acetaminophen (PubChem CID: 1983); 5, 5′ Dithiobis (2-nitrobenzoic acid) (PubChem CID: 6254); Taurine (PubChem CID: 1123); Thioacetamide (PubChem CID: 2723949); Thiobarbituric acid (PubChem CID: 2723628)
Keywords: Amino acid, Ammonia, Brain edema, Hepatic encephalopathy, Hepatic injury, Hepatoprotective
Abstract
Hyperammonemia is associated with chronic and acute liver injury. There is no promising therapeutic agent against ammonia-induced complications. Hence, finding therapeutic molecules with safe profile of administration has clinical value. The present study was conducted to evaluate the role of taurine (TA) administration on plasma and brain ammonia and its consequent events in different models of chronic and acute liver injury and hyperammonemia. Bile duct ligated (BDL) rats were used as a model of chronic liver injury. Thioacetamide and acetaminophen-induced acute liver failure were used as acute liver injury models. A high level of ammonia was detected in blood and brain of experimental groups. An increase in brain ammonia level coincided with a decreased total locomotor activity of animals and significant changes in the biochemistry of blood and also liver tissue. TA administration (500 and 1000 mg/kg, i.p), effectively alleviated liver injury and its consequent events including rise in plasma and brain ammonia and brain edema. The data suggested that TA is not only a useful and safe agent to preserve liver function, but also prevented hyperammonemia as a deleterious consequence of acute and chronic liver injury.
1. Introduction
Taurine (TA; β-amino sulfonic acid) is one of the most abundant amino acids in the human body [1]. TA is present in our daily foods and dietary supplements. A broad range of pharmacological and physiological effects are attributed to this amino acid [2], [3], [4], [5]. It has been reported that the hepatic TA level has been decreased in some liver disease and/or xenobiotics-induced hepatic injury [6]. TA concentration in plasma and urine is also reported as a biomarker of hepatic injury because of its release from pre-central hepatocytes [6], [7]. On the other hand, it has been shown that TA plays a protective role against many xenobiotics [8], [9], [10], [11], [12], [13], [14], [15]. Therefore, these results show that TA serves as a hepatoprotective agent to prevent liver injury [16]. TA also has been shown to have a profound effect in central nervous system (CNS) [17], [18]. It has been shown that TA acts as an osmoregulator, protects neurons, prevents astrocytes swelling and encounters oxidative stress in CNS [19], [20], [21], [22].
Hepatic encephalopathy (HE) is a serious complication associated with liver cirrhosis and/or acute liver failure [23]. Although the pathophysiology of HE is not fully understood, there is agreement on the importance of neurotoxins, especially ammonia [24]. Liver is the main organ responsible for ammonia detoxification via the urea cycle [25]. Hence, any defect in liver function and urea cycle might lead to hyperammonemia and conclusively HE. Ammonia is a neurotoxin which triggers a variety of neurological complications [24]. Ammonia causes oxidative stress and brain edema [24], [26], [27], induces neuroinflammation [28], [29], [30], and affects vital organelles such as mitochondria [24], [31].
Although the hepatic/neuroprotective properties of TA are well established, the effects of this amino acid on blood and brain ammonia, as a key factor in the consequences of acute and/or chronic liver injury and HE, has not been investigated so far. In the present study, we proposed to examine the effects of TA on hepatic injury, plasma and brain ammonia as well as brain damage in different experimental models of chronic and acute liver failure.
2. Material and methods
2.1. Chemicals
Taurine (2-amino-ethane sulfonic acid) was obtained from Acros (New Jersey, USA). Acetaminophen, thioacetamide, trichloroacetic acid (TCA), 5,5′-dithionitrobenzoic acid (DTNB), thiobarbituric acid (TBA), sodium citrate, sodium hypochlorite solution (5%), ammonium chloride, nicotinamide adenine dinucleotide phosphate (NADPH), phenol, sodium nitroprusside dihydrate, ethylenediamine tetra-acetic acid (EDTA), 4-nitrophenol, phosphoric acid, 2‐amino‐2-hydroxymethyl-propane-1,3-diol-hydrochloride (Tris–HCl), were obtained from Merck (Darmstadt, Germany). Kits for evaluating biomarkers of liver injury, including ALT, LDH, AST, ALP and bilirubin, were obtained from Pars Azmun® (Tehran, Iran). All salts used for preparing buffer solutions were of analytical grade and obtained from Merck (Darmstadt, Germany).
2.2. Animals
Male Sprague-Dawley rats (n = 54; 200–300 g weight) and male Swiss albino mice, (n = 30; 20–35 g weight) were obtained from the Center for Comparative and Experimental Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. Animals were housed in plastic cages over hardwood bedding. There was an environmental temperature of 24 °C and a 12L: 12D photoschedule along with a 40% of relative humidity. The rats were allowed ad-libitum access to a normal standard chow diet and water. Animals received humane care and all the experiments were performed in conformity with the guidance for care and use of experimental animals approved by a local ethic committee in Shiraz University of Medical Sciences, Shiraz, Iran (#93-01-36-7609).
2.3. Experimental setup
2.3.1. Bile duct ligation (BDL) in rat as a model for chronic liver injury and cirrhosis
Bile duct ligation (BDL) in rats is a very reproducible biliary cirrhosis animal model with all complications of hepatic encephalopathy including a rise in blood ammonia and its associated neurobiological disorders [32]. Animals (n = 24) were anesthetized (10 mg/kg of xylazine and 70 mg/kg of ketamine, i.p), a midline incision was made and the common bile duct was localized, doubly ligated, and cut between these two ligatures [33]. The sham operation consisted of laparotomy and bile duct identification and manipulation without ligation. Animals received TA (500 and 1000 mg/kg live weight/day, i.p) for 28 consecutive days. On day 29 after the BDL operation, rats were anesthetized with thiopental (80 mg/kg, i.p) and liver, brain and blood samples were collected for further studies [33], [34]. Sham-operated (control) animals received TA vehicle (normal saline).
2.3.2. Thioacetamide-induced acute liver injury in rats
Thioacetamide is extensively used as a model of acute hepatic failure [35]. Thioacetamide-induced fulminant hepatic failure was achieved by three consecutive i.p injections of thioacetamide (400 mg/kg) to rats (n = 30) at 24-h intervals [36]. TA (500 and 1000 mg/kg, i.p) was administered for three consecutive days, two hours after each dose of thioacetamide. On the fourth day (24 h after the last dose of thioacetamide), animals were anesthetized (thiopental, 80 mg/kg, i.p) and their blood, brain, and liver were collected. Supportive therapy by administering 5% dextrose (25 mL/kg body weight, S.C) containing 0.45% sodium chloride and 0.2% potassium chloride, was given to avoid weight loss, hypoglycemia and renal failure [36]. Control animals (Vehicle-treated) received normal saline as the thioacetamide solvent. The sole TA (1000 mg/kg, i.p) was administered to ensure its safety.
2.3.3. Acetaminophen-Induced acute liver injury in mice
Acetaminophen (paracetamol) (APAP) is a commonly used drug which can produce hepatic damage. Mice are more susceptible to acetaminophen hepatotoxicity than rats [37]. A high dose of acetaminophen (1 g/kg, i.p) was administered to mice (n = 30) and liver injury was assessed 24 h after drug administration [38]. TA (500 and 1000 mg/kg, i.p) was administered two hours after acetaminophen. Control animals received acetaminophen solvent (Dimethyl sulfoxide, 0.1 mL). A group of mice received TA (1000 mg/kg, i.p) alone.
2.4. Plasma biochemistry and tissue histopathology
A MindrayBS-200® auto analyzer and standard kits (Pars Azmun®) were used to measure plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), and bilirubin [39]. Plasma ammonia was measured with standard commercial kits with a method based on Bertholet phenate-hypochlorate reaction [40]. For histopathological assessments, samples of liver were fixed in buffered formalin solution (0.4% sodium phosphate monobasic, NaH2PO4, 0.64% sodium phosphate dibasic, Na2HPO4, and 10% formaldehyde in distilled water). Paraffin‐embedded sections of tissue (5 μm) were prepared and stained with hematoxylin and eosin (H&E) before light microscope viewing. Liver fibrotic changes were determined by Masson’s trichrome staining in BDL rats. The Ishak system which uses a six-point scale for fibrosis stage (0–6) was applied for scoring liver fibrosis in BDL rats [41], [42].
2.5. Brain ammonia level
Samples (100 mg) of the forebrain (cerebral cortex) were dissected, homogenized, and deproteinized in 3 mL of ice-cooled lysis solution containing trichloroacetic acid (6%, w/v). After centrifugation at 12,000g for 10 min at 4 °C, the supernatants were collected and neutralized with 2 mol/l KHCO3 (pH = 7). Brain ammonia content was measured using standard kits, based on the absorbance photometry method of phenate-hypochlorate reaction [40].
2.6. The open field test for locomotors activity assessment
Open field behavior is used as an index of animals’ locomotor activity in the animal models of hyperammonemia and hepatic encephalopathy [43], [44]. The open field test was conducted for each group five hours before animal anesthesia and blood and liver sample collection. The apparatus was made of white wood box (100 cm L × 100 cm W × 30 cm H, box floor was divided into 25 squares of 20 × 20 cm for rats and 20 cm L × 30 cm W × 15 cm H, which box floor was divided into 12 squares of equal size for mice) [45], [46], [47]. The open field arena was equipped with a webcam (2.0-Megapixel, Gigaware, UK) and all activities were monitored and recorded from a separate room. Animals behavior was recorded for fifteen minutes and the total number of crossed squares were counted (Total locomotion) [45], [46].
2.7. Brain edema
Brain water content was determined by the wet/dry weight method. A sample of 100 mg tissue of the cerebral cortex was separated. First, the wet weight of brain tissue was determined. Then, the tissue was dried overnight in an oven at 120 °C, and dry weights were determined. The difference in wet/dry weights was expressed as percent brain water content as an index of brain edema [48].
2.8. Hepatic glutathione content
Tissue samples (200 mg) were homogenized in 8 mL of ice-cooled EDTA (20 mM). Then, 5 mL of the prepared homogenate were added to 4 mL of distilled water and 1 mL of trichloroacetic acid (50% w/v). The mixture was shaken well and centrifuged (10,000g, 4 °C, 25 min). 2 mL of the supernatant was mixed with 4 mL of Tris buffer (pH = 8.9), and 100 μl of DTNB (0.01 M in methanol). The absorbance of the developed color was measured in 412 nm using an Ultrospec 2000®UV spectrophotometer [49].
2.9. Lipid peroxidation
As an index of lipid peroxidation, the thiobarbituric acid reactive substances (TBARS) test was used [49]. The reaction mixture consists of 500 μL of tissue homogenate (10% w/v in KCl, 1.15%), 1 mL of thiobarbituric acid (0.375%, w/v), and 3 mL of phosphoric acid (1% w/v, pH = 2). Samples were mixed well and heated in boiling water (100 °C) for 45 min. After the incubation period, the mixture was cooled, and then 2 mL of n-butanol was added. Samples were vigorously vortexed and centrifuged (10,000g for 5 min). Finally, the absorbance of developed color in n-butanol phase was read at 532 nm using an Ultrospec 2000®UV spectrophotometer [49].
2.10. Cytochrome 2E1 activity assessment
Hepatic microsomes were prepared by low-speed calcium aggregation method [50]. Briefly, 1 g of liver tissue was minced in 10 mL of Tris–EDTA buffer (50 mM Tris–HCl, 150 mM KCl, 2 mM EDTA, pH 7.4) and homogenized. The liver homogenate was centrifuged (10 min, 10,000g, 4 °C) and the supernatant was collected (crude homogenate). Then, the crude homogenate was diluted with calcium-containing (CaCl2, 8 mM) Tris–EDTA buffer to a final volume of 25 mL. After 4 min of incubation (25 °C), the samples were centrifuged (30 min, 25,000g, 4 °C) [50], [51]. Finally, the microsomal pellets were suspended in a buffer containing 50 mM Tris–HCl, 10 mM KH2PO4, 0.1 mM EDTA, and 20% glycerol (pH 7.4) and stored at −80 °C for later enzymatic assays.
CYP2E1 activity was measured by the rate of oxidation of p-nitrophenol to p‐nitrocatechol in the presence of NADPH according to the method of Reinke and Moyer [52]. Briefly, microsomes was mixed with a solution containing 0.2 mM p-nitrophenol, 50 mM Tris- HCl and 5 mM MgCl2 (pH = 7.4). The reaction was performed at 37 °C and was started by the addition of 1 mM NADPH. After 10 min, the reaction was stopped with 0.6 N perchloric acid. The precipitated proteins were removed by centrifugation at 3000 g for 5 min and the supernatants were mixed with NaOH(10 N) for the measurement of p-nitrocatechol at 510 nm using an Ultrospec 2000®UV spectrophotometer [53]. Protein content of the samples was determined using the Bradford method [54].
2.11. Statistical analysis
Data are given as Mean ± SEM. Comparison of data sets was performed by the one-way analysis of variance (ANOVA) with Tukey’s multiple comparison as a post hoc test. Values of P < 0.05 were considered statistically significant. The relationship between the locomotor activity and the brain ammonia were determined by the Pearson’s regression analysis.
3. Results
3.1. Effect of taurine on serum biochemistry in chronic and acute liver injury
The effects of BDL, paracetamol and thioacetamide and different doses of TA on the liver and plasma biochemical parameters are summarized in Tables 1. A significant increase in the activities of plasma enzyme levels (ALT, LDH, AST, and ALP), plasma total bilirubin, hepatic lipid peroxidation and a decrease in liver glutathione content were detected in thioacetamide and paracetamol-treated animals as well as in BDL rats (Table 1). It was found that TA administration (500 and 1000 mg/kg) significantly alleviated pathological changes in plasma and liver biochemistry in all investigated acute and chronic models of liver injury (Table 1).
Table 1.
Parameters assessed in different models of chronic and acute liver injury and the role of taurine administration.
| Treatment | Plasma ALT (U/l) |
Plasma AST (U/l) |
Plasma LDH (U/l) |
Plasma ALP (U/l) |
Plasma Bilirubin (mg/dl) | Plasma ammonia (μg/dl) |
Liver GSH (μmol/mg tissue) |
Liver TBARS (nmol/mg tissue) |
Brain edema (% brain water) |
Survival Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control (vehicle-treated rat) | 45 ± 3 | 84 ± 9 | 316 ± 68 | 571 ± 86 | 0.09 ± 0.01 | 223 ± 22 | 67.86 ± 9.35 | 1.87 ± 0.22 | 81 ± 3 | 100 |
| Control (vehicle-treated mice) | 17 ± 5 | 48 ± 7 | 164 ± 42 | 456 ± 106 | 0.03 ± 0.01 | 241 ± 36 | 75.80 ± 10.50 | 1.86 ± 0.23 | 79 ± 4 | 100 |
| Taurine-treated rats (1000 mg/kg) | 33 ± 7 | 72 ± 9 | 284 ± 57 | 396 ± 123 | 0.08 ± 0.03 | 186 ± 53 | 82.53 ± 7.45 | 1.04 ± 0.51 | 78 ± 5 | 100 |
| Taurine-treated mice (1000 mg/kg) | 22 ± 6 | 53 ± 8 | 209 ± 18 | 286 ± 98 | 0.07 ± 0.03 | 304 ± 56 | 84.31 ± 4.29 | 1.23 ± 0.61 | 82 ± 4 | 100 |
| TAA −treated rats | 751 ± 108 * | 1583 ± 366* | 3151 ± 269* | 755 ± 99 | 0.36 ± 0.04* | 625 ± 39* | 14.25 ± 2.30* | 9.57 ± 1.57* | 87 ± 1 | 100 |
| Bile duct ligated (BDL) ratsd | 335 ± 38* | 237 ± 55* | 606 ± 72* | 1738 ± 370* | 12.53 ± 0.45* | 938 ± 70* | 26.47 ± 3.95* | 3.93 ± 1.10* | 92 ± 2* | 66.66* |
| APAP-treated miced | 536 ± 130* | 627 ± 102* | 1083 ± 279* | 569 ± 65 | 0.22 ± 0.02* | 1110 ± 42* | 29.90 ± 3.70* | 10.85 ± 0.7* | 93 ± 3* | 66.66* |
| TAA + Taurine 500 mg/kg | 131 ± 29a | 476 ± 117 a | 890 ± 58a | 680 ± 75 | 0.20 ± 0.03a | 490 ± 41a | 17.75 ± 2.32 | 2.44 ± 0.13a | 82 ± 2 | 100 |
| TAA + Taurine 1000 mg/kg | 118 ± 34a | 324 ± 67a | 618 ± 143a | 596 ± 121 | 0.14 ± 0.02a | 220 ± 22a | 21.23 ± 1.45a | 2.60 ± 0.40a | 83 ± 2 | 100 |
| BDL + Taurine 500 mg/kg | 139 ± 31b | 114 ± 10b | 321 ± 40b | 583 ± 92b | 2.80 ± 0.05b | 278 ± 26b | 50.00 ± 5.51b | 2.60 ± 0.12b | 80 ± 3b | 100b |
| BDL + Taurine 1000 mg/kg | 134 ± 19b | 128 ± 40b | 295 ± 42b | 681 ± 80 b | 2.62 ± 0.08b | 228 ± 38b | 52.1 ± 4.28b | 2.2 ± 0.23 b | 78 ± 3b | 100b |
| APAP + Taurine 500 mg/kg | 98 ± 10c | 330 ± 30c | 226 ± 58c | 609 ± 111 | 0.06 ± 0.01c | 791 ± 63c | 50.00 ± 1.50c | 7.40 ± 0.28c | 81 ± 3c | 100 c |
| APAP + Taurine 1000 mg/kg | 79 ± 13c | 322 ± 80c | 280 ± 47c | 551 ± 54 | 0.026 ± 0.01c | 708 ± 52c | 53.13 ± 4.30 c | 6.61 ± 0.30 c | 83 ± 2 c | 100 c |
Data are given as Mean ± SEM for each group (n = 6). TAA: Thioacetamide, BDL: Bile duct ligated, APAP: Acetyl Para amino phenol, Acetaminophen.
*Indicates significantly different as compared to control animals (P < 0.05).
Shows significant difference as compared to thioacetamide-treated rats (P < 0.05).
Indicates significant difference as compared to BDL rats (P < 0.05).
Indicates significant difference as compared to APAP-treated mice (P < 0.05).
Two out of six animals were death (n = 4).
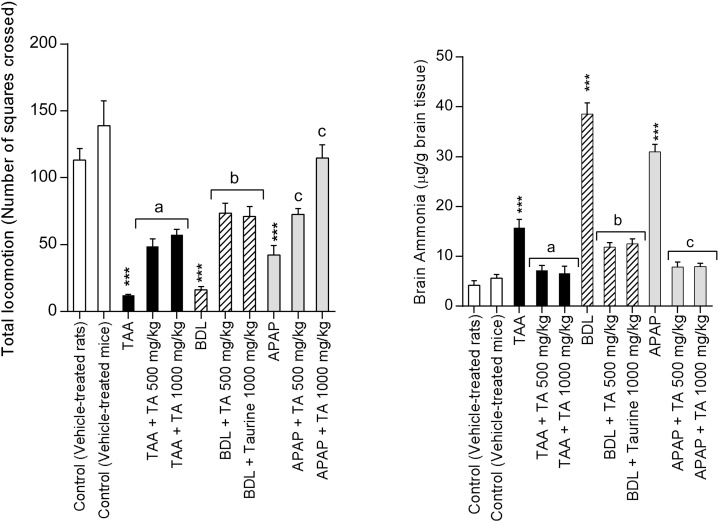
3.2. Effect of taurine on brain edema, plasma and brain ammonia, and animals’ locomotor activity after acute and chronic liver injury
The brain edema assessment revealed a significant increase in brain water in the acetaminophen-treated and BDL animals (Table 1). It was found that TA administration effectively mitigated brain edema (Table 1). A significant elevation in plasma ammonia was observed in all investigated liver injury models (Table 1). Elevation in plasma ammonia was accompanied with an increase in brain ammonia levels and a decrease in the total locomotor activity of animals (Fig. 1). Correlation analysis showed that the number of square crossings is negatively correlated with the brain ammonia levels in thioacetamide and acetaminophen-treated animals as well as in BDL rats (TAA-treated r = −0.823; p < 0.044; APAP-treated r = −0.939; p < 0.0001, BDL rats r = −0.951, p < 0.0036) (Fig. 1). This negative correlation means that as plasma ammonia increases, animals locomotor activity tends to decrease. Plasma and brain ammonia levels were significantly lower in TA-treated groups (Table 1 and Fig. 1). Furthermore, TA administration (500 and 1000 mg/kg) improved animals’ locomotor activity in the open-field test (Fig. 1). The correlation analysis of taurine-treated groups was as follows: APAP + Taurine 500 mg/kg; r = −0.653; p < 0.16; APAP + Taurine 1000 mg/kg; r = −0.936; p < 0.0061; TAA+ Taurine 500 mg/kg; r = −0.492, p < 0.322; TAA+ Taurine 1000 mg/kg; r = ‐0.984, p < 0.0004; BDL + Taurine 500 mg/kg; r = −0.982, p < 0.0005; BDL + Taurine 1000 mg/kg; r = −0.972, p < 0.0012 (Fig. 1).
Fig. 1.
Effect of taurine administration on brain ammonia level and animals’ locomotors activity in different models of liver injury. The relationship between the locomotor activity and the brain ammonia levels were determined by the Pearson’s regression analysis (Refer to result section for the correlation levels between groups). TAA: Thioacetamide, BDL: Bile duct ligated, APAP: Acetyl para amino phenol, Acetaminophen. TA: Taurine.
Data are expressed as Mean ± SEM for each group (n = 6).
***Indicates significantly different as compared to the control animals (P < 0.001).
aShows significant difference as compared to TTA-treated group (P < 0.05).
bIndicates significantly different as compared to BDL rats (P < 0.05).
cIndicates significant difference as compared to APAP-treated mice (P < 0.05).
3.3. Effect of taurine on liver histopathology
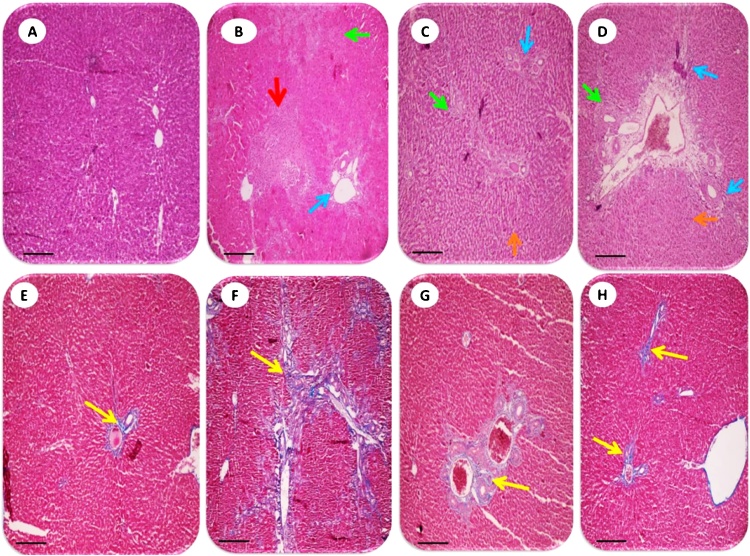
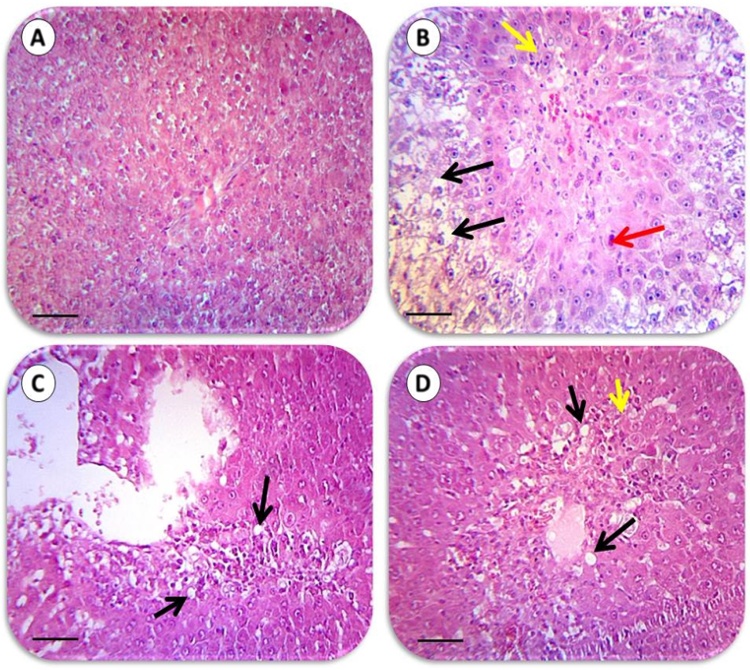
Liver histopathological changes in BDL rats revealed as extensive confluent and focal necrosis, inflammation and fibrotic changes of tissue as assessed by hematoxylin & eosin (Fig. 2 upper row and Table 2) and Masson’s trichrome staining (Fig. 2 lower row and Table 2). Liver necrosis, inflammation, and fibrogenesis were significantly inhibited in BDL rats when they were treated with TA (500 and 1000 mg/kg live weight) (Fig. 2) (Table 2). Liver histopathological lesions in thioacetamide-treated animals involved ballooning degeneration, fatty changes, tissue necrosis and inflammation (Fig. 3) (Table 2). TA administration effectively alleviated thioacetamide-induced histopathological changes (Fig. 3) (Table 2). A large amount of confluent and focal necrosis of liver tissue in addition of hemorrhage was detected in the liver of acetaminophen-treated animals (Fig. 4) (Table 2). TA (500 and 1000 mg/kg live weight, i.p) significantly blunted acetaminophen-induced hepatic injury (Fig. 4) (Table 2) and no sign of liver necrosis was detected when acetaminophen-intoxicated animals were treated with TA (Fig. 4) (Table 2).
Fig. 2.
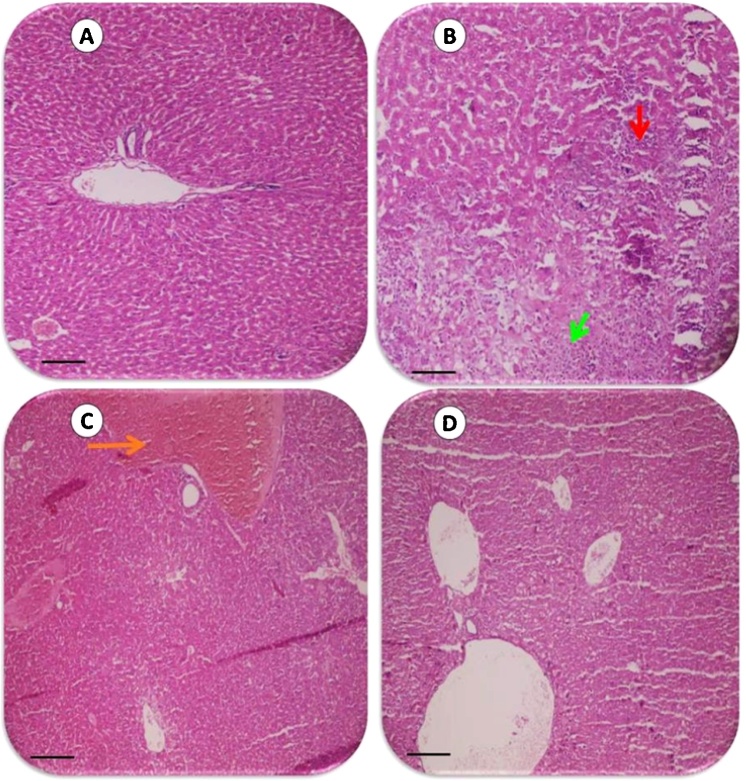
The histological photomicrographs of the liver in bile duct ligated rats and the effect of taurine administration (See also Table 2). Representative photomicrographs of liver sections processed for H&E (upper row) and Masson’s trichrome (lower row) staining (200×). Scale bar, 1060 μm. Sham (A&E), BDL (B&F), BDL+ Taurine 500 mg/kg (C&G), and BDL + Taurine 1000 mg/kg (D&H). Extensive tissue necrosis and bridging fibrosis in untreated BDL rats are shown in B and F (Yellow arrow; Ishak grade 5; Table 2). All these lesions were markedly attenuated in Taurine-treated BDL animals (C&D), G (Ishak grade 3; Table 2) and H (Ishak grade 2; Table 2). No significant pathological changes were observed in liver tissues with the sham operation (A) (Table 2). Red arrow: Confluent necrosis. Green arrow: focal necrosis. Blue arrow: Portal inflammation. Orange: Sinusoidal Congestion. BDL: Bile duct ligated.
Table 2.
Liver histopathological changes in different experimental groups.
| Pathologic assessment Treatment |
Confluent Necrosis |
Focal Necrosis | Portal Inflammation |
Interface Hepatitis |
Ballooning degeneration | Fatty changes | Total grade | Ishak stage of liver fibrosis |
|---|---|---|---|---|---|---|---|---|
| Control (vehicle-treated rats) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| Control (vehicle-treated mice) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| TAA −treated rats | 0 | 2 | 3 | 0 | 3 | 1 | 8 | – |
| Bile duct ligated (BDL) rats | 5 | 2 | 2 | 2 | 1 | 0 | 12 | 5 |
| APAP-treated mice | 3 | 3 | 0 | 0 | 0 | 1 | 7 | – |
| TAA + Taurine 500 mg/kg | 0 | 1 | 2 | 0 | 2 | 0 | 5 | – |
| TAA + Taurine 1000 mg/kg | 0 | 2 | 1 | 0 | 1 | 0 | 4 | – |
| BDL + Taurine 500 mg/kg | 0 | 0 | 2 | 2 | 1 | 0 | 5 | 3 |
| BDL + Taurine 1000 mg/kg | 0 | 0 | 1 | 1 | 1 | 0 | 3 | 2 |
| APAP+ Taurine 500 mg/kg | 0 | 0 | 1 | 1 | 1 | 1 | 4 | – |
| APAP+ Taurine 1000 mg/kg | 0 | 0 | 1 | 1 | 0 | 1 | 3 | – |
Fig. 3.
The effect of taurine administration on liver histopathological changes in thioacetamide-treated rats (See also Table 2 for grading of tissue histopathological changes). Representative photomicrographs of liver sections processed for H&E staining (400×). Scale bar, 530 μm. A: Control. B: Thioacetamide‐treated rats. C: Thioacetamide + Taurine 500 mg/kg D: Thioacetamide + Taurine 1000 mg/kg. Signs of hepatocytes apoptosis (red arrow), fatty changes and ballooning degeneration (black arrow), inflammation (yellow arrow), developed in thioacetamide-administered animals (B) (Table 2). Taurine administration significantly alleviated thioacetamide-induced lesions (C & D) and no tissue necrosis was observed in taurine-treated (1000 mg/kg, i.p) animals (D) (Table 2).
Fig. 4.
Liver histopathology of acetaminophen-treated mice. Acetaminophen (1000 mg/kg, i.p.) was administered to mice and animals were sacrificed 24 h after drug treatment. Liver sections were subjected to hematoxylin-eosin (H&E) staining (200 × ). Scale bar, 1060 μm. A: represents normal mice liver histopathology. B: Acetaminophen-challenged group. C: Acetaminophen + Taurine 500 mg/kg and D: Acetaminophen + Taurine 1000 mg/kg. Congestion (Orange arrow), tissue focal (green arrow) and confluent necrosis (red arrow) were detected in acetaminophen-treated animals (B) (Table 2). No sign of liver necrosis was detected when taurine was administered to acetaminophen-intoxicated mice (C&D) (Table 2).
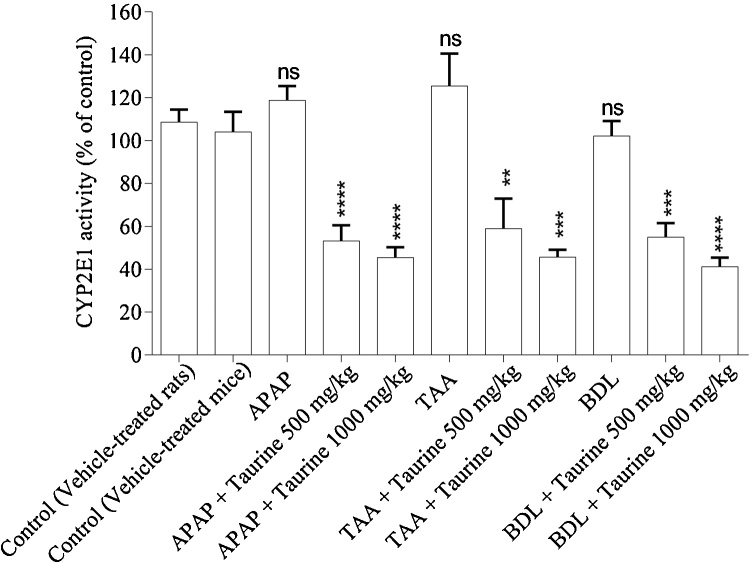
3.4. CYP2E1 inhibitory activity of taurine
It has been reported that taurine affects CYP2E1 enzyme [53]. Hence, we assessed the enzyme inhibitory potential of TA in our experimental models. We found that TA administration resulted in a significant decrease in p-nitrophenol metabolizing activity of CYP2E1 (Fig. 5), which indicates that this amino acid has an inhibitory effect on CYP2E1 activity in the liver (Fig. 5). BDL, APAP and thioacetamide did not affect CYP2E1 activity (Fig. 5). Moreover, there was no significant difference in CYP2E1 enzyme inhibitory effect of administered doses of taurine (500 and 1000 mf/kg) (Fig. 5).
Fig. 5.
Effect of taurine administration on CYP2E1 activity in liver. TAA: Thioacetamide, BDL: Bile duct ligated, APAP: Acetyl para-amino phenol, Acetaminophen.
Data are expressed as Mean ± SEM for each group (n = 6).
**Indicates significantly different as compared with control group (P < 0.01).
*** Indicates significantly different as compared with control group (P < 0.001).
**** Indicates significantly different as compared with control group (P < 0.0001).
ns: not significant as compared to the control group.
Finally, it should be mentioned that TA administration (500 and 1000 mg/kg) did not disturb any investigated parameters and animals showed no significant difference with control ones when they were treated with the sole TA at the mentioned doses.
4. Discussion
In spite of a good body of literature addressing the hepatoprotective properties of TA [16], no studies have been conducted on the effects of this amino acid on blood and brain ammonia, brain damage and neurological disorders ensued acute and chronic liver injury. The current investigation was designed to evaluate the role of TA administration on plasma and brain ammonia as the key factor associated with hepatic encephalopathy and its consequent neurological disorders.
Based on previous research, TA provides its protective properties through different mechanisms, including osmoregulation, anti-oxidation, membrane stabilization, xenobiotic detoxification, inhibition of fibrogenesis, enzyme inhibition, mitochondrial protection, anti-apoptosis, modulation of bile acids conjugation, and anti-inflammation [11], [13], [15], [16], [55], [56], [57].
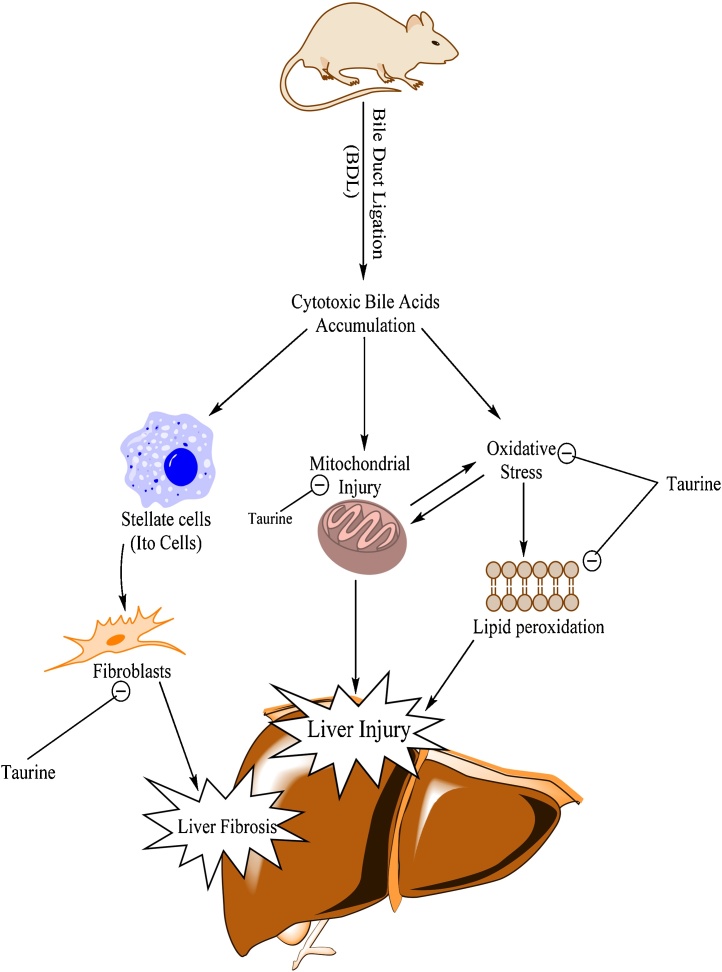
It has been shown that TA is a potent stellate cells inhibitor [55], [58]. Stellate cells have a great role in collagen formation during liver fibrosis process (Fig. 6) [59]. The anti-fibrotic properties of TA have been shown in different other models of liver injury [55], [58]. We found that TA (500 and 1000 mg/kg live weight) administration to bile duct ligated rats, effectively ameliorated liver fibrosis (Table 2; Ishak grade of liver fibrosis & Fig. 2, lower row). Hence, the anti-fibrotic properties of TA in BDL model might prevent liver failure and its consequent deleterious effects such as increase in blood and brain ammonia and finally tissue damage. On the other hand, bile acid conjugating and antioxidant properties of this amino acid might also play a role in its protective properties against toxic bile acids accumulated in the liver (Fig. 6).
Fig. 6.
The schematic diagram of the previously reported proposed mechanisms of hepatoprotection provided by taurine in chronic liver injury and cirrhosis.
It has been reported that TA is also a good mitochondrial protective agent [15], [56], [57], [60]. Toxic bile acids interact with hepatocytes mitochondrial function (Fig. 6) [61]. Hence, a part of TA hepatoprotective properties in BDL animals might be attributed to its protective properties against mitochondrial dysfunction in the liver (Fig. 6) [15], [62], [63]. We also evaluated the role of TA administration against ammonia-induced mitochondrial dysfunction in an in vitro model (Unpublished data). We found that, TA significantly alleviated ammonia-induced mitochondrial dysfunction and defect in energy metabolism in isolated brain and liver mitochondria (Unpublished data).
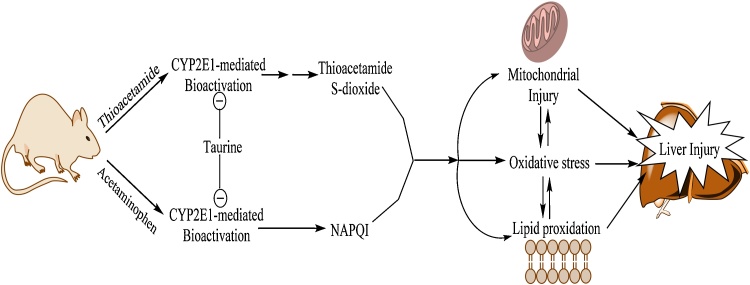
The situation in acute liver injury models and hepatic failure is somewhat different. We found that TA effectively inhibited acetaminophen and thioacetamide‐induced liver injury. These effects have also been reported previously [8], [9], [64]. Abbasoğlu et al. reported that TA administration effectively prevented thioacetamide-induced chronic liver injury [9]. We found that TA administration not only effectively inhibited thioacetamide-induced acute liver failure, but also it has a great effect on plasma and brain ammonia, and neurological disorders associated with thioacetamide-induced liver failure (Table 1, Fig. 1). As mentioned, the effect of TA on acetaminophen-induced liver injury has also been proved in different experimental models [8], [64]. In the current investigation, we found that TA effectively alleviated ammonia-induced complications in acetaminophen-treated animals. The antioxidative (Table 1), and more importantly the enzyme-inhibitory properties of TA (Fig. 5 and Fig. 7), might be important factors in TA hepatoprotective properties against acetaminophen or thioacetamide-induced liver injury. It has been shown that TA is a CYP2E1 inhibitor [53]. TA might inhibit thioacetamide and acetaminophen bioactivation through CYP2E1 inhibition and consequently prevent liver failure (Fig. 5 and Fig. 7). Moreover, oxidative stress has also been regarded as a major contributor to the development of various hepatic disorders including xenobiotics-induced acute hepatic failure and hepatic fibrosis [8], [65]. Hence, the antioxidant properties of TA [66], [67], might also have a role in its protective effect against acute liver injury as this amino acid effectively prevented lipid peroxidation and hepatic glutathione depletion (Fig. 7). Das et al. have been reported that TA might enhance the urinary excretion of acetaminophen [68]. Hence, other mechanisms such as enhancement of excretion of the hepatotoxic agents might also be involved in TA hepatoprotective properties.
Fig. 7.
The descriptive hepatoprotective mechanisms of taurine in experimental models of acute liver injury. NAPQI: N-acetyl p-quinone imine.
It has been shown that TA has several important roles in CNS [69]. Effects of this amino acid on CNS disorders also has been revealed [17]. Moreover, it has been shown that TA has protective properties against neurotoxins [70], [71]. As mentioned, ammonia is a neurotoxic agent and many consequences of hyper ammonia on the CNS have been previously described [26], [72], [73]. It has been found that elevated brain ammonia caused oxidative stress, bioenergetic failure, alterations in pH and Ca2+ homeostasis, and electrophysiological disturbances in CNS [26], [72], [73]. The antioxidative, mitochondrial protecting and calcium sequestering effects of TA might be involved in its protective properties against chronic and acute liver injury-induced hyperammonemia and its consequent brain injury.
The administered TA doses were chosen based on previous studies [74], [75]. We found no significant differences between the effects of administered TA doses (500 mg/kg and 1000 mg/kg) in most parameters assessed in the current investigation. This might be due to the pharmacokinetic parameters. TA transport through blood brain barrier and issues such as its saturation in different organs [76], [77] might contribute in the same effects of different high doses of TA (500 and 1000 mg/kg) in this study. As both doses of TA had significant effects on liver and brain we might be able to suggest lower doses of TA for future investigations to prevent adverse events, if any, associated with this amino acid.
Brain edema is a common finding in patients with acute/chronic liver injury [24]. TA effectively lowered ammonia levels and attenuated brain edema (%brain water) in our investigation (Table 1). Moreover, TA administration effectively mitigated the decrease in motor activities associated with chronic and acute liver injury. Interestingly, it has been shown that TA has inhibitory effects on astrocytes swelling [78]. Astrocytes are the major cells involved in hyperammonemia-induced brain edema [73]. It has been mentioned that brain edema might play a role in the neurologic complications of hyperammonemia [48], [79].
Although it was not statistically significant, brain edema (%brain water) was tended to rise in thioacetamide-treated animals (Table 1). Applying other more precise methods of edema assessment such as gradient protocol might provide different results [80]. The percent of brain water (edema) was significantly higher in BDL rats and APAP-treated animals (Table 1). We found that TA administration effectively mitigated brain edema in different experimental models of HE and hyperammonemia (Table 1, Fig. 7). The effect of TA on brain astrocytes might be involved in the mechanism by which this amino acid prevents brain edema in in vivo conditions. Several studies indicated the occurrence of brain edema in humans [81], and different animal models of acute and chronic liver injury [47], [82], [83]. On the other hand, some investigations mentioned the predominant role of neuroinflammation rather than brain edema in the pathogenesis of CNS injury in HE [84]. Neuroinflammation is one of the key factors in HE development and ammonia toxicity [28], [29]. As TA and its derivatives act as anti-inflammatory agents [85], [86], [87], one of the interesting mechanisms of neuroprotective properties of TA against hyperammonemia might be mediated by this pathway. Further investigations are needed to reveal the precise effect of TA on neuroinflammation in hyperammonemia.
It has been reported that in both acute and chronic liver failure, brain taurine concentrations are decreased [88]. TA plays an important role in the osmoregulation and ion (K+ and Ca2+) homeostasis in CNS [88], [89]. As the imbalance in CNS ions might lead to neuro-excitation and complications such as seizure [88], one of the important benefits of TA in preventing ammonia-induced brain injury might be mediated through CNS osmoregulation [88].
Indeed, the effect of TA on oxidative stress biomarkers and antioxidant enzymes along with other more precise techniques including immunohistochemical staining for detecting astrocytes swelling and brain inflammation [28], [80], will provide a more clear idea on the effect of TA on the brain in hyperammonemia and hepatic encephalopathy. Moreover, methods which exactly evaluate the pathological changes in the specific regions of the brain (e.g., stereologic methods which imply a detailed study of neuronal and glial changes in the brain) will clearly provide more information about brain injury induced by ammonia in HE and clear the mechanisms of neuroprotection provided by TA.
Effect of TA on the mitochondrial injury, bioenergetic and brain oxidative stress in hyperammonemia, as well as the effect of TA administration on the urea cycle enzymes in the liver has not been investigated so far. Effect of this amino acid on these enzymes might shed light on the mechanism of TA in decreasing plasma ammonia level and provide potential therapeutic options, especially in chronic liver injury and cirrhosis.
5. Conclusion
The current investigation focuses on the role of TA administration on the blood and brain ammonia level. Our data indicate that TA might be a useful agent to preserve liver function and prevent rise in blood and brain ammonia as a deleterious consequent of acute and chronic liver injury. Hence, TA supplementation might be considered as a therapeutic approach to ameliorate the severity of hepatic impairment and fibrosis in addition of brain damage and neurological disorders in acute and chronic cases of liver injury. Furthermore, more investigations on the effect of TA on cerebral energy metabolism and brain mitochondrial dysfunction will help to clear the mechanism(s) of its protection against hyperammonemia conditions.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
The instrumental facility providing of Pharmaceutical Sciences Research Center of Shiraz University of Medical Sciences is gratefully appreciated. This study was financially supported by the office of the Vice Chancellor of Research Affairs, Shiraz University of Medical Sciences (#93-01-36-7618/7220/7609).
References
- 1.Wright C.E., Tallan H.H., Taurine Lin Y.Y. Biological Update. Ann. Rev. Biochem. 1986;55(1):427–453. doi: 10.1146/annurev.bi.55.070186.002235. [DOI] [PubMed] [Google Scholar]
- 2.Islambulchilar M., Asvadi I., Sanaat Z., Esfahani A., Sattari M. Taurine attenuates chemotherapy-induced nausea and vomiting in acute lymphoblastic leukemia. Amino Acids. 2015;47(1):101–109. doi: 10.1007/s00726-014-1840-x. [DOI] [PubMed] [Google Scholar]
- 3.Wójcik O.P., Koenig K.L., Zeleniuch-Jacquotte A., Costa M., Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208(1):19–25. doi: 10.1016/j.atherosclerosis.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huxtable R.J. Others: physiological actions of taurine. Physiol. Rev. 1992;72(1):101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 5.Rashid K., Das J., Sil P.C. Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats. Food Chem. Toxicol. 2013;51:317–329. doi: 10.1016/j.fct.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Timbrell J.A., Waterfield C.J. Taurine 2: Springer; 1996. Changes in Taurine as an Indicator of Hepatic Dysfunction and Biochemical Perturbations; pp. 125–134. [DOI] [PubMed] [Google Scholar]
- 7.Ghandforoush-Sattari M., Mashayekhi S. Evaluation of taurine as a biomarker of liver damage in paracetamol poisoning. Eur. J. Pharmacol. 2008;581(1):171–176. doi: 10.1016/j.ejphar.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 8.Das J., Ghosh J., Manna P., Sil P.C. Acetaminophen induced acute liver failure via oxidative stress and JNK activation: protective role of taurine by the suppression of cytochrome P450 2E1. Free Rad. Res. 2010;44(3):340–355. doi: 10.3109/10715760903513017. [DOI] [PubMed] [Google Scholar]
- 9.Dorğru-Abbasoğlu S., Kanbağli Ö., Balkan J., Cevikbaş U., Aykaç-Tokerl G., Uysall M. The protective effect of taurine against thioacetamide hepatotoxicity of rats. Hum. Exp. Toxicol. 2001;20(1):23–27. doi: 10.1191/096032701673031525. [DOI] [PubMed] [Google Scholar]
- 10.Jagadeesan G., Pillai S.S. Hepatoprotective effects of taurine against mercury induced toxicity in rat. J. Environ. Biol. 2007;28(4):753. [PubMed] [Google Scholar]
- 11.Miyazaki T., Karube M., Matsuzaki Y., Ikegami T., Doy M., Tanaka N., Bouscarel B. Taurine inhibits oxidative damage and prevents fibrosis in carbon tetrachloride-induced hepatic fibrosis. J. Hepatol. 2005;43(1):117–125. doi: 10.1016/j.jhep.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Heidari R., Babaei H., Eghbal M.A. Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arch. Ind. Hyg. Toxicol. 2013;64(2):201–210. doi: 10.2478/10004-1254-64-2013-2297. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z., Liu D., Yi B., Liao Z., Tang L., Yin D., He M. Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol. Med. Rep. 2014;10(5):2255–2262. doi: 10.3892/mmr.2014.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balkan J., Parldar F.H., Dogru-Abbasoglu S., Aykaç-Toker G., Uysal M. The effect of taurine or betaine pretreatment on hepatotoxicity and prooxidant status induced by lipopolysaccharide treatment in the liver of rats. Eur. J. Gastroenterol. Hepatol. 2005;17(9):917–921. doi: 10.1097/00042737-200509000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Parvez S., Tabassum H., Banerjee B.D., Raisuddin S. Taurine prevents tamoxifen-Induced mitochondrial oxidative damage in mice. Basic Clin. Pharmacol. Toxicol. 2008;102(4):382–387. doi: 10.1111/j.1742-7843.2008.00208.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki T., Matsuzaki Y. Taurine and liver diseases: a focus on the heterogeneous protective properties of taurine. Amino Acids. 2014;46(1):101–110. doi: 10.1007/s00726-012-1381-0. [DOI] [PubMed] [Google Scholar]
- 17.Menzie J., Pan C., Prentice H., Wu J.-Y. Taurine and central nervous system disorders. Amino Acids. 2014;46(1):31–46. doi: 10.1007/s00726-012-1382-z. [DOI] [PubMed] [Google Scholar]
- 18.Huxtable R.J. Taurine in the central nervous system and the mammalian actions of taurine. Prog. Neurobiol. 1989;32(6):471–533. doi: 10.1016/0301-0082(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 19.Foos T.M., Wu J.-Y. The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem. Res. 2002;27(1–2):21–26. doi: 10.1023/a:1014890219513. [DOI] [PubMed] [Google Scholar]
- 20.Wu J.-Y., Prentice H. Role of taurine in the central nervous system. J. Biomed. Sci. 2010;17(Suppl. 1):S1. doi: 10.1186/1423-0127-17-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saransaari P., Oja S.S. Taurine and neural cell damage. Amino Acids. 2000;19(3–4):509–526. doi: 10.1007/s007260070003. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J., Li Y., Yan G., Bu Q., Lv L., Yang Y., Zhao J., Shao X., Deng Y., Zhu R. others: protective role of taurine against morphine-induced neurotoxicity in C6 cells via inhibition of oxidative stress. Neurotox. Res. 2011;20(4):334–342. doi: 10.1007/s12640-011-9247-x. [DOI] [PubMed] [Google Scholar]
- 23.Ferenci P., Lockwood A., Mullen K., Tarter R., Weissenborn K., Blei A.T. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 24.Butterworth R.F. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab. Brain Dis. 2002;17(4):221–227. doi: 10.1023/a:1021989230535. [DOI] [PubMed] [Google Scholar]
- 25.Dadsetan S., Waagepetersen H.S., Schousboe A., Bak L.K. Glutamine and ammonia in hepatic encephalopathy. In: Rajendram R., Preedy V.R., Patel V.B., editors. Glutamine in Clinical Nutrition. Springer; New York: 2015. pp. 219–227. [Google Scholar]
- 26.Norenberg M.D. Oxidative and nitrosative stress in ammonia neurotoxicity. Hepatology. 2003;37(2):245–248. doi: 10.1053/jhep.2003.50087. [DOI] [PubMed] [Google Scholar]
- 27.Häussinger D., Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr. Opin. Clin. Nut. Metab. Care. 2010;13(1):87–92. doi: 10.1097/MCO.0b013e328333b829. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigo R., Cauli O., Gomez-Pinedo U., Agusti A., Hernandez-Rabaza V., Garcia-Verdugo J.M., Felipo V. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139(2):675–684. doi: 10.1053/j.gastro.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Butterworth R.F. Neuroinflammation in acute liver failure: mechanisms and novel therapeutic targets. Neurochem. Int. 2011;59(6):830–836. doi: 10.1016/j.neuint.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Luo M., Liu H. Hu S-J, Bai F-H: Potential targeted therapies for the inflammatory pathogenesis of hepatic encephalopathy. Clin. Res. Hepatol. Gastroenterol. 2015;39(6):665–673. doi: 10.1016/j.clinre.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Rao K.V.R., Norenberg M.D. Cerebral energy metabolism in hepatic encephalopathy and hyperammonemia. Metab. Brain Dis. 2001;16(1–2):67–78. doi: 10.1023/a:1011666612822. [DOI] [PubMed] [Google Scholar]
- 32.Butterworth R.F., Norenberg M.D., Felipo V., Ferenci P., Albrecht J., Blei A.T. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009;29(6):783–788. doi: 10.1111/j.1478-3231.2009.02034.x. [DOI] [PubMed] [Google Scholar]
- 33.Moezi L., Heidari R., Amirghofran Z., Nekooeian A.A., Monabati A., Dehpour A.R. Enhanced anti-ulcer effect of pioglitazone on gastric ulcers in cirrhotic rats: the role of nitric oxide and IL-1b. Pharmacol. Rep. 2013;65(134):134–143. doi: 10.1016/s1734-1140(13)70971-x. [DOI] [PubMed] [Google Scholar]
- 34.Kountouras J., Billing B.H., Scheuer P.J. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Brit. J. Exp. Pathol. 1984;65(3) [PMC free article] [PubMed] [Google Scholar]
- 35.Tuñón M.J., Alvarez M., Culebras J.M., González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J. Gastroenterol. 2009;15(25) doi: 10.3748/wjg.15.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruck R., Aeed H., Shirin H., Matas Z., Zaidel L., Avni Y., Halpern Z. The hydroxyl radical scavengers dimethylsulfoxide and dimethylthiourea protect rats against thioacetamide-induced fulminant hepatic failure. J. Hepatol. 1999;31(1):27–38. doi: 10.1016/s0168-8278(99)80160-3. [DOI] [PubMed] [Google Scholar]
- 37.McGill M.R., Williams C.D., Xie Y., Ramachandran A., Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 2012;264(3):387–394. doi: 10.1016/j.taap.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L.-C., Wang C.-J., Lee C.-C., Su S.-C., Chen H.-L., Hsu J.-D., Lee H.-J. Aqueous extract of Hibiscus sabdariffa L. decelerates acetaminophen-induced acute liver damage by reducing cell death and oxidative stress in mouse experimental models. J. Sci. Food Agric. 2010;90(2):329–337. doi: 10.1002/jsfa.3821. [DOI] [PubMed] [Google Scholar]
- 39.Heidari R., Jamshidzadeh A., Keshavarz N., Azarpira N. Mitigation of methimazole-Induced hepatic injury by taurine in mice. Sci. Pharm. 2014;83(1):143–158. doi: 10.3797/scipharm.1408-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatauret N., Desjardins P., Zwingmann C., Rose C., Rao K.V.R., Butterworth R.F. Direct molecular and spectroscopic evidence for increased ammonia removal capacity of skeletal muscle in acute liver failure. J. Hepatol. 2006;44(6):1083–1088. doi: 10.1016/j.jhep.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 41.Goodman Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007;47(4):598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Brunt E.M. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31(1):241–246. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 43.Apelqvist G., Wikell C., Hindfelt B., Bergqvist P.B., Andersson G., Bengtsson F. Altered open-field behavior in experimental chronic hepatic encephalopathy after single venlafaxine and citalopram challenges. Psychopharmacology (Berl.) 1999;143(4):408–416. doi: 10.1007/s002130050966. [DOI] [PubMed] [Google Scholar]
- 44.Kugelberg F.C., Apelqvist G., Wikell C., Bengtsson F. Open-Field behavioural alterations in liver-Impaired and sham-Operated rats after acute exposure to the antidepressant venlafaxine. Basic Clin. Pharmacol. Toxicol. 2005;97(3):155–161. doi: 10.1111/j.1742-7843.2005.pto_97385.x. [DOI] [PubMed] [Google Scholar]
- 45.Amin B., Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L., and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia. 2012;83(5):888–895. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Hosseinzadeh H., Imenshahidi M., Hosseini M., Razavi B.M. Effect of linalool on morphine tolerance and dependence in mice. Phytother. Res. 2012;26(9):1399–1404. doi: 10.1002/ptr.3736. [DOI] [PubMed] [Google Scholar]
- 47.Avraham Y., Grigoriadis N.C., Poutahidis T., Vorobiev L., Magen I., Ilan Y., Mechoulam R., Berry E.M. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br. J. Pharmacol. 2011;162(7):1650–1658. doi: 10.1111/j.1476-5381.2010.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rama Rao K.V., Reddy P.V.B., Tong X., Norenberg M.D. Brain edema in acute liver failure: inhibition by l-Histidine. Am. J. Pathol. 2010;176(3):1400–1408. doi: 10.2353/ajpath.2010.090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heidari R., Babaei H., Roshangar L., Eghbal M.A. Effects of enzyme induction and/or glutathione depletion on methimazole-Induced hepatotoxicity in mice and the protective role of N-acetylcysteine. Adv. Pharm. Bull. 2014;4(1):21–28. doi: 10.5681/apb.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasmussen M.K., Ekstrand B., Zamaratskaia G. Comparison of cytochrome P450 concentrations and metabolic activities in porcine hepatic microsomes prepared with two different methods. Toxicol. In Vitro. 2011;25(1):343–346. doi: 10.1016/j.tiv.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Zamaratskaia G., Zlabek V. EROD and MROD as markers of cytochrome P450 1A activities in hepatic microsomes from entire and castrated male pigs. Sensors. 2009;9(3):2134–2147. doi: 10.3390/s90302134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinke L.A., Moyer M.J. p-Nitrophenol hydroxylation: a microsomal oxidation which is highly inducible by ethanol. Drug Metab. Dispos. 1985;13(5):548–552. [PubMed] [Google Scholar]
- 53.Yao H.-T., Lin P., Chang Y.-W., Chen C.-T., Chiang M.-T., Chang L., Kuo Y.-C., Tsai H.-T., Yeh T.-K. Effect of taurine supplementation on cytochrome P450 2E1 and oxidative stress in the liver and kidneys of rats with streptozotocin-induced diabetes. Food Chem. Toxicol. 2009;47(7):1703–1709. doi: 10.1016/j.fct.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 54.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976;72(1):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 55.Refik Mas M., Comert B., Oncu K., Vural S.A., Akay C., Tasci I., Ozkomur E., Serdar M., Mas N., Alcigir G., Yener N. The effect of taurine treatment on oxidative stress in experimental liver fibrosis. Hepatol. Res. 2004;28(4):207–215. doi: 10.1016/j.hepres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Hansen S.H., Grunnet N. Taurine, Glutathione and Bioenergetics. In: Idrissi A.E., L'Amoreaux W.J., editors. Taurine 8: Springer; New York: 2013. pp. 3–12. [Google Scholar]
- 57.El Idrissi A., Trenkner E. Taurine 5: Springer; 2003. Taurine Regulates Mitochondrial Calcium Homeostasis; pp. 527–536. [DOI] [PubMed] [Google Scholar]
- 58.Devi S.L., Viswanathan P., Anuradha C.V. Regression of liver fibrosis by taurine in rats fed alcohol: effects on collagen accumulation, selected cytokines and stellate cell activation. Eur. J. Pharmacol. 2010;647(1):161–170. doi: 10.1016/j.ejphar.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Safadi R., Friedman S.L. Hepatic fibrosis–role of hepatic stellate cell activation. Med. Gen. Med. 2002;4(3):27. [PubMed] [Google Scholar]
- 60.Hansen S.H., Andersen M.L., Cornett C., Gradinaru R., Grunnet N. A role for taurine in mitochondrial function. J. Biomed. Sci. 2010;17(Suppl. 1):S23. doi: 10.1186/1423-0127-17-S1-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmeira C.M., Rolo A.P. Mitochondrially-mediated toxicity of bile acids. Toxicology. 2004;203(1):1–15. doi: 10.1016/j.tox.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Palmi M., Youmbi G.T., Fusi F., Sgaragli G.P., Dixon H.B.F., Frosini M., Tipton K.F. Potentiation of mitochondrial Ca2+ sequestration by taurine. Biochem. Pharmacol. 1999;58(7):1123–1131. doi: 10.1016/s0006-2952(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh M., Pal S., Sil P.C. Taurine attenuates nano-copper-induced oxidative hepatic damage via mitochondria-dependent and NF-κB/TNF-α-mediated pathway. Toxicol. Res. 2014;3(6):474–486. [Google Scholar]
- 64.Acharya M., Lau-Cam C.A. Comparative Evaluation of the Effects of Taurine and Thiotaurine on Alterations of the Cellular Redox Status and Activities of Antioxidant and Glutathione-Related Enzymes by Acetaminophen in the Rat. In: Idrissi A.E., L'Amoreaux W.J., editors. Taurine 8: Springer; New York: 2013. pp. 199–215. [DOI] [PubMed] [Google Scholar]
- 65.Low T.Y., Leow C.K., Salto-Tellez M., Chung M.C.M. A proteomic analysis of thioacetamide-induced hepatotoxicity and cirrhosis in rat livers. Proteomics. 2004;4(12):3960–3974. doi: 10.1002/pmic.200400852. [DOI] [PubMed] [Google Scholar]
- 66.Cozzi R., Ricordy R., Bartolini F., Ramadori L., Perticone P., De Salvia R. Taurine and ellagic acid: two differently-acting natural antioxidants. Environ. Mol. Mutagen. 1995;26(3):248–254. doi: 10.1002/em.2850260310. [DOI] [PubMed] [Google Scholar]
- 67.Pushpakiran G., Mahalakshmi K., Anuradha C.V. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids. 2004;27(1):91–96. doi: 10.1007/s00726-004-0066-8. [DOI] [PubMed] [Google Scholar]
- 68.Das J., Ghosh J., Manna P., Sil P.C. Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology. 2010;269(1):24–34. doi: 10.1016/j.tox.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Oja S.S., Saransaari P. Taurine as osmoregulator and neuromodulator in the brain. Metab. Brain Dis. 1996;11(2):153–164. doi: 10.1007/BF02069502. [DOI] [PubMed] [Google Scholar]
- 70.O'Byrne M.B., Tipton K.F. Taurine-Induced attenuation of MPP+ neurotoxicity In vitro. J. Neurochem. 2000;74(5):2087–2093. doi: 10.1046/j.1471-4159.2000.0742087.x. [DOI] [PubMed] [Google Scholar]
- 71.Das J., Ghosh J., Manna P., Sinha M., Sil P.C. Arsenic-induced oxidative cerebral disorders: protection by taurine. Drug Chem. Toxicol. 2009;32(2):93–102. doi: 10.1080/01480540802564171. [DOI] [PubMed] [Google Scholar]
- 72.Lemberg A., Fernández M.A. Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann. Hepatol. 2009;8(2):95–102. [PubMed] [Google Scholar]
- 73.Felipo V., Butterworth R.F. Neurobiology of ammonia. Prog. Neurobiol. 2002;67(4):259–279. doi: 10.1016/s0301-0082(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 74.Son M., Kim H.K., Kim W.B., Yang J., Kim B.K. Protective Effect of Taurine on Indomethacin-Induced Gastric Mucosal Injury. In: Huxtable R.J., Azuma J., Kuriyama K., Nakagawa M., Baba A., editors. Taurine 2: Springer; US: 1996. pp. 147–155. [DOI] [PubMed] [Google Scholar]
- 75.Sochor J., Nejdl L., Ruttkay-Nedecky B., Bezdekova A., Lukesova K., Zitka O., Cernei N., Mares P., Pohanka M., Adam V., Babula P., Beklova M., Zeman L., Kizek R. Investigating the influence of taurine on thiol antioxidant status in Wistar rats with a multi-analytical approach. J. Appl. Biomed. 2014;12(2):97–110. [Google Scholar]
- 76.Lee N.-Y., Kang Y.-S. The brain-to-blood efflux transport of taurine and changes in the blood–brain barrier transport system by tumor necrosis factor-α. Brain Res. 2004;1023(1):141–147. doi: 10.1016/j.brainres.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 77.Kilberg M.S., Haussinger D. Springer US; Boston, MA: 1992. Mammalian Amino Acid Transport: Mechanisms and Control. [Google Scholar]
- 78.Law R.O., Zielinska M., Albrecht J. Taurine 5: Springer; 2003. Taurine Counteracts Cell Swelling in Rat Cerebrocortical Slices Exposed to Ammonia in Vitro and in Vivo; pp. 123–129. [DOI] [PubMed] [Google Scholar]
- 79.Rao K.V.R., Jayakumar A.R., Norenberg M.D. Brain edema in acute liver failure: mechanisms and concepts. Metab. Brain Dis. 2014;29(4):927–936. doi: 10.1007/s11011-014-9502-y. [DOI] [PubMed] [Google Scholar]
- 80.Jover R., Rodrigo R., Felipo V., Insausti R., Sáez-Valero J., García-Ayllón M.S., Suárez I., Candela A., Compañ A., Esteban A., Cauli O., Ausó E., Rodríguez E., Gutiérrez A., Girona E., Erceg S., Berbel P., Pérez-Mateo M. Brain edema and inflammatory activation in bile duct ligated rats with diet-induced hyperammonemia: a model of hepatic encephalopathy in cirrhosis. Hepatology. 2006;43(6):1257–1266. doi: 10.1002/hep.21180. [DOI] [PubMed] [Google Scholar]
- 81.Häussinger D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2006;43(6):1187–1190. doi: 10.1002/hep.21235. [DOI] [PubMed] [Google Scholar]
- 82.Bosoi C.R., Parent-Robitaille C., Anderson K., Tremblay M., Rose C.F. AST-120 (spherical carbon adsorbent) lowers ammonia levels and attenuates brain edema in bile duct–ligated rats. Hepatology. 2011;53(6):1995–2002. doi: 10.1002/hep.24273. [DOI] [PubMed] [Google Scholar]
- 83.Rama Rao K.V., Verkman A.S., Curtis K.M., Norenberg M.D. Aquaporin-4 deletion in mice reduces encephalopathy and brain edema in experimental acute liver failure. Neurobiol. Dis. 2014;63:222–228. doi: 10.1016/j.nbd.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cauli O., Llansola M., Agustí A., Rodrigo R., Hernández-Rabaza V., Rodrigues T.B., López-Larrubia P., Cerdán S., Felipo V. Cerebral oedema is not responsible for motor or cognitive deficits in rats with hepatic encephalopathy. Liver Int. 2014;34(3):379–387. doi: 10.1111/liv.12258. [DOI] [PubMed] [Google Scholar]
- 85.Marcinkiewicz J., Kurnyta M., Biedroń R., Bobek M., Kontny E., Maśliński W. Anti-inflammatory effects of taurine derivatives (taurine chloramine, taurine bromamine, and taurolidine) are mediated by different mechanisms. Adv. Exp. Med. Biol. 2006;583:481–492. doi: 10.1007/978-0-387-33504-9_54. [DOI] [PubMed] [Google Scholar]
- 86.Schuller-Levis G.B., Taurine Park E. New implications for an old amino acid. FEMS Microbiol. Lett. 2003;226(2):195–202. doi: 10.1016/S0378-1097(03)00611-6. [DOI] [PubMed] [Google Scholar]
- 87.Seabra V., Stachlewitz R.F., Thurman R.G. Taurine blunts LPS-induced increases in intracellular calcium and TNF-alpha production by Kupffer cells. J. Leuk. Biol. 1998;64(5):615–621. doi: 10.1002/jlb.64.5.615. [DOI] [PubMed] [Google Scholar]
- 88.Butterworth R.F. Taurine in Hepatic Encephalopathy. In: Huxtable R.J., Azuma J., Kuriyama K., Nakagawa M., Baba A., editors. Taurine 2: Springer; US: 2016. pp. 601–606. [Google Scholar]
- 89.Butterworth R.F. Neuroactive amino acids in hepatic encephalopathy. Metab. Brain Dis. 1996;11(2):165–173. doi: 10.1007/BF02069503. [DOI] [PubMed] [Google Scholar]