Abstract
The reproductive toxicity of combined fixed-dose first-line antituberculosis (CFDAT) regimen was assessed in rats. Thirty-two (32) Wistar rats weighing 168.1 ± 8.0 g were divided into four groups of eight rats per group. Two groups of male and female rats were administered oral distilled water (1.6 ml) and CFDAT drugs containing rifampicin, isoniazid, pyrazinamide and ethambutol (RIPE, 92.5 mg/m2 per body surface area) respectively for forty-five days. Serum follicle stimulating hormone, luteinizing and testosterone were reduced significantly (p < 0.05) in the treated male rats. Similarly, sperm count levels were decreased by 27.3% when compared with control. RIPE elevated serum oestrogen (p < 0.05), progesterone (p < 0.05) as well as prolactin (p > 0.05) levels in the treated females. In addition, RIPE reduced (p < 0.05) total proteins levels and increased (p < 0.05, 53%) catalase levels in male but not female animals. Superoxide dismutase, glutathione-S-transferase, glutathione peroxidase, reduced glutathione levels as well as lipid peroxidation were unaltered in all rats respectively. Histopathological studies revealed congested peritesticular vessels and no changes in the ovary when compared with control. Overall, our results demonstrate reproductive toxicity potentials of RIPE in the rat, thus, suggesting that these reproductive parameters be monitored during antituberculous chemotherapy.
Keywords: Fixed dose combined antituberculous drugs, Sub-chronic study, Reproductive toxicity, Rats
1. Introduction
There have been recent suggestions that anti-tuberculosis agents would produce adverse effects on reproductive health system [1], [2], [3], [4]. Tuberculosis (TB), a Mycobacterium tuberculosis (M. tuberculosis) remains an epidemic in many parts of the world. According to the WHO, an estimated 8.7 million new cases of TB and 1.4 million deaths from TB emerge in 2011[5]. TB is the leading cause of death from a curable infectious disease and is second only to HIV/AIDS as a cause of death from any infectious disease [7]. The rapid spread of drug-resistant TB in Asia, Eastern Europe, and Africa has limited the achievement of TB treatments goals among others [6], [7]. TB incidence has been falling globally for several years and fell at an average rate of about 1.5% per year between 2000 and 2014. For instance, the TB mortality rate has fallen by an estimated 47% between 1990 and 2015 [8]. It poses significant challenges to developing economies as it primarily affects people during their most productive years. The treatment protocol for drug-sensitive TB, however, varies slightly in different parts of the world, but they are based on a combination of three, or more typically, four drugs, i.e. isoniazid, rifampin, pyrazinamide, and ethambutol. This combination has been adjudged best for efficacy and tolerability amongst the available TB drugs and is, therefore, the mainstay “first line” therapy [8], [9]. There are historical and clinical proofs that these first-line antituberculosis agents are the most potent oral antituberculous medications [10], [11], [12]. In addition, in-vitro and in-vivo clinical data support the use of such individual agent [13], [14]. This may be because the combination has been found to be a beneficial and cost-effective treatment for TB [15], but, this is not without some systemic toxicity from the results from human data [1], [3], [5]. A study from Sharma [16] suggests that the high incidence of antituberculous drugs inducing toxicities may indicate difficulties with systematic steps in the prevention and management of tuberculosis. Evidence abound that most of the adverse effects due to the antituberculous and antiretroviral drugs are experienced during the first months of therapy [17]. For instance, two authors at different times have reported nephritis associated with rifampicin [18], [19]. Still, there exist cross adverse event among rifampicin, isoniazid, and pyrazinamide which is manifested as liver injury [20], [21], [22], [23] while ethambutol is widely known for its ocular toxicity [24]. Extensions of their adverse effects include skin rashes, gastrointestinal intolerance, central nervous system symptoms, peripheral neuropathy, and blood dyscrasias. In respect to the aforementioned, the concern about adverse drug effects of antituberculous agents has been advocated [25], [26] because of their negative impacts on the sustainability of public health system [6], [7]. Since tuberculosis affects people in their productive and reproductive age, but available data on adverse drug reactions in patients and animals experiments particularly with respect to reproduction are limited [27]. Although our previous studies showed synergy of fixed-dose combined antituberculous drugs in liver injury, its teratogenic potential in a first filial generation of animals model as well as the activity of antioxidants against the toxicity of rifampicin and the fixed-dose combined antituberculous [25], [28]. There is still a paucity of data on the reproductive toxicity of fixed-dose combined antituberculous drugs.
Therefore, in this present study, we assessed the reproductive toxicity of combined fixed-dose first-line anti-tuberculosis (CFDAT) drugs in rats.
2. Materials and methods
2.1. Drugs and chemicals
A Combined fixed-dose antituberculous drugs consisting of rifampicin (150 mg), isoniazid (75 mg), Pyrazinamide (400 mg) and ethambutol (275 mg) per tablet, was obtained from the DOTS clinic of Akerele Primary Healthcare Centre, Nigeria. Thiobarbituric acid (TBA) was purchased from Sigma Chemical Company (USA). Metaphosphoric acid, Reduced glutathione (GSH), and Trichloroacetic acid (TCA) were purchased from J.I. Baker (USA). Rat Follicle Stimulating Hormone (FSH) (Cat. No.: Rshakrfs-010R) and Luteinizing Hormone (LH) ELISA (Rshakrlh-010SR) kits were purchased from (Biovendor, Shibayagi Co., Ltd. (Japan). RAT Testosterone (RTC001R) and Prolactin ELISA were obtained from Biovendor, Laboratorni, medicina a.s Karasek (Czech Republic). Rat oestrogen ELISA (CSB-E07279r) (Cusabio Biotech CO., LTD.) and Progesterone (RTC008R) kit was purchased from Biovendor, Laboratorni, medicina a.s Karasek (Czech Republic) and Sodium hydroxide from MERCK (Germany). All other chemicals and reagents used were of analytical grades. Atomic UV/Visible Spectrophotometer obtained from JENWAY, Bibby Scientific (Model 7300 and 7305) (USA).
2.2. Animals
Albino rats of the Wistar strain weighing between 120 and 250 g were purchased from inbreeding animal house of the Redeemers University, Mowe, Ogun State, Nigeria. The rats were housed in the experimental animal handling facility of the University of Lagos, College of Medicine, Idi-Araba Campus, Lagos State, Nigeria, at temperature of the experimental animal rooms of 22 ± 3 °C, under controlled conditions with a 12 h light/12 h dark schedule and fed with commercially available rat pelleted diet (Ladoke Akintola Growers Mash) and water ad libitum throughout the period of the experiment. Animals were acclimatized for a period of two weeks before the experiment. The experimental protocols were approved by the Institutional Animal Care and Use Committee, Department of Pharmacology, Therapeutic and Toxicology, College of Medicine, University of Lagos, and all of the experimental procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The institution animal health officers certify the animals fit for the experiment. The beddings of the animals were changed on alternate days and the animals were sacrificed in a humane manner at the end of the experiment by cervical dislocation. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the U. S. National Institutes of Health (NIH Publication No. 85-23, revised 1996)” for studies involving experimental animals and the procedures as documented by Kilkenny et al. [29] for reporting animal research.
2.3. Sub-chronic study
Thirty-two (32) (n: male or female = 8) Wistar rats weighing 168.1 ± 8.0 g were divided into 4 groups of 8 rats per group for each sex and treatment group. The control groups were administered 1.6 ml distilled water by oral administration daily. A combined fixed-dose anti-TB drugs (92.5 mg/m2 per body surface area, p.o) [30] was administered to the test groups (male and female separately). The rats were weighed weekly and treatments lasted for Forty-five (45)-days.
2.4. Collection of blood samples and tissues
All treatments were terminated on day 45 and Twenty-four (24) hours following the last administration, blood samples were obtained via ocular puncture into lithium heparinized bottles and animals were sacrificed via cervical dislocation. The blood samples containing anticoagulant were centrifuged at 4200 rpm for 5 min to obtain the clear supernatant (i.e. serum) from which all biochemical analyses were carried out. In addition, the reproductive organs (testis, epididymis and ovaries) were removed, weighed and stored for histological examinations.
2.5. Sperm function analysis
The testes from each rat were carefully exposed and removed along with its adjoining epididymis. The left testis was separated from the epididymis, and the caudal epididymal tissue was removed and placed in a petri dish containing 1 ml normal saline solution. An incision of about 1 mm was made in the caudal epididymis to liberate its spermatozoa into the saline solution. Progressive sperm motility, sperm count, and sperm viability were then examined under the microscope attached to a Celestron® digital microscope imager (Torrance, CA 90503) and viewed under X40 objective according to the method described by Raji et al. [31] Epididymal sperm motility was assessed by calculating motile spermatozoa per unit area and was expressed as percentage motility. Epididymal sperm counts were made using the improved Neubauer hemocytometer and were expressed as million/ml of suspension. The sperm viability was also determined using Eosin/Nigrosin stain. The motile (live) sperm cells were unstained while the non-motile (dead) sperms absorbed the stain. The stained and unstained sperm cells were counted and an average value for each was recorded from which percentage viability was calculated. Sperm morphology was evaluated by staining the sperm smears on microscope slides with two drops of Walls and Ewa stain after they were air-dried. The slides were examined under the microscope under oil immersion with X 100 objectives.
2.6. Reproductive hormone assessments
Serum concentrations of male reproductive hormones were measured using micro plate enzyme-linked immunosorbent assay (ELISA) and expressed as Units/l. Studies protocols were discussed below. Direct immune-enzymatic determination of the Luteinizing (LH) and Follicle-Stimulating Hormones (FSH) in human serum were determined by the methods of Odell et al. [32] based on the manufacturer’s manual. Testosterone activity was determined following the principle described by Joshi et al. [33]. The method of DeVilla et al. [34] was followed in estimating the levels of progesterone and oestrogen levels while prolactin levels were determined according to the method of Shome and Parlow [35] based on the manufacturer’s manual.
2.6.1. Rat FSH and LH ELISA
Briefly, in Rat FSH or LH ELISA Kit, biotin-conjugated anti-FSH/anti-LH and standard or sample were incubated in monoclonal anti-FSH antibody-coated wells. After 15 h incubation and washing, HRP (horse radish peroxidase)-conjugated avidin was added, and incubated for 30 min. After washing, HRP-complex remaining in wells was reacted with a chromogenic substrate (TMB) for 20 min, and reaction was stopped by addition of acidic solution, and absorbance of yellow product was measured spectrophotometrically at 450 nm. The absorbance is nearly proportional to FSH or LH concentration. FSH or LH concentrations in unknown samples were then exptrapolated via given their respected standard curve [32].
2.6.2. Rat testosterone ELISA
A 10 μl of each of sample with new disposable tips into appropriate wells was dispensed in a 100 μl of incubation Buffer into each well. Added was a 50 μl enzyme Conjugate into each well which was incubated for 60 min at room temperature on a microplate mixer. This was discarded and the well rinsed 4 times with diluted washing solution (300 μl per well). Then 200 μl was added of substrate solution to each well and incubated standing for 30 min in the dark. The reaction was stopped by adding 50 μl of stop solution to each well and the absorbance determined for each well at 450 nm [33].
2.6.3. Rat estrogen ELISA
The blank well and or 50 μl of standard in triplicate or sample per well were prepared. Then added was a 50 μl of HRP-conjugate to each well except for the blank. Then 50 μl antibody was added to each well. The solution was thorough mixed, and then incubated for 3 h at 37 °C. Each well was washed with buffer (350 μl), wait 10 s and spin. Following the last wash, remaining wash buffer was aspirated. Plate was inverted and blotted against clean paper towels. A 50 μl of substrate A was added and substrate B to each well, and mixed. Solution was incubated for 15 min at 37 °C. In a dark environment, a 50 μl of stop solution was added to each well. The color change appeared uniform. Determination of the optical density was carried out within 10 min, using a microplate reader set to 450 nm [34].
2.6.4. Rat progesterone ELISA
A dispensed 25 μl of each sample with new disposable tips into appropriate wells and added 50 μl of incubation Buffer into each well. Added was a 100 μl enzyme conjugate into each well and incubated for 1 h at 22 ± 2 °C on a microplate mixer. Following a thorough rinsing up to 4 times with diluted wash solution, a 200 μl of substrate solution was added to each well and incubated for 30 min in the dark. This reaction was terminated by the addition of a 50 μl of stop solution to each well. The progesterone level was measured at 450 nm within 15 min [34].
2.6.5. Rat prolactin ELISA
A pipetted 25 μl of each sample into the wells was prepared and added 50 μl of rat prolactin sample buffer to every well. Shake for 2 h at room temperature (22 ± 1 °C), rinsed 4 times with 300 μl buffered wash solution. A 200 μl was added of enzyme-labeled anti-rat prolactin antibody to all wells and shaken for 1 h, rinsed again for 4 times with 300 μl buffered wash solution. A 200 μl of liquid TMB/substrate solution was added to all wells. This was then incubated standing for 30 min in the dark. Then 50 μl of stop solution was added to each well, mixed carefully and measured at 450 nm [35].
2.7. Antioxidants and oxidative stress
The method of Beutler et al. [36] was followed in estimating the levels of glutathione (GSH). Glutathione-S-transferase (GST) activity was determined according to Habig et al. [37]. The level of superoxide dismutase (SOD) activity was determined by the method of Misra and Fridovich [38]. Catalase (CAT) activity was determined according to the method of Sinha [39]. Glutathione Peroxidase (GPx) activity was determined according to the method of Ellerby and Bredesen [40]. Lipid peroxidation was determined by measuring the formation of thiobarbituric acid reactive substances (TBARS) according to the method of Varshney and Kale [41].
2.8. Histological assessment
The tissue samples from the liver, kidney and heart for histological examination were passed through the process of fixation, dehydration, clearing, infiltration, embedding, sectioning and staining. To ensure good fixation, the tissues were trimmed to about 5 mm thickness, so as to obtain good fixation. The tissues were then fixed in 10% formol saline and were then transferred to 50% alcohol (70%, 80%, 85%, 95 and 100%), for two hours. Alcohol was removed from the treated tissues by titrating them through first an equal mixture 100% (absolute) alcohol and xylene for one hour each in that order. Infiltration was carried out twice by passing each tissue through molten paraffin wax in an oven at a temperature of 30 °C for one and a half hours each. The tissues so embedded in molten paraffin wax were later placed on a wooden block and trimmed to size. Serial sections 10 μms thick were made using a rotatory microtome. The cut sections were then floated in a warm water bath at a temperature of 30–40 °C and were placed slides. Eight sections were obtained from each treated organ from each animal. Four samples were placed on each slides. Microscopic examination was done by using varying magnifications of 10, 40, 100 and 400 to determine if the samples were properly fixed on the slide. Following staining, mounting of sections was carried out using dimethyl paraffinate xylene (DPX) as a mounting agent, after which microscopic examination was done.
3. Statistical analysis of data
All data were expressed as mean ± standard error of mean (SEM). Significant differences among the group were determined by one-way analysis of variance (ANOVA) and student T-test using the statistical analysis programme for social sciences (SPSS). Post hoc testing was performed for inter-group comparisons using the least significant difference (LSD) [42]. Graphical presentations were achieved using GraphPad Prism 6. Results were considered to be significant at p ≤ 0.05.
3.1. Male reproductive hormones
Table 1 shows the effects of RIPE in normal and treated rats. RIPE produces a significantly (p < 0.05) reduced serum luteinizing, follicle stimulating hormone and testosterone by 46.2%, 66.7% and 42.7% respectively when compared with control.
Table 1.
Effects of Antituberculous Drugs on Male Reproductive Hormones in Rats.
| FSH (U/l) | LH (U/l) | Testosterone (U/l) | |
|---|---|---|---|
| Control | 0.18 ± 0.10 | 0.26 ± 0.10 | 1.57 ± 0.30 |
| RIPE | 0.06 ± 0.02*(66.7)[80] | 0.14 ± 0.04*(46.2)[60] | 0.90 ± 0.10*(42.8)[66.7] |
NB: Result expressed as Mean ± SEM; n = 8; Control (DW, Distilled water, 1.6 ml); Values in parenthesis represent% change; (−) increase; (+) decrease. () % mean, when compared with control distilled water; [] SEM when compared with control. *p < 0.05 change relative to control group. RIPE: Rifampicin + Isoniazid + Pyrazinamide + Ethambutol (92.5 mg/m2 body surface area, body weight 60 kg man; Body Surface Area (BSA): man = 1.6; BSA rat (0.16 kg) = 0.025; km = Weight/BSA; km rat: 6; Km man: 37). FSH = Follicle Stimulating Hormone; LH = Luteinizing Hormone.
3.2. Assessment of female reproductive hormones in rats
The results of the female reproductive hormones in rats are presented in Table 2. From the result obtained serum oestrogen, prolactin and progesterone levels were increased in animals administered with RIPE by 191.7% (p < 0.05), 33.3% (p > 0.05) and 350% (p < 0.05) respectively when compared with control distilled water group.
Table 2.
Effects of Antituberculous Drugs on Female Reproductive Hormones in Rats.
| Oestroge (U/l) | Prolactin (U/l) | Progesterone (U/l) | |
|---|---|---|---|
| Control | 0.36 ± 0.12 | 0.06 ± 0.03 | 0.12 ± 0.05 |
| RIPE | 1.05 ± 0.21*(−191.7) [−75] | 0.08 ± 0.03 (−33.3) [0] | 0.54 ± 0.25*(−350.0)[−400] |
NB: Result expressed as Mean ± SEM; n = 8; Control (DW: Distilled Water, 1.6 ml); Values in parenthesis represent% change; (−) increase; (+) decrease. () % mean, when compared with control distilled water; [] SEM when compared with control. *p < 0.05 change relative to control group. RIPE: (Rifampicin + Isoniazid + Pyrazinamide + Ethambutol, (92.5 mg/m2 body surface area, body weight 60 kg man; Body Surface Area (BSA): man = 1.6; BSA rat (0.16 kg) = 0.025; km = Weight/BSA; km rat: 6; Km man: 37).
3.3. Antioxidants activities
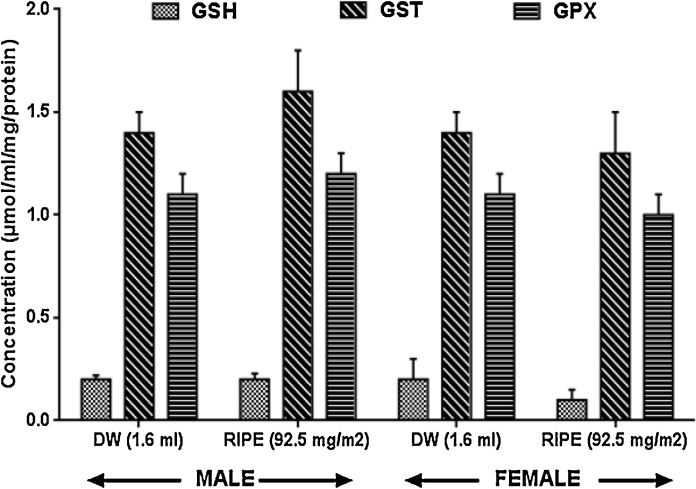
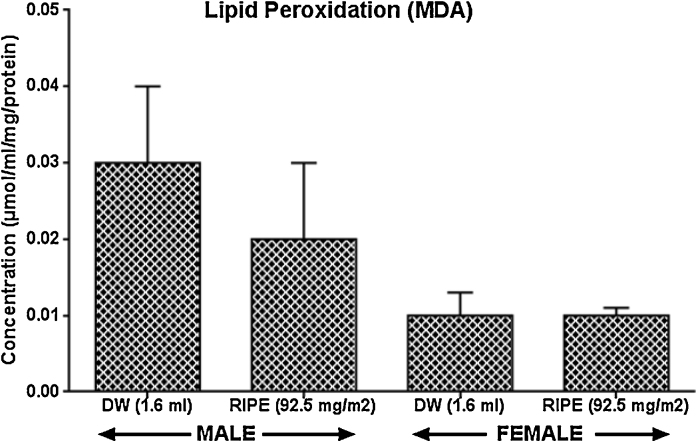
Fig. 1, Fig. 2: show that superoxide dismutase, glutathione-S-transferase, glutathione peroxidase, reduced glutathione, as well as lipid peroxidation were not significantly (p > 0.05) altered in RIPE-treated rats this study experiment.
Fig. 1.
Effects of anti-tuberculous drugs on anti-oxidant activities in rats, Results expressed as Mean ± SEM; n = 8. Control (DW: Distilled Water,); RIPE: Rifampicin + Isoniazid + Pyrazinamide + Ethambutol), GSH: Reduced glutathione, GST: Glutathione-S-transferase GPX: Glutathione peroxidase.
Fig. 2.
Effects of anti-tuberculous drugs on anti-oxidant activities in rats, Result expressed as Mean ± SEM; n = 8. Control (DW: Distilled Water); RIPE: Rifampicin + Isoniazid + Pyrazinamide + Ethambutol), MDA: Malondialdehyde.
3.4. Total protein and catalase
Fig. 3: shows the results of the effect of RIPE on total protein and catalase levels in male and female rats. There were decreased (p < 0.05) in total protein by 24% (male) while catalase increases by 53% in RIPE treated male but not female rats.
Fig. 3.
Effects of anti-tuberculous drugs on anti-oxidant activities in rats, Result expressed as Mean ± SEM; n = 8. Control (DW: Distilled Water); RIPE: Rifampicin + Isoniazid + Pyrazinamide + Ethambutol), SOD: Superoxide dismutase, CAT: Catalase, TP: Total Protein.
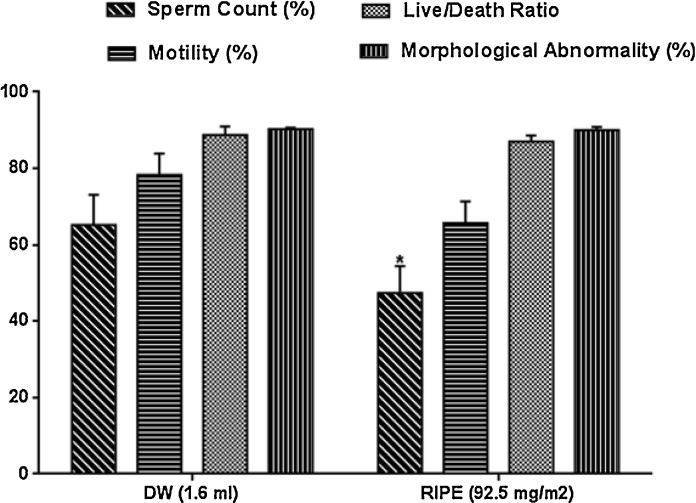
3.5. Sperm characteristics
Fig. 4: shows the result of RIPE on sperm characteristics and morphology in normal and treated male rats. Sperm counts and the percentage of spermatozoa moving actively (motility) forward decreased by 27.3% and 16.1% respectively when compared with control distilled water group.
Fig. 4.
Effects of anti-tuberculous drugs on anti-oxidant activities in male rats, Result expressed as Mean ± SEM; n = 8. Control (DW: Distilled Water); RIPE: Rifampicin + Isoniazid + Pyrazinamide + Ethambutol).
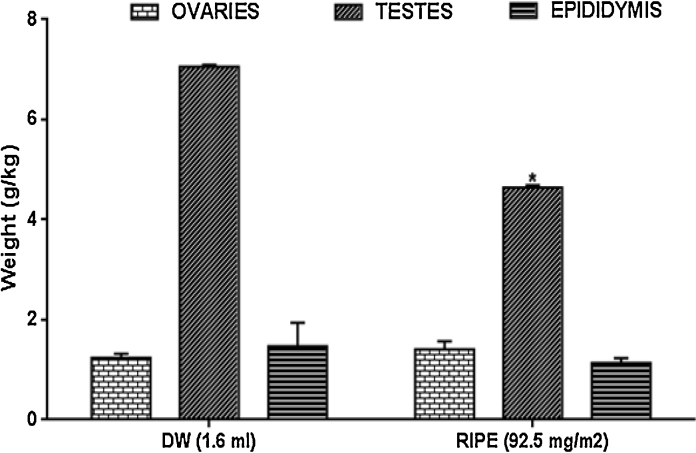
3.6. Organ system weights in rats
Fig. 5: shows ovary, testis, and epididymis weights expressed per body weight. Treatments with RIPE caused an increased ovary weight by 13.8% significantly (p > 0.05). Testis weight was also decreased, although, insignificantly by 33.6% (p < 0.05). However, the epididymis was unaltered in all animals.
Fig. 5.
Effects of anti-tuberculous drugs on anti-oxidant activities in rats, Result expressed as Mean ± SEM; n = 8; Control (DW: Distilled Water); RIPE: Rifampicin + Isoniazid + Pyrazinamide + Ethambutol).
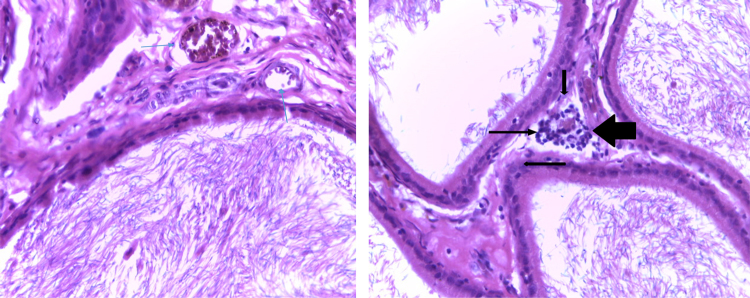
Fig. 6 shows the photomicrograph showing testes of the control rat. (1.6 ml distilled water) (left) and treated rat (RIPE; 92.5 mg/m2 per body surface area) (right). The treated rat shows congested vessels below the tunica as indicated by their dilation and their engorgement, as shown by the arrows.
Fig. 6.
Photomicrograph showing testes of the control rat (1.6 ml distilled water) (left) and treated rat (RIPE; 92.5 mg/m2 per body surface area) (right). The treated rat shows congested vessels below the tunica, as shown by the arrows. [H&E, x400]. RIPE: Combined fixed-dose Rifampicin, Isoniazid, Pyrazinamide, Ethambutol.
Fig. 7 shows the epididymis showing sperm cells, sterocilia, basal cells, expanded stroma with proliferated blood vessels (thin arrows) control rat (1.6 ml distilled water) (left) and increased stroma and intra-epithelial lymphocytes (large arrows, more pronounced) in the treated rat (RIPE; 92.5 mg/m2 per body surface area) (right).
Fig. 7.
The epididymis showing sperm cells, sterocilia, basal cells, expanded stroma with proliferated blood vessels (thin arrows) Control (1.6 ml distilled water) (left) and increased stroma and intra-epithelial lymphocytes (large arrows, more pronounced) in the treated rat (RIPE; 92.5 mg/m2 per body surface area) (right). [H&E, x400]. RIPE: Combined fixed-dose Rifampicin, Isoniazid, Pyrazinamide, Ethambutol.
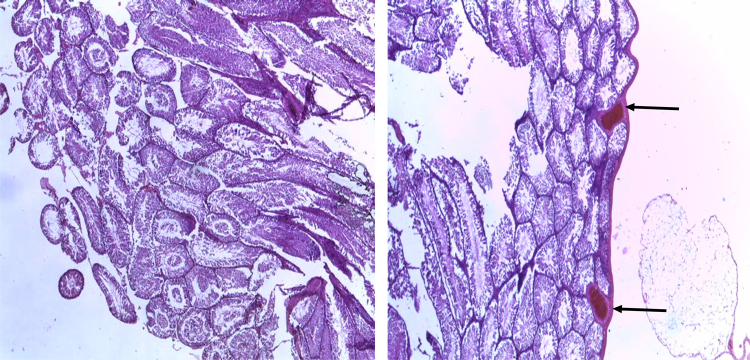
Fig. 8 shows normal ovaries (thin arrows) and normal fallopian tubes (double headed arrows) of the control rat (1.6 ml distilled water) (left) and treated rat.
Fig. 8.
Normal ovaries (thin arrows) and normal fallopian tubes (double headed arrows) of the control rat Control (1.6 ml distilled water) (left) and treated rat (RIPE; 92.5 mg/m2 per body surface area) (right). [H&E, x400]. RIPE: Combined fixed-dose Rifampicin, Isoniazid, Pyrazinamide, Ethambutol.
4. Discussion
The combination therapy used in TB management has in no doubt significantly improved the outcome of the management [43], although, individual agent retain its known functional activities. Nevertheless, there are major challenges in the treatment options which have been reviewed by WHO [7]. Several of such setbacks also have contributed in recent years to a resurgence of interest in developing improved therapies to treat TB. Thus, in a pragmatic matter, comparative studies have pondered on the issue of toxicity in order to add to database information [6] related to survival, disability and capacity for functioning in daily life [7]. However, when information indicates that a particular species is more relevant for assessing human risk, the human equivalent dose for that species is suggested to be used in subsequent calculations, regardless of whether this species is the most sensitive [44]. This aforementioned has translated into an advocacy that the body surface area correlates well across several mammalian species with several parameters of biology, including oxygen utilization, caloric expenditure, basal metabolism, blood volume, circulating plasma proteins, and renal function [44]. Thus, if serious toxicities are observed in an animal species considered less relevant, those toxicities might be taken into consideration in determining the species to be used to calculate an human equivalent dose [44], [45].
Previous reports of some notable adverse drug reactions include weight increase and hepatotoxicity [29], [45], reproductive toxicity [29], exanthema, hematological [46] among others when RIPE is used either as the sole agent or together. In fact, antituberculosis drug-induced hepatotoxicity has been reviewed [47]. According to WHO report on the Global project on antituberculous drug Resistance Surveillance, adverse effects of RIPE have chopped up into diminishing treatment effectiveness because they significantly contribute to treatment failure while relapse or the emergence of drug-resistance are now routinely encountered [48]. Furthermore, weight increase or reduction can be clinically relevant in the prediction of TB treatment outcome because changes in weight have been observed from the first month of therapy [49], [50], [51]. Thus, weight gain is often used to predict the TB treatment outcomes. In respect, we also observed weight changes with respect to the combined fixed-dose first-line antituberculosis (CFDAT). There were reduced testicular and epididymis weights in RIPE treated animals, (Fig. 5), whereas, contrastingly, the ovaries increased. Although, our study did not assess the mechanism involved in weight alterations, however, it is hoped that such alteration in weights during treatment might have been due, in part, to responses triggered by anti-TB drugs (4). Also, we obtained decreased in sperm counts and motility (Fig. 4), although, insignificant statistically, with no alteration in sperm morphological abnormality in RIPE treated rats. This decreased in sperm quality was confirmed by the histological changes due to congested vessels in peritesticular and Leydig cells of the stroma in the testes of the experimental rats treated with RIPE (Fig. 7). It agrees also with our previous report on RIPE [25]. A study by Joffe [52] has however linked male fertility with sperm counts while asking the question to whether such would reflect a general reduction in male fertility, especially among the TB patients. In animals receiving RIPE, several intraepithelial lymphocytes and macrophages were scattered throughout the tissue and the stroma became expanded with proliferated small vessels as opposed to few present in normal rats. Also, in Fig. 6, the testes of the treated rat show congested vessels below the tunica while the epididymis shows increased stroma and intra-epithelial lymphocytes in the treated male rat (Fig. 7). The mild inflammation can predispose to a mother disease when cause is not maximized. However, both the ovaries and fallopian tubes of the treated rat were unaltered throughout the experiment (Fig. 8 shows). Thus, indicates further that caution must be exercise due to the tendency to obtain toxicity during a prolong use. More so, Serum LH and FSH and testosterone levels were reduced in treated male rats (Table 1). Thus, indicating the potential role of RIPE in the general reduction of male fertility, although, our study is limited in that it falls below such a duration that would adjudge RIPE anti-gonadal properties and did not evaluate reproductive behavior.
Conversely, serum oestrogen, prolactin, and progesterone levels were elevated in treated female rats (Table 2). This brings an insight to further ponder on Peloquin [53] suggestion on the essentiality of therapeutic drug monitoring in the treatment of tuberculosis which may help identify peak levels of biological enzymes due to the use of CFDAT drugs while providing some solutions and thereby resolve issues of drug interactions prior to treatment failure relapse or toxicity. Reports of different authors from different parts of the world have at different times reported Isoniazid-induced gynaecomastia [54], [55], [56], [57]. Similarly, such cross activity has been found associated with thioacetazone and ethionamide [58], [59], [60]. Isoniazid-induced gynaecomastia has been linked in part to modulation of weight, gonadotrophin secretion, and gonadal functions [56]. Presently, antituberculous chemotherapy is rarely known to cause gynaecomastia unlike the sex hormones, antiandrogens, spironolactone, cimetidine, verapamil and alkylating agents. In addition, studies indicating this abnormality are few and scanty.
Further, oxidative stress results from an imbalance between oxidants and antioxidants in favor of the oxidants, but, whether this is involved in antituberculous drug-induced toxicity has been debated. Some studies from basic to clinical applications have concluded that both non-enzymatic and enzymatic antioxidant systems play major roles in the detoxification of reactive oxygen species [61]. In addition, reports that diminishing levels of activity of any of these enzymes such as glutathione-S-transferase, catalase, and superoxide dismutase activities and glutathione levels following antituberculous agents administration in rats may indicate oxidative stress and may confer thereafter, in part, some levels of toxicity [62], [63], [64]. From the result obtained given RIPE, catalase levels and total protein levels in male but not female rats decreased significantly (Fig. 3). We have previously reported a condition of diminished catalase and other oxidative parameters during hepatotoxicity achieved through a combination of zidovudine and combined antituberculous agents in rats [28]. A study by Chowdhury et al. [64] has reported that TB patients on receiving antituberculous therapy have been shown to have reduced plasma levels of glutathione and higher malondialdehyde due to oxidative stress from the antituberculous therapy, although, the mechanism for the later was not apparent. In this study, reduced glutathione, superoxide dismutase, lipid peroxidation, glutathione-S-transferase, glutathione peroxidase were altered in the treated rats. RIPE modulation of antioxidant status in vivo in this study is typical as most antituberculous agents have been reported to interfere with liver biotransformation (28,62,63,64)
5. Conclusion
The present study demonstrates the tendency of RIPE to cause reproductive toxicity in the rat. Thus, this may suggest that parameters associated with reproductive activity be monitored during antituberculous chemotherapy.
Conflict of interest
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgement
The technical assistant of Chijoke M. of Pharmacology, Therapeutics and Toxicology Department, College of Medicine, University of Lagos, Lagos State, Nigeria, is gratefully acknowledged.
References
- 1.Ghosh K., Ghosh K., Chowdhury J.R. Tuberculosis and female reproductive health. J. Postgrad. Med. 2011;57(4):307. doi: 10.4103/0022-3859.90082. [DOI] [PubMed] [Google Scholar]
- 2.Caliskan E., Cakiroglu Y., Sofuoglu K., Doger E., Akar M.E., Ozkan S.O. Effects of salpingectomy and antituberculosis treatments on fertility results in patients with genital tuberculosis. J. Obstet. Gynaecol. Res. 2014;40(10):2104–2109. doi: 10.1111/jog.12450. [DOI] [PubMed] [Google Scholar]
- 3.Kuwabara K. Anti-tuberculosis chemotherapy and management of adverse reactions. Nihon Rinsho. Jap. J. Clin. Med. 2011;69(8):1389–1393. [PubMed] [Google Scholar]
- 4.Kulchavenia E.V., Brizhitiuk E.V., Medvedev S.A. Toxic effect of antituberculous drugs on spermatogenesis. Probl. Tuberk. 2011;(5):29–32. [PubMed] [Google Scholar]
- 5.World Health Organization: WHO . WHO; Geneva, Switzerland: 2011. Towards Universal Access to Diagnosis and Treatment of Multidrug-resistant and Extensively Drug-resistant Tuberculosis by 2015; p. 119. [Google Scholar]
- 6.World Health Organization : WHO . WHO; Geneva, Switzerland: 2010. Global Tuberculosis Control 2010; p. 100. [Google Scholar]
- 7.Prasad R. Multidrug and extensively drug-resistant TB (M/XDR-TB): problems and solutions. Indian J. Tuberc. 2010;57:180–191. [PubMed] [Google Scholar]
- 8.Mathers C.D., Stevens G.A., Boerma T., White R.A., Tobias M.I. Causes of international increases in older age life expectancy. Lancet. 2015;9967(385):540–548. doi: 10.1016/S0140-6736(14)60569-9. [DOI] [PubMed] [Google Scholar]
- 9.Shah N.S., Richardson J., Moodley P., Moodley S., Babaria P., Ramtahal M., Heysell S.K., Li X., Moll A.P., Friedland A.W. Sturm, Gandhi N.R. Increasing drug resistance in extensively drug-resistant tuberculosis. S. Afr. Emerg. Infect Dis. 2011;17:510–513. doi: 10.3201/eid1703.101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moulding T., Dutt A.K., Reichman L.B. Fixed-dose combinations of antituberculous medications to prevent drug resistance. Ann. Intern. Med. 1995;122(12):951–954. doi: 10.7326/0003-4819-122-12-199506150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Chaulet P. Implementation of fixed-dose combinations in tuberculosis control: outline of responsibilities. Int. J. Tuberc. Lung Dis. 1999;3 (11 Suppl. 3):S353-7; discussion S381-7. [PubMed] [Google Scholar]
- 12.Small P.M. Tuberculosis: a new vision for the 21st century. Kekkaku. 2009;84(11):721–726. [PubMed] [Google Scholar]
- 13.Mitnick C., Bayona J., Palacios E., Shin S., Furin J., Alcántara F., Sánchez E., Sarria M., Becerra M., Fawzi M.C., Kapiga S., Neuberg D., Maguire J.H., Kim J.Y., Farmer P. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N. Engl. J. Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 14.Franzblau S.G., DeGroote M.A., Cho S.H., Andries K., Nuermberger E., Orme I.M., Mdluli K., Angulo-Barturen I., Dick T., Dartois V., Lenaerts A.J. Comprehensive analysis of methods used for the evaluation of compounds against Mycobacterium tuberculosis. Tuberculosis (Edinb) 2012;92(6):453–488. doi: 10.1016/j.tube.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Monedero I., Caminero J.A. Evidence for promoting fixed-dose combination drugs in tuberculosis treatment and control: a review. Int. J. Tuberc. Lung Dis. 2011;15(4):433–439. doi: 10.5588/ijtld.09.0439. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S.K. Antituberculosis drugs and hepatotoxicity. Infect. Genet. Evol. 2004;4:167–170. doi: 10.1016/j.meegid.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Dean G.L., Edwards S.G., Ives N.J., Matthews G., Fox E.F., Navaratne L., Fisher M., Taylor G.P., Miller R., Taylor C.B., de Ruiter A., Pozniak A.L. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS. 2002;16:75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 18.Katz M.D., Lor E. Acute interstitial nephritis associated with intermittent rifampicin use. Drug Intell. Clin. Pharm. 1986;20:789–792. doi: 10.1177/106002808602001014. [DOI] [PubMed] [Google Scholar]
- 19.Rekha V.V., Santha T., Jawahar M.S. Rifampicin induced renal toxicity during retreatment of patients with pulmonary tuberculosis. J. Assoc. Physician India. 2005;53:811–813. [PubMed] [Google Scholar]
- 20.Yee D., Valiquette C., Pelletier M., Parisien I., Rocher I., Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am. J. Respir. Crit. Care Med. 2003;167:1472–1477. doi: 10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 21.Shih T.Y., Pai C.Y., Yang P., Chang W.L., Wang N.C., Hu O.Y. A novel mechanism underlies the hepatotoxicity of pyrazinamide. Antimicrob. Agents Chemother. 2013;57(4):1685–1690. doi: 10.1128/AAC.01866-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiba S., Tsuchiya K., Sakashita H., Ito E., Inase N. Rifampicin-induced acute kidney injury during the initial treatment for pulmonary tuberculosis: a case report and literature review. Intern. Med. 2013;52(21):2457–2460. doi: 10.2169/internalmedicine.52.0634. [DOI] [PubMed] [Google Scholar]
- 23.Miyazawa S., Matsuoka S., Hamana S., Nagai S., Nakamura H., Nirei K., Moriyama M. Isoniazid-induced acute liver failure during preventive therapy for latent tuberculosis infection. Intern. Med. 2015;54(6):591–595. doi: 10.2169/internalmedicine.54.3669. [DOI] [PubMed] [Google Scholar]
- 24.Garg P., Garg R., Prasad R., Mishra A.K. A prospective study of ocular toxicity in patients receiving ethambutol as a part of directly observed treatment strategy therapy. Lung India. 2015;32(1):16–19. doi: 10.4103/0970-2113.148428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awodele O., Osunkalu V.O., Adejumo I.A., Odeyemi A.A., Ebuehi O.A., Akintonwa A. Haematotoxic and reproductive toxicity of fixed dose combined antituberculous agents: protective role of antioxidants in rats. Nig. Q. J. Hosp. Med. 2013;23(1):17–21. [PubMed] [Google Scholar]
- 26.Wells W.A., Konduri N., Chen C., Lee D., Ignatius H.R., Gardiner E., Schwalbe N.R. Implications of the current tuberculosis treatment landscape for future regimen change. Int. J. Tuberc. Lung Dis. 2011;15(6):746–753. doi: 10.5588/ijtld.10.0094. [DOI] [PubMed] [Google Scholar]
- 27.Gwaza L., Gordon J., Welink J., Potthast H., Leufkens H., Stahl M., Garcia-Arieta A. Adjusted indirect treatment comparison of the bioavailability of WHO-prequalified first-line generic antituberculosis medicines. Clin. Pharmacol. Ther. 2014;96(5):580–588. doi: 10.1038/clpt.2014.144. [DOI] [PubMed] [Google Scholar]
- 28.Awodele Olufunsho, Agbaje E.O., Adesina E.A., Akintonwa A. Hepatoprotective role of neutrosecR on hepatic damage induced by combination of zidovudine and combined antituberculous agents in rats. Tokai J. Exp. Clin. Med. 2011;36(2):31–36. [PubMed] [Google Scholar]
- 29.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Animals. 2014;4(1):35–44. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Pinkel D. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res. 1958;18:853–856. [PubMed] [Google Scholar]
- 31.Raji Y., Oloyo A.K., Morakinyo A.O. Studies on the reproductive activities of Ricinus communis seed in male albino rats. Asian J. Androl. 2006;8:115–121. doi: 10.1111/j.1745-7262.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 32.Odell W.D., Parlow A.F., Cargille C.M., Ross G.T. Radioimmunoassay for human follicle-stimulating hormone: physiological studies. J. Clin. Invest. 1968;47(12):2551. doi: 10.1172/JCI105937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi U.M., Shah H.P., Sudhama S.P. A sensitive and specific enzymeimmunoassay for serum testosteone. Steroids. 1979;34(1):35–46. doi: 10.1016/0039-128x(79)90124-7. [DOI] [PubMed] [Google Scholar]
- 34.DeVilla G.O., Roberts K., Wiest W.G., Mikhail G., Flickinger G. A specific radioimmunoassay of plasma progesterone. J. Clin. Endocrinol. Metab. 1972;35(3):458–460. doi: 10.1210/jcem-35-3-458. [DOI] [PubMed] [Google Scholar]
- 35.Shome B., Parlow A.F. Human follicle stimulating hormone (hFSH): first proposal for the amino acid sequence of the alpha-subunit (hFSHa) and first demonstration of its identity with the alpha-subunit of human luteinizing hormone (hLHa) J. Clin. Endocrinol. Metab. 1974;39(1):199–202. doi: 10.1210/jcem-39-1-199. [DOI] [PubMed] [Google Scholar]
- 36.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 37.Habig W.J., Pabst M.J., Jacoby W.B. Glutathione-S-transferase, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 38.Misra H.P., Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 39.Sinha A. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–395. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 40.Ellerby D.E., Bredesen L.M. Measurement of cellular oxidation, reactive oxygen species and antioxidant enzymes during apoptosis. Methods Enzymol. 2000;322:413–421. doi: 10.1016/s0076-6879(00)22040-5. [DOI] [PubMed] [Google Scholar]
- 41.Varshney R., Kale R.K. Effect of calmodulin antagonist on radiation induced lipid peroxidation in microsomes. Int. J. Radiat. Biol. 1990;58(5):733–743. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 42.Levine G. Lawrence Erlbaum Associates Inc., Publishers; Hillsdale, NJ: 1991. A Guide to SPSS for Analysis of Variance; pp. 65–67. [Google Scholar]
- 43.Cobelens F., Van den Hof S., Pai M., Squire S.B., Ramsay A., Kimerling M.E. Which new diagnostics for tuberculosis, and when? J. Infect. Dis. 2012;205(Suppl. 2):S191–S198. doi: 10.1093/infdis/jis188. [DOI] [PubMed] [Google Scholar]
- 44.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 45.Mahmood Iftekhar, Green Martin David, Fisher J. Edward. Selection of the first-time dose in humans: comparison of different approaches based on interspecies scaling of clearance. J. Clin. Pharmacol. 2003;43(7):692–697. [PubMed] [Google Scholar]
- 46.Damasceno G.S., Guaraldo L., Engstrom E.M., Theme Filha M.M., Souza-Santos R., Vasconcelos A.G., Rozenfeld S. Adverse reactions to antituberculosis drugs in Manguinhos, Rio de Janeiro, Brazil. Clinics. 2013;68(3):329–337. doi: 10.6061/clinics/2013(03)OA08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kargar M., Mansouri A., Hadjibabaie M., Javadi M., Radfar M., Gholami K. Anti-tuberculosis drugs adverse reactions: a review of the Iranian literature. Expert Opin. Drug Saf. 2014;13(7):875–891. doi: 10.1517/14740338.2014.925443. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization . Third Global Report. WHO/HTM/TB/2004.343; Geneva: 2004. World health Organization/IUATLD global project onantituberculous drug resistance surveillance. antituberculous drug resistance in the world. [Google Scholar]
- 49.Khan A., Sterling T.R., Reves R., Vernon A., Horsburgh C.R. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am. J. Respir. Crit. Care Med. 2006;174:344–348. doi: 10.1164/rccm.200511-1834OC. [DOI] [PubMed] [Google Scholar]
- 50.Krapp F., Veliz J.C., Cornejo E., Gotuzzo E., Seas C. Bodyweight gain to predict treatment outcome in patients with pulmonary tuberculosis in Peru. Int. J. Tuberc. Lung Dis. 2008;12:1153–1159. [PubMed] [Google Scholar]
- 51.Bernabe-Ortiz A., Carcamo C.P., Sanchez J.F., Rios J. Weight variation over time and its association with tuberculosis treatment outcome: a longitudinal analysis. PLoS One. 2011;6:e18474. doi: 10.1371/journal.pone.0018474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joffe M. Decreased fertility in Britain compared with Finland. Lancet. 1996;347(9014):1519–1522. doi: 10.1016/s0140-6736(96)90673-x. [DOI] [PubMed] [Google Scholar]
- 53.Peloquin C.A. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62:2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 54.Guinet P., Garin J.P., Morpex A. Un cas de gynecomastie chez un tuberculeux pulmonaire grave en cours de traitment par I'hydrazide de I'acide isonicotinique. Lyon Med. 1953;85:281–284. [PubMed] [Google Scholar]
- 55.Bergogne-Berezin E., Nouhouayi A., Letonturier P., Tourneur R. Gynaecomastia caused by isoniazid: value of determination of inactivation of phenotype. Nouv. Presse Med. 1976;5:213–214. [PubMed] [Google Scholar]
- 56.Garg R., Mehra S., Prasad R. Isoniazid induced gynaecomastia: a case report. Internet J. Pharmacol. 2007;5(2) [PubMed] [Google Scholar]
- 57.Kumar L., Gupta R., Puri M.M., Jaiswal A., Srinath Murar A., Behera D. Unilateral and painless development of isoniazid induced gynecomastia during re-treatment of pulmonary tuberculosis. J. Assoc. Physicians India. 2011;59:733–735. [PubMed] [Google Scholar]
- 58.Chunhaswasdikul B. Gynaecomastia in association with administration of thiacetazone in the treatment of tuberculosis. J. Med. Assoc. Thai. 1974;57(6):323–327. [PubMed] [Google Scholar]
- 59.Park A.J., Lamberty B.G. Gynaecomastia: have Webster’s lessons been ignored? J. R. Coll. Surg. Edinb. 1998;43(2):89. [PubMed] [Google Scholar]
- 60.Sharma P.K., Bansal R. Gynecomastia caused by ethionamide. Indian J. Pharmacol. 2012;44(5):654–655. doi: 10.4103/0253-7613.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bembo S.A., Carlson H.E. Gynaecomastia: Its feature, and when and how to treat it. Cleve Clin. J. Med. 2004;7(6):511–517. doi: 10.3949/ccjm.71.6.511. [DOI] [PubMed] [Google Scholar]
- 62.Sies H. Oxidative stress: from basic research to clinical application. Am. J. Med. 1991;91:31S–38S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- 63.Sodhi C.P., Rana S.V., Mehta S.K., Vaiphei K., Attari S., Mehta S. Study of oxidative-stress in isoniazid-rifampicin induced hepatic injury in young rats. Drug Chem. Toxicol. 1997;20:255–269. doi: 10.3109/01480549709003881. [DOI] [PubMed] [Google Scholar]
- 64.Chowdhury A., Santra A., Kundu S., Mukherjee A., Pandit A., Chaudhuri S., Dhali G.K. Induction of oxidative stress in antituberculous drug-induced hepatotoxicity. Indian J. Gastroenterol. 2001;20:97–100. [PubMed] [Google Scholar]