Abstract
A potentially useful means of predicting the pulmonary risk posed by new forms of nano-structured titanium dioxide (nano-TiO2) is to use the associations between the physicochemical properties and pulmonary toxicity of characterized forms of TiO2. In the present study, we conducted intratracheal administration studies in rats to clarify the associations between the physicochemical characteristics of seven characterized forms of TiO2 and their acute or subacute pulmonary inflammatory toxicity. Examination of the associations between the physicochemical characteristics of the TiO2 and the pulmonary inflammatory responses they induced revealed (1) that differences in the crystallinity or shape of the TiO2 particles were not associated with the acute pulmonary inflammatory response; (2) that particle size was associated with the acute pulmonary inflammatory response; and (3) that TiO2 particles coated with Al(OH)3 induced a greater pulmonary inflammatory response than did non-coated particles. We separated the seven TiO2 into two groups: a group containing the six TiO2 with no surface coating and a group containing the one TiO2 with a surface coating. Intratracheal administration to rats of TiO2 from the first group (i.e., non-coated TiO2) induced only acute pulmonary inflammatory responses, and within this group, the acute pulmonary inflammatory response was equivalent when the particle size was the same, regardless of crystallinity or shape. In contrast, intratracheal administration to rats of the TiO2 from the second group (i.e., the coated TiO2) induced a more severe, subacute pulmonary inflammatory response compared with that produced by the non-coated TiO2. Since alteration of the pulmonary inflammatory response by surface treatment may depend on the coating material used, the pulmonary toxicities of coated TiO2 need to be further evaluated. Overall, the present results demonstrate that physicochemical properties may be useful for predicting the pulmonary risk posed by new nano-TiO2 materials.
Keywords: Nano materials, Titanium dioxide, Intratracheal administration, Pulmonary toxicity, Risk assessment
1. Introduction
The European Commission defines a nanomaterial as “a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm–100 nm” [1]. Due to this small particle size, the surface area per unit mass of nanomaterials is greater than that of the corresponding bulk materials, and it is this characteristic that gives nanomaterials their unique properties.
Nanomaterials are predicted to soon become the cornerstone of the microelectronics, materials, textiles, energy, healthcare, and cosmetics industries [2]. Indeed, nano-structured titanium dioxide (nano-TiO2) is one of the most widely used nanomaterials in the world, and the production volume of nano-TiO2, which is increasing annually, is expected to reach nearly 2.5 million metric tons per year in 2025 [3]. Traditionally, TiO2 fine particles have been considered to have low toxicity; however, concerns have been raised recently regarding the potential health risks posed by nano-TiO2 to consumers, workers, and the environment [4]. In particular, a recently published toxicological review showed that inhalation was the primary route of nano-TiO2 exposure and thus highlighted the need for more information on the safety of nano-TiO2 [5]. Many researchers and regulators are now trying to understand the hazards of nano-TiO2 and determine appropriate strategies for the assessment of the pulmonary risk associated with exposure to nano-TiO2 materials. Nano-TiO2 can be manufactured in various forms that have different physicochemical characteristics (e.g., crystallinity, shape, particle size, surface area, and surface modification), which can lead to nanomaterials with the same chemical formula but different pulmonary toxicities [6]. Therefore, from a regulatory standpoint, it would be beneficial to assess the pulmonary risk of all newly developed nano-TiO2 materials; however, a program of this size is unrealistic due to the time and money that would be required.
One potential means of predicting the pulmonary risk of new forms of nano-TiO2 is to use the associations between the physicochemical properties and pulmonary toxicity of characterized forms of TiO2. Several researchers have already investigated the relationships between the physicochemical characteristics of different forms of nano-TiO2 and their pulmonary toxicities in rats, including the relationship between pulmonary toxicity and particle crystallinity [7], particle size [8], [9], [10], [11], [12], particle surface area [13], [10], [12], and particle surface modification [14], [15], [16]. For example, smaller TiO2 particles have been shown to induce a greater acute inflammatory response compared with larger TiO2 particles after intratracheal administration in rats [8], [9], [11]. However, Warheit et al. [12] have also shown that the acute inflammatory and cell-injury effects of three TiO2 materials with different particle sizes did not differ. Furthermore, in most of these previous studies only two or three test materials were examined and the studies were conducted under different test conditions, thus hampering comparison of the results.
In the present study, to clarify the associations between the physicochemical characteristics of TiO2-based materials and their pulmonary toxicity, intratracheal administration studies were conducted in rats by using seven different characterized TiO2. To allow a quantitative and statistical approach to be used, all physicochemical characterizations and in vivo studies were performed in the same laboratory under the same test conditions.
2. Materials and methods
2.1. Test materials and preparation of administration formulations
Seven forms of TiO2 were selected for inclusion in this study: AMT-100, MT-150AW, and MP-100 (Tayca Co. Ltd., Japan); TTO-S-3, TTO-S-3 (Coated), and FTL-100 (Ishihara Sangyo Kaisha, LTD., Japan); and P25 (Evonik Industries, Germany). These materials were chosen to allow examination of as many different physicochemical characteristics as possible, i.e., three different types of crystallinity, three different particle shapes, surface coating (untreated or Al[OH]3 coating), and a range of different particle sizes (Table 1). Representative scanning electron microscope images (S-4800, Hitachi High-Technologies Co., Japan) of each test material are shown in Fig. S1.
Table 1.
Physicochemical characteristics of the seven forms of TiO2.
| Material | Crystallinitya | Shapea | Primary particle sizea(nm) | Surface area(m2/g) | Surface coatinga | Particle sizeb(nm) |
|
|---|---|---|---|---|---|---|---|
| Volume average diameter | Number average diameter | ||||||
| AMT-100 | anatase | spherical | 6 | 250–300 | No | 185(161–198) | 68.5(60.6–82.6) |
| MT-150AW | rutile | spindle | long axis:28.8 short axis:7.6 | 100–120 | No | 58.5(52.3–65.8) | 28.7(19.5–42.6) |
| TTO-S-3 | rutile | spindle | long axis:50–100 short axis:10–20 | 102 | No | 61.9(57.2–64.7) | 45.8(41.2–48.9) |
| TTO-S-3 (Coated) | rutile | spindle | long axis:50–100 short axis:10–20 | 93.0 | Al(OH)3 coating | 241(202–289) | 125(119–133) |
| P25 | rutile/anatase(20/80) | spherical | 21 | 50 ± 15 | No | 99.2(95.6–101) | 73.8(65.5–79.8) |
| MP-100 | rutile | spherical | 1000 | 6 | No | 531(501–549) | 289(89.8–393) |
| FTL-100 | rutile | needle | long axis:1680 short axis:130 | 12.0 | No | −c | −c |
Data from catalog or obtained from the manufacturer.
Average particle diameter was measured in the three different administration formulations by means of dynamic light scattering (Zetasizer Nano ZS; Malvern Instruments Ltd., UK). Figures in parentheses show the smallest to largest value among the three different concentrations. Intensity size distributions measured by dynamic light scattering was converted into volume and number size distributions using Mie theory [23].
Particle size was not calculated because dynamic light scattering gives little effective information for needle-shaped particles with a large aspect ratio.
Administration formulations were prepared as described in Refs. [17], [18] (Table S1). Briefly, 2 g of test material was dispersed in 50 mL of 2 mg/mL of disodium phosphate (food additive grade; Wako Pure Chemical Industries, Ltd., Japan), which was prepared by using endotoxin-free pure water. The suspension was sonicated in a glass bottle by using an ultrasonic bath (5510J-MT; Branson Ultrasonics Co., USA) for one to three hours and then centrifuged at 20–1000g for 5–40 min at 20 °C (CF16RXII and T15A41; Hitachi Koki Co., Ltd., Japan). The supernatant was collected as a stock suspension. Administration formulations were prepared by diluting the stock suspension to the appropriate concentration with 2 mg/mL disodium phosphate. The concentration of test material in the administration formulations was determined by using a weight analysis method, where the weight loss of the suspension was measured with a balance scale (AUW220D; Shimadzu Co., Japan) after drying at 200 °C in a thermostatic chamber (ON-300S; As One Co., Japan). The particle size and size distribution of the test materials in the administration formulations were measured by means of dynamic light scattering (Zetasizer Nano ZS; Malvern Instruments Ltd., UK). In principle, particle size and size distribution can be expressed in terms of mass, volume, or number of particles [19]. However, since a robust understanding of which means of expression is the best for understanding the pulmonary toxicity of TiO2 in rats is yet to be obtained, two different values—volume average diameter and number average diameter—obtained from the dynamic light scattering analysis were used in the present study (Table 1 and Fig. S2). The particle size of FTL-100 was not determined because dynamic light scattering gives little effective information for needle-shaped particles with a large aspect ratio.
2.2. Test animals
Male F344/DuCrlCrlj rats were obtained from Charles River Laboratories Japan, Inc. Animals at 12 weeks of age with body weights of 212.3–282.1 g on the day of administration were used in the in vivo studies. The animals were housed in animal rooms equipped with a local barrier system and were maintained at 21–25 °C and 40% to 70% relative humidity with 10–15 air changes per hour and a photoperiod of 12 h of light per day (lights on, 7:00; lights off, 19:00). The study was approved by the Institutional Animal Care and Use Committee prior to the start of the study.
2.3. Test conditions
In the in vivo studies, one vehicle control group (2 mg/mL of disodium phosphate aqueous solution without test material) and three treatment groups (0.67, 2, or 6 mg/kg TiO2) were used for each test material. The majority of previous studies using intratracheal administration of TiO2 in rats were conducted with doses in the range of 0.75–6 mg/kg TiO2 [8], [9], [20], [11], [12], [7]. Therefore, based on the results of these previous studies, the doses in the present study were chosen so that toxic effects but not death or severe suffering were induced at the highest dose and so that adverse effects were avoided at the lowest dose. Forty rats were used for each test material (i.e., ten test animals/dose). Test animals were anesthetized by means of isoflurane inhalation and angled approximately 45° on a restraining stand. Administrations were conducted by using a stomach sonde (Natsume Seisakusho Co., Ltd., Japan) or MicroSprayer Aerosolizer (Model IA-1B-R for Rat, Penn-Century, Inc., USA) inserted transorally into the tracheal lumen at a depth of approximately 6 cm from the angle of the mouth. The volumes of the administrations were 1 mL/kg for AMT-100, MT-150AW, TTO-S-3, TTO-S-3 (Coated), P25 and MP-100, and 2 mL/kg for FTL-100, based on the animal’s body weight on the day of administration. To assess the acute and subacute pulmonary toxicity of each test material, bronchoalveolar lavage fluid (BALF) examination and pathological examination were conducted at three days (acute phase) and at four weeks (subacute phase) after intratracheal administration. The examinations were not conducted earlier than three days after administration because the results would likely reflect the initial pulmonary inflammatory response to the bolus administration of liquid into the lungs [8]. On the day of examination, ten test animals per dose were euthanized by means of bleeding via the ventral aorta under pentobarbital anesthesia. Five animals were used for the BALF examination, and five animals were used for the pathological examination. Test animals were fasted for 16–20 h prior to euthanasia and dissection. In all animals, clinical signs were observed daily after intratracheal administration, and body weight was recorded before the administration of the test materials, at three and seven days after administration, and once per week thereafter until the end of the study.
2.4. BALF examination
The trachea of each rat was cannulated and 7 mL of saline (Otsuka Pharmaceutical Co., Ltd, Japan) was instilled into the lungs by means of gravity; that is, saline was instilled into the lungs from 30 cm above the test animal and then drained from the lungs to a bottle that was placed 30 cm under the test animal. The lavage procedure was conducted twice (i.e., a total of 14 mL of saline was instilled into the lungs). Approximately 90% of the instilled saline volume was retrieved. Total cell counts were determined in a portion of the BALF by using an ADVIA 120 hematology analyzer (Siemens Healthcare Diagnostics, USA). The remaining BALF was centrifuged (400g, 10 min, 4 °C), and the total protein content, concentrations of albumin, lactate dehydrogenase (LDH) activity, and alkaline phosphatase (ALP) activity in the supernatant were determined by using a Hitachi Automatic Clinical Analyzer 7170 (Hitachi High-Technologies Corporation Co., Japan). The cell pellets were re-suspended in phosphate-buffered saline and smeared onto glass slides; 200 cells per specimen were counted to determine the leukocyte differential (neutrophils, macrophages, lymphocytes, basophils, and eosinophils).
2.5. Pathological examinations
The lungs, trachea, parathymic lymph nodes, posterior mediastinal lymph node, liver, kidneys, spleen, and brain were removed from the rats. The weights of the lungs, liver, kidneys, spleen, and brain were measured to calculate relative organ weights per body weight (g/100 g). The organs were fixed with 10% (v/v) neutral phosphate-buffered formalin solution, stained with hematoxylin–eosin, and examined histopathologically under a light microscope.
2.6. Associations between the physicochemical characteristics of TiO2 and the pulmonary inflammatory response in rats
To investigate the associations between the physicochemical characteristics of the TiO2 (i.e., crystallinity, shape, particle size, and surface coating) and the pulmonary inflammatory response to the TiO2 in rats (i.e., acute or subacute), we used three BALF parameters—differential neutrophil ratio (an indicator of inflammatory response), total protein content (an indicator of permeability of vessels to protein in blood), and LDH activity (an indicator of cell injury). These BALF parameters have previously been used to evaluate the pulmonary toxicity of nano-TiO2 in rats [14], [8], [21], [22], [11], [16], [12], [7]. When analysing the dose-dependency of the three BALF parameters, we suspected that at three days after intratracheal administration of the TiO2, some of the parameters might be saturated in the rats administered 6 mg/kg TiO2. Therefore, the values of the BALF parameters obtained for the rats administered 2 mg/kg TiO2 were used. To standardize the results obtained for each test material, the results of the BALF examinations are expressed as the difference between the value in the rats administered 2 mg/kg TiO2 and that in the control rats.
2.7. Statistical analysis
Statistical analyses of body weight, BALF parameters, and relative organ weight were conducted by using the StatLight software (Yukms Co., Ltd., Japan). Bartlett’s test for homogeneity of variance was conducted, and when the variances were homogeneous at a significance level of 5%, Dunnett’s test was conducted. When the variances were not homogeneous, Dunnett’s test for nonparametric data was conducted. Statistical significances were judged at the 5% probability level. Regression analyses were performed by using Microsoft Office Excel 2013 and GraphPad Prism 6 version 6.01 (GraphPad Software, Inc., USA) for Windows.
3. Results
3.1. Clinical signs and body weight
No mortalities or abnormalities in clinical signs were observed in either the control or treatment animals for all test materials. The body weight of all rats increased with increasing age, and there were no significant differences in body weight between the control and treatment groups for all test materials (data not shown).
3.2. BALF examinations
3.2.1. Cells in BALF
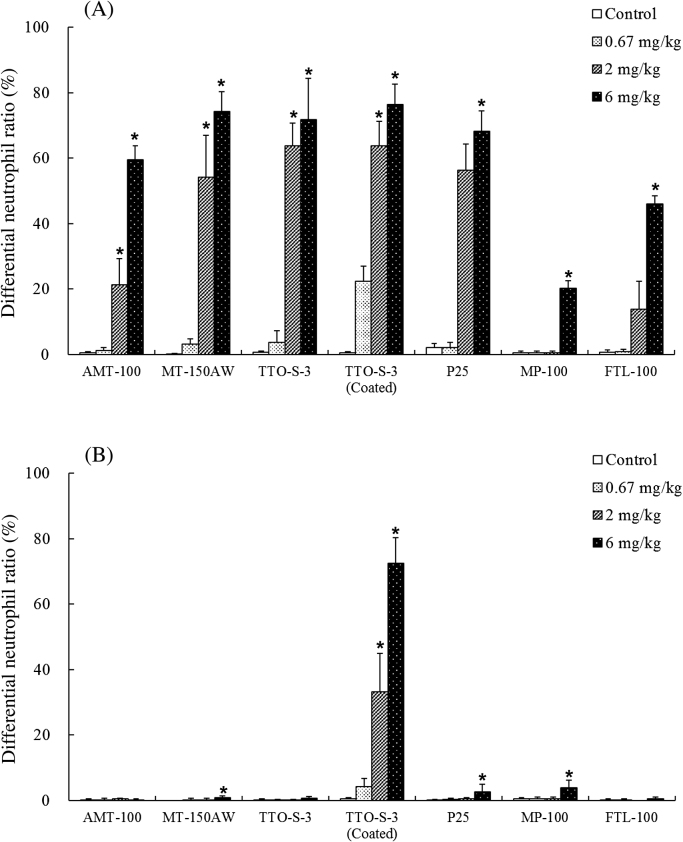
The results of the BALF examinations at three days and at four weeks after administration of the test materials are shown in Figs. 1 and S3−6. At three days after administration, dose-dependent increases in the differential neutrophil ratio were observed for all of the test materials (Fig. 1A). At four weeks after administration, although significant increases in differential neutrophil ratio remained in the animals administered 6 mg/kg MT-150AW, P25, or MP-100, the increases in differential neutrophil ratio had recovered to levels comparable with those in the control groups, except for in the animals administered 2 or 6 mg/kg TTO-S-3 (Coated) (Fig. 1B). Similar results were observed for total cell count (Fig. S3), differential macrophage ratio (Fig. S4), and differential lymphocyte ratio (Fig. S5). No significant differences in differential eosinophil ratio at three days or at four weeks after administration were observed for any of the test materials (Fig. S6).
Fig. 1.
Differential neutrophil ratio in bronchoalveolar lavage fluid at three days (A) and at four weeks (B) after intratracheal administration of various forms of TiO2. Values are presented as average ± SD. * significant difference from control group (P < 0.05).
3.2.2. Biochemical examination of BALF
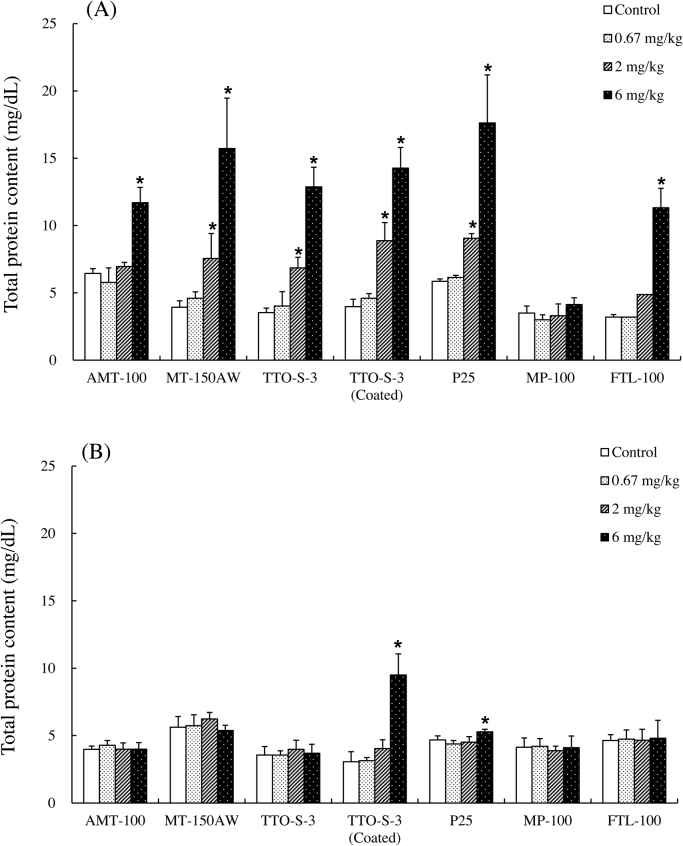
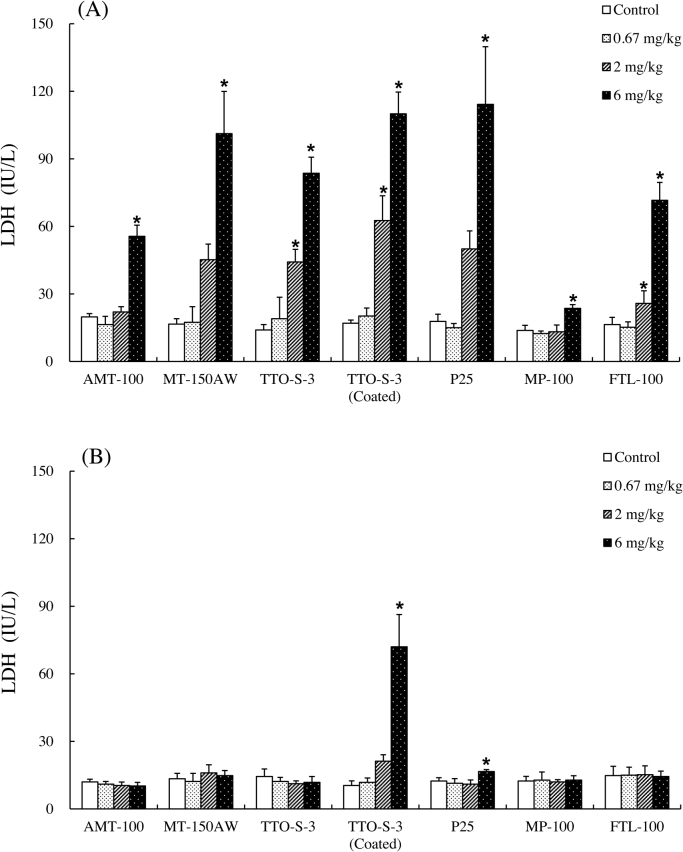
The results of the biochemical examination of BALF are shown in Figs. 2, 3, S7, and S8. At three days after administration, dose-dependent increases in total protein content were observed for all of the test materials except MP-100 (Fig. 2A). At four weeks after administration, although significant increases in total protein content remained in the animals administered 6 mg/kg P25, the increases in total protein content had recovered to levels comparable with those in the control groups for all test materials, except for in the rats administered 6 mg/kg TTO-S-3 (Coated) (Fig. 2B). Similar trends were observed for LDH activity (Fig. 3), albumin concentration (Fig. S7), and ALP activity (Fig. S8).
Fig. 2.
Total protein content in bronchoalveolar lavage fluid at three days (A) and at four weeks (B) after intratracheal administration of various forms of TiO2. Values are presented as average ± SD. * significant difference from control group (P < 0.05).
Fig. 3.
Lactate dehydrogenase (LDH) activity in bronchoalveolar lavage fluid at three days (A) and at four weeks (B) after intratracheal administration of various forms of TiO2. Values are presented as average ± SD. * significant difference from control group (P < 0.05).
3.3. Pathological examinations
3.3.1. Relative organ weight
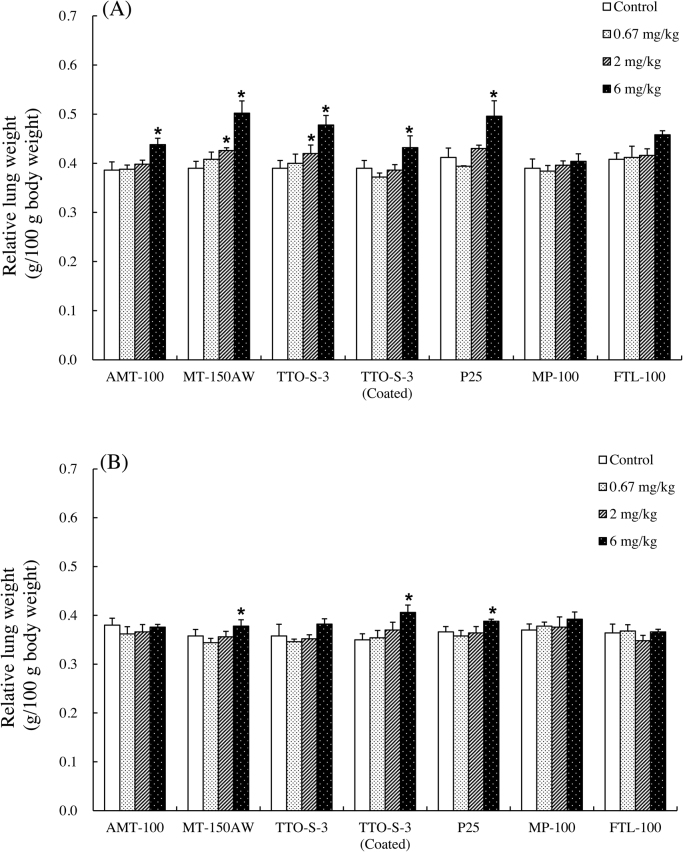
At three days after administration, significant increases in relative lung weight were observed in the rats administered 6 mg/kg TiO2, except in those administered MP-100 or FTL-100 (Fig. 4A). For MT-150AW or TTO-S-3, significant increases were also observed in the animals administered 2 mg/kg. At four weeks after administration, significant increases in relative lung weight remained in the animals administered 6 mg/kg MT-150AW, TTO-S-3 (Coated), or P25 (Fig. 4B). No significant differences were observed in the relative organ weight of liver, kidney, spleen, and brain for any of the test materials (data not shown).
Fig. 4.
Relative lung weight at three days (A) and at four weeks (B) after intratracheal administration of various forms of TiO2. Values are presented as average ± SD. * significant difference from control group (P < 0.05).
3.3.2. Histopathological examinations
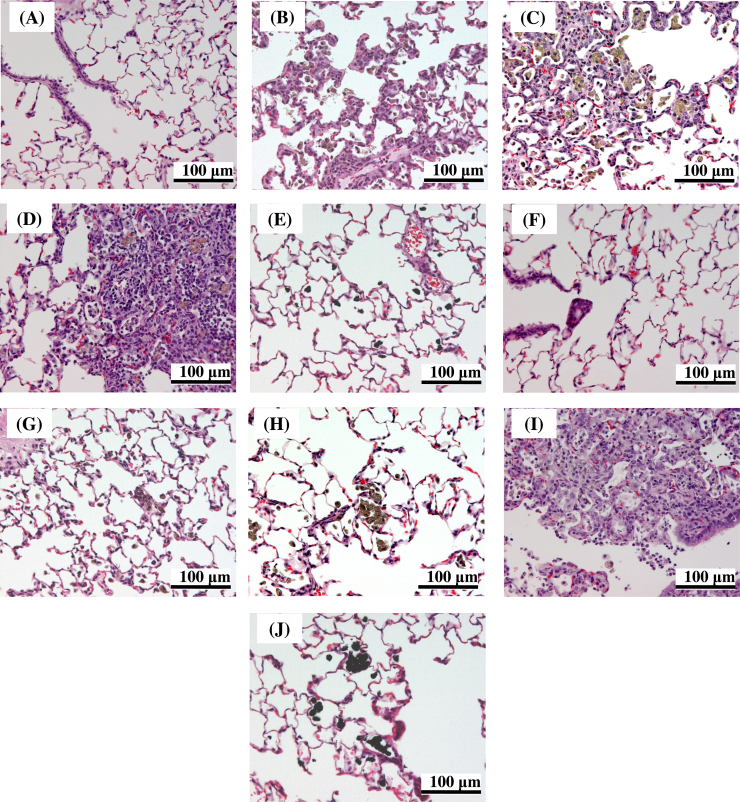
At three days after administration, inflammation in the lung was observed for all test materials. Infiltration of inflammatory cells (neutrophils and alveolar macrophages) or hyperplasia of the alveolar epithelium with thickening of the alveolar wall, or both, were observed for all test materials (Table 2 and Fig. 5). Most of the histopathological changes were very slight with only MT-150AW and P25 inducing mild changes at the highest dose at three days after administration. The frequency and grade of the histopathological changes increased in a dose-dependent manner. At four weeks after administration, the inflammatory responses had subsided in all groups, except for in the animals administered TTO-S-3 (Coated). In the animals administered TTO-S-3 (Coated), infiltration of inflammatory cells (neutrophils and foamy alveolar macrophages) or hyperplasia of the alveolar epithelium with thickening of the alveolar wall were observed (Table 2 and Fig. 5). These findings are consistent with the results of the BALF examinations, in which six of the seven test materials induced acute responses, whereas TTO-S-3 (Coated) induced a subacute response. No toxicological findings were observed in any of the other organs examined for any of the test materials.
Table 2.
Comparison of frequency and grade of histopathological responses.
| Test material | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMT-100 | MT-150AW | TTO-S-3 | TTO-S-3 (Coated) | P25 | MP-100 | FTL-100 | |||||||||||||||
| Administration dose (mg/kg) | |||||||||||||||||||||
| 0.67 | 2 | 6 | 0.67 | 2 | 6 | 0.67 | 2 | 6 | 0.67 | 2 | 6 | 0.67 | 2 | 6 | 0.67 | 2 | 6 | 0.67 | 2 | 6 | |
| (At three days after administration) | |||||||||||||||||||||
| Infiltration of inflammatory cells (neutrophils and alveolar macrophages) | |||||||||||||||||||||
| ± | 1 | 1 | 3 | 0 | 2 | 0 | 1 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 3 |
| + | 0 | 0 | 1 | 0 | 3 | 5 | 0 | 2 | 5 | 0 | 0 | 3 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hyperplasia of alveolar epithelium | |||||||||||||||||||||
| ± | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 2 |
| ++ | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| (At four weeks after administration) | |||||||||||||||||||||
| Infiltration of inflammatory cells (neutrophils and foamy alveolar macrophages) | |||||||||||||||||||||
| ± | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperplasia of alveolar epithelium | |||||||||||||||||||||
| + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Five animals were observed in each group.
±, very slight change; +, slight change; ++, mild change.
Fig. 5.
Representative light micrographs of lung tissue from rats after intratracheal administration of 6 mg/kg of the indicated form of TiO2. At three days after intratracheal administration: (A) Control, (B) MT-150AW, (C) TTO-S-3, (D) TTO-S-3 (Coated), (E) MP-100. At four weeks after intratracheal administration: (F) Control, (G) MT-150AW, (H) TTO-S-3, (I) TTO-S-3 (Coated), (J) MP-100.
3.4. Associations between physicochemical characteristics of TiO2 and pulmonary inflammatory responses in rats
3.4.1. Crystallinity
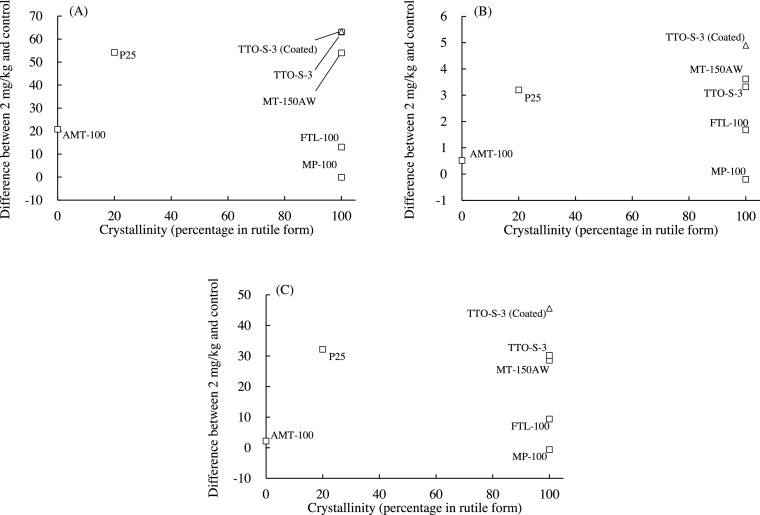
The associations between the crystallinity of the test materials and the values of three BALF parameters (differential neutrophil ratio, total protein content, and LDH activity) determined at three days after administration are shown in Fig. 6. To examine these associations quantitatively and statistically, the percentage of the materials in rutile form, as determined by the manufacturer, was used as an index of crystallinity (Table 1). For example, the percentage of materials in the rutile form for AMT-100 was 0%, that of MT-150AW was 100%, and that of P25 was 20%. The BALF parameter values for the predominantly rutile materials varied greatly, and no clear trends were observed between the percentage of material in the rutile form and any of the BALF parameter values. Similarly, there were also no clear associations between the percentage of material in the rutile form and the values of the BALF parameters at four weeks after administration (data not shown). Therefore, it is unlikely that the crystallinity of the test materials was associated with the acute pulmonary inflammatory responses observed in the rats.
Fig. 6.
Associations between crystallinity (percentage of TiO2 in rutile form) and bronchoalveolar lavage fluid parameter values at three days after intratracheal administration of indicated form of TiO2. (A) Differential neutrophil ratio, (B) Total protein content, (C) Lactate dehydrogenase (LDH). Values are presented as the average of the difference between the value for the 2-mg/kg group and that of the control group. Percentage of TiO2 in rutile form: AMT-100, 0%; MT-150AW, 100%; TTO-S-3, 100%; TTO-S-3 (Coated), 100%; P25, 20%; MP-100, 100%; FTL-100, 100%.
3.4.2. Shape
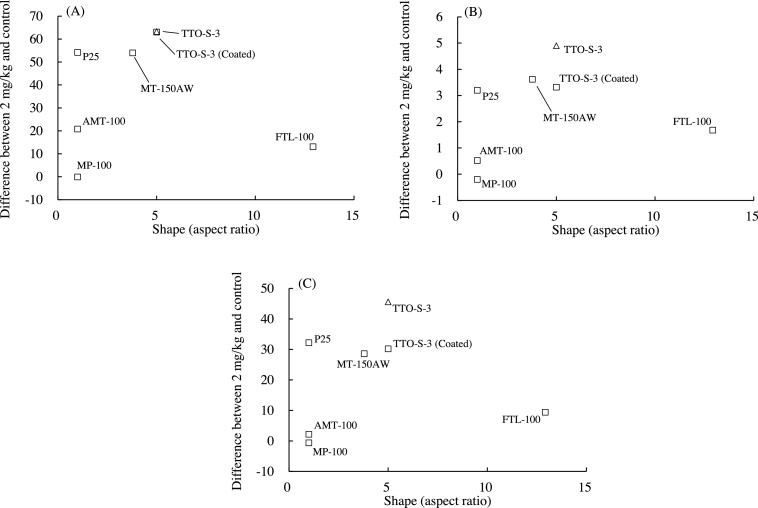
The associations between the shape of the test materials and the values of the three BALF parameters determined at three days after administration are shown in Fig. 7. To examine these associations quantitatively and statistically, the shape of each test material was expressed as its aspect ratio (i.e., long axis/short axis). No clear associations were found between aspect ratio and inflammatory response at three days (Fig. 7) or at four weeks after administration of the test materials (data not shown). Therefore, it is unlikely that particle shape was associated with the acute pulmonary inflammatory responses observed in the rats.
Fig. 7.
Associations between aspect ratio and bronchoalveolar lavage fluid parameter values three days after intratracheal administration of various forms of TiO2. (A) Differential neutrophil ratio, (B) Total protein content, (C) Lactate dehydrogenase (LDH). Values are presented as the average of the difference between the value for the 2-mg/kg group and that of the control group. Aspect ratios: AMT-100, 1.00; MT-150AW, 3.79; TTO-S-3, 5.00; TTO-S-3 (Coated), 5.00; P25, 1.00; MP-100, 1.00; FTL-100, 12.9.
3.4.3. Particle size
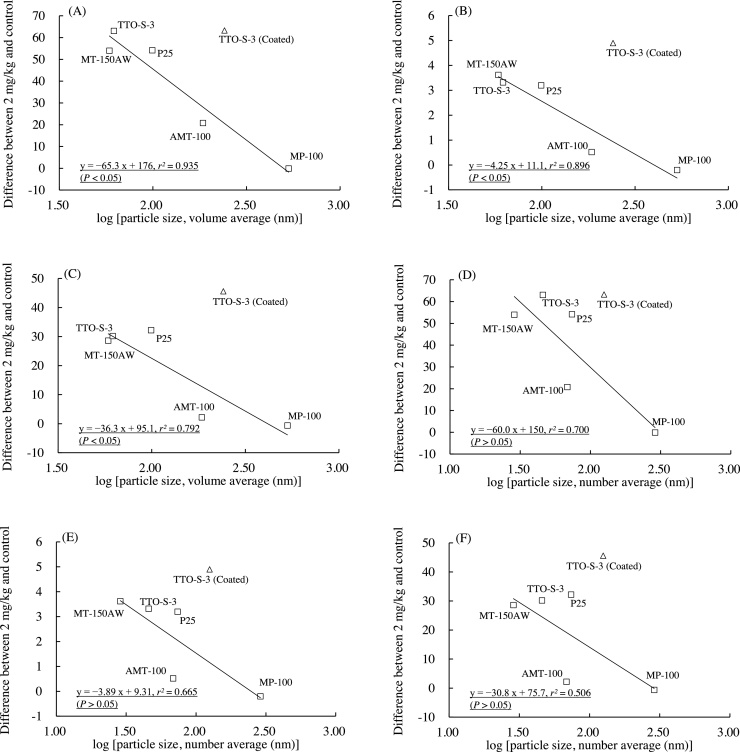
The associations between the particle size of the test materials and the values of the three BALF parameters determined at three days after administration are shown in Fig. 8. The data for FTL-100 were not plotted because the particle size measured by means of dynamic light scattering would have given little effective information due to the material’s large aspect ratio. In Fig. 8, linear relationships are presented between the logarithm of particle size and BALF parameter value. Strong correlations were observed when the data for TTO-S-3 (Coated) were excluded from the regression analysis expressed by using Eqs. (1)–(6):
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
Fig. 8.
Associations between particle size (nm) and bronchoalveolar lavage fluid parameter values three days after intratracheal administration of various forms of TiO2. Volume average diameter: (A) Differential neutrophil ratio, (B) Total protein content, (C) Lactate dehydrogenase (LDH). Number average diameter: (D) Differential neutrophil ratio, (E) Total protein content, (F) LDH. Solid line is the linear regression line derived from five test materials (excluding the data for TTO-S-3 (Coated) and FTL-100). Values are presented as the average of the difference between the value for the 2-mg/kg group and that of the control group.
The correlations between BALF parameter value and logarithm of particle size at three days after administration were strong for all three BALF parameters examined, that is, the r2 values for volume average diameter were high, and the correlations were statistically significant (P < 0.05). No clear associations between BALF parameter value and particle size were observed at four weeks after administration (data not shown). Therefore, it is likely that particle size, especially volume average diameter, was associated with the acute—but not the subacute—pulmonary inflammatory response observed in the rats.
3.4.4. Surface coating
In the plots of BALF parameter value versus particle size at three days after administration, the plot of TTO-S-3 (Coated) was located separate from the plots for the other test materials (Fig. 8A–F). Furthermore, the results of the BALF examinations and histopathological examinations at four weeks after administration showed that TTO-S-3 (Coated) continued to induce an inflammatory response whereas the other test materials did not (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). Therefore, it is likely that the Al(OH)3 coating the surface of the particles in TTO-S-3 (Coated) had a marked effect on the pulmonary inflammatory response in rats.
3.4.5. Categorization of seven TiO2
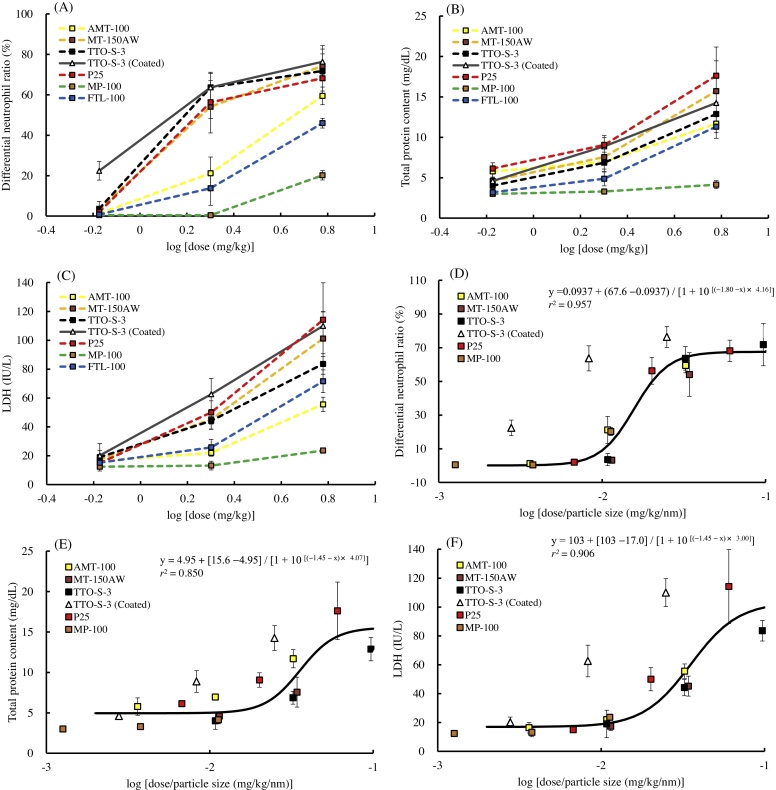
Using the present results, we categorized the seven TiO2 into two groups: a group containing TiO2 with no surface coating (i.e., AMT-100, MT-150AW, TTO-S-3, P25, MP-100, and FTL-100) and a group containing the TiO2 with surface coating (i.e., TTO-S-3 [Coated]). The dose–response curves for the BALF parameters (differential neutrophil ratio, total protein content, and LDH activity) at three days after administration are shown in Fig. 9. When the administration doses were expressed as mass (mg/kg), the curves for the BALF parameter values varied greatly (Fig. 9A–C); however, when the administration doses of each test material were normalized by particle size (e.g., volume average), that is, by dividing the mass administration dose (mg/kg) by the particle size (nm) (Fig. 9D–F), the curves for the BALF parameters after administration of the TiO2 from the first group of materials (i.e., the materials with no surface coating) all fitted the same sigmoidal dose–response curve (r2 = 0.957 [differential neutrophil ratio], 0.850 [total protein content], 0.906 [LDH]). This suggests that for the first group of TiO2, the non-coated TiO2, the acute pulmonary inflammatory response (at three days after intratracheal administration) is equivalent when the particle size is the same, regardless of crystallinity or shape.
Fig. 9.
Dose—response curves for bronchoalveolar lavage fluid parameters three days after intratracheal administration of various forms of TiO2. Administration doses expressed as mass (mg/kg): (A) Differential neutrophil ratio, (B) Total protein content, (C) Lactate dehydrogenase (LDH). Administration doses normalized by particle size (mg/kg/nm): (D) Differential neutrophil ratio, (E) Total protein content, (F) LDH. Solid lines are the sigmoidal dose—response curve derived from five test materials (excluding the data for TTO-S-3 (Coated) and FTL-100). Values were taken from the 2-mg/kg group and are presented as average ± SD.
4. Discussion
In the present study, all seven TiO2 induced acute pulmonary inflammatory responses at three days after intratracheal administration in rats, which included increases in the differential neutrophil ratio, total protein content, and LDH activity in BALF; infiltration of inflammatory cells in the lung; and the development of hyperplasia of the alveolar epithelium. For six of the seven test materials, these inflammatory responses had recovered at four weeks after administration to levels comparable to those in the control groups, which is consistent with the results of a previous study Nöel et al. [21]. However, rats administered TTO-S-3 (Coated) continued to exhibit inflammatory responses at four weeks after administration, indicating that this TiO2 also induced subacute pulmonary toxicity.
The relationships between the physicochemical characteristics of the TiO2 and the results of the BALF examination showed that smaller TiO2 particles induced a greater acute pulmonary inflammatory response than did larger TiO2 particles, irrespective of particle crystallinity or shape (Fig. 8A–8F). Consistent with this result, the frequencies and grades of the histopathological findings (infiltration of inflammatory cells and hyperplasia of the alveolar epithelium) at three days after administration of the test materials were also greater for the smaller TiO2 particles than for the larger TiO2 particles (Table 2 and Fig. 5). Several previous studies have shown that smaller TiO2 particles induce a greater acute inflammatory response than do larger TiO2 particles after intratracheal administration [8], [9], [11]. However, Warheit et al. [12] evaluated the acute lung toxicity in rats after intratracheal administration of three TiO2 with different particle sizes (primary particle size: approx. 300 nm [particles], 200 nm × 35 nm [rods], and approx. 10 nm [dots]) and showed that there were no differences in the acute inflammatory response and cell injury induced by these three materials. A robust understanding of the effect of TiO2 particle size on acute pulmonary toxicity in rats is yet to be obtained; however, in the present study, we characterized and tested seven forms of TiO2 in the same laboratory under identical test conditions, and our results suggest that particle size, especially volume average diameter, may be associated with the acute pulmonary inflammatory response to intratracheal administration of TiO2 in rats.
Our results also showed that the surface coating of TiO2 particles might have a marked effect on the pulmonary inflammatory response to intratracheal administration of TiO2 in rats. Several previous studies have shown that surface treatment affects the pulmonary toxicity of TiO2 in rats. For example, Oberdörster [15] showed that silane-coated TiO2 induces a much lower pulmonary inflammatory response compared with untreated TiO2, and Höhr et al. [14] showed that hydrophobic surface treatment of TiO2 (achieved through methylation) reduces total cell numbers and induces less influx of neutrophils into BALF compared with untreated TiO2. Similarly, Warheit et al. [16] showed that TiO2 surface-treated with alumina (Al2O3) and amorphous silica (SiO2) (82% TiO2 + 7% Al2O3 + 11% SiO2) induces mild adverse pulmonary effects when compared with the effects induced by untreated TiO2. Together with these previous results, the present results indicate that altering the surface structure of TiO2 particles affects the rat pulmonary inflammatory response to those particles. Furthermore, the present results also show that the type of pulmonary inflammatory response may be related to the coating material used. That is, in the present study, TTO-S-3 (Coated), which is surface-coated with Al(OH)3, induced both an acute and a subacute pulmonary inflammatory response, whereas uncoated TTO-S-3 induced only an acute pulmonary inflammatory response. Further studies are needed to determine the relationships between surface treatments and pulmonary inflammatory response.
It is known that nanomaterial-induced pulmonary inflammation depends largely on the extent of deposition and clearance of the material in the lungs [4], [22]. Our group previously examined the pulmonary clearance kinetics of TiO2 in rats by using the same seven test materials assessed in the present study and reported that the pulmonary clearance rate constant of TTO-S-3 (Coated) (0.00018–0.011/day) was much lower than those for the other six uncoated TiO2 (0.0073–0.020/day) [18]. Therefore, slower clearance from the lung may account for the subacute pulmonary inflammatory response observed after administration of TTO-S-3 (Coated) in the present study, although further studies are required to elucidate the detailed toxicological mechanism.
Together, the present results allow the following three conclusions to be drawn regarding the relationship between the physicochemical characteristics of nano-TiO2 and the pulmonary inflammatory response after intratracheal administration in rats: (1) crystallinity and shape are unlikely to be associated with acute pulmonary inflammatory responses to TiO2; (2) particle size is likely associated with the acute pulmonary inflammatory response to TiO2; and (3) surface coating has the potential to alter the pulmonary inflammatory response to TiO2.
We next categorized the seven TiO2 into two groups: a group containing the six TiO2 with no surface coating (i.e., AMT-100, MT-150AW, TTO-S-3, P25, MP-100, and FTL-100) and a group containing the one TiO2 with a surface coating (i.e., TTO-S-3 [Coated]). Intratracheal administration to rats of the TiO2 from the first group of materials induced acute pulmonary inflammatory responses, and within this group, the acute pulmonary inflammatory response (at three days after intratracheal administration) was equivalent when the particle size was the same, regardless of crystallinity or shape. Therefore, the acute pulmonary inflammatory responses in this group may be predicted by using the correlation between the particle size of and inflammatory responses induced by characterized forms of TiO2. In contrast, intratracheal administration to rats of the TiO2 from the second group (i.e., TTO-S-3 [Coated]) induced a more severe, subacute pulmonary inflammatory response. The pulmonary toxicity of coated TiO2 needs to be further evaluated since the alteration of the pulmonary inflammatory response by surface treatment may depend on the coating material used.
In summary, the present study provides a possible means of predicting the pulmonary risk of new nano-TiO2 by using the known associations between the physicochemical characteristics and pulmonary toxicity of characterized forms of TiO2. Additional studies using TiO2 with different surface treatments, and examination of the toxicological mechanisms behind the alteration of the pulmonary inflammatory response by surface treatment, will help in constructing a robust rationale for predicting the pulmonary toxicity of nano-TiO2 based on their physicochemical characteristics.
Conflict of interest
This study was conducted under the “Development of Innovative Methodology for Safety Assessment of Industrial Nanomaterials” supported by the Ministry of Economy, Trade and Industry (METI) of Japan.
Transparency document
Acknowledgements
We thank Dr. Makoto Ema (National Institute of Advanced Industrial Science and Technology [AIST]) and Dr. Toru Takebayashi (Keio University School of Medicine, Tokyo, Japan) for their assistance in interpreting the data and for providing their comments on the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2016.05.005.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.The European Commission Commission recommendation of 18 October 2011 on the definition of nanomaterial. Off. J. Eur. Union. 2011 (2011/696/EU) [Google Scholar]
- 2.McIntyre R.A. Common nano-materials and their use in real world applications. Sci. Prog. 2012;95:1–22. doi: 10.3184/003685012X13294715456431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robichaud C.O., Uyar A.E., Darby M.R., Zucker L.G., Wiesner M.R. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ. Sci. Technol. 2009;43:4227–4233. doi: 10.1021/es8032549. [DOI] [PubMed] [Google Scholar]
- 4.Braakhuis H.M., Park M.V., Gosens I., De Jong W.H., Cassee F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014;11:18. doi: 10.1186/1743-8977-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part. Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Fan Y. Lung injury induced by TiO2 nanoparticles depends on their structural features: size, shape, crystal phases, and surface coating. Int. J. Mol. Sci. 2014;15:22258–22278. doi: 10.3390/ijms151222258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warheit D.B., Webb T.R., Reed K.L., Frerichs S., Sayes C.M. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology. 2007;230:90–104. doi: 10.1016/j.tox.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi N., Naya M., Endoh S., Maru J., Yamamoto K., Nakanishi J. Comparative pulmonary toxicity study of nano-TiO2 particles of different sizes and agglomerations in rats: different short-and long-term post-instillation results. Toxicology. 2009;264:110–118. doi: 10.1016/j.tox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Oberdörster G., Ferin J., Gelein R., Soderholm A.C., Finkelstein J. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ. Health Perspect. 1992;97:193–199. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renwick L.C., Brown D., Clouter A., Donaldson K. Increased inflammation and altered macrophage. Occup. Environ. Med. 2004;61:442–447. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warheit D.B., Webb T.R., Sayes C.M., Colvin V.L., Reed K.L. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol. Sci. 2006;91:227–236. doi: 10.1093/toxsci/kfj140. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson K., Stone V., Clouter A., Renwick L., MacNee W. Ultrafine particles. Occup. Environ. Med. 2001;58:211–216. doi: 10.1136/oem.58.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Höhr D., Steinfartz Y., Schins R.P., Knaapen A.M., Martra G., Fubini B., Borm P.J. The surface area rather than the surface coating determines the acute inflammatory response after instillation of fine and ultrafine TiO2 in the rat. Int. J. Hyg. Environ. Health. 2002;205:239–244. doi: 10.1078/1438-4639-00123. [DOI] [PubMed] [Google Scholar]
- 15.Oberdörster G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health. 2001;74:1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- 16.Warheit D.B., Brock W.J., Lee K.P., Webb T.R., Reed K.L. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol. Sci. 2005;88:514–524. doi: 10.1093/toxsci/kfi331. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara N., Oshima Y., Kobayashi T., Imatanaka N., Nakai M., Ichinose T., Sasaki T., Zhang G., Fukui H., Gamo M. Dose-dependent clearance kinetics of intratracheally administered titanium dioxide nanoparticles in rat lung. Toxicology. 2014;325:1–11. doi: 10.1016/j.tox.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara N., Oshima Y., Kobayashi T., Imatanaka N., Nakai M., Ichinose T., Sasaki T., Kawaguchi K., Zhang G., Gamo M. Pulmonary clearance kinetics and extrapulmonary translocation of seven titanium dioxide nano- and submicron materials following intratracheal administration in rats. Nanotoxicology. 2015;9:1050–1058. doi: 10.3109/17435390.2015.1015644. [DOI] [PubMed] [Google Scholar]
- 19.Organisation for economic cooperation and development Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials. Series on the Safety of Manufactured Nanomaterials No. 36, 2012
- 20.Rehn B., Seiler F., Rehn S., Bruch J., Maier M. Investigations on the inflammatory and genotoxic lung effects of two types of titanium dioxide: untreated and surface treated. Toxicol. Appl. Pharmacol. 2003;189:84–95. doi: 10.1016/s0041-008x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 21.Noël A., Charbonneau M., Cloutier Y., Tardif R., Truchon G. Rat pulmonary responses to inhaled nano-TiO2: effect of primary particle size and agglomeration state. Part. Fibre Toxicol. 2013;10:48. doi: 10.1186/1743-8977-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyabu T., Morimoto Y., Hirohashi M., Horie M., Kambara T., Lee B.W., Hashiba M., Mizuguchi Y., Myojo T., Kuroda E. Dose-dependent pulmonary response of well-dispersed titanium dioxide nanoparticles following intratracheal instillation. J. Nanopart. Res. 2013;15:1600–1610. [Google Scholar]
- 23.Mie G. B Eiträge Zur Optik Trüber Medien, Speziell Kolloidaler. Metallösungen. Ann. Phys. 1908;25:377–445. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.