Abstract
Objective:
Cardiovascular events are the most frequent cause of death or disability among people with systemic lupus erythematosus (SLE). However, the causes of this increased the risk of major adverse cardiovascular events (MACEs) are not completely understood. The Age-adjusted Charlson Comorbidity Index (ACCI) is a prognostic classification that was initially developed for patients who have a number of comorbid conditions and the ACCI has been validated in many clinical settings.
Materials and Methods:
In this study, 5998 patients were enrolled from the National Health Research Institute Database of Taiwan. All the patients' sequential clinical data related to their diagnosis of SLE were reviewed from 2004 to 2007 to determine their risk of MACE occurrence and of all-cause mortality using their ACCI scores.
Results:
The predictive accuracy of the ACCI scores in relation to MACE occurrence among SLE patients was estimated and the C-statistic for this curve was found to be 0.687 (95% confidence interval [CI]: 0.664–0.709). The distribution of ACCI scores for MACE patients was 4.7%, 10.3%, 11.4%, and 21.5% for those with ACCI scores in the ranges of 0–1, 2–3, 4–5, and >6, respectively. A plot of the cumulative risk also showed a much higher risk among SLE patients with an ACCI score of >6. When patients were divided into different groups based on their ACCI scores, those with ACCI scores of >6 had an adjusted hazards ratio of 4.88 (95% CI: 3.84–6.19; P < 0.001) as compared to those with ACCI scores of 0–1.
Conclusion:
SLE patients with higher ACCI scores have a significantly higher risk of a MACE and of all-cause mortality. Our results suggested that ACCI scores may be useful as an index for estimating global cardiovascular risk among patients with SLE.
KEYWORDS: Age-adjusted Charlson Comorbidity Index, Major adverse cardiovascular events, National Health Research Institute Database, Systemic lupus erythematosus
INTRODUCTION
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that is characterized by the production of auto-antibodies; these can affect many parts of the body causing tissue inflammation and damage. The heart, joints, skin, lungs, blood vessels, liver, kidneys, and nervous system are the most frequently affected organs when SLE is present [1]. In Taiwan, the average annual incidence rate of SLE has been reported to range from 4.9 to 8.1/100,000 populations with an average female-to-male incidence ratio of 7:1 [2,3]. Cardiovascular events, including pericarditis, myocarditis, endocarditis, and coronary heart disease, are the most frequent cause of death among individuals with SLE [4,5]. Atherosclerosis also tends to occur more often among SLE patients than in the general population and also advances more rapidly [6].
It has been proposed that individuals with SLE have an increased risk of coronary heart disease [7,8] and this is possibly caused by the presence of kidney disease, problems related to the use of corticosteroids [6], including elevated cholesterol and/or blood glucose levels and a sedentary lifestyle due to joint problems, fatigue, and/or muscle pain. However, even after controlling for all known risk factors, individuals with SLE are still more likely to develop coronary heart disease than controls. Thus, the exact mechanism by which the increased risk of coronary heart disease is brought about is not completely understood.
The Charlson Comorbidity Index is a prognostic classification that was initially developed for patients who may have a number of comorbid conditions, and this index has been validated in many clinical settings [9,10,11,12,13,14]. This index includes 19 comorbidity categories, and each condition is assigned a score of 1, 2, 3, or 6 depending on the risk of dying due to this particular condition. The overall score is the sum of the weighted scores for all comorbidity conditions. Higher scores indicate a greater comorbidity and patients with scores of >5 have essentially a 100% mortality risk over 1 year. During its validation, age was found to be a significant contributing factor to overall survival, and this was subsequently incorporated into the Charlson comorbidity score to create a single index that accounted for both age and any medical comorbidities present; this became the Age-adjusted Charlson Comorbidity Index (ACCI) [9].
In this study, patients with clinically diagnosed SLE were identified using the National Health Insurance Research Database (NHIRD) of Taiwan. This database currently enrolls about 99% of the 23 million residents of Taiwan who receive medical care through the National Health Insurance (NHI) program. Accumulating evidence has confirmed the validity of the NHIRD [15]. Data regarding the effects of medical comorbidities on major adverse cardiovascular events (MACEs) among SLE patients have been scarce until now and therefore, the primary objective of this population-based cohort study was to determine the value of the ACCI scores among SLE patients in relation to prognosis regarding MACE as well as all-cause mortality.
MATERIALS AND METHODS
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Buddhist Dalin Tzu Chi Hospital, Taiwan. Informed written consent was waived because the study was a retrospective data analysis. All data were retrieved from the reimbursement database of the NHI Bureau and the study cohort was acquired from the whole population NHIRD from 2004 to 2007. SLE was identified by the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 710.0. Patients were enrolled when this ICD code was first entered as a primary diagnosis for any in-patient admission or out-patient department visit. We verified these patients in the insurance file using records containing their encrypted patient ID numbers and insurance fees. If their encrypted ID numbers could not be found in this insurance file, then these patients were excluded from the study. We did not use the catastrophic illness database for the identification of the SLE patients due to differences in time between the diagnosis of SLE and the approval of a catastrophic illness certificate for a given patients; this could have introduced errors into our study due to the study's relatively short follow-up period. In addition, it is possible that some patients with SLE did not applied for a catastrophic illness certificate. Enrolled patients were then followed up until the end of 2007, the termination of insurance (death), or the occurrence of MACE.
Age-adjusted Charlson Comorbidity Index scores
The ACCI scores were derived from in- and out-patient diagnostic data that had been collected during the 6 months before SLE enrollment. Weights for these scores were determined using the methods previously reported by Charlson et al. [9]. Comorbid conditions were weighted and scored, and 1 point was added for each decade after 40 years of age (i.e., 1 point for 41–50 years, 2 points for 51–60 years, 3 points for 61–70 years, and 4 points for 71 years or older [10,11] [Table 1].
Table 1.
Age-adjusted Charlson Comorbidity Index
| Score | Comorbid condition |
|---|---|
| 1 | Myocardial infarction |
| Congestive heart failure | |
| Cerebral vascular disease | |
| Peripheral vascular disease | |
| Dementia | |
| Chronic obstructive pulmonary disease | |
| Connective tissue disease | |
| Peptic ulcer disease | |
| Mild liver disease | |
| Agea | |
| 2 | Diabetes |
| Hemiplegia | |
| Moderate/severe renal disease | |
| Diabetes with end-organ damage | |
| Any solid tumor | |
| Leukemia | |
| Lymphoma | |
| 3 | Moderate/severe liver disease |
| 6 | Metastatic solid tumor |
| Acquired immunodeficiency syndrome |
a1 point is added to patients aged 41-50 years, 2 points for those aged 51-60 years, 3 points for those 61-70 years, and 4 points for those 71 years or older
Major adverse cardiovascular events
A MACE was defined as including an outcome that occurred after the date of SLE enrollment and these outcomes consisted a nonfatal myocardial infarction (ICD-9-CM code 410.x), a nonfatal cerebrovascular event/stroke (ICD-9-CM codes 43x.x, 430.x, 431.x, 432.x, 434.x, 435.x, 436.x, 438.x), and all-cause of death. All diagnoses were based on both primary diagnoses during any inpatient admission or primary diagnosis during any outpatient department visit.
Other variables
Patients' demographic information, including age, sex, and comorbidities/complications other than those included in the ACCI, were also collected. These complications/comorbidities other than those included in the ACCI were also obtained from in- and out-patient diagnostic data that had been collected during the 6 months before SLE enrollment.
Statistical analysis
The SAS statistical package version 9.2 (SAS Institute, Inc., Cary, NC, USA) was used for statistical analysis. Receiver operative characteristic curves were generated to assess the prediction accuracy for any MACE using the ACCI scores and plots of observed and predicted MACEs were prepared. The cumulative risk of any MACE and of all-cause mortality among the various ACCI score groups were plotted across 2004–2007 and their statistical significance was assessed using a log-rank test. For the multivariate analysis, a Cox proportional hazards model was used to adjust for age, sex, comorbidities, complications, and any other variables available from the database. The value of P < 0.05 was considered statistically significant.
RESULTS
From 2004 to 2007, a total of 6556 patients were given the ICD code 710.0 as their primary diagnosis. Among these patients, 558 could not be verified in the insurance ID file and were excluded. Thus, 5998 patients with an SLE diagnosis were enrolled in the present study [Figure 1]. The average age of these patients was 36 ± 15 years, and they were predominantly females (88.4%). The distribution of their ACCI scores was 2364 (39.4%) with a score of 0–1, 1451 (24.2%) with a score of 2–3, 1212 (20.2%) with a score of 4–5, and 971 (16.2%) with a score of >6. Their other basic characteristics and the comorbidities are presented in Table 2.
Figure 1.
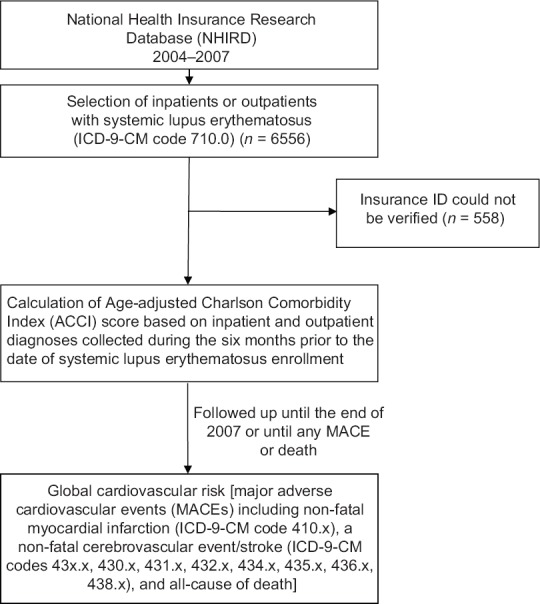
Flowchart for the sample collection and selection in this study
Table 2.
Baseline characteristics of patients with systemic lupus erythematosus in the National Health Insurance Research Database from 2004-2007
| Variable | n (%) |
|---|---|
| Mean age (years)±SD | 36±15 |
| Sex | |
| Male | 693 (11.6) |
| Female | 5305 (88.4) |
| ACCI score category | |
| 0-1 | 2364 (39.4) |
| 2-3 | 1451 (24.2) |
| 4-5 | 1212 (20.2) |
| >6 | 971 (16.2) |
| Comorbidities | |
| Hyperlipidemia | 169 (2.8) |
| Coronary artery disease | 63 (1.1) |
| Atrial fibrillation | 21 (0.4) |
| Scleroderma | 63 (1.1) |
| Dermatomyositis | 27 (0.5) |
| Polymyositis | 19 (0.3) |
| Rheumatoid arthritis | 93 (1.6) |
| Autoimmune thyroid diseases | 32 (0.5) |
| Primary Sjögren's syndrome | 372 (6.2) |
| Deep vein thrombosis | 57 (1.0) |
| Pulmonary embolism | 30 (0.5) |
SD: Standard deviation, ACCI: Age-adjusted Charlson Comorbidity Index
The predictive accuracy of the ACCI scores for MACE among the SLE patients was estimated using the receiver operating characteristic curve approach [Figure 2]. The C-statistic for this curve was 0.687 (95% confidence interval: 0.664–0.709). As shown in Table 3, a total of 607 patients with MACE and all-cause mortality were identified and the distribution of ACCI scores was 110 (4.7%) with a score of 0–1, 150 (10.3%) with a score of 2–3, 138 (11.4%) with a score of 4–5, and 209 (21.5%) with a score of >6. A plot of the cumulative risk showed a much higher risk when a SLE patient has an ACCI score of >6 [Figure 3].
Figure 2.
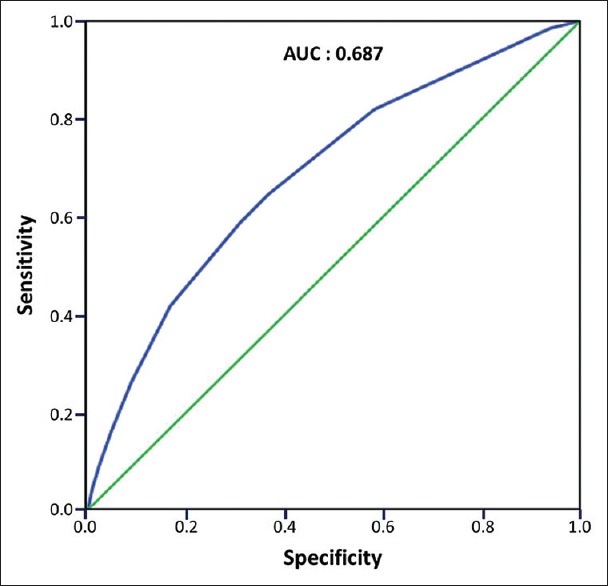
Receiver operating characteristic curve for Age-adjusted Charlson Comorbidity Index (ACCI) scores when used to predict global cardiovascular risk among patients with systemic lupus erythematosus
Table 3.
Global cardiovascular risk among patients with systemic lupus erythematosus by Age-adjusted Charlson Comorbidity Index score (n=5998; 2004-2007)
| Variable | Events (%) | P |
|---|---|---|
| ACCI score category | ||
| 0-1 (n=2364) | 110 (4.7) | <0.001 |
| 2-3 (n=1451) | 150 (10.3) | |
| 4-5 (n=1212) | 138 (11.4) | |
| >6 (n=971) | 209 (21.5) | |
ACCI: Age-adjusted Charlson Comorbidity Index
Figure 3.
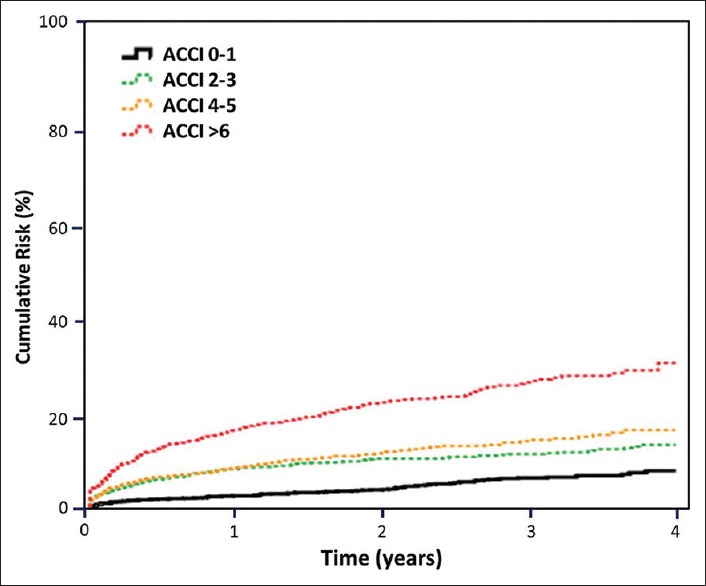
Global cardiovascular risk stratified by Age-adjusted Charlson Comorbidity Index score category among patients with systemic lupus erythematosus
The results of the Cox proportional hazards analysis showed that when the ACCI scores were treated as a continuous variable, there was a 25% increase in the risk of MACE and all-cause mortality for each one-point increment in the ACCI score. When the ACCI scores were analyzed as an ordinal variable, patients with an ACCI score of >6 showed an adjusted hazards ratio of 4.88 (95% CI: 3.84–6.19; P < 0.001) when compared to those with an ACCI score of 0–1 [Table 4].
Table 4.
Hazard ratios for the Age-adjusted Charlson Comorbidity Index score categories according to global cardiovascular risk among patients with systemic lupus erythematosus
| Model | Adjusted hazard ratio | 95% CI | P |
|---|---|---|---|
| Model A | |||
| ACCI score (per 1-unit increment) | 1.25 | 1.21-1.29 | <0.001 |
| Model B | |||
| ACCI score category | |||
| 0-1 | 1 | ||
| 2-3 | 2.11 | 1.64-2.71 | <0.001 |
| 4-5 | 2.60 | 2.02-3.35 | <0.001 |
| >6 | 4.88 | 3.84-6.19 | <0.001 |
Model A: ACCI treated as a continuous variable; adjusted for the patients’ age, sex, and comorbidities or complications other than those already included in the ACCI, Model B: ACCI treated as an ordinary variable; adjusted for the patients’ age, sex, and comorbidities or complications other than those already included in the ACCI, ACCI: Age-adjusted Charlson Comorbidity Index, CI: Confidence interval
DISCUSSION
This is the first population-based cohort study to evaluate the risk of any MACE among SLE patients. The major finding of this study was that ACCI scores could be applied when evaluating global cardiovascular risk, that is any MACE and all-cause mortality, among patients with SLE. Patients with higher ACCI scores are at a higher risk of MACE and all-cause mortality.
Our findings show that, among SLE patients, the frequencies of MACE occurrence were 4.7%, 10.3%, 11.4%, and 21.5% for those with ACCI scores of 0–1, 2–3, 4–5, and >6, respectively. Similar studies focusing on this comorbidity index among SLE patients have been published [16,17]. However, most of those studies were conducted using hospital-based cohorts. Our study uses a population-based database to assess prognostic ability of this comorbidity index among SLE patients. Furthermore, in addition to mortality as a particular outcome, central nervous system involvement, and cardiovascular disease are also severe complications among SLE patients, and in this context, our study has also included MACE evaluations for the SLE patients.
Although overall mortality has improved in recent decades due to the development of more advanced medical therapies [18,19], cardiovascular events remain the most frequent comorbidity among SLE patients [4,18]. In addition, the occurrence of any MACE is an important long-term issue for these patients, their families, and even society as a whole. From the perspective of medical providers, MACE prevention is more important than the evaluation of a mortality rate among patients. Our study is the only one to identify the impact of comorbidities on MACE occurrence among SLE patients. Clinically, ACCI scoring is easy to do via a retrospective chart review and it is able to account for those disease processes that have been shown to be most important to the occurrence of MACE.
The main strength of our study is that our data are derived from a nationwide population-based, longitudinal study. The important follow-up information that was needed to carry out this study could be accessed from the NHIRD. In addition, because all diagnosis information was collected longitudinally, recall bias should not be present. Nevertheless, there are a number of limitations that must be considered. First, the diagnosis of SLE, coronary arterial disease, stroke, and other types of MACE was based on the ICD codes recorded in the NHIRD database. Therefore, information bias as a result of misclassification is possible. Second, our study is based on retrospective data and it is not possible to validate the ACCI scoring and thus, again, misclassification of the ACCI scores is a possibility. Third, the NHIRD database does not contain any information on tobacco use, dietary habits, metabolic profiles, or other behavioral factors, all of which are possible risk factors for MACE occurrence among SLE patients and the possibility of confounding effects due to these factors cannot be ruled out. Nonetheless, given the magnitude and statistical significance of the observed effects in this study, these limitations are unlikely to have compromised our results.
CONCLUSION
In this population-based cohort study, the ACCI was identified as a good tool for predicting MACE occurrence and all-cause mortality among SLE patients. SLE patients with a higher ACCI score are thus at a higher risk of a global cardiovascular event.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fei Y, Shi X, Gan F, Li X, Zhang W, Li M, et al. Death causes and pathogens analysis of systemic lupus erythematosus during the past 26 years. Clin Rheumatol. 2014;33:57–63. doi: 10.1007/s10067-013-2383-3. [DOI] [PubMed] [Google Scholar]
- 2.Chiu YM, Lai CH. Nationwide population-based epidemiologic study of systemic lupus erythematosus in Taiwan. Lupus. 2010;19:1250–5. doi: 10.1177/0961203310373780. [DOI] [PubMed] [Google Scholar]
- 3.Yeh KW, Yu CH, Chan PC, Horng JT, Huang JL. Burden of systemic lupus erythematosus in Taiwan: A population-based survey. Rheumatol Int. 2013;33:1805–11. doi: 10.1007/s00296-012-2643-6. [DOI] [PubMed] [Google Scholar]
- 4.Nossent J, Cikes N, Kiss E, Marchesoni A, Nassonova V, Mosca M, et al. Current causes of death in systemic lupus erythematosus in Europe, 2000-2004: Relation to disease activity and damage accrual. Lupus. 2007;16:309–17. doi: 10.1177/0961203307077987. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen S, Petersen J, Ullman S, Junker P, Voss A, Rasmussen JM, et al. Mortality and causes of death of 513 Danish patients with systemic lupus erythematosus. Scand J Rheumatol. 1999;28:75–80. doi: 10.1080/030097499442522. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Tian X, Zhang F. Premature atherosclerosis-related central nervous system involvement in two cases of systemic lupus erythematosus. J Clin Rheumatol. 2007;13:211–2. doi: 10.1097/RHU.0b013e318133476d. [DOI] [PubMed] [Google Scholar]
- 7.Mattu A, Petrini J, Swencki S, Chaudhari C, Brady WJ. Premature atherosclerosis and acute coronary syndrome in systemic lupus erythematosus. Am J Emerg Med. 2005;23:696–703. doi: 10.1016/j.ajem.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Nikpour M, Urowitz MB, Gladman DD. Premature atherosclerosis in systemic lupus erythematosus. Rheum Dis Clin North Am. 2005;31:329–54. doi: 10.1016/j.rdc.2005.01.001. vii. [DOI] [PubMed] [Google Scholar]
- 9.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 10.Robbins JR, Gayar OH, Zaki M, Mahan M, Buekers T, Elshaikh MA. Impact of age-adjusted Charlson comorbidity score on outcomes for patients with early-stage endometrial cancer. Gynecol Oncol. 2013;131:593–7. doi: 10.1016/j.ygyno.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Kim SK, Choe JY, Lee SS. Charlson comorbidity index is related to organ damage in systemic lupus erythematosus: Data from KORean lupus network (KORNET) registry. J Rheumatol. 2017;44:452–8. doi: 10.3899/jrheum.160900. [DOI] [PubMed] [Google Scholar]
- 12.Lu KJ, Kearney LG, Ord M, Jones E, Burrell LM, Srivastava PM, et al. Age adjusted Charlson co-morbidity index is an independent predictor of mortality over long-term follow-up in infective endocarditis. Int J Cardiol. 2013;168:5243–8. doi: 10.1016/j.ijcard.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Koppie TM, Serio AM, Vickers AJ, Vora K, Dalbagni G, Donat SM, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384–92. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- 14.Kastner C, Armitage J, Kimble A, Rawal J, Carter PG, Venn S, et al. The Charlson comorbidity score: A superior comorbidity assessment tool for the prostate cancer multidisciplinary meeting. Prostate Cancer Prostatic Dis. 2006;9:270–4. doi: 10.1038/sj.pcan.4500889. [DOI] [PubMed] [Google Scholar]
- 15.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236–42. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Thumboo J, Earnest A, Yong SL, Fong KY. The effect of comorbidity on hospital mortality in patients with SLE from an Asian tertiary hospital. Lupus. 2014;23:714–20. doi: 10.1177/0961203314522340. [DOI] [PubMed] [Google Scholar]
- 17.Jönsen A, Clarke AE, Joseph L, Belisle P, Bernatsky S, Nived O, et al. Association of the Charlson comorbidity index with mortality in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011;63:1233–7. doi: 10.1002/acr.20506. [DOI] [PubMed] [Google Scholar]
- 18.Elfving P, Puolakka K, Kautiainen H, Virta LJ, Pohjolainen T, Kaipiainen-Seppänen O, et al. Mortality and causes of death among incident cases of systemic lupus erythematosus in Finland 2000-2008. Lupus. 2014;23:1430–4. doi: 10.1177/0961203314543919. [DOI] [PubMed] [Google Scholar]
- 19.Uramoto KM, Michet CJ, Jr, Thumboo J, Sunku J, O'Fallon WM, Gabriel SE, et al. Trends in the incidence and mortality of systemic lupus erythematosus, 1950-1992. Arthritis Rheum. 1999;42:46–50. doi: 10.1002/1529-0131(199901)42:1<46::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]


