Abstract
Cadmium is a natural element found in the earth’s crust; it is usually associated with other metals, but due to the impacts caused by human activity, its concentration has increased in the aquatic environment. This metal may damage aquatic animal reproduction, decreasing the rate of fertilization of organisms such as fish. Thus, this study aimed to evaluate the in vitro toxicity of different concentrations of cadmium (0 (control), 0.5, 5, and 10 μg/L) using sperm cells of model organism zebrafish, Danio rerio. Structural parameters, including integrity and fluidity of the plasma membrane, concentration of oxygen species, mitochondrial function and DNA fragmentation were measured by flow cytometry. The following sperm movement parameters were also measured using the computer assisted sperm analysis (CASA) system: motility, time of motility, curvilinear velocity, average path velocity and straight line velocity in μm/s. Significant effects were observed on path speed, straight speed, curvilinear velocity, motility time, progressive and total motility, and plasma and DNA integrity. The results showed that cadmium can negatively affect some reproductive parameters in D. rerio, which may reduce the fertility rate of these animals.
Keywords: Cadmium, Reproduction, Danio rerio, Spermatozoa, CASA motility
1. Introduction
Cadmium (Cd) is a major heavy metal present in the earth’s crust and is usually associated with other metals such as zinc, copper and lead. Cadmium bioaccumulates in phytoplankton and complex food webs involving aquatic animals as mollusks, fish and crustaceans [5]. Because it is a pollutant at high concentrations, there are regulatory rules that stipulate safe concentrations of cadmium in water resources. In Brazil, for example, the maximum concentration permitted by CONAMA (National Environmental Council) in fresh water for classes I, II and III (consumption, recreation, irrigation, and protection of aquatic and aquaculture communities) is 0.01 mg/L (10 g/L) [8]. However, in the US and Europe the limits are 1 and 5 mg/L of cadmium, respectively [14], [13].
Cadmium's toxicity is related to its ability to replace calcium (Ca2+) in biological reactions, which is due to the similar characteristics of these two elements such as their similar ionic radii. Therefore, the cellular uptake of Cd occurs mainly through Ca2+ channels, inhibiting the uptake of Ca2+. Hence, Cd acts as a potent blocker of these channels [26], leading to deleterious effects because Ca2+ in critical to many cell signaling pathways [25].
The mechanisms of Cd-dependent reproductive toxicity have been reported in in vivo and in vitro studies. This metal can decrease testosterone levels in rats [27], significantly affecting the testicular tissue by changing the hematotesticular barrier [6]. It can also reduce sperm concentration and motility in rice fish, Oryziaslatipes, and zebrafish, Danio rerio [9], increase lipid peroxidation in carp, Cyprinus carpio (Tale Cinier et al., 1998), and affect sperm maturation in common snook, Centropomus undecimalis [1].
Aquatic organisms are commonly used for toxicological studies of various contaminants because they are affected both directly through contact with contaminated water and indirectly through their diet, whether it includes plants, invertebrates, or various species of smaller fish [3]. Thus, fish may reflect contamination in other organisms and trophic levels within the aquatic ecosystem and are an important part of the diets of mammals and waterfowls [20]. Zebrafish, Danio rerio, has been used extensively as an experimental model because of its advantageous features such as its small size, which allows easy handling, and its rapid absorption of substances added directly to the water [34].
D. rerio uses external fertilization like most teleost fish, the male and female gametes are released into the aquatic environment and they need to come in contact for fertilization to occur [9]. Thus, while the fish itself has defenses against toxicants that can cause reproductive effects, the release of spermatozoa into the water exposes the gamete directly to pollutants such as cadmium. The spermatozoa have a poor antioxidant defense system and are highly prone to oxidative stress induced by pollutants [36]. Furthermore, zebrafish spermatozoa do not contain metallothionein, which has been identified in other fish sperm cells and reduces the toxicity of Cd (Tale Cinier et al., 1998). Therefore, experiments to assess the direct exposure of spermatozoa to cadmium that closely mimic the reproductive biology of teleost fish exposed to cadmium in nature are environmentally relevant. This study aimed to analyze the effects of different concentrations of cadmium on various in vitro parameters of Danio rerio sperm cells.
2. Materials and methods
The animals were euthanized by sectioning of the spinal cord, an accepted method, with restrictions according to the Federal Council of Veterinary Medicine (Resolution no. 05/2012 1000) because the use of anesthetics could affect the results of sperm analysis. The methodology used in this study was approved by the Ethics Committee at the Federal University of Pelotas/Rs, Brazil under number 10016. The gonads were withdrawn from 10 reproductive-phase, male zebrafish adults aged 4–6 months by dissection using an abdominal incision. The gonads were placed individually in Eppendorf tubes containing 1.5 mL of Beltsville Thawing Solution (BTS) [37] at pH 7.4 and an osmolarity of 350 mOsm and sectioned to aid the release of the spermatozoa. Sperm motility was assessed to determine motility rate and motility time for the pre-treatment samples (only fresh semen diluted with BTS was assessed before the incubation period) by activation using Milli-Q water; non-motile samples, indicative of sperm death, were excluded. Then, each semen sample was diluted at a 1:1 ratio (v/v) with four different concentrations of cadmium for treatment with final concentrations of 0 (control, BTS only), 0.5, 5.0 and 10 μg/L cadmium, osmolality of 300–320 mOsm/kg.
The samples were incubated at 20 °C for 10 min. This temperature was determined to be acceptable for the survival of zebrafish, which live at temperatures between 18 and 26 °C [18], and was also chosen because higher temperatures, such as 22 and 25 °C, had deleterious effects on sperm survival in previous experiments conducted by our research group (unpublished data).
After the incubation period, sperm analysis was performed to measure total motility; progressive sperm velocity parameters, straight line velocity (VSL) curvilinear velocity (VCL) and average path velocity (VAP); time of motility; fluidity and integrity of the plasma membrane; production of reactive oxygen species; mitochondrial function; and the DNA fragmentation index
2.1. Assessment of sperm motility using computer-aided semen analysis (CASA)
To estimate sperm motility, 1 μL of diluted semen and 4 μL of Milli-Q water containing cadmium (at concentrations of 0.5, 5 and 10 μg/L cadmium) were placed on slides under coverslips and analyzed using CASA [7].
The images generated were reproduced and efficiently and objectively analyzed using the Sperm Class Analyzer software (SCA) to assess overall motility parameters, progressive motility, VSL, VCL and VAP [38]. The time or duration of motility after sperm activation was determined based on the time of complete arrest of the progressive movement of the spermatozoa following the method described by [33]. Each image (n = 10) was analyzed using the standard settings for fish by SCA. Sperm was considered immotile when velocity was <10 m/s. Although SCA simultaneously assessed more than 15 sperm motility end points, for brevity only curvilinear velocity (VCL), straight line velocity (VSL), and average path velocity (VAP) were considered for further analysis, as similar effects were observed for all end points. To determine these velocities, each individual sperm cell (n = at least 1000 sperm/concentrations Cd) was followed throughout the 10 images and a sperm trajectory was calculated.
2.2. Flow cytometry
We used the Attune® Acoustic Focusing Flow Cytometer (Applied Biosystems). To detect the sperm population, non-sperm cells were removed based on the FSC x SSC scatter plots [28], [29] and debris was eliminated by staining of the cells with Hoechst 33342 at a concentration of 16.2 μM (Sigma-Aldrich Co., St. Louis, MO, USA), except for those samples used to measure the DNA fragmentation index. A total of 10,000 events per sperm sample with a flow of 200 cells/s were analyzed using the Cytometric Attune Software V2.1 program.
2.3. Integrity of the plasma membrane
To verify the integrity of the plasma membrane, we used 20.0 μM carboxyfluorescein diacetate (DCF), which fluoresces green, and 7.3 μM propidium iodide (PI), which fluoresces red (Sigma-Aldrich Co., St. Louis, MO, USA). The sperm were classified as non-injured (DCF + /IP-) and injured (DCF + /IP+; DCF-/IP+; DCF-/IP-) [19], [17].
2.4. Fluidity of the plasma membrane
Plasma membrane fluidity was analyzed using hydrophobic merocyanine 540 dye (M540) at a final concentration of 2.7 μM (Sigma-Aldrich Co., St. Louis, MO, USA) and YO-PRO, which fluoresces green, at a final concentration of 0.1 μM (Invitrogen, Eugene, OR, USA). Only live sperm (YO-PRO negative) were selected and classified into high fluidity cells (high M540 concentration) and low fluidity cells (low M540 concentration) [17].
2.5. Mitochondrial functionality
The fluorescent dyes Rhodamine 123 (13 μM), which fluoresces green, and PI (7.3 μM) (Sigma-Aldrich Co., St. Louis, MO, USA) were used to assess mitochondrial function. Only intact sperm (IP-) were selected and classified into cells with high functionality (high fluorescence, high accumulation of Rhodamine) and low functionality (low fluorescence, low accumulation of Rhodamine) [19].
2.6. DNA fragmentation index
DNA integrity and fragmentation were measured using the sperm chromatin structure assay (SCSA). For this evaluation, 10 μL of semen were mixed with 5 μL TNE (0.01 M Tris-HCl, 0.15 M NaCl, 0.001 M EDTA, pH 7.2). Then, after a 30 s incubation, 10 μL of Triton (0.1% Triton X-100) (v/v) were added, followed by another 30 s incubation, and finally, the addition of 5 μL acridine orange (Sigma-Aldrich Co., St. Louis, MO, USA). This reaction was then incubated for 5 min. The results of the flow cytometry are expressed as the percentage of sperm with fragmented DNA [16].
2.7. Concentration of reactive oxygen species (ROS)
ROS concentration was determined using the fluorescent dye 2’7-dichlorofluorescein diacetate at a final concentration of 1.0 μM, which emits green fluorescence when oxidized by intracellular ROS, and IP (7.3 μM final concentration). As our reported measurement, we used the median intensity of green fluorescence of the living sperm (IP-) only [12].
2.8. Statistical analysis
In this study, descriptive data (mean and standard error of the mean) were generated for each of the dependent variables: total sperm motility, progressive sperm velocity (VCL, VAP and VSL), mitochondrial functionality, reactive oxygen species, plasma membrane integrity and fluidity, and the DNA fragmentation index.
For all of the dependent variables, normality was tested using the Shapiro-Wilk test. Subsequently, a Kruskal Wallis test for nonparametric data was used because none of the variables exhibited a normal distribution. Statistix® 2009 software was used for the analyses.
3. Results
Total and progressive motility and motility time showed similar patterns; all of the cadmium-treated samples differed from the pre-treatment controls and the BTS-only controls (P < 0.01) (Table 1).
Table 1.
Progressive motility (PM), total motility (TM) and time of motility (TeMot) of D. rerio sperm exposed to Beltsville Thawing Solution (BTS) with 0, 0.5, 5.0 or 10 μg/L Cadmium at 20 °C for 10 min analyzed by CASA.
| Fresh semen | 10 min incubation at 20 °C |
||||
|---|---|---|---|---|---|
| 0 (only BTS) | 0,5 | 5,0 | 10 | ||
| PM (%) | 46.0 ± 3.63A | 37.7 ± 2.95A | 26.3 ± 3.1B | 27.1 ± 2.86B | 25.2 ± 3.56B |
| TM (%) | 53.9 ± 3.6A | 52.3 ± 2.5A | 33.9 ± 3.3B | 33.8 ± 3.07B | 34.6 ± 3.6B |
| TeMot (s) | 133.3 ± 11.5A | 103.2 ± 13.1A | 77.5 ± 16.48B | 68.7 ± 8.45B | 71.2 ± 13.3B |
Data are expressed as the mean and standard error of the mean. Different letters in the same line indicate significant differences based on the nonparametric Kruskal-Wallis test with a 0.01 significance threshold.
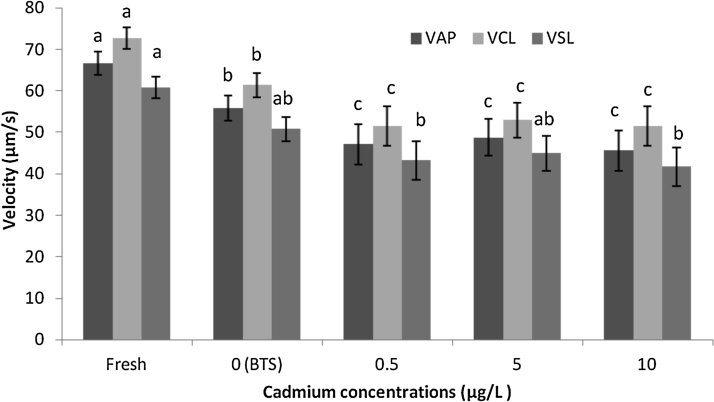
Although the VAP and VCL variables were not different among the samples treated with various cadmium concentrations (P > 0.01), differences were observed between the treated samples and the untreated and BTS-only controls (P < 0.01) (Fig. 1). For the VSL parameter, no differences were observed among the various cadmium concentrations or the BTS-only control; however, all of the treated samples were significantly different than the untreated semen samples (Fig. 1).
Fig. 1.
Straight line velocity (VSL) curvilinear velocity (VCL) and average path velocity (VAP) in μm/s of D. rerio sperm cells exposed to Beltsville Thawing Solution (BTS) with 0, 0.5, 5, or 10 μg/L cadmium at 20 °C for 10 min. Data are expressed as the mean and standard error of the mean. Different letters indicate significant differences based on the nonparametric Kruskal-Wallis test with a 0.01 significance threshold.
For DNA fragmentation index and plasma membrane integrity (IME), the samples treated with 5.0 and 10 μg/L cadmium differed from the controls (P < 0.05). Regarding mitochondrial functionality (FMI), there were no significant differences between the treated samples and the controls. For plasma membrane fluidity (FLU), the cadmium-treated samples were all similar and did not differ from the controls. ROS concentration was slightly reduced in the cadmium-treated samples compared with the controls, although this difference was not statistically significant (Table 2).
Table 2.
Spermatozoa of D. rerio exposed to Beltsville Thawing Solution (BTS) alone (control) or 0.5, 5, or 10 μg/L cadmium at 20 °C for 10 min were evaluated for high fluidity (FLU) and integrity of the plasma membrane, high functionality mitochondrial (FMI), the sperm chromatin structure assay (SCSA) and the production of reactive oxygen species (ROS) (n = 10). * ROS expression is based on the median fluorescence intensity.
| [Cd +2] | SCSA | Membrane Integrity (%) | FMI (%) | FLU (%) | ROS |
|---|---|---|---|---|---|
| 0 (BTS) | 7.27 ± 1.0A | 83.40 ± 8.5A | 74.87 ± 8.3A | 81.60 ± 3.1A | 3428.8 ± 886.2A |
| 0.5 | 9.53 ± 1.2A | 73.93 ± 8.7AB | 69.04 ± 6.1A | 78.54 ± 3.7A | 2584.3 ± 654.5A |
| 5 | 13.72 ± 3.0B | 47.85 ± 9.0B | 65.82 ± 5.2A | 77.39 ± 4.3A | 2145.0 ± 356.9A |
| 10 | 14.29 ±3.3B | 41.52 ± 10.1B | 64.47 ± 5.8A | 77.11 ± 3.3A | 2279.5 ± 428.2A |
Data are expressed as the mean and standard error of the mean. Different letters in the same column indicate significant differences based on the nonparametric Kruskal-Wallis test with a 0.05 significance threshold.
4. Discussion
Upon exposure at a controlled temperature for 10 min, the total and progressive motilities as well as the time of motility of sperm treated with different concentrations of cadmium were similar, but all of the treated samples exhibited motility characteristics different from those of the untreated and BTS-only controls. The similarities between the two controls demonstrate that the diluent medium did not affect semen quality, which was only affected by the addition of cadmium at any concentration used.
The other spermatozoa movement variables, VCL and VAP, were negatively affected by cadmium exposure. VCL and VAP were lower in the cadmium-treated samples compared with both the BTS-only and untreated controls; however, VSL was lower in all of the treated samples than in the untreated control. A similar result was observed by [11] for VAP in rainbow trout treated with low concentrations of cadmium (up to 10 μg/L). According to the review by [4], the sperm speed is essential for longevity in external fertilization fish because high-speed sperm can rapidly locate oocytes in open water before the end of their motility time.
These results corroborate those observed in the sperm of rainbow trout, Oncorhynchus mykiss, in which doses in excess of 1 μg/L of Cd+2 significantly affected motility, demonstrating the increased susceptibility of speed parameters of motility to environmental stressors. Furthermore, VAP and VCL, which declined significantly upon cadmium exposure in the current study, are strongly correlated with fertilization rates in A. Crassispina (0.928 and 0.902, respectively) [2]. Thus, these parameters can be useful in predicting reproductive harm.
In addition to the automated CASA analysis, this study presents analyses by flow cytometry that have been largely unexplored in reproductive toxicology studies of different sperm structures that are fundamental to the maintenance and viability of sperm fertilization capacity. In our analyses of plasma membrane integrity and the DNA fragmentation index, we observed dose-dependent decreases relative to the controls. However, a similar statistically significant, dose-dependent decrease was not observed for mitochondrial functionality and plasma membrane fluidity, possibly because the semen samples are biological samples with a high degree of variability that have been shown to exhibit non-normal distributions in several experiments evaluating sperm quality [23], [31], [32].
Treatment with 5 and 10 μg/L cadmium led to a significant decrease in plasma membrane integrity compared to the controls. This result may help explain the loss of fertilizing capacity in teleost fish [22]. It is possible that the plasma membrane of sperm, as the first barrier, is more sensitive to different types of pollutants. This idea was demonstrated by [21] and [24] in Poecilia vivipara and D. rerio sperm, respectively, treated with different glyphosate concentrations, leading to a decline in membrane integrity. The significant loss of membrane integrity is important because the plasma membrane maintains the selective permeability of the spermatozoa, supporting metabolism. The membrane may be impaired due to the anionic character of this structure [15], causing a severe interaction with the cadmium and breakages due to the blockage of calcium channels by cadmium that cause cell death due to low cytosolic calcium concentrations. Similar results were obtained with Sinopotamon henanense by [10], in which sperm cells exposed to cadmium at concentrations above 7.5 mg/L exhibited lesions in the plasma membrane.
Sea urchin sperm (Anthocidaris crassispina) exposed to cadmium exhibited a deformation of the mitochondrial crest [2], no observations in we studies. This effect of cadmium is important because mitochondrial injuries can decrease fertilization capacity because the mitochondria are required for ATP production, which provides the energy for sperm motility.
A significant increase in DNA fragmentation was observed in sperm cells exposed to cadmium at concentrations above 5 μg/L. The maintenance of DNA integrity, or maintenance of the genetic code to be transmitted to the offspring, is of paramount importance as it decreases the chance of mutations or death during the hatching stage of fish [30]. These data suggest that perhaps because the plasma membrane was damaged, the DNA was exposed to the effects of cadmium.
The percentage of cells with low membrane fluidity and the concentrations of reactive oxygen species did not differ among the treated samples or between the treated samples and the controls. According to Ercal et al. (2001), the anionic nature of biological membranes facilitates cadmium injury because cadmium penetrates the cell directly via calcium channels. Cadmium blocks calcium channels and is not allowed entry into the cell; it also inhibits the function of potassium channels [35] without altering membrane fluidity, but affecting sperm membrane integrity.
The concentration of ROS was similar in all samples, most likely because the mitochondrial chain was not affected. However, when there is an excess of calcium in the cytosol due to cadmium exposure, mitochondrial function should be inhibited and hydroxyl radicals should not be produced. This discrepancy between the expected result and our actual observations suggests that the species under study may be more complex than other species.
5. Conclusion
The in vitro experiments performed on D. rerio semen demonstrated that even cadmium concentrations of 5 and 10 μg/L have harmful effects on sperm cells.
Transparency document
Acknowledgements
Financial support for this study was from ‘Coordenacão de Aperfeicoamento de Pessoal de Ensino Superior’ (CAPES, Programa Ciências do Mar, Brasília, DF, Brazil) and Universidade Federal do Rio Grande, Rio Grande, RS, Brazil. A.S. Varela Junior and C.D. Corcini are research fellows from the Brazilian “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, DF, Brazil).
Contributor Information
Izani Bonel Acosta, Email: izanibonel@hotmail.com.
Carine Dahl Corcini, Email: corcinicd@gmail.com.
References
- 1.Abascal F.J., Cosson J., Fauvel C. Characterization of sperm motility in sea bass: the effect of heavy metals and physicochemical variables on sperm motility. J. Fish. Biol. 2007;70:509–522. [Google Scholar]
- 2.Au D.W.T., Reunovb A.A., Wua R.S.S. Reproductive impairment of sea urchin upon chronic exposure to cadmium. Part II: effects on sperm development. Environ. Pollut. 2001;111:11–20. doi: 10.1016/s0269-7491(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi M.M., Moraes R.C., Varoli F.M.F., Osti S.C. Ecotoxicologia. In: Spinosa H.S., Gómiak S.L., Palermo-neto J., editors. Toxicologia Aplicada à Medicina Veterinária. Manole; São Paulo: 2008. 942 p. [Google Scholar]
- 4.Browne R.K., Kaurova S.A., Uteshev V.K., Shishova N.V., McGinnity D., Figiel C.R., Mansour N., Agnew D., Wu M., Gakhova P.T., Dzyuba B., Cosson J. Sperm motility of externally fertilizing fish and amphibians. Theriogenology. 2015;83:1–13. doi: 10.1016/j.theriogenology.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso, L.M.N., Chasin, A.A.M, 2001. Ecotoxicologia do cádmio e seus compostos. Série Cadernos de Referência Ambiental. Vol. 6.
- 6.Chung N., Cheng Y. Is cadmium chloride-induced inter-sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- 7.Chyb J., Kime D.E., Szczerbik P., Mikołajczyk T., Epler P. Computer-assisted analysis (CASA) of common carp Cyprinus carpio L. spermatozoa motility in the presence of cadmium. Arch. Pol. Fish. 2001;9:173–181. [Google Scholar]
- 8.CONAMA n° 357, 2005, Classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e outras providências. Brasília.
- 9.Coward K., Bromage N.R., Hibitt O., Parrington J. Gamete physiology, fertilization and egg activation in teleost fish. Rev. Fish Biol. Fish. 2002;12:33–58. [Google Scholar]
- 10.Dandan M., Yuhua H., Lijun D., Li N., Xuan R., Wang F., Jing W., Wang Lan. Oxidative damages and ultrastructural changes in the sperm of freshwater crab Sinopotamon henanense exposed to cadmium. Ecotoxicol. Environ. Saf. 2013;98:244–249. doi: 10.1016/j.ecoenv.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich M.A., Żmijewski D., Karol H., Hejmej A., Bilińska B., Jurecka P., Irnazarow I., Słowińska M., Hliwa P., Ciereszko A. Isolation and characterization of transferrin from common carp (Cyprinus carpio L) seminal plasma. Fish Shellfish Immunol. 2010;29:66–74. doi: 10.1016/j.fsi.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Domínguez-Rebolledo A.E., Martínez-Pastor F., Bisbal A.F., Ros-Santaella J.L., García-Álvarez O., Maroto-Morales A., Soler A.J., Garde J.J., Fernández-Santos M.R. Response of thawed epididymal red deer spermatozoa to increasing concentrations of hydrogen peroxide, and importance of individual male variability. Reprod. Domest. Anim. 2011;46:393–403. doi: 10.1111/j.1439-0531.2010.01677.x. [DOI] [PubMed] [Google Scholar]
- 13.EEC, European Environment Council, 1983, On limit values and quality objectives for cadmium discharges. Last accession: 01.12.15.
- 14.EPA, 2001, United States Environmental Protection Agency, Update of ambient water quality criteria for cadmium, Last accession: 01.12.15
- 15.Ercal N., Gurer-Orhan H., Aykin-Burns N. Toxic metals andioxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 16.Evenson D.P., Thompson L., Jost L. Flow cytometric evaluation of boar semen by the sperm chromatin structure assay as related to cryopreservation and fertility. Theriogenology. 1994;41:637–651. doi: 10.1016/0093-691x(94)90174-h. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Gago R., Domínguez J.C., Martínez-Pastor F. Seminal plasma applied post-thawing affects boar sperm physiology: a flow cytometry study. Theriogenology. 2013;80:400–410. doi: 10.1016/j.theriogenology.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Froese R., Pauly D., 2014, FishBase. World Wide Web electronic publication, www.fishbase.org, version (11/2014). www.fishbase.org, versão, (11/2014)
- 19.Gillan L., Evans G., Maxwell W.M.C. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology. 2005;63:445–457. doi: 10.1016/j.theriogenology.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Han S., Fan Y., Xu X., Qin J., Wu B., Wang X., Aglioti S.M., Mao L. Empathic neural responses to others’ pain are modulated by emotional contexts. Hum. Brain Mapp. 2009;30:3227–3237. doi: 10.1002/hbm.20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harayashiki C.A.Y., Junior A.S.V., Machado A.A.S., Cabrera L.C., Primel E.G., Bianchini A., Corcini C.D. Toxic effects of the herbicide roundup in the guppy Poecilia vivipara acclimated to fresh water. Aquat. Toxicol. 2013;142–143:176–184. doi: 10.1016/j.aquatox.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 22.He S., Woods C. Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology. 2004;48:254–262. doi: 10.1016/j.cryobiol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Hernández M., Roca J., Gil M.A., Vázquez J.M., Martínez E.A. Adjustments on the cryopreservation conditions reduce the incidence of boar ejaculates with poor sperm freezability. Theriogenology. 2007;67:1436–1445. doi: 10.1016/j.theriogenology.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Lopes F.M., Varela Junior A.S., Corcini C.D., Silva A.C., Guazzelli V.G., Tavares G., Rosa C.E. Effect of glyphosate on the sperm quality of zebrafish Danio rerio. Aquat. Toxicol. 2014;155:322–326. doi: 10.1016/j.aquatox.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Marchetti C. Role of calcium channels in heavy metal toxicity. ISRN Toxicol. 2013;184:369. doi: 10.1155/2013/184360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGeer J.C., Niyogi S., Smith D.S. Cadmium. Fish Physiol. 2011;31:125–184. [Google Scholar]
- 27.Monsefi M., Alaee S., Moradshahi A., Rohani L. Cadmium induced male infertility in male mice. Environ. Toxicol. 2009;25:94–102. doi: 10.1002/tox.20468. [DOI] [PubMed] [Google Scholar]
- 28.Petrunkina A.M., Volker G., Weitze K.F., Beyerbach M., Töpfen-Petersen E., Waberski D. Detection of cooling-induced membrane changes in the response of boar sperm to capacitating conditions. Theriogenology. 2005;63:2278–2299. doi: 10.1016/j.theriogenology.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Piehler E., Petrunkina A.M., Ekhlasi-Hundrieser M., Töpfer-Petersen E. Dynamic quantification of the tyrosine phosphorylation of the sperm surface proteins during capacitation in vitro. Cytometry. 2006;69:1062–1070. doi: 10.1002/cyto.a.20338. [DOI] [PubMed] [Google Scholar]
- 30.Santos R., Palos-Ladeiro M., Besnard A., Porcher J.M., Bony S., Sanchez W. Relationship between DNA damage in sperm after ex vivo exposure and abnormal embryo development in the progeny of the three-spined stickleback. Reprod. Toxicol. 2013;36:6–11. doi: 10.1016/j.reprotox.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Satorre M.M., Breininger E., Beconi M.T. Cryopreservation with α-tocopherol and Sephadex filtration improved the quality of boar sperm. Theriogenology. 2012;78:1548–1556. doi: 10.1016/j.theriogenology.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Silva E.C.B., Cajueiro J.F.P., Silva S.V., Vidal A.H., Soares P.C., Guerra M.M.P. In vitro evaluation of ram sperm frozen with glycerol, ethylene glycol or acetamide. Anim. Reprod. Sci. 2012;132:155–158. doi: 10.1016/j.anireprosci.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen A.M. 4ª ed. American Press; Massachusetts: 1979. A laboratory for animal reproduction. [Google Scholar]
- 34.Spence R., Gerlach G., Lawrence C., Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 35.Swandulla D., Armstrons C.D. Calcium channel block by cadmium in chicken sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 1989;86:1736–1740. doi: 10.1073/pnas.86.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valença, R.M.B., Guerra, M.M.P., 2007. Espécies Reativas ao Oxigênio (ROS) e a utilização de antioxidantes na criopreservação do sêmen suíno. Revista Brasileira de Reprodução Animal, Belo Horizonte. 31, 47-53.
- 37.Varela Junior A.S., Corcini C.D., Gheller S.M.M., Jardim R.D., Lucia T., Jr., Streit D.P., Jr., Figueiredo M.R.C. Use of amides as cryoprotectants in extend-ers for frozen sperm of tambaqui, Colossoma macropomum. Theriogenology. 2012;78:244–251. doi: 10.1016/j.theriogenology.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Verstegen J., Iguer-Ouada M., Oclin K. Computer assisted sêmen analyzers in andrology research and veterinary practice. Theriogenology. 2002;57:149–179. doi: 10.1016/s0093-691x(01)00664-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.