Graphical abstract
Keywords: Cadmium (Cd), Oxidative stress, Lipid peroxidation, Nephrotoxicity, PAGE analysis
Abstract
This study was aimed to examine the protective effects of supplementation with calcium + zinc (Ca + Zn) or vitamin E (Vit-E) on Cd-induced renal oxidative damage. Young albino Wistar rats (180 ± 10 g) (n = 6) control rats, Cd, Cd + Ca + Zn, and Cd + Vit-E experimental groups and the experimental period was 30 days. Rats were exposed to Cd (20 mg/kg body weight) alone treated as Cd treated group and the absence or presence of Ca + Zn (2 mg/kg each) or Vit-E (20 mg/kg body weight) supplementation treated as two separate groups. The activities of the stress marker enzymes superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), glutathione-S-transferase (GST) and lipid peroxidase (LPx) were determined in renal mitochondrial fractions of experimental rats. We observed quantitative changes in SOD isoenzymatic patterns by non-denaturing PAGE analysis, and quantified band densities. These results showed that Cd exposure leads to decreases in SOD, CAT, GR, and GPx activities and a concomitant increase in LPx and GST activities. Ca + Zn and Vit-E administration with Cd significantly reversed Cd-induced perturbations in oxidative stress marker enzymes. However, Vit-E showed more inhibitory activity against Cd than did Ca + Zn, and it protected against Cd-induced nephrotoxicity.
1. Introduction
Disclosure to heavy metals is a routine experience due to their environmental pervasiveness. Studies on mammals revealed that cadmium (Cd) induces several renal injuries, mainly tubular dysfunction, marked reduction of renal energy metabolism. Although the exact mechanism of action of Cd-induced kidney damage is poorly understood, Cd is one of the most damaging heavy metal pollutants and affects almost all vital organs in animal systems. It has been claimed that one effect of Cd is the induction of oxidative stress in target vital organs. It was previously suggested that Cd exposure results in the generation of superoxide radicals, hydroxyl radicals and nitric oxide radicals, thereby altering antioxidant metabolism and eventually leading to oxidative stress [15], [46], [13]. Among the vital organs, kidneys are believed to be the most critical because after absorption, Cd binds to metallothionein and is filtered through the glomerulus into the injured space, where the metallothionein-Cd complex is endocytosed by the proximal tubule cells and degraded by lysosomes, resulting in the release of Cd. Then, the intracellular release of Cd decreases the levels of reduced glutathione, thereby altering the activity of glutathione-dependent antioxidant enzymes by changing the structure of the membrane cellular via lipid peroxidation [12], [25]. Currently, research related to improving the antioxidant status after Cd intoxication is of major importance [47], [20]. It is well known that endogenous antioxidants may play an imperative role in antioxidative defense against oxidative impairment, possibly by protecting the biological cells function [16]. Among a range of antioxidants, zinc (Zn) and calcium (Ca) play an important role in the regulation of the antioxidant system [35], [32], [31]. However, the effect of Zn and Ca on Cd intoxication in the kidneys has not yet been studied. Vitamin E (Vit-E) protects critical cellular structures against oxidative damage caused by oxygen free radicals and reactive products of lipid peroxidation. During the Cd exposure it has been reported that lipid peroxidation is prevented by Vit-E [27]. Vit-E inhibits the peroxidation of membrane lipids by hunting lipid peroxyl radicals, and this process converts it into a tocopheroxyl radical. In fact, α-tocopherylquinone may act as a potent an antioxidant through its reduction to hydroquinone [2]. Additionally, [6] reported that the protective role of Vit-E against the toxicity of oxidants may be due to the quenching of hydroxyl radicals. Therefore, this report addresses the oxidative stress marker enzymes that constitute an important part of antioxidant defense mechanisms in cellular systems and also examines whether the combination of Ca, Zn and/or Vit-E can reverse Cd-induced renal oxidative damage in rats using oxidative marker enzymes as oxidative defense biomarkers.
2. Materials and methods
2.1. Materials
The chemicals used in this study were purchased from Sigma Chemicals (St. Louis, MO, USA) and Merck (India).
2.2. Animal exposure
Male albino Wistar rats, aged 3 months (180 ± 20 g body weight) were used for the present study, rats were exposed to Cd salt sample (20 mg/kg body weight, each) LD50 for cadmium dosage the experimental rats were treated with Cd as Cdcl2 at the dose of 1/10th LD50 i.e. 20 mg/kg/BW, Ca + Zn salt sample (2 mg/kg) and Vit-E (20 mg/kg body weight) through daily oral gavage for a period of 30 days. Animals were housed at a constant temperature (28 ± 2 °C) and relative humidity 60 ± 10% with a 12-h light/dark cycle. Standard rat chow (Sri Venkateswara Traders, Bangalore, India) and water were made available ad libitum. All protocols for animal care and use were approved by the Animal Ethical Clearance Committee, Sri Venkateswara University (No. 01/2011-2012/(i)/a/CPCSEA/IAEC/SVU/MB-SSR/Dt 20/06/2011).
2.3. Estimation of antioxidant enzymes
Mitochondrial fractions of kidneys from different groups were isolated using differential centrifugation [10]. The brief protocol includes homogenization of kidney tissues in sucrose solution with a motor-driven Teflon and glass Potter-Elvehjem homogenizer. The homogenate was transferred into a centrifuge tube for differential centrifugation. The total volume was adjusted to 30 ml by adding 1 × MS homogenization buffer (0.65 M sorbitol, 5 mM MES, pH 5.5) and was then centrifuged at 1300 × g for 5 min. The pellet containing nuclei, unbroken cells, and large membrane fragments were discarded, and the supernatant was transferred into a clean centrifuge tube and centrifuged at 7000–17,000 × g for 15 min. The supernatant was discarded, and the pellet containing the mitochondrial fraction was washed by suspending the pellet in 1 × MS buffer and repeating the 7000 g–17,000 g sedimentation. Finally, the pellet containing the mitochondrial fraction was suspended in buffer and used for further analysis [10]. The activities of antioxidant enzymes, specifically superoxide dismutase (SOD) [28], catalase (CAT) [9], glutathione-S-transferase (GST) [17], glutathione reductase (GR) [41], and glutathione peroxidase (GPx) [36], and lipid peroxidase (LPx) [19] were estimated in the mitochondrial fractions and normal tissue fractions of kidneys. Assays for all the enzymes studied in this investigation were standardized in both experimental and control tissues by conducting preliminary tests to determine the optimal pH, temperature, enzyme concentration and substrate concentration; these optimal conditions were subsequently used for each enzyme assay. Appropriate control animals were utilized. The activities of enzymes in experimental tissue were compared with their activities in the appropriate control.
2.4. Analysis of SOD isoenzymes
Nitro blue tetrazolium (NBT) was used as a superoxide radical competitor and a color indicator to qualitatively locate SOD on polyacrylamide gels when cytochrome c was replaced by NBT. In the NBT negative-staining system, after gels were soaked with NBT and then riboflavin, exposing them to light caused the riboflavin to generate a superoxide radical flux in the presence of oxygen and TEMED. Meanwhile, NBT and SOD in the gels competed for the superoxide radicals. In the areas where SOD is present, the gel remains transparent, whereas in the areas without SOD, the gel becomes purple-blue due to reduced NBT. To separate SOD isoenzymes, non-denaturing PAGE was performed on 10% acrylamide slab mini gels (Mini Protean II, Bio-Rad). SOD isoenzymes were detected in the gels by a photochemical NBT staining method [4]. The different types of SODs were differentiated by performing activity stains in gels previously incubated for 20 min at 25 °C in 50 mM potassium phosphate buffer, pH 7.8, containing either 50 mM KCN or 5 mM H2O2. Cu/Zn-SOD was inhibited by KCN and H2O2, Fe-SOD was resistant to CN− but inactivated by H2O2, and Mn-SOD was resistant to both inhibitors [14].
2.5. Statistical results
The data obtained from samples were analyzed and are expressed as the mean ± SD. Data were analyzed by one-way analysis of variance (ANOVA) using the standard statistical software SPSS 16.0.1 to compare the effects among various groups. A probability value of 0.05 was used as the criterion for significance.
2.6. Histopathology examinations
kidneys tissue attained from each rat group were fixed in 10% buffered neutral formalin, dehydrated in ascending grades of alcohol, and embedded in paraffin. Sections approximately 5 μm thick were taken, stained with hematoxylin and eosin (H&E), and examined under a light microscope (Olympus, Japan).
3. Results
3.1. Superoxide dismutase activity
3.1.1. (a). Mn-SOD activity
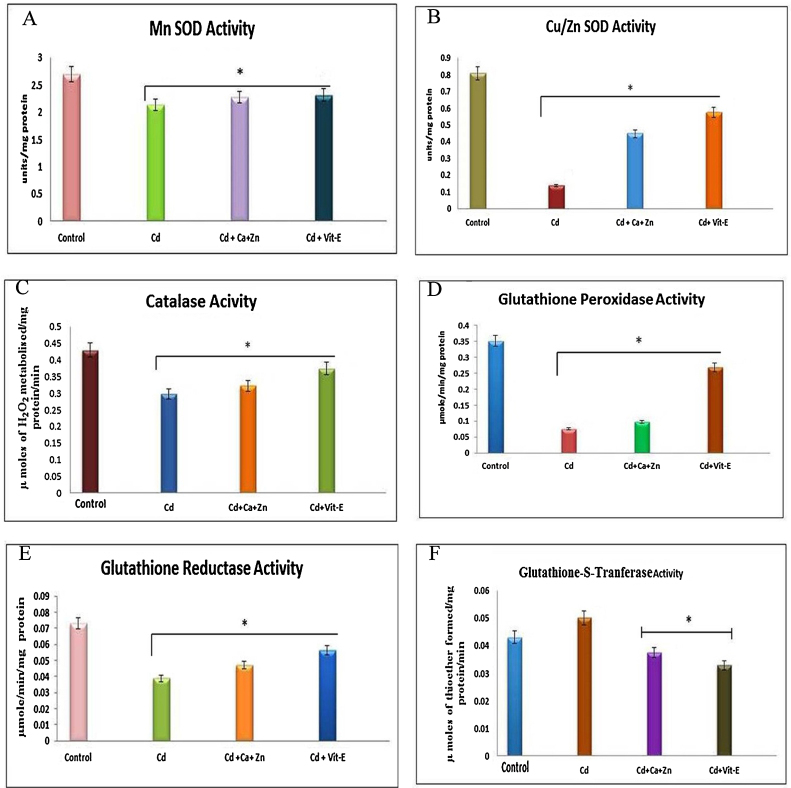
The specific activity of Mn-SOD was determined in kidney mitochondrial fractions from control, Cd-exposed, Cd + Ca + Zn-supplemented, and Cd + Vit-E supplemented rats. Cd exposure led to pronounced decreases in Mn-SOD activity (21% compared with the control). Supplementation with Ca + Zn and Vit-E ameliorated the effects induced by Cd exposure, causing 2.5 U/mg and 0.5 U/mg reductions in Mn-SOD activity compared with the control. Notably, greater recovery was observed with Vit-E supplementation compared with Ca + Zn supplementation (Fig. 1A).
Fig. 1.
A. Effects of Cd and cotreatment with Ca-Zn or Vit-E on oxidative defense enzymes in mitochondrial kidney fractions. Each bar represents the mean ± SD (n = 6). B. Effects of Cd and cotreatments on Cu/Zn-SOD activity in the kidneys. Each bar represents the mean ± SD (n = 6). C. Effects of Cd exposure on CAT activity in the kidneys. Each bar represents the mean ± SD (n = 6). D. Effects of Cd exposure on GPx activity in the kidneys. Each bar represents the mean ± SD (n = 6). E. Effects of Cd exposure on GR activity in the kidneys. Each bar represents the mean ± SD (n = 6). F. Effects of Cd exposure on GST activity in the kidneys. Each bar represents the mean ± SD (n = 6). *, Significant.
3.1.2. (b). Cu/Zn-SOD activity
The specific activity of Cu/Zn-SOD was determined in kidneys mitochondrial fractions from control rats, Cd-exposed rats, Cd-exposed/Ca + Zn-supplemented rats, and Cd-exposed/Vit-E-supplemented rats. Cd exposure produced a 0.1 mg protein decrease in Cu/Zn-SOD activity compared with the control. Supplementation with Ca + Zn and Vit-E ameliorated the effects of Cd exposure, causing 0.2 U/ mg and 0.4 U/mg reductions in Cu/Zn-SOD activity compared with the control, respectively. Greater recovery was observed with Vit-E supplementation than with Ca + Zn supplementation (Fig. 1B).
3.2. Catalase activity (CAT) activity
The specific activity of CAT was determined in kidneys mitochondrial fractions from control rats, Cd-exposed rats, Cd-exposed/Ca + Zn-supplemented rats, and Cd-exposed/Vit-E-supplemented rats. Cd exposure produced a 0.25 μ mg/protein decrease in CAT activity compared with the control. Supplementation with Ca + Zn and Vit-E ameliorated the effects of Cd exposure, causing 0.3 U/ mg/protein and 0.4 U/ mg/protein reductions in CAT activity compared with the control, respectively. Greater recovery occurred with Vit-E supplementation than with Ca + Zn supplementation (Fig. 1C).
3.3. Glutathione peroxidase activity (GPX) activity
The specific activity of GPx was determined in kidneys mitochondrial fractions from control rats, Cd-exposed rats, Cd-exposed/Ca + Zn-supplemented rats, and Cd-exposed/Vit-E-supplemented rats. Cd exposure decreased GPX activity by 0.06 μ mole/min/mg/protein compared with the control. Supplementation with Ca + Zn and Vit-E ameliorated the effects of Cd exposure, causing 0.10 U//mg/protein and 0.27 U/mg/protein reductions in GPx activity compared with the control, respectively. Greater recovery was observed with Vit-E supplementation than with Ca + Zn supplementation (Fig. 1D).
3.4. Glutathione reductase activity (GR) activity
The specific activity of GR was determined in kidney mitochondrial fractions from control rats, Cd-exposed rats, Cd-exposed/Ca + Zn-supplemented rats, and Cd-exposed/Vit-E-supplemented rats. GR activity decreased in Cd-exposed rats. Cd exposure decreased GR activity by 0.06 μ mol/ mg protein compared with the control. Supplementation with Ca + Zn and Vit-E ameliorated the effects of Cd exposure, causing 0.05 U/mg/protein and 0.06 U/mg/protein decreases GR activity levels compared with the control, respectively. Greater recovery was observed with Vit-E supplementation than with Ca + Zn supplementation (Fig. 1E).
3.5. Glutathione-S-transferase (GST) activity
The specific activity of GST was determined in kidney mitochondrial fractions from control rats, Cd-exposed rats, Cd-exposed/Ca + Zn-supplemented rats, and Cd-exposed/Vit-E-supplemented rats. GST activity was increased by 0.05 U/mg/protein/min in Cd-exposed rats compared with control rats. Supplementation with Ca + Zn and Vit-E decreased the GST activity to 0.04 U/mg/protein/min and 0.03 mol of U/mg/protein/min, respectively, of the levels observed during Cd exposure. Greater recovery was observed with Vit-E supplementation than with Ca + Zn supplementation (Fig. 1F).
3.6. Lipid peroxidase activity (LPX) activity
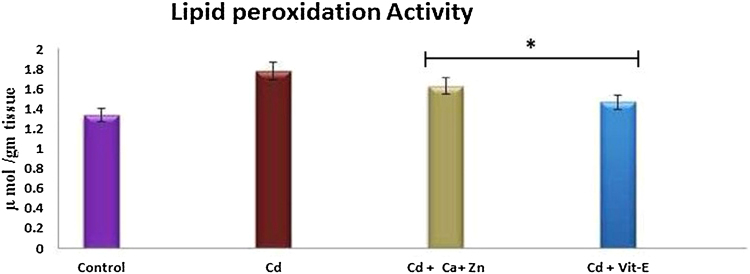
The specific level of LPx activity was determined in kidney mitochondrial fractions from control rats, Cd-exposed rats, Cd-exposed/Ca + Zn-supplemented rats, and Cd-exposed/Vit-E-supplemented rats. LPx activity increased in Cd-exposed rats. The LPX level was increased by 1.8 U/mg/protein/min in Cd-exposed rats compared with control rats. Supplementation with Ca + Zn and Vit-E ameliorated the effects of Cd exposure, causing increases of 1.6 U/mg/protein/min and 1.4 U/mg/protein/min, respectively, compared with the control. Notably, greater recovery was observed with Vit-E supplementation than with Ca + Zn supplementation (Fig. 2).
Fig. 2.
The effects of Cd and cotreatment with Ca-Zn or Vit-E on lipid peroxidation in mitochondrial kidney fractions. Each bar represents the mean ± SD (n = 6). *, Significant.
3.7. Superoxide dismutase (SOD) PAGE analysis
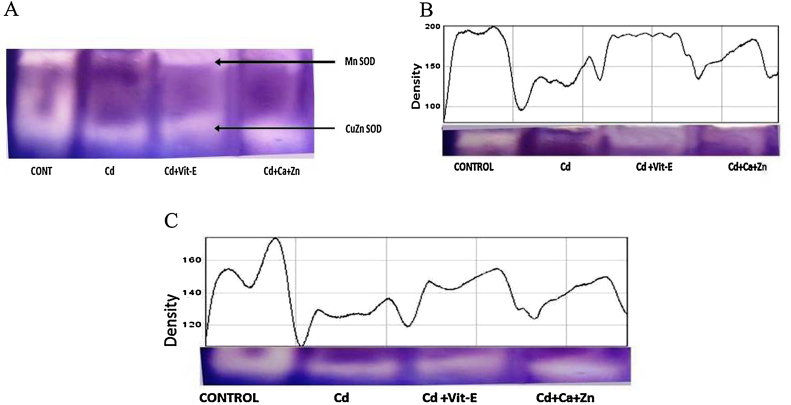
Quantitative changes in kidneys SOD isoenzyme patterns were found in all groups which showed two activity bands. After incubation with specific inhibitors, we determined that the slower migrating band corresponded to Mn-SOD while the faster migrating band corresponded to Cu/Zn-SOD, based on their respective increases in electrophoretic mobility (Fig. 3). The readings were proportional to the concentration of enzyme present in the sample. The band densities of Mn-SOD (140) and Cu/Zn-SOD (130) were lower in Cd-exposed rats than in control rats. Supplementation with Ca + Zn and Vit-E increased the band densities of Mn-SOD (170 and 190, respectively) and Cu/Zn-SOD (150 and 170, respectively) compared with Cd exposure alone. However, comparing the Ca + Zn-supplemented and Vit-E supplemented rats, Vit-E-supplemented rats showed higher band densities for both Mn-SOD and Cu/Zn-SOD (Fig. 3A–C).
Fig. 3.
A. PAGE profile of SOD isoenzymes (Mn-SOD, Cu/Zn-SOD) in the kidneys. Control, Cd-exposed, Cd + Ca + Zn supplementation, and Cd + Vit-E supplementation conditions are indicated as control, Cd, Cd + Ca + Zn and Cd + Vit-E, respectively. B. PAGE profile and densitometry graph of the Mn-SOD isoenzyme in the kidneys of all experimental groups. C. PAGE profile and densitometry graph of the Cu/Zn-SOD isoenzyme in the kidneys of all experimental groups.
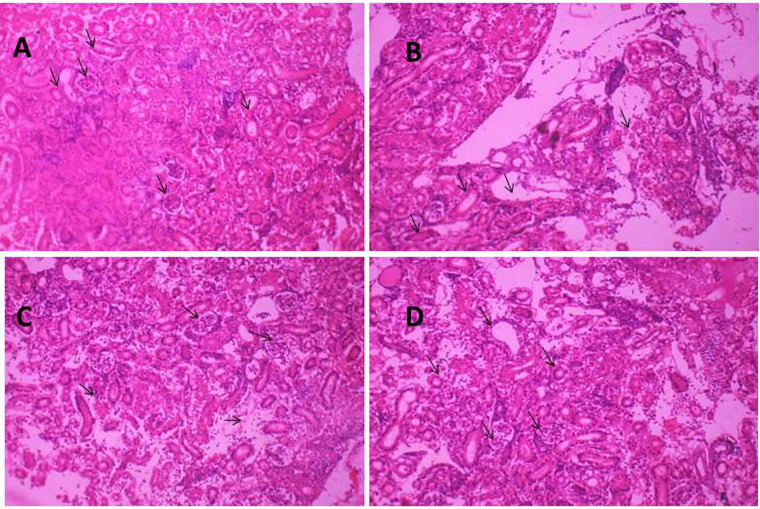
3.8. Histopathological changes of kidneys
Histopathology of the kidneys in the control rats showed a normal nephritic architecture with regular proximal convoluted tubule, distal convoluted tubule, and glomerular epithelial (Fig. 4A). Rats treated with Cd exhibited obvious kidneys lesions with swollen proximal convoluted tubule epithelium and glomerular epithelium, vacuolar formation, fibrosis of glomerulus, disappearance of part of the lumen, and severe necrosis in part of the renal tubular epithelial cells (Fig. 4B). Ca + Zn administration during Cd exposure markedly attenuated the Cd-induced histological changes of the kidneys and displayed slight cytoplasmic vacuolation and coagulative necrosis of the kidney (Fig. 4C). Vit-E alone administration during Cd exposure significantly exhibits the normal nephritic architecture as like in control(Fig. 4D).
Fig. 4.
A. Photomicrograph of longitudinal section of control rat kidneys (H and E) showing normal glomeruli with an intact Bowman's capsule, Proximal convoluted tubules, Distal convoluted tubules. The kidneys of the control group B. The kidneys of the Cd group showing severe degenerated structure of Cd intoxicated atrophied glomeruli, albuminous material in dilated urinary space, fibroblasts and perivascular edema. C. Effects of Ca + Zn administration on the ultrastructural changes in the kidneys of Cd-induced rats showing the recovery longitudinal section of Cd + Ca + Zn treated group. D. The kidneys of the Cd + Vit-E co-treated group showing a better recovery longitudinal section of Vit-E treated group.
4. Discussion
Cadmium, which is recognized as one of the most toxic environmental and industrial pollutants, is a ubiquitous toxic metal that induces oxidative damage by disturbing pro-oxidant/antioxidant balances in tissue. Cd induced oxidative damage was previously observed in rats treated with this metal [23], [48], [34]. The seriousness of intoxication be governed by on the route, dose, and duration of exposure to Cd [30], [42]. In cells, Cd mainly accumulates in the cytosol (70%), followed by the nucleus (15%), and small amounts are found in the mitochondria and endoplasmic reticulum [7]. However, administration of Cd with chemical and mineral or vitamin supplements was previously shown to diminish the toxic effects of Cd and increase the accumulation of Cd in the kidney [44]. Our data showed significant decreases in the activities of SOD, CAT, GPX, and GR with increased activity of LPX and GST. These data show that exposure to Cd decreases the activity of redox cycling antioxidant enzymes and rises lipid peroxidation in the kidneys of rats. This effect has been correlated with oxidative stress [1]. The activities of SOD and CAT were decreased in Cd-exposed rats and increased in rats given supplements. The decreased activities of SOD and CAT may be due to the concomitant increase in the generation of free radicals, in the kidneys of Cd-exposed rats. The interaction between Cd and essential trace elements may be one of the reasons for the decrease in antioxidant enzymes in the rat kidney because Cd can occupy the Zn site in Cu/Zn-SOD and creates inactive forms of the enzyme (Cu/Cd-SOD) [3]. However, the activity of Cu/Zn-SOD was increased in rats supplemented with Ca + Zn or Vit-E; this may occur because the supplements protect against the cytotoxicity of Cd, permitting the maintenance of the normal cellular redox balance by blocking free radical generation [35], [40]. Based on our experience and previous reports, SOD activity can be easily quantified. The isozymes of the SOD family can be separated by electrophoresis; under consistent conditions, their band intensities are proportional to the amount of SOD in the loaded sample [4]. Significant decreases in the activity of GPX in Cd-exposed rats may be due to the enhancement of peroxidative damage to polyunsaturated fatty acids, which would result in higher levels of lipid peroxidation in different tissues, or to the accumulation of ROS with subsequent development of kidney injury. This suggests that free radicals could be involved in the damage caused by long-term exposure to Cd [33]. Cd may reduce the efficiency of GPx only in part via its direct inhibitory effect on the enzyme, which indirectly leads to a shortage of the reduced glutathione and NADPH required for its activity [39]. However, changes in GPx activity caused by Ca-Zn or vitamin E supplementation with co-administration of Cd may occur because the supplements enhance the antioxidative defense system, thereby providing protection against Cd toxicity [38]. GR activity was reduced in Cd-exposed rats and increased in Ca-Zn-supplemented and Vit-E-supplemented rats. This may be due to the sensitivity of GR to Cd, even at low levels. GR was present in several tissues in which reduction of glutathione depends on reduction of peroxides. Cd exposure may slow the process of removing cytotoxic organic peroxides and cause damage to tissues [26]. However, GR activity was increased in naturally supplemented rats compared with chemically supplemented rats. This may be due to the ability of mineral and vitamin supplements to enhance the efflux and decrease the accumulation of Cd. The increased activity of GST in the kidney is consistent with our previous findings that exposure to Cd causes increased activity of GST in the kidney, liver, plasma, heart and skeletal muscle [23], [34], [30]. Other authors showed that Cd exposure increased the activity of this enzyme in kidney [8], [21]. The enzyme GST has an important role in the detoxification of xenobiotics, drugs and carcinogens and thus protects cells against redox cycling and oxidative stress [24], [8]. Cd roots significant increase in LPX concentrations in the rat kidneys and causes lipid peroxidation in numerous tissues [23], [37], [11], which suggests that Cd may persuade oxidative stress by producing hydroxyl radicals [29], superoxide ions, nitric oxide and H2O2 [22], [45]. The activity of LPX was significantly decreased in rats co-supplemented with chemical (Ca + Zn) and natural (Vit-E) supplements due to the effects of the antioxidant defense system, which protects cells from Cd-induced toxicity [38], [43], [8]. Thus, supplements have roles in maintaining the LPX level and in protecting the integrity and function of tissues [18]. The LPx content was much lower in rats supplemented with Vit-E, a natural supplement. This may be because Vit-E is a liposoluble antioxidant that has an important role in scavenging free radicals and stabilizing cell membranes, thereby maintaining membrane permeability. Therefore, Vit-E functions to break free radical chains, interfering with the initiation and progression of Cd-induced oxidative damage [5]. Moreover, it is known that antioxidants (e.g.,Vit-E) may act synergistically to prevent lipid peroxidation and cell destruction [11].
Histopathology results showed normal nephritic ultrastructure in the control and the Vit-E co-treated groups. The Cd and the Vit-E groups displayed the normal appearance of microvillus, smooth rounded nucleus, and undamaged mitochondria with regular structure (Fig. 4A) in the renal tubular epithelial cells. Cd treatment caused wide-ranging kidney injury with fractured microvillus and dilated cisternae of the smooth endoplasmic reticulum (SER), The glomerular epithelial cells showed nuclear membrane damage, nuclear chromatin condensation, and margination in Cd-induced kidneys (Fig. 4B).The Ca + Zn co-treated group displayed slight regenerative nephritic ultrastructure(Fig. 4C) The Cd + Vit-E co-treated group exhibited normal nephritic ultrastructure of mitochondria in the renal tubular epithelial cells as like in the control group (Fig. 4D).
5. Conclusions
In summary, our study explored how Cd exposure leads to significant decreases in the activities of SOD, CAT, GPx, and GR and to increases in the activities of LPx and GST. The decreased activities were due to the concomitant increases in the generation of free radicals in the kidney of Cd-exposed rats. Thus, Cd accumulation in the kidney, mainly increased the level of nascent oxygen species and enhanced the oxidative stress during the subsequent development of renal injury. The activities of the scavenger enzymes SOD, CAT, GPx, and GR were increased in Cd-exposed rats supplemented with Ca + Zn or Vit-E. This essential metals and vitamins can reduce the heavy metal burden by competing with Cd for intestinal absorption and prevent heavy metal induced tissue damage by competitive binding to active sites of the enzymes. These supplements protect against the cytotoxicity of Cd by maintaining the cellular redox balance via blockage of free radical generation. Histopathological and ultrastructural damages, and apoptosis in the rat kidneys observed in Cd induced group. Therefore, supplementation with Vit-E alone may have pronounced inhibitory effects against Cd-induced nephrotoxicity and regenerate structural changes of kidneys observed than Ca + Zn supplemented group rats.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We are very thankful to the UGC-BSR for providing the meritorious fellowship to carry out this work; we also thank our lab mates who encouraged us to carry out this work.
References
- 1.Amara S., Abdelmelek H., Garrel C., Guiraud P., Douki T., Ravanat J.L., Favier A., Sakly M., Ben Rhouma K. Influence of static magnetic field on cadmium toxicity: Study of oxidative stress and DNA damage in rat tissuesInfluence of static magnetic field on cadmium toxicity: study of oxidative stress and DNA damage in rat tissues. J. Trace Elem. Med. Biol. 2006;20:263–269. doi: 10.1016/j.jtemb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Arita M., Sato Y., Arai H., Inoue K. Binding of atocopherylquinone, an oxidized form of a-tocopherol, to glutathione S-transferase in the liver cytosol. FEBS Lett. 1998;436:424–426. doi: 10.1016/s0014-5793(98)01176-4. [DOI] [PubMed] [Google Scholar]
- 3.Bauer R., Demeter I., Hasemann V., Johansen J.T. Structural properties of the zinc site in Cu, Zn-superoxide dismutase; perturbed angular correlation of gamma ray spectroscopy on the Cu, 111Cd-superoxide dismutase derivative. Biochem. Biophys. Res. Commun. 1980;94:1296–1302. doi: 10.1016/0006-291x(80)90560-4. [DOI] [PubMed] [Google Scholar]
- 4.Beauchamp C.O., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 5.Beyer R.E. The role of ascorbate in antioxidant protection of biomolecules: interaction with vitamin E and coenzyme Q. J. Bioenerg. Biomembr. 1994;26:349–358. doi: 10.1007/BF00762775. [DOI] [PubMed] [Google Scholar]
- 6.Boldyrev A.A., Bulygina E.R., Volynskaya E.A., Kurella E.G., Tiulina O.V. The effect of hydrogen peroxide and hypochlorite on brain Na,K-ATPase activity. Biokhimiia. 1995;60:1688–1696. [PubMed] [Google Scholar]
- 7.Casalino E., Sblano C., Landriscina C. Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement. Arch. Biochem. Biophys. 1997;346:171–179. doi: 10.1006/abbi.1997.0197. [DOI] [PubMed] [Google Scholar]
- 8.Casalino E., Sblano C., Landriscina V., Calzaretti G., Landriscina C. Rat liver glutathione S transferase activity stimulation following acute cadmium or manganese intoxication. Toxicology. 2004;200:29–38. doi: 10.1016/j.tox.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Chance B., Machly A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- 10.Clayton D.A., Shadel G.S. Cold Spring Harb Protoc; 2014. Isolation of mitochondria from tissue culture cellsIsolation of Mitochondria from Tissue Culture Cells. pdb.prot080002. [DOI] [PubMed] [Google Scholar]
- 11.El-Demerdash F.M., Yousef M.I., Kedwany F.S., Baghdadi H.H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem. Toxicol. 2004;42:1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 12.El-Sharaky A.S., Newairy A.A., Badreldeen M.M., Eweda S.M., Sheweita S.A. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology. 2007;235:185–193. doi: 10.1016/j.tox.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Eriyamremu G.E., Ojimogho S.E., Asagaba S.O., Lolodi O. Changes in brain, liver, and kidney lipid peroxidation, antioxidant enzymes and ATPases of rabbits exposed to cadmium ocularly. J. Biol. Sci. 2008;8:67–73. [Google Scholar]
- 14.Fridovich I. Superoxide Dismutase. In: Meister A., editor. Advances in Enzymology and Related Areas of Molecular Biology. Wiley; New York: 1986. pp. 61–97. [DOI] [PubMed] [Google Scholar]
- 15.Galan C., Garcia B.L., Troyano A., Vilaboa N.E., Fernandez C., Blas D.E. The role of Intracellular oxidation in death induction (apoptosis and necrosis) in human promonocytic cells treated with stress inducers (cadmium, heat, x-rays) Eur. J. Cell Biol. 2001;80:312–320. doi: 10.1078/0171-9335-00159. [DOI] [PubMed] [Google Scholar]
- 16.Gunther M.R. Probing the free radicals formed in the metmyoglobin-hydrogen peroxide reaction. Free Radic. Biol. Med. 2004;36:1345–1354. doi: 10.1016/j.freeradbiomed.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formationGlutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7713. [PubMed] [Google Scholar]
- 18.Halliwell B., Gutteridge J.M.C. third edition. Oxford University Press; 1999. Free Radicals in Biology and Medicine. [Google Scholar]
- 19.Hiroshi O. Assay for Lipid peroxides in animal tissue by Thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa H., Wang X., Konishi T. Role of component herbs in antioxidant activity of shengmai an- a traditional Chinese medicine formula preventing oxidative damage in rat. Am. J. Chin. Med. 2003;31:509–521. doi: 10.1142/S0192415X03001193. [DOI] [PubMed] [Google Scholar]
- 21.Iscan M., Coban T., Eke B.C. Differential combined effect of cadmium and nickel on hepatic and renal glutathione S-transferase of the guinea pig. Environ. Health Perspect. 1994;102:69–72. doi: 10.1289/ehp.94102s969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koizumi T., Shirakura G., Kumagai H., Tatsumoto H., Suzuki K.T. Mechanism of cadmium-induced cytotoxicity in rat hepatocytes: cadmium-induced active oxygenrelated permeability changes of the plasma membrane. Toxicology. 1996;114:124–134. doi: 10.1016/s0300-483x(96)03477-4. [DOI] [PubMed] [Google Scholar]
- 23.Kostic M.M., Ognjanovic B., Dimitrijevic S., Zikic R.V., Stajn A., Rosic G.L. Cadmium induced changes of antioxidant and metabolic status in red blood cells of rats: in vivo effects. Eur. J. Haematol. 1993;51:86–92. doi: 10.1111/j.1600-0609.1993.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 24.Matés M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 25.Matovic V., Buha A., Dukic-Cosic D., Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015;78:130–140. doi: 10.1016/j.fct.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Mercurio S.D., Combs G.F., Jr. Selenium-dependent glutathione peroxidase inhibitors increase toxicity of pro-oxidant compounds. J. Nutr. 1986;116:1726–1734. doi: 10.1093/jn/116.9.1726. [DOI] [PubMed] [Google Scholar]
- 27.Meydani M. Vitamin E. Lancet. 1995;345:170–175. doi: 10.1016/s0140-6736(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 28.Misra H.P., Fridovich I. The role of superoxideanion in the autooxidation of epinephrine and simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 29.O'brien P., Salasinski H.J. Evidence that the reactions of cadmium in the presence of metallothionein can produce hydroxyl radicals. Arch. Toxicol. 1998;72:690–700. doi: 10.1007/s002040050562. [DOI] [PubMed] [Google Scholar]
- 30.Ognjanović B., Žikić R.V., Štajn A., Saičić Z.S., Kostić M.M., Petrović V.M. The effects of selenium on the antioxidant defense system in the liver of rats exposed to cadmium. Physiol. Res. 1995;44:293–300. [PubMed] [Google Scholar]
- 31.Ozdemir G., Inanc F. Zinc may protect remote ocular injury caused by intestinal ischemia reperfusion in rats. Tohoku J. Exp. Med. 2005;206:247–251. doi: 10.1620/tjem.206.247. [DOI] [PubMed] [Google Scholar]
- 32.Ozturk A., Baltaci A.K., Bediz C.S., Mogulkoc R., Gungor S. Effects of zinc and melatonin deficiency on testicular tissue of rats. Biol. Trace Elem. Res. 2003;96:255–262. doi: 10.1385/BTER:96:1-3:255. [DOI] [PubMed] [Google Scholar]
- 33.Patra R.C., Swarup D., Senapati S.K. Effects of cadmium on lipid peroxides and superoxide dismutase in hepatic, renal and testicular tissue of rats. Vet. Hum. Toxicol. 1999;41:65–67. [PubMed] [Google Scholar]
- 34.Pavlović S.Z. Antioxidant defense system in skeletal muscle of rats treated with cadmium A possible protective role of coenzyme Q10. Jugoslav. Med. Biochem. 2001;20:229–235. [Google Scholar]
- 35.Powell S.R. The antioxidant properties of zinc. J. Nutr. 2000;130:1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 36.Rotruck J.T. Selenium: biochemical roles as a component of glutathione peroxidase. Science. 1973;9:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar S., Yadov P., Bhatnagar D. Lipid peroxidative damage on cadmium exposure and alterations in antioxidant system in rat erythrocytes: A study with relation to timeLipid peroxidative damage on cadmium exposure and alterations in antioxidant system in rat erythrocytes: a study with relation to time. BioMetals. 1998;11:153–157. doi: 10.1023/a:1009286130324. [DOI] [PubMed] [Google Scholar]
- 38.Shaikh Z.A., Vu T.T., Zaman K. Oxidative stress as a mechanism of chronic cadmium induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999;154:256–263. doi: 10.1006/taap.1998.8586. [DOI] [PubMed] [Google Scholar]
- 39.Shukla G.S., Chandra S.V. Cadmium toxicity and bioantioxidants: status of vitamin-E and ascorbic acid of selected organs in rat. J. Appl. Toxicol. 1989;9:119–122. doi: 10.1002/jat.2550090209. [DOI] [PubMed] [Google Scholar]
- 40.Souza V., Escobar Mdel C., Bucio L., Hernandez E., Gutierrez-Ruiz M.C. Zinc pretreatment prevents hepatic stellate cells from cadmium-produced oxidative damage. Cell Biol. Toxicol. 2004;20:241–251. doi: 10.1023/b:cbto.0000038462.39859.2f. [DOI] [PubMed] [Google Scholar]
- 41.Staal G.E.J., Veeger C. The reaction mechanism of glutathione reductase from human erythrocytes. Biochim. Biophys. Acta. 1969;185:49–62. doi: 10.1016/0005-2744(69)90281-2. [DOI] [PubMed] [Google Scholar]
- 42.Stajn A., Zikić R.V., Stajn A., Saicić Z.S., Kostić M.M., Petrović V.M. Effect of cadmium and selenium on the antioxidant defense system in rat kidneys. Comp. Biochem. Physiol. 1997;117:167–172. doi: 10.1016/s0742-8413(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 43.Tandon S.K., Singh S., Prasad S., Khandekar K., Dwivedi V.K., Chatterjee M., Mathur N. Reversal of cadmium induced oxidative stress by chelating agent, antioxidant or their combination in rat. Toxicol. Lett. 2003;145:211–217. doi: 10.1016/s0378-4274(03)00265-0. [DOI] [PubMed] [Google Scholar]
- 44.Wahba Z.Z., Coogan T.R., Rhodes S.W., Waalkes M.P. Protective effects of selenium on cadmium toxicity in rats − Role of altered toxicokinetics and metallothionein. J. Toxicol. Environ. Health. 1993;38:171–182. doi: 10.1080/15287399309531710. [DOI] [PubMed] [Google Scholar]
- 45.Waisberg M., Joseph P., Hale B., Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis: a review. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe M., Henmi K., Ogwa K., Suzuki T. Cadmium-dependent generation of reactive oxygen species and mitochondrial DNA breaks in photosynthetic and nonphotosynthetic strains of Euglena gracitis. Comp. Biochem. Physiol. C. 2003;134:227–234. doi: 10.1016/s1532-0456(02)00253-3. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W., Yu B.Y., Kou J.P., Wang J.R. Studies on the correlation between toxicity and activities of realgar. Chin. J. Nat. Med. 2004;2:123–125. [Google Scholar]
- 48.Zikic R.V., Stajn A.S., Ognjanović B.I., Saicić Z.S., Kostić M.M., Pavlović S.Z., Petrovic V.M. The effect of cadmium and selenium on the antioxidant enzyme activities in rat heart. J. Environ. Pathol. Toxicol. Oncol. 1998;17:259–264. [PubMed] [Google Scholar]