Abstract
There is a growing concern on heavy metals in consumer products due to their potential human health risks and environmental effects. In this study, the levels of zinc, cadmium, lead and nickel were assessed in 3 different classes of personal care products commonly used in Ibadan, Nigeria. Samples were analysed for heavy metals using Atomic Absorption Spectrophotometer (AAS) after acid digestion. Estimated daily intake (EDI) of the metals and Health Risk Index (HRI) were calculated to assess the human health risks associated with the use of these PCPs. The concentrations (mg/kg) of zinc ranged from 3.75 to 19.3, 1.88 to 112,000 and 19.8 to 217 respectively in creams, powders and eyeliners. Cadmium ranged from ND—0.50, ND—36.3 and ND—0.50 mg/kg while lead ranged from ND—6.25, ND—468 and 3.73–27.5 mg/kg and nickel ranged from ND—6.25, 0.13–107 and 2.75–22.7 mg/kg respectively. There were high concentrations of Cd, Pb and Ni in some of the samples when compared with the available permissible limits in cosmetics (Cd: 0.3 ppm, Pb: 10 ppm and Ni: 0.6 ppm while there is no permissible limit for Zn in cosmetics currently available). Prolonged use of PCPs may pose human health and environmental risks due to toxic metal loading through dermal contact and accumulation over a period of time. Hence, the need for necessary government agencies to regulate and enforce toxic metals in consumer products including cosmetics produced and imported into Nigeria to safeguard public health and the environment, which is the final sink.
Keywords: Heavy metals, Personal care products, Health effects, Dermal contact, Exposure
1. Introduction
The quest for physical beauty by more people has promoted the use of personal care products (PCPs) globally. Millions of people use one or two types of PCPs and their ingredients on a daily basis [1]. This, of course, exposes them to different chemicals contained in the PCPs via direct skin contact which often involves exposure of a large area of the body. This is coupled with prolonged duration of contact, which may occur due to repeated use of the products [33]. There are different types of PCPs which include lipstick and lip gloss (used to colour the lips); powder and rouge (used to colour the face, lighten and remove flaws); mascara, eyeliners and eyeshadow (used to colour the eyelids); nail polish (used to colour the fingernails and toenails) and different types of both moisturizing and lightening/toning creams [58].
Studies have described human exposure to heavy metals from different sources in Nigeria [63], [54], [6], [53], [38], [48], [47], [68], [4], but little is known about human exposure to heavy metals contained in PCPs [11], [2], [8], [44], [5], [46], [24], [52], [69], [27] and the associated human health risks. Data on the exposure of humans to metal toxins through dermal contact is very scanty. There is equally a paucity of data on the concentrations of cadmium, nickel, lead, and zinc in PCPs commonly available and used in Ibadan, Nigeria and their potential human health risk. At high concentrations, some of the metals may damage the internal body organs in animals and humans. Various forms of mammalian cancers, respiratory diseases, organ failure and learning disabilities had been traced to metal poisoning [39], [40], [32]; and [59].
Chronic exposure to cadmium causes kidney dysfunction and high levels of exposure will result in death [18], [55], [3], [26], [29]. Zinc has been reported to cause the same illness as lead [36], and can cause other health problems such as stomach cramps, vomiting, nausea, skin irritation, and anemia [55]. Other health effects associated with cadmium, copper and zinc poisoning includes gastrointestinal disorder, diarrhea, stomatities, tremor, ataxia, paralysis, vomiting and convulsion, depression, and pneumonia [37], [22], [39]. Nickel is a human carcinogen that can lead to kidney problems, gastrointestinal distress, pulmonary fibrosis and skin dermatitis [18].
Lead can easily cross the placenta of mothers and damage the brain of fetuses. Children exposure to lead and mercury can result in learning difficulties, memory impairment, damage to the nervous system, and behavioural problems such as aggressiveness and hyperactivity [49], [56]. The nervous systems of children are especially sensitive to Pb [15], [7], and can damage the kidney, liver, reproductive system, basic cellular processes and brain functions. Symptoms include anemia, insomnia, headache, dizziness and irritability, weakness of muscles, hallucination and renal damages [3], [42].
Studies have shown that there is an association between some ingredients of PCPs and various health problems like cancer [31], renal and kidney damages [40], [20], nephrotic syndrome or impaired renal function, neurological, nephrological and dermatological damage in fetuses [21]. Physiological changes can also alter the bioaccumulation of these metals in human body [36]. Exogenous ochronosis, impaired wound healing and wound dehiscence, fish odour syndrome, nephropathy, steroid addiction syndrome, predisposition to infections, broad spectrum of cutaneous and endocrinologic complications of corticosteroids, including suppression of hypothalamic-pituitary-adrenal axis [51] are some of the complications linked to some of the ingredients in PCPs especially the skin lightening ones.
There are currently no stringent regulations to control the levels of heavy metals in consumer products manufactured and sold in Nigeria and no national permissible limits are presently available. Hence, this study assesses the levels of lead, cadmium, nickel and zinc in three different categories of personal care products (i.e., creams, powders and eyeliners) commonly available and used in Ibadan, Nigeria. It evaluates the human health risks associated with using the PCPs by comparing the levels of the metals in the products with the available international permissible limits so as to estimate the risks associated with human exposure and to assess the characteristics of the samples such as moisture and ash contents.
2. Materials and methods
2.1. Sample collection and preservation
Thirty five (35) different samples of PCPs from different manufacturers commonly used in Ibadan, Nigeria were collected randomly by purchasing them in their sealed form from six (6) major markets in Ibadan, Oyo State. The PCPs collected were 20 body creams and lotions, 10 powders and 5 eyeliners (Table 1, Table 2). After purchase, prior to analysis, the samples were kept in a cool dry place as there was no special preservation method for the samples.
Table 1.
List of some PCPs (creams and powders) commonly used in Ibadan, Nigeria.
| S/N | Sample code | Names of PCPs | Names of manufacturer | Country of manufacture | NAFDAC reg. No | Date of manufacture | Expiry date | Quantity in pack |
|---|---|---|---|---|---|---|---|---|
| Creams | ||||||||
| 01 | 01CC | Skin perfection | Zenon Laboratories and Chemicals Ind. Ltd. | Lagos, Nigeria | 02−1587 | April 2014 | April 2017 | 150 mL |
| 02 | 02CC | Balila Beauty cream | Geydekosoun Nig. Ltd. | Lagos, Nigeria | 02−3831 | March 2014 | March 2017 | 150 mL |
| 03 | 03CC | Cussons baby Jelly | PZ cussons Nig. Plc | Ibadan, Nigeria | 02−3811 | March 2014 | February 2017 | 90 g |
| 04 | 04CC | Caro white | Dream cosmetic | Abidjan 23, Cole de’ voire | LOT: 13/7606:13 | February 2014 | February 2016 | 50 mL |
| 05 | 05CC | Authentic herbal cream | Bright Future Hope Ent. | Nigeria | 04-8617L | April 2014 | March 2018 | |
| 06 | 06CC | Peau Claire | SIVOP perfume and cosmetiques | Lagos, Nigeria | 02−7756 | March 2014 | March 2016 | 150 mL |
| 07 | 07CC | Baby and me Jelly | Aspira Nig. Ltd. | Kano, Nigeria | 02−5979 | February 2013 | February 2018 | 150 g |
| 08 | 08CC | Zenon cocoa butter body cream | Zenon Laboratories and Chemical Industries | Lagos, Nigeria | 02−5642 | May 2014 | May 2017 | 150 g |
| 09 | 09CC | Cocoderm | Sivop Perfume and Cosmetics | Lagos, Nigeria | 02−6990 | May 2014 | May 2016 | 300 mL |
| 10 | 10CC | Clair- Liss | Sivop Perfume and Cosmetics | Lagos, Nigeria | 02−1510 | June 2014 | June 2016 | 150 mL |
| 11 | 11CC | Vaseline blue seal | Unilever South Africa (pty) Ltd. | South Africa | 02−0385 | September 2013 | September 2016 | 50 mL |
| 12 | 12CC | Tony Montana | Gey de Kognogn Nigeria | Lagos, Nigeria | 02−1310 | July 2014 | July 2016 | 150 g |
| 13 | 13CC | Cacatin Herbal Antiseptic Cream | Iayus Investment Ltd. | Lagos, Nigeria | 04−8701 | August 2014 | July 2017 | 50 g |
| 14 | 14CC | Nouveaute | Gey de Kognogn Nigeria | Lagos, Nigeria | 02−1291 | June 2014 | June 2017 | 75 g |
| 15 | 15CC | Dettol | Cybele Cosmetic Ltd. | Lagos, Nigeria | 02−6942 | May 2014 | May 2016 | 40 g |
| 16 | 16CC | Drya’s Sporting Wave Hair Cream | Nigeria | 1B-0832L | November 2012 | November 2016 | 115 g | |
| 17 | 17CC | Zoleen Hair Cream | Jabman Industries Ltd. | Lagos, Nigeria | 02−3209 | August 2011 | July 2016 | 50 g |
| 18 | 18CC | Golden Perfume Baby Jelly | Ebesun Company Nig. Ltd. | Lagos, Nigeria | 02-0029L | March 2014 | June 2016 | 80 g |
| 19 | 19CC | Debby Baby Jelly | Ejyson Ind. Ltd. | Ibadan, Nigeria | November 2012 | November 2014 | 80 g | |
| 20 | 20CC | Sweet mother Jelly | Stico laboratory Ltd. | Lagos, Nigeria | 02−1739 | November 2013 | November 2017 | 80 g |
| Powders | ||||||||
| 01 | 01PP | Passion Talcum powder | Cybele cosmetics Ltd. | Lagos, Nigeria | 02−1434 | May 2014 | May 2017 | 30 g |
| 02 | 002PP | April Beauty Talc | Negatrex Nig. Ltd. | Lagos, Nigeria | 02−6634 | March 2014 | March 2017 | 100 g |
| 03 | 003PP | Mp3Cool Refreshing powder | Imperio Int. Ltd. | Lagos, Nigeria | 02−5729 | May 2014 | May 2017 | 100 g |
| 04 | 04PP | Salga Cool Refreshing Talcum powder | Imperio Int. Ltd. | Lagos, Nigeria | 02−8492 | May 2014 | May 2017 | 100 g |
| 05 | 05PP | MYK flower face powder | MYK cosmetics Ltd. | Lagos, Nigeria | July 2014 | June 2017 | 50 g | |
| 06 | 06PP | Balila powder | Grey de Kourou GDK | Lagos, Nigeria | 02−3830 | April 2014 | April 2017 | 50 g |
| 07 | 07PP | Rising Raving baby powder | Rising Ventures | Edo state, Nigeria | 004 | April 2014 | March 2018 | 100 g |
| 08 | 08PP | MYK Lilo baby powder | MYK cosmetics Ltd. | Lagos, Nigeria | 02−6599 | August 2014 | July 2017 | – |
| 09 | 09PP | POP facial powder | Corporative Beauty Nig. Ltd. | Nigeria | 30 g | |||
| 10 | 10PP | Euchauteur powder | UNZA Singapore Ltd. | Malaysia | 02.1392 | December 2013 | December 2016 | 125 g |
Note: Reg. = registration.
Table 2.
List of PCPs (eyeliners) commonly used in Ibadan, Nigeria.
| S/N | Sample code | Name of PCPs | Country of manufacture |
|---|---|---|---|
| 01 | 01EE | Davis | Nigeria |
| 02 | 02EE | VIC Mary | Nigeria |
| 03 | 03EE | Absolute | Nigeria |
| 04 | 04EE | Fair maid eye | Nigeria |
| 05 | 05EE | Air woman | Nigeria |
2.2. Sample pre-treatment and analysis
Eyeliners were ground to powder to increase the surface areas and to increase their susceptibility to reaction with the mixture of acids (nitric acid and hydrochloric acid) used in the digestion. Sub-sample errors were reduced by grinding and sieving the samples to <2 mm which enhanced sample homogenization and also assisted in complete dissolution. All the samples were digested using Q–block wireless digester system. Solid samples like eyeliners were dried in an oven at 105 °C for 2 h to remove moisture and to obtain a constant weight and then cooled in a desiccator. Creamy samples liable to charring were dried at 70–80 °C. About 0.5 g each of the dried samples was weighed and 10 mL aqua regia added. The samples were digested for 30 min in the Q-block digester at 150 °C and the digested samples were allowed to cool to room temperature and then filtered. The filtrates were made up to mark with distilled water and then analysed for lead (Pb), cadmium (Cd) nickel (Ni) and zinc (Zn) using a Buck Scientific 210/211 VGP AAS Model 220 GF version 3.94C. 50% duplicate analysis was considered. An inter-laboratory analysis was carried out on some of the samples to ascertain the accuracy and reliability of the results especially those samples that the concentrations of some of the metals were too high or too low. Recovery study was also carried out.
2.3. Health risk assessment
The adverse health effects of the metals in the PCPs considered in this study were estimated by calculating the estimated daily intake of the metals using the formula:
Where; EDI is the estimated daily intake, C is the mean concentration of the metals obtained in this study, and W is the average human body weight (70 kg) [23], [61], [50], [3].
The human health risks posed by heavy metal exposure in PCPs considered in this study were estimated using the Health Risk Index (HRI), which was calculated by dividing the estimated daily intake by the dermal reference doses of the metals. Hazard indices must be less than 1 in order for it not to pose any health hazards. When the hazard index values exceed 1, there may be concerns for potential health risks associated with over exposure [57], [61], [50], [3], [66].
2.4. Statistical analysis
The data obtained in this study were subjected to statistical analysis using Microsoft Office Excel (8). Coefficient of Variation (CV) was used to show the distribution patterns of the heavy metals in the PCPs. CV were calculated using the formula:
where; SD is the standard deviation.
Also, Analysis of Variance (ANOVA) was used to compare the mean concentrations of the metals between the classes of PCPs.
3. Results and discussion
3.1. Concentrations of heavy metals in personal care products (PCPs) commonly available and used in Ibadan, Nigeria
The results of heavy metals determined in this study (Cd, Pb, Ni and Zn) are presented in Table 3, Table 4, Table 5. There were great variations in the concentrations of these heavy metals in the PCPs considered in this study. The range of zinc concentrations were 3.75–19.3 mg/kg, 1.88–112,000 mg/kg and 19.8–217.0 mg/kg in the creams, powders and eyeliners with mean concentrations of 8.11 ± 4.3, 11,715 ± 35,270 and 90.5 ± 77 mg/kg, respectively (Table 6). The concentrations in some of the creams were 11.9 mg/kg, 15.3 mg/kg, 16.5 mg/kg and 19.3 mg/kg respectively in samples 12CC, 03CC, 10CC and 04CC while some of the powder samples had very high concentrations of zinc. The values were 114 mg/kg (5PP), 4890 mg/kg (1PP) and 112,000 mg/kg (7PP). Among the selected eyeliners, sample 1EE had the highest concentration of zinc (217 mg/kg). The order of Zn in the samples are powders > eyeliners > creams. Zinc was present in all the samples and its concentrations were generally high. There is no national and international guidelines and limits for this element in cosmetics and PCPs generally. Thus, one could not ascertain the health impact of this element on the consumers. However, accumulation over time is likely to have negative impacts on humans. Some of the human health effects associated with zinc exposure includes inhibition of oxygen and calcium transportation in the body; inhibition of nerve transmission in the brain, reduced IQ and learning disabilities, intrauterine fatal death, premature delivery and low birth weight. In the workplace environment, zinc contamination can lead to a flu-like condition known as metal fever. This condition will pass after two days and is caused by over sensitivity. Zinc can pose a danger to unborn and newborn which may be exposed through mother’s blood or milk [39].
Table 3.
Concentrations (mg/kg) of selected heavy metals in some cream samples commonly used in Ibadan, Nigeria.
| S/N | Sample codes | Zn | Cd | Pb | Ni |
|---|---|---|---|---|---|
| 1 | 1CC | 7.25 | 0.25 | 0.25 | 3.25 |
| 2 | 2CCa | 6.75 ± 0.0 | ND | 5.00 ± 0.0 | ND |
| 3 | 3CC | 16.5 | ND | ND | 1.75 |
| 4 | 4CCa | 19.3 ± 0.25 | 0.13 ± 0.25 | 6.25 ± 0.18 | 5.00 ± 0.0 |
| 5 | 5CC | 8.25 | 0.25 | ND | 6.25 |
| 6 | 6CCa | 4.50 ± 0.0 | ND | 0.13 ± 0.18 | 2.50 ± 0.0 |
| 7 | 7CC | 7.50 | ND | ND | 4.00 |
| 8 | 8CCa | 8.75 ± 0.25 | ND | 2.50 ± 0.0 | 3.63 ± 0.18 |
| 9 | 9CC | 3.75 | 0.500 | ND | 0.75 |
| 10 | 10CCa | 15.3 ± 0.25 | 0.50 ± 0.0 | ND | 2.63 ± 0.18 |
| 11 | 11CC | 5.25 | ND | 2.50 | 2.50 |
| 12 | 12CCa | 11.9 ± 0.13 | ND | 5.00 ± 0.54 | 2.50 ± 0.35 |
| 13 | 13CC | 5.50 | ND | 2.50 | 1.50 |
| 14 | 14CCa | 6.13 ± 0.13 | 0.25 ± 0.0 | 0.13 ± 0.18 | 4.50 ± 0.0 |
| 15 | 15CC | 6.25 | ND | 5.00 | ND |
| 16 | 16CCa | 5.50 ± 0.25 | ND | 5.00 ± 0.00 | 1.50 ± 0.35 |
| 17 | 17CC | 5.75 | ND | 5.00 | ND |
| 18 | 18CCa | 5.50 ± 0.0 | 0.13 ± 0.18 | 5.00 ± 0.0 | 0.13 ± 0.18 |
| 19 | 19CC | 6.75 | ND | 5.00 | ND |
| 20 | 20CCa | 5.88 ± 0.13 | 0.13± 0.18 | 2.61 ± 0.18 | ND |
| Range | 3.75–19.3 | ND—0.50 | ND—6.25 | ND—6.25 |
Note: ND = Not Detected.
Mean concentrations and standard deviation of duplicate analysis.
Table 4.
Concentrations (mg/kg) of selected heavy metals in some powder samples commonly used in Ibadan, Nigeria.
| S/N | Sample code | Zn | Cd | Pb | Ni |
|---|---|---|---|---|---|
| 1 | 1PPa | 4890 ± 130 | 0.13 ± 0.2 | 6.25 ± 1.8 | 4.63 ± 2.7 |
| 2 | 2PP | 22.8 | 0.25 | 2.50 | 0.50 |
| 3 | 3PPa | 20.3 ± 2.8 | 0.25 ± 0.0 | 3.75 ± 1.8 | 2.75 ± 0.4 |
| 4 | 4PP | 24.8 | ND | 2.50 | 4.25 |
| 5 | 5PP | 114 | ND | 2.50 | 3.25 |
| 6 | 6PPa | 26.5 ± 3.5 | ND | 5.0 ± 3.5 | 4.88 ± 0.5 |
| 7 | 7PP | 112,000 | ND | 468 | 5.75 |
| 8 | 8PPa | 33.9 ± 0.1 | 36.3 ± 0.0 | 5.0 ± 0.0 | 0.13 ± 0.2 |
| 9 | 9PP | 14.0 | ND | ND | 107 |
| 10 | 10PPa | 1.88 ± 0.1 | ND | 0.13 ± 0.2 | 1.50 ± 0.0 |
| Range | 1.88–112,000 | ND—36.3 | ND—468 | 0.13–107 |
Note: ND = Not Detected.
Mean concentrations and standard deviation of duplicate analysis.
Table 5.
Concentrations (mg/kg) of selected heavy metals in some eyeliner commonly used in Ibadan, Nigeria.
| S/N | Sample code | Zn | Cd | Pb | Ni |
|---|---|---|---|---|---|
| 1 | 1EEa | 217 ± 18 | 0.25 ± 0.4 | 5.0 ± 0.0 | 22.6 ± 2.7 |
| 2 | 2EEa | 38.4 ± 2.1 | ND | 3.75 ± 1.8 | 8.63 ± 0.9 |
| 3 | 3EE | 19.8 | 0.50 | 5.00 | 2.75 |
| 4 | 4EE | 92.0 | ND | 27.5 | 10.3 |
| 5 | 5EE | 85.5 | ND | 5.25 | 6.50 |
| Range | 19.8–217 | ND—0.50 | 3.75–27.5 | 2.75–22.6 |
Note: ND = Not Detected.
Mean concentrations and standard deviation of duplicate analysis.
Table 6.
Comparison of the mean concentrations (mg/kg), standard deviation (SD) and Coefficient of variation (CV) of heavy metals in the different products.
| Metals | Concentrations (mean ± SD in mg/kg) (CV) |
||
|---|---|---|---|
| Creams (n = 20) | Powders (n = 5) | Eyeliners (n = 5) | |
| Zn | 8.11 ± 4.3 (53) | 11,715± 35,270 (301) | 90.5 ±77 (85.1) |
| Cd | 0.11 ± 0.2 (153) | 3.69 ± 11 (310) | 0.15 ± 0.2 (149) |
| Pb | 2.59 ± 2.4 (90.7) | 49.6 ±147 (297) | 9.3 ± 10 (110) |
| Ni | 2.12 ± 1.9 (89.3) | 13.5 ± 33 (245) | 10.2 ± 7.5 (73.9) |
The range of cadmium concentrations in the three categories of PCPs were ND—0.50 mg/kg, ND—36.3 mg/kg and ND—0.50 mg/kg in the creams, powders and eyeliners with an average concentrations of 0.11 ± 0.2 mg/kg, 3.69 ± 11 mg/kg and 0.15 ± 0.2 mg/kg respectively (Table 6). The results obtained in this study were compared with the available international permissible limits as there are no national guidelines on metals in cosmetics in Nigeria. The values obtained in this study were above the permissible limits of 0.3 ppm by the WHO (Table 7). 10% of the creams and powders and 20% of the eyeliners were above the permissible limits. The PCPs considered in this study, which are below the permissible limits, are unlikely to present any health risk. Those above the limits may cause harm to the consumers due to the accumulation over time through dermal exposure thus a significant source of cadmium loading to the users. The order of cadmium concentrations in the samples were powders > eyeliners > creams. Skin absorption of Cd is rare [62] but when absorbed into the body, cadmium accumulates in the kidney and the liver. Although cadmium can be found in almost all adult tissues, the total amount absorbed by humans has been estimated to be between 0.2–0.5 μg/day, with absorption via skin estimated to be 0.5% [28]. Cadmium and cadmium compounds are considered to be carcinogenic to humans by the IARC and are also classified as known human carcinogens by the United States Department of Health and Human Services.
Table 7.
Comparison of concentrations of selected heavy metals obtained in this study with international allowable limits in PCPs.
| Zn | Cd | Pb | Ni | |
|---|---|---|---|---|
| This study: Creams (mg/kg) Powders (mg/kg) Eyeliners (mg/kg) |
8.11 ± 4.3 11,715 ± 35,270 90.5 ± 77 |
0.11 ± 0.2 3.69 ± 11 0.15 ± 0.2 |
2.59 ± 2.4 49.6 ± 147 9.3 ± 10 |
2.12 ± 1.9 13.5 ± 33 10.2 ± 7.5 |
| WHO Health Canada |
– | 0.3 ppm 3 ppm |
10 ppm 10 ppm |
– – |
| EU US |
– – |
0.5 ppm 0.08 ppm |
0.5 ppm 1.0 ppm |
– 0.6 ppm |
| Germany | – | 0.1 ppm | 1.0 ppm | – |
The concentrations of Pb in the samples ranged from ND—6.25 mg/kg, ND– 467 mg/kg and 3.75–27.5 mg/kg with mean concentrations of 2.59 ± 2.4 mg/kg, 49.6 ± 147 mg/kg and 9.3 ± 10 mg/kg in the creams, powders and eyeliners respectively (Table 6). The results obtained were compared with the available international limits of 10 ppm in cosmetics by WHO (Table 7). 100%, 90% and 80% of the creams, powders, and eyeliners considered in this study were below the permissible limits, thus they are unlikely to pose any risk to the consumer through dermal exposure. The order of lead concentrations in the samples were powders > eyeliners > creams. Lead is a neurotoxin that is found in cosmetics, plastics, batteries, gasoline, insecticides, pottery glaze, soldered pipes, and paints [13]. In the body, lead will either accumulate in the tissues, especially bones, and also in the livers, kidneys, pancreas, and lungs. Pregnant women and young children are particularly vulnerable because lead can cross the placenta with ease and enter the fetal brain [17]. Lead can also be transferred to infants via breast feeding and lead stored in the bone is a source of fetal lead exposure [35]. After immediate exposure, humans are able to get rid of 50% of the lead within two to six weeks, but it takes 25–30 years to get rid of 50% of lead that has accumulated in the body over time [45].
No safe blood level of lead is known, with even the lowest levels having shown to affect the fetus and the central nervous system in children [41]. Small amounts are recognized as being hazardous to human health. Infants, toddlers, children, fetuses, and pregnant women are most susceptible to its chronic low-dose effects [17]. Chronic low-level exposure may affect the kidneys, cardiovascular systems, blood, immune systems, and especially the central and peripheral nervous systems Agency of Toxic Substances and Disease Registry [9]. IQ deficits have been associated with both low and high blood lead levels. Lead exposure has also been linked to miscarriage, hormonal changes, reduced fertility in men and women, menstrual irregularities, delays in puberty onset in girls, memory loss, mood swings; nerve, joint and muscle disorders; cardiovascular, skeletal, kidney and renal problems [31]. Lead and inorganic lead compounds have been classified as possible and probable carcinogens to humans Agency of Toxic Substances and Disease Registry [10]. Exposure to lead results in damage to cognitive development in children and damage to the nervous system. The use of leaded eye powders (Sumar, Kohl, Alkol and Tiro) has been associated with increased blood- lead levels in children and women [39], [67].
Nickel concentrations in the creams ranged from ND—6.25 mg/kg, 0.13–107 mg/kg in the powder and 2.75–22.3 mg/kg in the eyeliners with mean concentrations of 2.12 ± 1.9 mg/kg, 13.5 ± 33 mg/kg and 10.2 ± 7.5 mg/kg respectively (Table 6). When the results were compared with the 0.6 ppm permissible limits in cosmetics by the U.S (Table 7), 70%, 80%, and 100% of the creams, powders and eyeliners were above the permissible limits and hence likely to cause health risk to the consumers from dermal exposure. The order of nickel concentrations in the samples are powders > eyeliners > creams. Nickel is naturally occurring element and may be an essential element in humans. Fetal exposures can occur, which can be passed to breast-feeding infants [16]. High levels of exposure can lead to health effects depending on the route and the kind of nickel exposed to [60]. Nickel represents the main cause of contact dermatitis and minimal amount of other toxic metals can trigger pre-existing allergy. Nickel dermatitis produces erythema, eczema and lichenification of the hands and other areas of the skin that is in contact with nickel [52]. The first case of Ni allergy caused by eyeshadow has been reported and 1 ppm of it may trigger a pre-existing allergy [60]. The primary targets of toxicity appear to be the respiratory tract following inhalation exposure, the immune system following inhalation, oral, or dermal exposure; and possibly the reproductive system following oral exposure. Based on the mean heavy metal concentrations, the order of metals in all the products are
| Creams and powders: Zn > Pb > Ni > Cd. |
| Eyeliners: Zn > Ni > Pb > Cd. |
3.2. Comparison of metal levels in this study with those from previous works
The concentration of zinc (13.8–19.3 mg/kg) in creams obtained in this study was lower than 0.646–32.8 mg/kg obtained by [64] and higher than 0.136–0.355 mg/kg reported by [8] while 1.88–112000 mg/kg obtained in powders was higher than 1.82–1070 mg/kg reported by [64], and 19.8–217 mg/kg in eyeliners was higher than 72.00–128.50 mg/kg reported by [44] (Table 8). The range of cadmium (ND—0.50 mg/kg) obtained in this study was higher than 0.041–0.058 mg/kg reported by [64], and 0.016–0.082 mg/kg reported by Onwardi et al. (2011). ND—36.3 mg/kg obtained in powders analysed in this study was higher than 0.258–0.360 mg/kg reported by [64] and ND—8.1 mg/kg reported by [43]. ND—0.50 mg/kg obtained in eyeliners was lower than 0.3–1.8 mg/kg reported by [44] and ND—0.266 mg/kg reported by [14] (Table 8). Also, the range of lead (ND—6.25 mg/kg) obtained in the creams was higher than 1.741–3.708 mg/kg reported by [64] while ND—468 mg/kg obtained in powders was higher than 2.325–3.975 mg/kg reported by [64] and 0.4–41 mg/kg reported by [43]. The 3.75–27.5 mg/kg in eyeliners was lower than 66.4–213.6 mg/kg reported by [15]. The range of nickel concentrations, ND—6.25 mg/kg, in the creams was higher than 0.258–0.308 mg/kg reported by [64] while 0.13–107 mg/kg in the powders was higher than 0.72–1.425 mg/kg reported by [64] and 2.75–22.6 mg/kg in eyeliners was lower than 78.00–325.2 mg/kg reported by [44] (Table 8).
Table 8.
Comparative values (mg/kg) of heavy metals in different cosmetic products from different countries with similar work reported in the literature.
| Product name | Origin | Pb | Cd | Ni | Zn | References |
|---|---|---|---|---|---|---|
| Creams | Nigeria | ND—6.25 | ND—0.50 | ND—6.25 | 3.75–19.3 | This study |
| Powders | Nigeria | ND—468 | ND—36.3 | 0.13–107 | 1.88–112,000 | This study |
| Eyeliners | Nigeria | 3.75–27.5 | ND—0.50 | 2.75–22.6 | 19.8–217 | This study |
| Facial powder | Nigeria | 0.4–4.1 | ND—8.1 | – | – | [43] |
| Medicated cream | Nigeria | – | 0.063–0.361 | – | 0.539–1.104 | [8] |
| Non- Medicated cream | Nigeria | – | 0.016–0.082 | – | 0.136–0.355 | [8] |
| Hair cream | Nigeria | – | 0.279–0.781 | – | 0.531–0.811 | [8] |
| Eyeliner | Nigeria | 66.4–213 | 0.3–1.8 | 78.0–325.2 | 72.0–128.8 | [44] |
| Eye pencil | Nigeria | 66.0–187.1 | 0.5–1.1 | 4.9–21.5 | 36.3–198.7 | [44] |
| Cream | Nigeria | ND | ND- 1.33 | 1.82−8.43 | – | [52] |
| Mascara Eye Shade |
Saudi Arabia Saudi Arabia |
ND—2.18 4.41–11.9 |
0.002−0.035 14.4–37.3 |
5–31.4 6.01−46.8 |
ND—63.1 38.6–2000 |
[12] [12] |
| Lipsticks | Japan, China, Pakistan | 2.58–11.53 | 0.2–0.430 | 0.696–1.610 | 0.696–1.610 | [64] |
| Powder | Pakistan | 2.325–3.975 | 0.258–0.36 | 0.72–1.425 | 1.818–1067 | [64] |
| Cream Lipstick Powder Lipstick Eye pencil Lipstick |
Pakistan India Nigeria Nigeria Nigeria Pakistan |
1.741–3.708 ND—42.0323 0.10 ND-0.10 ND-2.10 1.10–48.0 |
0.041–0.058 ND—0.0246 ND—0.02 0.03–0.07 0.03–0.05 1.49–3.72 |
0.258–0.308 ND—1.0626 – – – 2.20–3.64 |
0.646–32.83 – – – – – |
[64] [19] [24] [24] [24] [34] |
3.3. Results of risk assessment
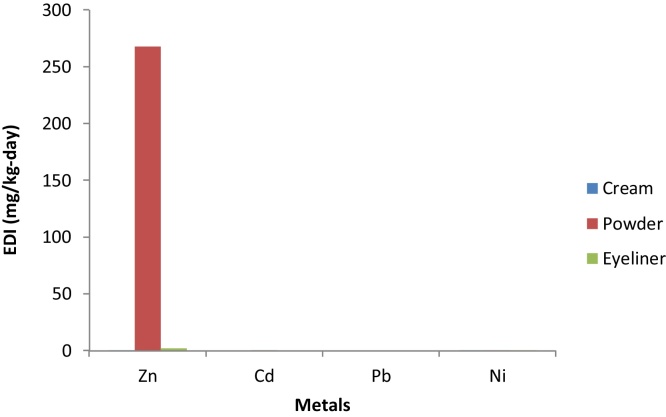
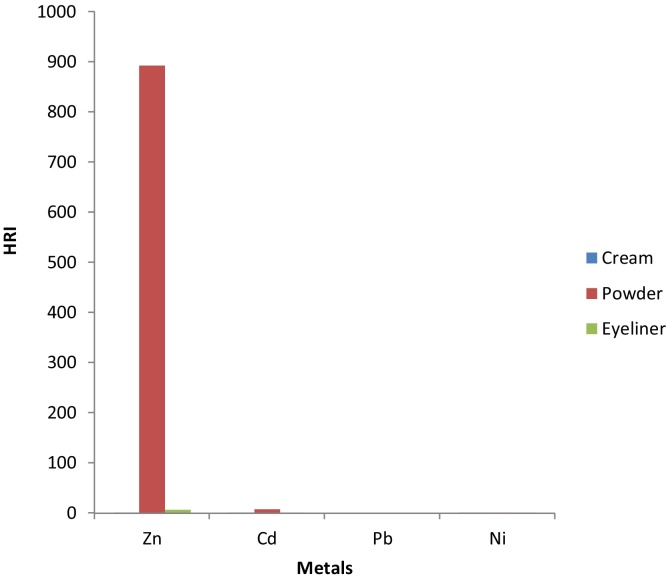
The estimated daily intake (EDI) of the metals from the use of the PCPs considered in this study is presented in Fig. 1. The EDI values ranged from 7.3 × 10−6- 268 mg/day and were very low in all the metals in all the categories of the PCPs considered in this study except Zn whose highest EDI was 268 mg/day in powders. The order of the metals in the PCPs are Zn > Cd >Ni as EDI was not calculated for Pb. Hazard risk indices (HRI) must be less than 1 for it not to pose any health hazard. When the hazard index values exceed 1, there may be concerns for potential health risks associated with over exposure [57]. The HI obtained in this study ranged from 8.52 × 10−2-893. The order of HRI followed the same trend as observed in EDI as shown in Fig. 2. The HRI values estimated for Zn was 893, which exceeded 1 and calls for urgent concern.
Fig. 1.
Estimated Daily Intake of Metals via Dermal Exposure from PCPs commonly used in Ibadan.
Fig. 2.
Health Risk Index (HRI) of Metals.
3.4. Results of statistical analysis
The coefficient of variation (CV) was used to explain the distribution patterns of heavy metals in the three different classes of PCPs (i.e., creams, powders and eyeliners) commonly used in Ibadan. The higher the CV values, the greater the uneven distributions of the parameters under consideration [3]. Thus, in the creams, the CV values ranged from 52.6 in Zn to 153 in Cd, and 245 in Ni to 310 in Cd in the powders and 73.9 in Ni to 149 in Cd in the eyeliners (Table 6). These showed that there are no guidelines and or strict regulations in the use of different raw materials in the production of these PCPs. There were great variations in the concentrations of heavy metals in the products hence the uneven distribution of the metals, especially Cd and Pb. One-way ANOVA results showed that there were no significant differences between and within groups (Table 9).
Table 9.
Results of One-way ANOVA of the PCPs Commonly Used in Ibadan.
| Sum of Squares | df | Mean Square | F | Sig. | ||
|---|---|---|---|---|---|---|
| Zn | Between Groups (Combined) | 976178850.386 | 2 | 488089425.193 | 1.395 | 0.262 |
| Within Groups | 11195583174.114 | 32 | 349861974.191 | |||
| Total | 12171762024.500 | 34 | ||||
| Cd | Between Groups (Combined) | 91.420 | 2 | 45.710 | 1.237 | 0.304 |
| Within Groups | 1182.159 | 32 | 36.942 | |||
| Total | 1273.579 | 34 | ||||
| Pb | Between Groups (Combined) | 16247.295 | 2 | 8123.648 | 1.235 | 0.304 |
| Within Groups | 210456.935 | 32 | 6576.779 | |||
| Total | 226704.231 | 34 | ||||
| Ni | Between Groups (Combined) | 936.719 | 2 | 468.360 | 1.492 | 0.240 |
| Within Groups | 10047.191 | 32 | 313.975 | |||
| Total | 10983.910 | 34 | ||||
3.5. Percentage ash and moisture contents of personal care products
The percentage ash content of the PCPs considered in this study is shown in Table 10. The percentage ash content of the creams ranged from 1.14–7.10% while it was 91.6%–99.2% in the powders and 11.7–19.2% in the eyeliners. The percentage ash indicates the levels of inorganic minerals present in the samples. Hence, the results showed that powder with the highest percentage ash content (91.59–99.2%) has a large percentage of inorganic components therein. The percentage moisture content in the creams ranged from 0.10–87.8%, 0.36%–1.20% in the powders and 9.05%–16.7% in the eyeliners (Table 10). The results showed that creams have the highest water content, followed by powders and eyeliners.
Table 10.
Percentage Ash and Moisture Content of PCPs Commonly Used in Ibadan, Nigeria.
| S/N | Sample code | Ash content Mean ± SD | Moisture content Mean ± SD |
|---|---|---|---|
| 1 | 01PP | 91.6 ± 0.01 | 0.55 ± 0.01 |
| 2 | 02PP | 97.5 ± 0.1 | 1.20 ± 0.0 |
| 3 | 03PP | 94.9 ± 0.1 | 0.95 ± 0.1 |
| 4 | 04PP | 96.2 ± 0.2 | 1.10 ± 0.1 |
| 5 | 05PP | 97.6 ± 0.01 | 0.45 ± 0.1 |
| 6 | 06PP | 95.9 ± 0.2 | 0.40 ± 0.01 |
| 7 | 07PP | 95.9 ± 0.1 | 0.36 ± 0.01 |
| 8 | 08PP | 96.4 ± 0.1 | 0.99 ± 0.01 |
| 9 | 09PP | 98.6 ± 0.9 | 0.96 ± 0.1 |
| 10 | 10PP | 99.2 ± 0.1 | 1.04 ± 0.02 |
| 11 | 01CC | 4.4 ± 0.1 | 12.2 ± 0.1 |
| 12 | 02CC | 6.06 ± 0.1 | 17.2 ± 0.1 |
| 13 | 03CC | 3.74 ± 0.2 | 14.7 ± 0.1 |
| 14 | 04CC | 5.95 ± 0.1 | 15.7 ± 0.01 |
| 15 | 05CC | 4.16 ± 0.1 | 17.2 ± 0.01 |
| 16 | 06CC | 5.15 ± 0.1 | 10.2 ± 0.02 |
| 17 | 07CC | 7.1 ± 0.1 | 12.3 ± 0.02 |
| 18 | 08CC | 4.99 ± 0.01 | 9.74 ± 0.01 |
| 19 | 09CC | 5.65 ± 0.1 | 11.3 ± 0.01 |
| 20 | 10CC | 6.76 ± 0.1 | 13.3 ± 0.01 |
| 21 | 11CC | 1.48 ± 0.04 | 0.47 ± 0.02 |
| 22 | 12CC | 1.58 ± 0.03 | 82.0 ±0.01 |
| 23 | 13CC | 2.40 ± 0.1 | 87.8 ± 0.01 |
| 24 | 14CC | 2.37 ± 0.02 | 79.4 ± 0.1 |
| 25 | 15CC | 2.00 ± 0.1 | 0.71 ± 0.01 |
| 26 | 16CC | 1.15 ± 0.1 | 2.60 ± 0.02 |
| 27 | 17CC | 1.40 ± 0.02 | 0.52 ± 0.02 |
| 28 | 18CC | 3.67 ± 0.01 | 0.12 ± 0.01 |
| 29 | 19CC | 1.79 ± 0.1 | 4.10 ± 0.1 |
| 30 | 20CC | 1.62 ± 0.1 | 3.78 ± 0.1 |
| 31 | 01EE | 15.1 ± 0.1 | 16.7 ± 0.01 |
| 32 | 02EE | 13.6 ± 0.2 | 15.9 ± 0.1 |
| 33 | 03EE | 19.2 ± 0.1 | 9.05 ± 0.1 |
| 34 | 04EE | 11.7 ± 0.1 | 13.1 ± 0.01 |
| 35 | 05EE | 16.9 ± 0.1 | 16.4 ± 0.6 |
Note: PP = powder, CC = cream, EE = eyeliner
3.6. Results of recovery study of heavy metals and validation
In order to ascertain the efficiency of the analytical results, recovery study was carried out for lead and cadmium in two of the cream samples (01CC and 02CC). This was achieved by spiking the two cream samples with known concentrations of pure standards. The spiked samples were then passed through the analytical procedure and analysed to determine the concentrations of the metals. The results showed 98% and 91% recovery for Pb and Cd respectively in 01CC and 92% and 95% respectively in 02CC. The high results obtained in some of the products were validated using inter-laboratory analysis as shown in Table 11. There were no significant differences in the results obtained from the different laboratories at p < 0.05.
Table 11.
Inter-laboratory comparison of outrageous results.
| S/N | sample codes | Concentration of zinc in the samples in (mg/kg) |
Concentration of cadmium in the samples in (mg/kg) |
Concentrations of lead in the samples in (mg/kg) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LAB 1 | LAB.2 | LAB.3 | LAB.1 | LAB.2 | LAB.3 | LAB.1 | LAB.2 | LAB.3 | ||
| 1 | 01pp | 4890 | 4750.5 | 2204.5 | 0.13 | ND | 0.13 | 6.23 | ND | 17.375 |
| 2 | 02pp | 114 | 39.75 | 81.75 | ND | ND | 0.13 | 2.5 | ND | 3.375 |
| 3 | 07pp | 112000 | 103750.8 | 41872.6 | ND | ND | 40.75 | 468 | 508.5 | 364.63 |
| 4 | o8pp | 33.9 | 234 | 69 | 36.3 | 25 | 0.38 | 5 | ND | 10.375 |
4. Conclusion
This study investigated the levels of heavy metals such as lead, cadmium, zinc, and nickel in Personal Care Products commonly used in Ibadan, Nigeria. In some of the PCPs, the levels of the metals were higher than the available permissible limits and reported studies. The metal concentrations in the samples were in the order: powder > eyeliners > creams while the order of metals in creams and powders were: Zn > Pb > Ni > Cd, and Zn > Ni > Pb > Cd in the eyeliners. This study revealed that human exposure to heavy metals is not only from food, water, mining activity, toys, plastics, jewelry, but also from personal care products. Continuous use of personal care products may result in an increase in the trace metal levels in the ocular system and the human body. The use of personal care products may be considered as a source of toxic metal poisoning. Though, the metals are from the contamination of raw materials and use of sub-standard raw materials, lack of compliance by small scale manufacturers, and lack of strict regulations. Currently, there are no national regulations and permissible limits of heavy metals and other toxins in most consumer products including personal care products in Nigeria. This study therefore emphasize the need to monitor the presence of these toxins in personal care products commonly used in Nigeria to avert heavy metal poisoning through dermal exposure to humans especially the vulnerable groups such as children and women. Further studies on heavy metals in personal care products are recommended and should include other toxic metals such as aluminium and mercury, among others, in different categories of PCPs commonly used in Nigeria to prevent associated human health risks.
Contributor Information
Sunday Samuel Omenka, Email: s.omenka@gmail.com.
Adebola Abosede Adeyi, Email: aa.oketola@ui.edu.ng, bolaoketola@yahoo.com.
References
- 1.Abdel A.F., Pingitor N.E. Low levels of toxic elements in dead sea black mud and smud derived cosmetic products. Environ. Geochem. Health. 2009;31(4):487–492. doi: 10.1007/s10653-008-9201-x. [DOI] [PubMed] [Google Scholar]
- 2.Adebajo S.B. An epidemiological survey of the use of skin lightening cosmetics among traders in Lagos, Nigeria. West Afr. J. Med. 2002;21(1):51–55. [PubMed] [Google Scholar]
- 3.Adekunle A.S. Speciation study of the heavy metals in commercially available recharge cards coatings in Nigeria and the health implication. Toxicol. Rep. 2014;1:243–251. doi: 10.1016/j.toxrep.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adekunle A.S. Removal of heavy metals from industrial effluents by water hyacinth (Eichornia crassipes) J. Environ. Chem. Ecotoxicol. 2012;4(11):203–211. [Google Scholar]
- 5.Adepoju-Bello A.A., Oguntibeju O. Evaluation of the concentration of toxic metals in cosmetic products in Nigeria. Afr. J. Biotechnol. 2012;11(3):1630–1664. [Google Scholar]
- 6.Adeyi A.A., Torto N. Profiling heavy metal distribution and contamination in soil of old power generation station in Lagos, Nigeria. Am. J. Sci. Technol. 2014;1(1):1–10. [Google Scholar]
- 7.Adeyi A.A., Omidiran O.M., Osibanjo O. Assessment of soil contamination of a cattle market around river ogun basin, Isheri, Nigerian. In: Maria C., Soriano Hernandez, editors. Environmental Risk Assessment of Soil Contamination. Intech Publisher; 2014. pp. 225–255. Chapter 7 Croatia ISBN: 978-953-51-1235-8. [Google Scholar]
- 8.Ayenimo J.G., Yusuf A.M., Adekunle A.S., Makinde O.W. Heavy metal exposure from personal care products. Bull. Environ. Contam. Toxicol. 2010;84:8–14. doi: 10.1007/s00128-009-9867-5. [DOI] [PubMed] [Google Scholar]
- 9.Agency of Toxic Substances and Disease Registry ATSDR . U.S. Department of Health and Human Services. Public Health Service; 2005. Toxicological Profile for Lead. [Google Scholar]
- 10.Agency of Toxic Substances and Disease Registry ATSDR . U.S. Department of Health and Human Services. Public Health Service; 2008. Toxicological Profile for Cadmium. [Google Scholar]
- 11.Ajayi S.O., Oladipo M.O., Ogunsayi H.O., Adebayo A.O. Determination of the minor and trace elements in biriniwa tin pyrite and ornamental lead/zinc ore using neutron activation analysis. Bull. Chem. Soc. Ethiop. 2002;16(2):207–211. [Google Scholar]
- 12.Al-Dayel O., Hefne J., Al-Ajyan T. Human exposure to heavy metals from cosmetics. Orient. J. Chem. 2011;27(1):1–11. [Google Scholar]
- 13.Ali A.R., Smales O.R., Aslam M. Surma and lead poisoning. Br. Med. J. 1978;2:915–916. doi: 10.1136/bmj.2.6142.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Saleh I.A., Coate L. Lead exposure in Saudi Arabia from the use of traditional cosmetics and medical remedies. Environ. Geochem. Health. 1995;170(1):29–31. doi: 10.1007/BF00188629. [DOI] [PubMed] [Google Scholar]
- 15.Al-Saleh I., Al-Enazi S., Shinwari N. Assessment of lead in cosmetics products. Regul. Toxicol. Pharm. 2009;54:105–113. doi: 10.1016/j.yrtph.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Bakeller D.A., Lally L., Bontinck W.J. Nickel, cobalt and chromium in consumer products; a role in allergic. Contact Dermatitis. 1993;28(1):15–25. doi: 10.1111/j.1600-0536.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 17.Bellinger D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- 18.Borba C.E., Guirardello R., Silva E.A., Veit M.T., Tavares C.R.G. Removal of nickel(II) ions from aqueous solution by biosorption in a fixed bed column: experimental and theoretical breakthrough curves. Biochem. Eng. J. 2006;30:184–191. [Google Scholar]
- 19.Chauhan S.B., Chandak A., Agrawal S.S. Evaluation of heavy metals contamination in marketed lipsticks. Int. J. Adv. Res. 2014;2(4):257–262. [Google Scholar]
- 20.Clarkson T.W., Magos L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 21.Counter S.A., Buchanan L.H. Mercury exposure in children: a review. Toxicol. Appl. Pharmacol. 2004;198:209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Duruibe J.O., Ogwuegbu M.O.C., Egwurugwu J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007;2:112–118. [Google Scholar]
- 23.EBRC, Health Risk Assessment Guidance for Metals. Indirect Exposure via the Environment and Consumer Exposure, Fact sheet 03 (2007).
- 24.Ekere N.R., Ihedioha J.N., Oparanozie1 T.I., Ogbuefi-Chima F.I., Ayogu J. Assessment of some heavy metals in facial cosmetic products. J. Chem. Pharm. Res. 2014;6(8):561–564. [Google Scholar]
- 26.Eltaweel Y., Nassef E.M., Hazza R. Recovery of copper from wastewater by cementation technique. World Environ. 2014;4(5):199–205. [Google Scholar]
- 27.Faruruwa M.D., Bartholomew S.P. Study of heavy metals content in facial cosmetics obtained from open markets and superstores within Kaduna Metropolis, Nigeria. Am. J. Chem. Appl. 2014;1(2):27–33. [Google Scholar]
- 28.Ferro V.H., Carpenter S.J. Tetratogenic effects of cadmium and its inhibition by zinc. Nature. 2000;216:112. doi: 10.1038/2161123a0. [DOI] [PubMed] [Google Scholar]
- 29.Fu F., Wang Q. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manag. 2011;92:407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert S.G., Weiss B. A rationale for lowering the blood lead action level from 10 to 2 mg/dl. Neurotoxicology. 2006;27:693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu W., Chen Y., Huang B., Niedermann S. Health risk assessment of heavy metals in soils and vegetables from a typical greenhouse vegetable production system in China. Hum. Ecol. Risk Assess. 2014;20(5):1264–1280. [Google Scholar]
- 33.IPCS International Programme on Chemical Safety . vol. 242. WHO; 2014. (Dermal Exposure. Environmental Health Criteria). [Google Scholar]
- 34.Khalid A., Bukhari I.H., Riaz M., Rehman G., Ain Q.U., Bokhari T.H., Rasool N., Zubair M., Munir S. Determination of lead, cadmium, chromium, and nickel in different brands of lipsticks. Int. J. Biol. Pharm. Allied Sci. 2013;2(5):1003–1009. [Google Scholar]
- 35.Koller K., Brown T., Spurgeon A., Levy L. Recent developments in low- level lead exposure and intellectual impairment in children. Environ. Health Perspect. 2004;112:987–994. doi: 10.1289/ehp.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liden C., Skare L., Vauter M. Assessment of skin exposure to nickel, chromium and by acid wipe sampling and LCP-MS. Contact Dermatitis. 2006;54(5):233–238. doi: 10.1111/j.0105-1873.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu S., Hammond S.K., Rojas A. Concentrations and potential health risks of metals in lip products. Environ. Health Perspect. 2013;l12:705–710. doi: 10.1289/ehp.1205518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majolagbe A.O., Adeyi A.A., Osibanjo O. Vulnerability assessment of groundwater pollution in the vicinity of an active dumpsite (Olusosun), Lagos, Nigeria. Chem. Int. 2016;2(4):232–241. [Google Scholar]
- 39.Mayildurai R., Velmani N., Ramasubbu A. Systematic investigation of heavy metal assays in Indian fashion jewellery. Int. J. Sci. Technol. 2015;3(8):8–16. [Google Scholar]
- 40.Murphy T. Mercury contamination of skin whiteners in Cambodia. Hum. Ecol. Risk Assess. 2009;15(6):1286–1303. [Google Scholar]
- 41.Needleman H.L., Gunnoe C., Leviton A., Reed R., Peresie H., Maher C., Barrett P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N. Engl. J. Med. 1979;300(13):689–695. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- 42.Naseem R., Tahir S.S. Removal of Pb2+ from aqueous solution by using bentonite as an adsorbent. Water Res. 2001;35:3982–3986. doi: 10.1016/s0043-1354(01)00130-0. [DOI] [PubMed] [Google Scholar]
- 43.Nnorom I.C. Trace metals in cosmetics facial talcum powders marketed in Nigeria. Toxicol. Environ. Chem. 2011;93(5/6):1135–1148. [Google Scholar]
- 44.Nnorom I.C., Igwe J.C., Oji-Nnorom C.G. Trace metal contents of facial (make-up) cosmetics commonly used in Nigeria. Afr. J. Biotechnol. 2005;4(10):1133–1138. [Google Scholar]
- 45.Nriagu J., Jinabhai C., Naidoo R., Contoudis A. Atmospheric lead pollution in Kwazulu Natal South Africa. Sci. Total Environ. 1996;191:69–76. doi: 10.1016/0048-9697(96)05249-7. [DOI] [PubMed] [Google Scholar]
- 46.Okereke J.N. Possible health implications associated with cosmetics: a review. Sci. J. Pub. Health. 2015;3(5-1):58–63. [Google Scholar]
- 47.Oketola A.A., Adebisi A.A., Morakinyo O. Distribution and bioavailability of metals in gasoline contaminated sites in Lagos, Nigeria. J. Solid Waste Technol. Manag. 2013;39(3):161–172. [Google Scholar]
- 48.Oketola A.A., Akpotu S.O. Assessment of solid waste and dumpsite leachate and topsoil. Chem. Ecol. 2015;31(2):134–146. [Google Scholar]
- 49.Oliver M.A. Soil and human health: a review. Eur. J. Soil Sci. 1997;48(4):573–592. [Google Scholar]
- 50.Okunola O.J., Alhassan Y., Yebpella G.G., Uzairu A., Tsafe A.I., Abechi E.S., Apene E. Risk assessment of using coated mobile recharge cards in Nigeria. J. Environm. Chem. Ecotoxicol. 2011;3(4):80–85. [Google Scholar]
- 51.Olumide Y.M., Akinkugbe A.O., Altraide D. Complications of chronic use of skin lightening cosmetics. Int. J. Dermatol. 2008;47(4):344–353. doi: 10.1111/j.1365-4632.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 52.Onwordi C.T., Orizu C.O., Wusu A.D., Ogunwande I.A. Potentially toxic metals exposure from body creams sold in Lagos, Nigeria. Researcher. 2011;3(1):30–37. [Google Scholar]
- 53.Osibanjo O., Adeyi A.A., Majolagbe A.O. Characterisation of groundwater quality around soluos dumpsite in Lagos, Nigeria. Int. J. Water. 2016 in press. [Google Scholar]
- 54.Oti Wilberforce J.O., Nwabue F.I. Heavy metals effect due to contamination of vegetables from enyigba lead mine in Ebonyi State, Nigeria. Environ. Pollut. 2013;2(1):19–26. [Google Scholar]
- 55.Oyaro N., Juddy O., Murago E.N.M., Gitonga E. The contents of Pb, Cu, Zn and Cd in meat in Nairobi, Kenya. Int. J. Food Agric. Environ. 2007;5:119–121. [Google Scholar]
- 56.Puschenreiter M., Horak O., Friesel W., Hartl W. Low-cost agricultural measures to reduce heavy metal transfer into the food chain-a review. Plant Soil Environ. 2005;51(1):1–11. [Google Scholar]
- 57.Qu C.S., Ma Z.W., Yang J., Liu Y., Bi J. Human exposure pathways of heavy metals in a lead-Zinc mining area, Jiangsu Province, China. PLoS ONE. 2012;7(11):e46793. doi: 10.1371/journal.pone.0046793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed S. Department of Health and Human Services; USA: 2007. Cosmetics and Your Health; pp. 22–25. [Google Scholar]
- 59.Rocheleau C.M. Inter-rater reliability of assessed prenatal maternal occupational exposures to solvents, polycyclic aromatic hydrocarbons, and heavy metals. J. Occup. Environ. Hyg. 2011;8(12):718–728. doi: 10.1080/15459624.2011.627293. [DOI] [PubMed] [Google Scholar]
- 60.Sainio E.L., Jolanki R., Hakala E., Kanerva L. Metals and arsenic in eyeshadows. Contact Dermatitis. 2001;42(1):5–10. doi: 10.1034/j.1600-0536.2000.042001005.x. [DOI] [PubMed] [Google Scholar]
- 61.Sajjad K., Robina F., Shagufta S., Mohammad A.K., Maria S. Health risk assessment of heavy metals for population via consumption of vegetables. World Appl. Sci. J. 2009;6(12):1602–1606. [Google Scholar]
- 62.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metals toxicity and the environment. EXS. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Udiba U.U., Ogabiela E.E., Hammuel C. Post remediation assessment of contaminants levels in soil, dareta village, Zamfara, Nigeria. Trends Adv. Sci. Eng. 2012;4(1):70–79. [Google Scholar]
- 64.Ullah H. Comparative study of heavy metals content in cosmetic products of different countries marketed in Khyber Pakhtunkhwa, Pakistan. Arab. J. Chem. 2013 (in press) [Google Scholar]
- 66.USEPA, Integrated Risk Information System (2007).
- 67.WHO Lead exposure in african children: contemporary sources and concerns. World Health Organ. Reg. Office Afr. 2015:20. ISBN: 978-0-86970-787-6. [Google Scholar]
- 68.Yahaya M.I. Analysis of heavy metals concentration in road sides soil in Yauri, Nigeria. Afr. J. Pure Appl. Chem. 2010;4(3):22–30. [Google Scholar]
- 69.Yebpella G.G., Magomya A.M., Lawal U., Gauje B., Oko O.J. Assessment of trace metals in imported cosmetics marketed in Nigeria. J. Nat. Sci. Res. 2014;4(14):11–14. [Google Scholar]