Abstract
C-Glycosyl flavones are present in different plant tissues and they exhibit health benefits. In the present study, it was found that C-glycosyl flavones are distributed in different milled fractions of black gram and among these fractions, husk had the highest content of C-glycosyl flavones. Two C-glycosyl flavones from black gram husk were extracted and purified by preparative high-performance liquid chromatography (HPLC) column. The purity of each compound was assessed by analytical C18 column. The structure of each compound was confirmed by LC–MS/MS, NMR. The molecular mass of these compounds were found to be [M−H]−, m/z 431.36 and [M−H]−, m/z 431.35 and were identified as vitexin and isovitexin, respectively. Content of vitexin and isovitexin in aqueous ethanol extract was found to be 76 and 65 mg/g of extract, respectively. These C-glycosyl flavones protected DNA and erythrocytes from oxidative damage. The IC50 values for vitexin, isovitexin and quercetin for hemolysis were 6, 5.7 and 2.37 μg, respectively. These compounds also triggered the process of apoptosis in HeLa cells by downregulating Bcl-2 level with the simultaneous upregulation of Bax and caspase-3 protein expression. Thus, C-glycosyl flavones from black gram husk protected DNA and erythrocytes from oxidative damage and exhibited anticancer activity.
Keywords: C-Glycosyl flavones, DNA damage protection, Erythrocyte damage protection, Scatchard plot, Melting temperature of DNA, Scanning electron microscopy
1. Introduction
Excessive formation of reactive oxygen species (ROS) induces oxidative stress leading to cell damage that can culminate in cell death. It has been reported that free radicals and radical mediated oxidation play a role in many pathological processes. ROS are capable of oxidizing cellular proteins, nucleic acids and lipids. Lipid peroxidation is a free-radical mediated propagation of oxidative insult to polyunsaturated fatty acids, and its termination occurs through enzymatic means or by free radical scavenging by antioxidants [28], [29]. Severe oxidative stress is caused due to imbalance between the antioxidative defence systems and the formation of ROS that may alter intracellular signalling processes.
Polyphenols are potent antioxidants found ubiquitously in plants and consumed in relatively high quantities in the human diet. Extensive work has been carried out to understand the health benefits of several classes of polyphenolic compounds, in particular flavonoids [26]. Epidemiological studies have suggested the associations between the consumption of flavonoids and flavonoid-rich foods and the incidence of coronary heart disease, various cancers, stroke and osteoporosis [12]. Rather than exerting direct antioxidant effects, these polyphenols shows beneficial properties by various mechanisms and it involves their cellular interaction and signalling pathways related machinery that mediate cell function under both normal and pathological conditions [26].
Polyphenols are the class of secondary metabolites which are apparently absorbed from the upper gastrointestinal tract by a number of mechanisms [25]. Flavonoid glucosides interact with the sodium dependent glucose transporters (SGLT1) and transported across the apical membrane of enterocytes [9], [27]. Beneficial effects of flavonoids have been strongly demonstrated in in vitro as well as in vivo systems including human studies. Flavonoids are a broad class of plant secondary metabolites with low molecular weight, characterized by the flavan nucleus. Naturally occurring flavonoids usually exist as O- or C-glycosides of flavonoid moiety. The O-glycosides possess sugar substituents bound to a hydroxyl of aglycone, usually at 3 or 7 positions, whereas C-glycosides possess sugar groups linked to the carbon of aglycone usually at C-6 or C-8 by forming a C—C bond, giving them more resistance to acid hydrolysis [4]. On the other hand, flavone C-glycosides such as vitexin and isovitexin frequently occur in many edible or medicinal plants including mung beans, pigeon pea leaves, bamboo leaves and ficus deltoidea leaves as main constituents [5], [8], [15], [30].
Black gram is widely used pulse crop in India and also in different parts of the world. Dehusked black gram (dhal) is used for various food preparations. During milling of black gram into dhal, husk is one of the major fractions in the by-product. Earlier report from our laboratory indicated that aqueous extract of black gram husk exhibited antioxidant properties [11]. In the present study, we have extracted the husk bioactive components using 50% aqueous ethanol and fractionated in to two peaks by preparative RP-HPLC. Compounds present in these peaks were identified as vitexin and isovitexin using NMR and LC–MS/MS, and determined their antioxidant activity, protection against oxidative damage of DNA and erythrocyte and cytotoxic efficacy in cervical cancer cells.
2. Materials and methods
2.1. Chemicals
λ-DNA was purchased from Bangalore Genei, India. Agarose, H2O2, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), dimethyl sulfoxide (DMSO) were purchased from Sisco Research Laboratories. FeSO4·7H2O, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, ascorbic acid, Tris base, ethidium bromide (EtBr), ethylenediaminetetraacetic acid (EDTA), Enhanced Chemiluminescence solution (ECL), vitexin, isovitexin and quercetin were purchased from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA). Polyvinylidene fluoride membrane (PVDF) was purchased from pall corporation, (New York, USA). BCA protein assay kit was purchased from Thermo Fisher Scientific (Massachusetts, USA). All other chemicals used were of analytical grade.
2.2. Milling of black gram and separation of milled products
Black gram (10 kg) was pitted in Versatile Dhal Mill (designed and developed by CSIR-CFTRI, Mysore, India) mixed with 30 ml of oil, kept overnight for tempering and dried at 60 °C for 8 h. The black gram thus obtained after treatment was milled using Versatile Dhal Mill. Black gram was milled into cotyledon, seed coat, and mixture of germ, aleurone, seed coat powder, and plumule. The husk was further fractionated by air classification as described earlier [10].
2.3. Extraction and isolation of bioactive components from black gram husk using different solvents
Powdered black gram husk was packed in a glass column, retained for 2 h in hexane and later gradually eluted with hexane till the eluent became colourless. The material remained after complete removal of hexane was further soaked and eluted with different solvents sequentially with increase in polarity such as chloroform, acetone, ethyl acetate, ethanol, aqueous ethanol (50%) and finally with water. The each fraction thus obtained was concentrated under reduced pressure.
2.4. Detection and quantification of c-glycosyl flavones in BGBP fractions
C-Glycosyl flavones like vitexin and isovitexin in black gram milled fractions were separated and quantified by analytical reversed-phase C18 column (4.6 × 250 mm) using HPLC system (Agilent- Model 1200 series) according to the method described by Kim et al. [16] using a diode array detector. A gradient solvent system consisting of solvent A water: acetic acid (99:1) and solvent B methanol: acetic acid (99:1) was used as mobile phase at a flow rate of 1 ml/min for a total run time of 50 min. The gradient elution used was as follows: 90–65% A in 10 min, 65–58% A in 25 min, 58–25% A in 35 min, 25% A in 40 min, 25–90% A in 45 min and 90% A in 50 min. Quantitative determination of the eluted flavones was detected at 337 nm and known quantities of vitexin and isovitexin standards were used for identification and quantification.
2.5. Purification of vitexin and isovitexin of aqueous ethanol fraction by preparative HPLC
The chromatographic separation was performed according to the method described in Section 2.4, on a Shimadzu Prep LC8A Preparative Chromatography system equipped with SCL-10AVP system controller (Shimadzu). The preparative HPLC was performed on a Varian, Pursuit XRs 10 C18 preparative column (250 mm × 21.2 mm). The flow rate was 8 ml/min and the wavelength used for detection was 337 nm. The sample volume injected was 2 ml. Two flavone C-glycoside peaks were collected manually, concentrated and evaporated to dryness by rotary evaporator.
2.6. Identification of vitexin and isovitexin of aqueous ethanol fraction
2.6.1. Evaluation of purity of purified flavone C-glycosides by HPLC and their identification by LC–MS/MS
Manually collected C-glycosyl flavones in aqueous ethanol fraction were tested for their purity according to the method described in Section 2.4. HPLC–ESI–MS analysis was done on a Waters platform ZMD 4000 system composed of a micro ZMD mass spectrophotometer, a Waters 2690 HPLC and a Waters 996 photo diode array detector (Waters corporation, MA, USA). Data were collected and processed via a personal computer running Mass Lynx software version 3.1 (Micromass, a diversion of Waters corporation, MA, USA). The samples in 10 μl diluted aliquot were separated on a reversed phase C18 column (4.6 × 250 mm), using a diode array detector (operating at 337 nm). Solvent A water: formic acid (99:1) and solvent B methanol: formic acid (99:1) was used as mobile phase at a flow rate of 1 ml/min for a total run time of 40 min. UV–vis absorption spectra were recorded on-line during HPLC analysis. The following ion optics was used- capillary voltage 3.5 kV, cone voltage 100 V and collision voltage 10 V. The source block temperature was 120 °C and the desolvation temperature was 350 °C. ESI–MS was performed using argon as cone gas (50 l/h) and nitrogen as desolvation gas (500 l/h). The electron spray probe flow was adjusted to 70 ml/min. Continuous mass spectra were recorded over the range of m/z 100–1000 with scan time 1 s and inter scan delay 0.1 s.
2.6.2. NMR analysis of flavone C-glycosides
1H &13C NMR spectra for the compounds were recorded on a Bruker Avance 500 MHz spectrometer (Bruker biospin, Reinstetten, Germany) using CD3OD solvent.
2.7. Determination of free radical-scavenging activity
The effect of two isolated C-glycosyl flavones on DPPH radical scavenging activity was determined using earlier described method [1]. A 100 μM solution of DPPH in methanol was prepared and vitexin and isovitexin (200 μl) containing 5–30 μg were mixed with 1 ml of DPPH solution. The mixture was shaken vigorously and left in the dark at room temperature for 20 min. The absorbance of the resulting solution was measured at 517 nm. The control contained all the reagents except compounds/ascorbic acid. The capacity to scavenge DPPH radical was calculated by following equation:
| Scavenging activity (%) = 1 − (As/A0) × 100 |
where A0 is the absorbance at 517 nm of the control and As is the absorbance in the presence of compounds/ascorbic acid. The results were plotted as the% of scavenging activity against concentration of the sample. The half-inhibition concentration (IC50) was defined as the amount of compound required for 50% of free radical scavenging activity. The IC50 value was calculated from the plots as the antioxidant concentration required for providing 50% free radical scavenging activity.
2.8. Prevention of λ-DNA damage by flavone C-glycosides
2.8.1. Agarose gel electrophoresis
Agarose gel electrophoresis was carried out to know the prevention of oxidative λ-DNA damage by vitexin and isovitexin as previously described by Girish et al. [11] with slight modifications. λ-DNA (1 μg), with and without vitexin and isovitexin (2.5 and 10 μg), was incubated with 2 mM FeSO4, 30 mM H2O2 in Tris buffer (10 mM, pH 7.4) in a final reaction volume of 20 μl for 1 h at 37 °C. Samples were analyzed on 1% agarose gel prepared in Tris–acetate–EDTA buffer (pH 8.5) at 50 V at room temperature.
2.8.2. EtBr binding to DNA by fluorescence analysis
Efficacy of vitexin and isovitexin against oxidative λ-DNA damage was analyzed by measuring the changes in fluorescence of EtBr binding to DNA. λ-DNA (2 μg) was incubated with 2 mM FeSO4, 30 mM H2O2 in 10 mM Tris-HCl (pH 7.4) for 1 h forthe oxidative λ-DNA damage and the protection against oxidative damage of λ-DNA was analyzed in the presence of vitexin and isovitexin (2.5 and 10 μg)for 1 h. The samples thus prepared were mixed with 5 μg of EtBr and the fluorescence was recorded by exciting at 535 nm and emission at 600 nm.
2.8.3. Ethidium bromide binding analysis by scatchard plots
λ-DNA (1 μg) was incubated with 2 mM FeSO4, 30 mM H2O2 in Tris buffer (10 mM, pH 7.4) with and without vitexin and isovitexin (2.5 and 10 μg) in a reaction volume of 20 μl for 1 h at 37 °C. The fluorescence was measured by titrating with increasing EtBr against constant amount of λ-DNA. The maximum amount of EtBr boundper base pair ofλ-DNA was calculated using Scatchard plots of ‘r’ vs. ‘r/Cf’ in the DNA–EtBr reaction mixture at various titration intervals [3], [24]. The concentration of bound EtBr in 1 ml dye-DNA mixture (Cbꞌ) was calculated using the following formula.
| Cbꞌ = [Coꞌ(F-FO)/(V × Fo)] |
Where,
Coꞌ = Concentration of EtBr (pmoles) in the dye complex mixture
F = Observed fluorescence of EtBr at any point of dye-DNA mixture
Fo = Observed fluorescence of EtBr with no DNA
V = Experimental value, ratio of bound EtBr to free EtBr at saturation point.
The concentration of free EtBr (Cfꞌ) was then calculated by using the formula
| Cfꞌ = Coꞌ − Cbꞌ |
Where, Cfꞌ, Coꞌ and Cbꞌ were expressed in pmoles. The amount of bound EtBr bound per base pair was calculated by r = Cbꞌ (pmoles)/DNA concentration (pmoles of base pair). A plot was made for r vs. r/Cf and the point where the straight line interacts the axis r was defined as the maximum amount of dye bound per base pair (n), where Cf = Cfꞌ × 10−12 moles.
2.8.4. Melting temperature studies
Thermal denaturation studies were performed to know the λ-DNA integrity. λ-DNA (2 μg) was incubated with 2 mM FeSO4, 30 mM H2O2 in 10 mM Tris-HCl (pH 7.4) for 1 h for monitoring the oxidative λ-DNA damage, with and without vitexin and isovitexin (2.5and 10 μg), for 1 h. The melting profiles (Tm) of λ-DNA were recorded at different temperatures ranging from 25 to 95 °C using a Spectrophotometer (Ultraspec, 4300 probe) equipped with thermo-programmer and data processor (Amersham Pharmacia Biotech, Hong Kong). Tm values were determined graphically from the absorbance vs. temperature plots.
2.9. Prevention of erythrocyte damage by flavone C-glycosides
2.9.1. Preparation of erythrocytes
All the animal experiments were carried out with the approval of institutional animal ethical committee. Male wistar rats in the body weight range of 180–220 g were housed in individual polypropylene cages and had free access to food and water. The animals were fed with standard diet. The animals were sacrificed under anesthesia and blood was collected by heart puncture in heparinized tubes. Erythrocytes were isolated and stored according to the method described by Girish et al. [11]. Briefly, blood samples collected were centrifuged (1500 × g, 5 min) at 4 °C, erythrocytes were separated from the plasma and buffy coat, and were washed three times by using 10 vol of 20 mM phosphate buffered saline (pH 7.4; PBS). Each time, the cell suspension centrifuged at 1500 × g for 5 min. The supernatant and buffy coats of white cells were carefully removed with each wash. Erythrocytes thus obtained were stored at 4 °C and used within 6 h for further studies.
2.9.2. In vitro assay of inhibition of rat erythrocyte hemolysis
The inhibition of rat erythrocyte hemolysis by the vitexin and isovitexin was evaluated according to the procedure described by Ajila and Prasada Rao [2] with slight modifications. The rat erythrocyte hemolysis was performed with hydrogen peroxide. To 200 μl of 10% (v/v) suspension of erythrocytes in PBS, 50 μl of vitexin or isovitexin with different concentrations (2–10 μg) was added. To this, 100 μl of 200 μM H2O2 (in PBS pH7.4) was added. The reaction mixture was incubated at 37 °C for 30 min and was centrifuged at 2000 × g for 10 min. The absorbance of the resulting supernatant was measured at 410 nm by taking 200 μl of reaction mixture with 800 μl PBS to determine the hemolysis. Likewise, the erythrocytes were treated with hydrogen peroxide and without inhibitors to obtain a complete hemolysis. The absorbance of the supernatant was measured at the same condition. The inhibitory effect of the vitexin and isovitexin was compared with standard quercetin. Percentage of hemolysis was calculated by taking hemolysis caused by 200 μM hydrogen peroxide as 100%. The IC50 values were calculated from the plots as the antioxidant concentration required for the inhibition of 50% hemolysis.
2.9.3. Protective effect on erythrocytes structural morphology
Erythrocytes (50 μl) were incubated with and without vitexin and isovitexin (2.5and 10 μg) and treated with 100 μl of 200 μM H2O2 for 30 min at 37 °C. After incubation, the incubate was centrifuged at 1500 × g for 10 min and the cell pellets were processed and were fixed in 3% glutaraldehyde on a coverslip [11]. After fixing on the coverslip, the cells were dehydrated in an ascending series of acetone (30–100%). The dried samples were mounted on an aluminum stubb (100–200 Ǻ) using double sided tape and coated with gold film with a thickness of 10–20 nm using sputter coater (Polaron, E 5000, SEM coating system). The cells were examined under a scanning electron microscope (Model No, LEO 425 VP, Electron microscopy LTD, Cambridge, UK).
2.10. Cytotoxic effect of flavone C-glycosides on HeLa (cervical cancer) cells
2.10.1. Effect of flavone C-glycosides on cell viability
Effect of C-glycosyl flavones on cell viability was assessed by MTT assay [13]. Aliquots of 1 × 104 exponentially growing HeLa (cervical cancer, procured from NCCS, Pune)cells were seeded on 96-well microtitre plates. After 4 h of incubation at 37 °C in 5% CO2, the growth medium was replaced by media containing different concentrations of vitexin and isovitexin; the plates were incubated for 48 h again. The reaction was terminated by addition of MTT solution (5 mg/mL; 20 μl) to each well, and the cells were incubated at 37 °C for 3 h. Then 100 μl of DMSO was added and measured at 570 nm in a microplate reader (Model 680 Bio-Rad Laboratories, Hercules, CA). Absorbance in the untreated wells were considered as 100% and relative percent values are expressed for cells in wells treated with different doses of vitexin and isovitexin.
2.10.2. Nuclear fragmentation assay by staining with 4ʹ, 6-diaminidino-2-phenylindole (DAPI)
Nuclear fragmentation assay after DAPI staining and apoptotic evaluation was carried out according to the method followed by Jayaram et al. [13]. HeLa cells were plated into 6 well plates (2 × 104 cells/chamber) overnight and treated with vitexin and isovitexin (30, 40 and 50 μg) and incubated for 48 h. The cells were washed with PBS and stained with DAPI for 30 min at 37 °C in dark. Cells were viewed using a fluorescent microscope with ultraviolet excitation at 300–500 nm.
2.10.3. Western blot analysis
The method used for Western blot analysis for protein levels in C-glycosyl flavone treated HeLa cells was as follows. Total protein was prepared by homogenization of cells in RIPA buffer (50 mM Tris-HCl, 1% NP-40, 0.5% sodium-deoxycholate, 1% SDS, 300 mM NaCl, 2 mM EDTA and 50 mM NaF) supplemented with protease inhibitor cocktail (Sigma-Aldrich Co.) on ice. Tissue lysate was centrifuged and the supernatant was harvested for protein for BCA kit. Protein denaturation was done by mixing the lysate with the loading buffer and boiling at 95 °C for 5 min. Equal quantity of proteins were loaded per lane and separated on SDS-polyacrylamide gel electrophoresis (12%), and transferred to polyvinylidene difluoride (PVDF) membrane. The membranes were then separately incubated overnight at 4 °C with antibodies against Bcl2, Bax and caspase 3 in 2.5% skimmed milk in Tris buffered saline (TBS) for 2 h at room temperature. The membrane was further incubated with HRP conjugated secondary antibody in 2.5% skimmed milk. β-Actin was used as the loading control. After washing with TBS, the immune reactive bands were visualized by using enhanced chemiluminiscence as substrate.
2.11. Statistical analysis
Three independent experiments were conducted in triplicate and the data were reported as mean ± SD. DMRT was used to determine the difference of means, and P < 0.05 was considered to statistically significant.
3. Result and discussion
3.1. Content of vitexin and isovitexin in BGBP fractions
Extraction with suitable solvents is the crucial first step in the analysis of a sample, because it is necessary to extract the desired chemical components from the sample for further separation and characterization. Due to the fact, that the plant extracts generally occur as a combination of different type of bioactive compounds or phytochemicals with different polarities, their separation still remains a big challenge for the process of identification and characterization of bioactive compounds. As mentioned in Section 2.3, in the present study, we have used different solvent with varying polarities. Among all the solvent systems employed, aqueous ethanol (50%) yielded maximum solids and therefore, aqueous ethanol fraction of different milled tissues was subjected for further analysis.
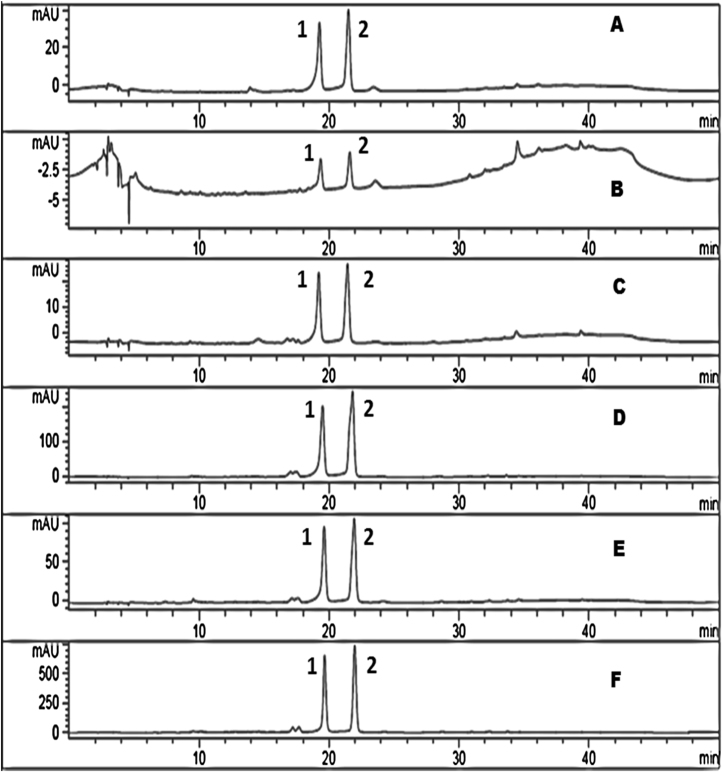
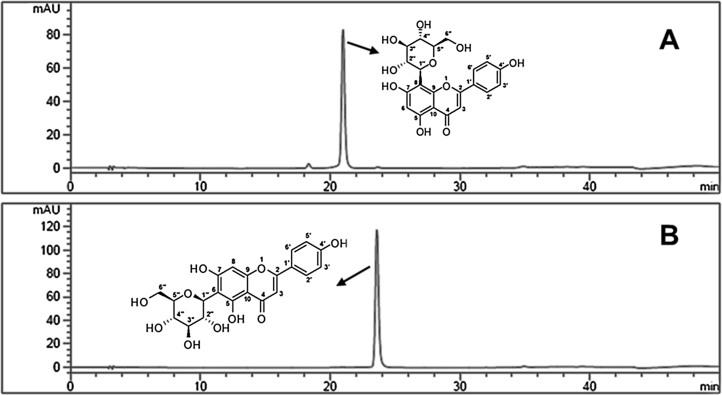
Vitexin and isovitexin were the two major C-glycosyl flavones identified in all the fractions of BGBP (Table 1). The quantitative analysis of the C-glycosyl flavones in different fraction of black gram milled by-products was performed by HPLC-PDA. The content of C-glycosyl flavones in all the fractions was ranged from 2.5 to 536 μg/g and 5–1221 μg/g for vitexin and isovitexin, respectively. Among all the fractions, husk contained the highest content of C-glycosyl flavones followed by aleurone and plumule fractions, and dhal had the least. In all the fractions, isovitexin content was the highest followed by vitexin (Fig. 1). As husk had more amount of vitexin and isovitexin, husk was used for further isolation and purification of C-glycosyl flavones. Sequential extraction with different solvents such as hexane, chloroform, acetone, ethyl acetate, ethanol and aqueous ethanolyielded solid content of 7.82%, 3.63%, 3.49%, 0.64%, 14.77% and 44.81% respectively. As more solids were extracted with aqueous ethanol, vitexin (1) and isovitexin (2) in aqueous ethanol extract were quantified by analytical HPLC and they were found to be 76 and 65 mg/g of extract, respectively (Fig. 2).
Table 1.
Vitexin and isovitexin contents in different fractions of black gram milled by-product (μg/ g).
| Sample | Vitexin μg/g | Isovitexin μg/g |
|---|---|---|
| Whole black gram | 42.31 ± 2.79c | 84.61 ± 4.36c |
| Dhal | 2.55 ± 0.21a | 5.06 ± 0.15a |
| Germ | 29.99 ± 2.36b | 71.94 ± 4.34b |
| Aleurone | 202.89 ± 5.57e | 518.61 ± 10.13e |
| Plumule | 91.46 ± 1.39d | 214.51 ± 5.64d |
| Husk | 536.54 ± 7.94f | 1221 ± 15.47f |
Values are mean ± SD (n = 3). Means with different letters within a column for different fraction are significantly different at p < 0.05.
Fig 1.
HPLC chromatogram of black gram milled by-product fractions showing vitexin (1) and isovitexin (2). (A) Whole gram; (B) Dhal; (C) Germ; (D) Aleurone; (E) Plumule; (F) Husk.
Fig. 2.
RP-HPLC chromatograms of flavone C-glycosides purified from husk extract. (A) vitexin (B) isovitexin.
3.2. Identification and characterization of C glycosyl flavones
3.2.1. LC–MS/MS analysis
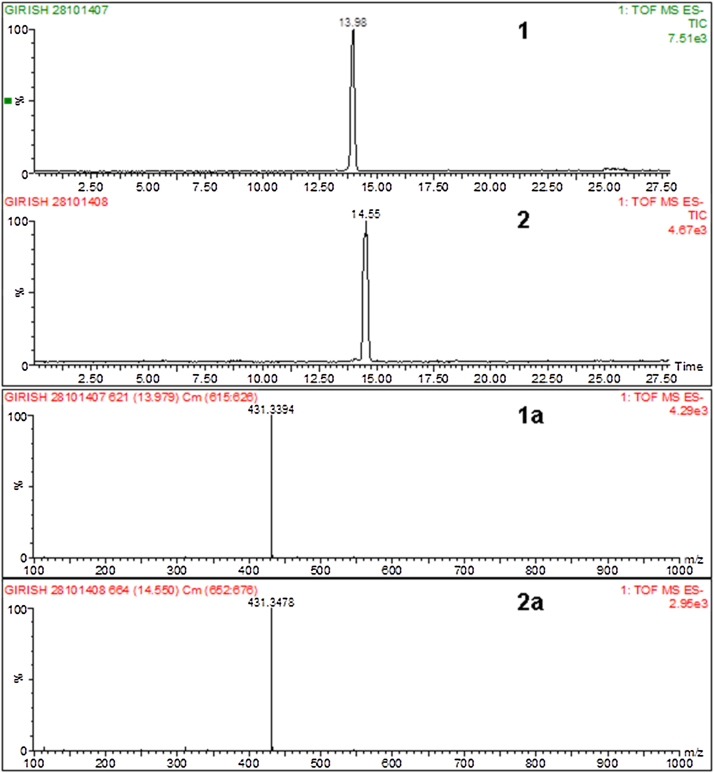
HPLC-PDA-ESI–MS Analysis.The compounds purified by preparative HPLC from aqueous ethanolextract were subjected to HPLC-PDA-ESI–MS analysis in negative ionization mode. Both the tested compounds showed similar UV spectra with the absorption maxima (λmax) at 337 nm. The MS spectra for peaks 1 and 2 showed a characteristic peak in negative ion mode, [M−H]− at m/z 431. Moreover, they exhibited the same molecular mass. These data suggested that peaks 1 and 2 were C-glycosyl isomers of Apigenin, and tentatively identified as 8-C-glucosylapigenin (vitexin) and peak 2 as 6-C-glucosylapigenin (isovitexin), respectively (Fig. 3).
Fig. 3.
LC–MS chromatogram showing retention time 13.98, vitexin (1) 14.51 isovitexin (2) purified from husk aqueous alcoholic extract at 337 nm. ESI–MS spectra of peaks (1a) vitexin ([M−H]−, m/z 431.36), (2a) isovitexin ([M−H]−, m/z 431.35).
3.2.2. NMR analysis
Peak 1 was obtained as a yellow powder. 1H NMR (500 MHz, CD3OD): δ 8.01 (1H at C-2′), 7.91 (1H at C-6′), 6.94 (1H at C-3′), 6.91 (1H at C-5′), 6.76 (1H at C-6), 6.33 (1H at C-3), 5.18 (1H at C-1′′), 4.68 (1H at C-C-2′′), 4.58 (1H at C-5′′), 3.74-3.87 (2H at C-6′′), 3.61–3.67 (1H at C-4′′), 3.54-3.60 (1H at C-3′′). 13C NMR (125 MHz, CD3OD): d 182.22 (C-4), 164.51 (C-2), 162.75 (C-7), 160.25 (C-4′), 159.95 (C-5), 156.13 (C-9), 128.84 (C-2′ & 6′), 121.79 (C-1′), 115.93 (C-3′ & 5′), 104.03 (C-8), 103.76 (C-10), 102.37 (C-3), 98.54 (C-6), 80.77 (C-5′′), 77.90 (C-3′′), 73.19 (C-1′′), 70.97 (C-2′′), 70.25 (C-4′′), 60.97 (C-6′′). Peak 1 was, therefore, identified as vitexin, by NMR analysis, and comparison with its literature data.
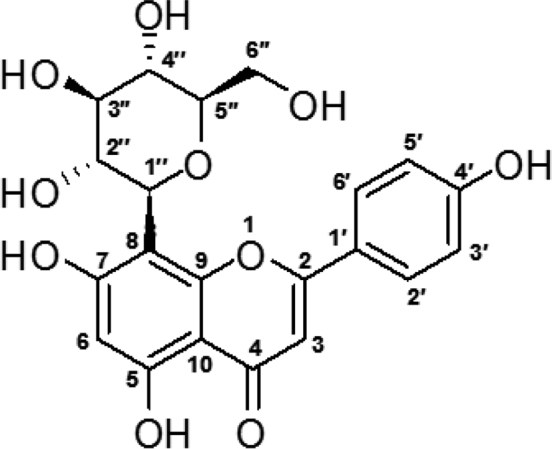
Peak 2 was obtained as a yellow powder. 1H NMR (500 MHz, CD3OD): δ 7.82 (2H at C-2′ & 6′), 6.91 (2H at C-3′ & 5′), 6.57 (1H at C-8), 6.48 (1H at C-3), 4.89 (1H at C-1′′), 4.16 (1H at C-2′′), 3.87 and 3.73 (2H at C-6′′), 3.44-3.52 (2H at C-4′′ and C-3′′), 3.38–3.44 (1H at C-5′′). 13C NMR (125 MHz, CD3OD): d 182.34 (C-4), 164.50 (C-7), 163.18 (C-2), 161.06 (C-9), 160.31 (C-4′), 157.02 (C-5), 127.73 (C-2′ & 6′), 121.44 (C-1′), 115.34 (C-3′ & 5′), 107.49 (C-6), 103.52 (C-10), 102.21 (C-3), 93.57 (C-8), 80.89 (C-5′′), 78.43 (C-3′′), 73.62 (C-1′′), 70.94 (C-2′′), 70.08 (C-4′′), 61.15 (C-6′′). Peak 2 was, therefore, identified as isovitexin, by NMR analysis, and comparison with its literature data.
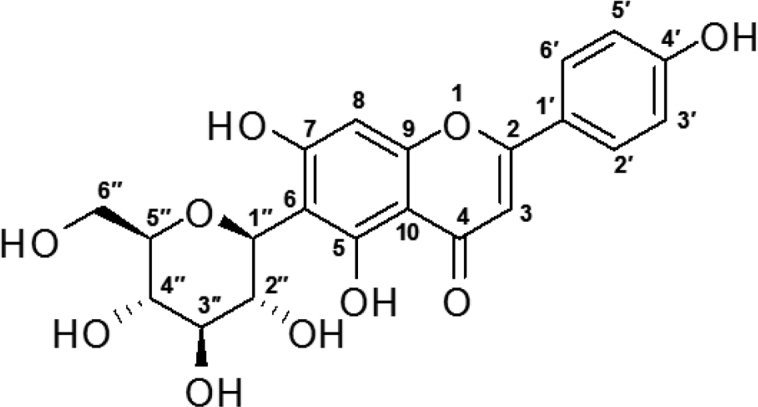
These compounds were confirmed by NMR. All the spectral data obtained by using HPLC, MS spectra and NMR also confirmed the purified compounds as vitexin and isovitexin.
3.3. Antioxidant activity of flavone C-glycosides
The DPPH radical-scavenging activities of the isolated flavonoid glycosides,vitexin, isovitexin and standard ascorbic acid had IC50 values of 19.2, 21, and 14.2 μM, respectively. Both vitexin and isovitexin had antioxidant properties, but lower then ascorbic acid. Antioxidant ability of ascorbic acid was found to be less than the isoflavonoids, vitexin had the high antioxidant potential when compared to isovitexin. Both the compounds showed a concentration dependent DPPH radical scavenging activity, which may be attributed due to their hydrogen donating ability.
3.4. Inhibition of Fe2+ induced λ-DNA damage by C glycosyl flavones
3.4.1. Agarose gel electrophoresis
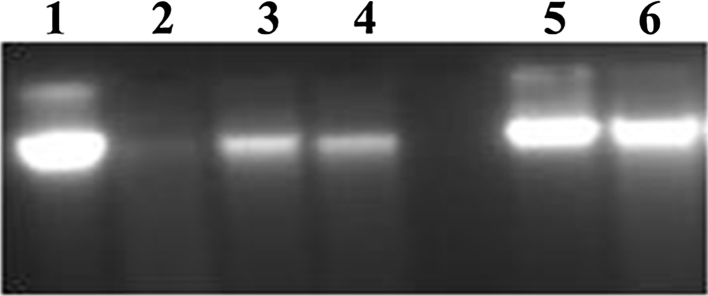
DNA damage process is a common event in the life of a cell and as a results it may lead to mutation, cancer, and ultimately cell death [23]. DNA integrity is always under attack from internal as well as external/environmental factors. As the vitexin and isovitexin showed potential antioxidant properties, it was tested for its capability to prevent DNA damage caused by hydroxyl radicals that are produced by FeSO4 and H2O2 using agarose gel electrophoresis. The hydroxyl radicals attack hydrogen atoms of λ-DNA leading to the formation of nicks in DNA which results in single strand and double strand breaks in λ-DNA [11]. Incubation of λ-DNA with FeSO4 and H2O2 for 1 h resulted in a decrease in λ-DNA band intensity by 90% (Fig. 4, lane 2). Indicating damage of λ-DNA. However, in the presence of C-glycosyl flavones, the λ-DNA damage by FeSO4/H2O2 system was not observed. As can be seen from Fig. 4 (lanes 3 and 4), DNA treated with the FeSO4/H2O2 system for 1 h in the presence of vitexin and isovitexin (2.5 μg), the DNA band intensity was decreased by 50% indicating the prevention of λ-DNA damage, whereas, in the presence of vitexin and isovitexin at 10 μg concentration, λ-DNA band intensity was comparable to control (Fig. 4, lane 5 and 6) indicating that C-glycosyl flavones offered maximum protection against radical induced λ-DNA at 10 μg concentration. Thus, the results suggest that both vitexin and isovitexin prevent DNA damage against radical induced oxidative damage. The protective ability may be due to free radical scavenging activity of C-glycosyl flavones.
Fig. 4.
Agarose gel electrophoresis pattern of DNA damage inhibition by vitexin and isovitexin. After 1 h incubation of reaction mixture. Lanes 1: 0.5 μg DNA alone; Lane 2: 0.5 μg DNA + 2 mM FeSO4 + 30 mM H2O2; Lane 3: 0.5 μg DNA + 2 mM FeSO4 + 30 mM H2O2 + isovitexin, 2.5 μg; Lane 4: 0.5 μg DNA + 2 mM FeSO4 + 30 mM H2O2 + vitexin, 2.5 μg; Lane 5: 0.5 μg DNA + 2 mM FeSO4 + 30 mM H2O2 + isovitexin 10 μg; Lane 6: 0.5 μg DNA + 2 mM FeSO4 + 30 mM H2O2 + vitexin 10 μg.
3.4.2. EtBr binding and scatchard plots
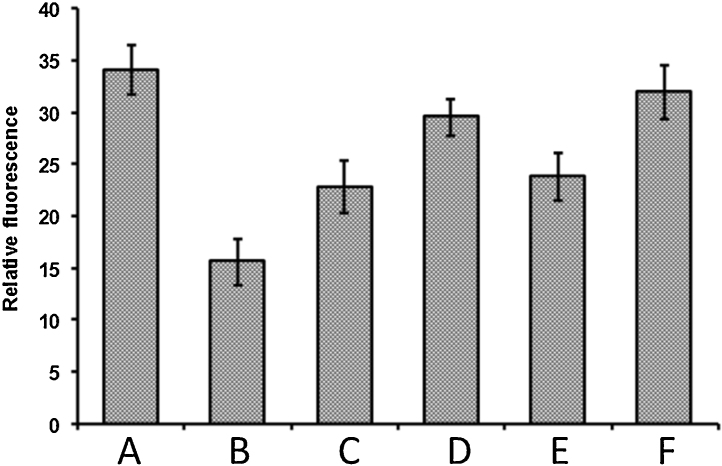
In the previous experiment we have verified that FeSO4/H2O2 system has caused damage in λ-DNA. To further characterize the λ-DNA damage by FeSO4/H2O2 system and its protection by C-glycosyl flavones, changes in EtBr fluorescence upon binding to λ-DNA were carried out (Fig. 5). EtBr fluorescence in the presence of intact λ-DNA was 34.1, whereas it was 15.6 for λ-DNA treated with FeSO4 in the presence of H2O2. The significant decrease in the fluorescence intensity in the case of λ-DNA treated with FeSO4 and H2O2 was due to the damage of DNA by Fenton's mediated hydroxyl radicals. However, the fluorescence intensity for DNA treated with the FeSO4/H2O2 system for 1 h in the presence of vitexin and isovitexin (2.5 μg) increased the fluorescence from 15.6 to 22.8 and 23.8, respectively, whereas at 10 μg level it was increased to 29.5 and 31.9. The increased fluorescence intensity may be due to the prevention of DNA damage by free radical scavenging activity of C-glycosyl flavones.
Fig. 5.
EtBr binding to DNA. (A) DNA alone; (B) DNA + FeSO4 + H2O2; (C) DNA + FeSO4 + H2O2 + vitexin, 2.5 μg; (D) DNA + FeSO4 + H2O2 + vitexin, 10 μg; (E) DNA + FeSO4 + H2O2 + isovitexin, 2.5 μg; (F) DNA + FeSO4 + H2O2 + isovitexin, 10 μg.
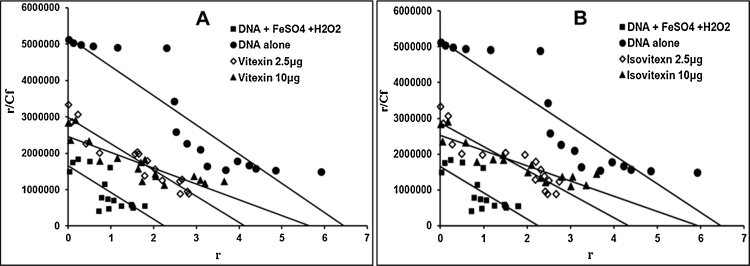
To substantiate the λ-DNA damage protection by C-glycosyl flavones, the differences in the binding pattern of EtBr to intact λ-DNA and damaged λ-DNA was measured. EtBr titration studies were carried out to determine the amount of EtBr molecules bound per base pair (bp) of DNA. Four representative Scatchard plots of ‘r vs. r/Cf' for λ-DNA were shown in Fig. 6A and B. The number of EtBr molecules bound/bp of DNA (control) was 6.4 (Fig. 6●), whereas it was 2.28 (Fig. 6■) for λ-DNA treated with FeSO4/H2O2. The number of EtBr molecules bound per base pair of λ-DNA decreased because of oxidative damage to DNA. However, λ-DNA with FeSO4/H2O2 in the presence of vitexin and isovitexin (2.5 μg), the number of EtBr molecules bound per base pair was found to be 4.1 (Fig. 6A◊) and 4.28 (Fig. 6B◊) respectively. λ-DNA with FeSO4/H2O2 in the presence of vitexin and isovitexin (10 μg) was comparable to the value obtained with intact λ-DNA and it was found to be 5.6 (Fig. 6A▲) and 5.9 (Fig. 6B▲) respectively. Thus, the results confirm that the C-glycosyl flavones prevent the DNA damage by FeSO4/H2O2.
Fig. 6.
Scatchard plot of ethidium bromide binding to DNA. (A) (●) DNA, (■) DNA + FeSO4 + H2O2, (◊) DNA + Vitexin, 2.5 μg + FeSO4 + H2O2, (▲) DNA + Vitexin, 10 μg + FeSO4 + H2O2. (B) (●) DNA, (■) DNA + FeSO4 + H2O2, (◊) DNA + Isovitexin, 2.5 μg + FeSO4 + H2O2, (▲) DNA + Isovitexin, 10 μg + FeSO4 + H2O2. Fluorescent measurements were done at room temperature setting excitation at 535 nm and emission at 600 nm. The Scatchard plots were drawn using least square method.
3.4.3. DNA stability assessment by melting temperature (Tm)
The melting temperature of DNA provides an insight on the integrity of DNA. The melting temperature (Tm) of λ-DNA was 76.1 °C, whereas FeSO4/H2O2 potentially decreased the Tm of DNA to 56.6 °C (Table 2). Low Tm in the case of λ-DNA treated with FeSO4/H2O2 was due to the damage to DNA in the presence of hydroxyl radicals. However, the Tm value for λ-DNA with FeSO4/H2O2 in the presence of vitexin and isovitexin (2.5 μg) was 63.2 and 65.5 °C, respectively. The higher Tm of λ-DNA in the presence of vitexin and isovitexin indicates that the compounds prevented oxidative damage. Tm value for DNA with FeSO4/H2O2 in the presence of vitexin and isovitexin (10 μg) was found to be 75.8 and 77.9 °C, respectively indicating λ-DNA is much more stabilized at higher concentration of C-glycosyl flavones. Thus, the studies revealed that C-glycosyl flavones prevented the oxidative DNA damage caused by free radicals. The oxidative damage prevention mechanism of λ-DNA may be due to the scavenging of free radicals.
Table 2.
Protective effect of husk flavonoids on thermal denaturation of DNA.
| Sample | Tm value |
|---|---|
| DNA alone | 76.16 ± 2.47c |
| DNA + FeSO4 + H2O2 | 56.64 ± 3.22a |
| DNA + FeSO4 + H2O2 + vitexin 2.5 μg | 62.31 ± 1.91b |
| DNA + FeSO4 + H2O2 + vitexin 10 μg | 75.86 ± 1.37c |
| DNA + FeSO4 + H2O2 + isovitexin 2.5 μg | 65.52 ± 2.61b |
| DNA + FeSO4 + H2O2 + isovitexin 10 μg | 77.96 ± 2.95c |
Values are mean ± SD (n = 3). Means with different letters within a column are significantly different at p < 0.05.
3.5. Inhibition of rat erythrocyte hemolysis
Erythrocytes are highly susceptible to attack by reactive oxygen species because of the high amount of polyunsaturated fatty acid content in their membranes and the metal catalyzed oxidation reactions mediated by Fe present in haemoglobin [20]. Hydrogen peroxide generated during the autoxidation of oxyhemoglobin contributes to heme degradation leading to the damage of erythrocytes, which in turn leads to change in whole red blood cell structural conformation and its functioning [18], [19], [2], [10], [11], [14].
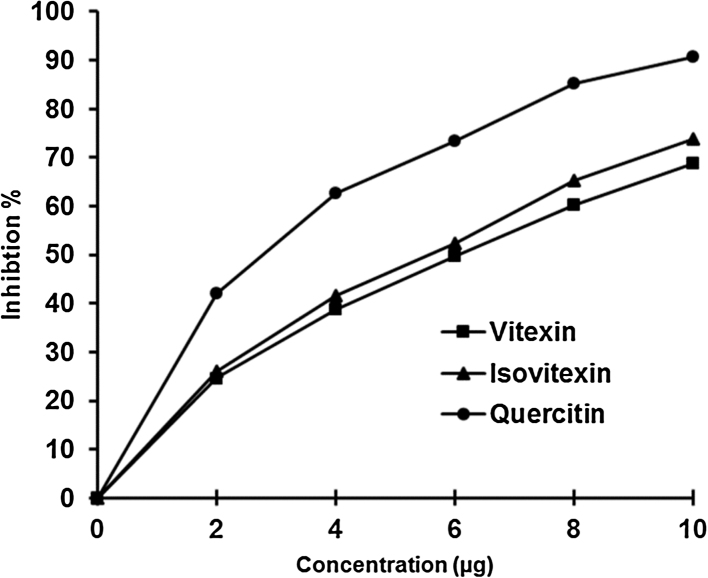
In the present study, H2O2 hemolysis was induced in erythrocytes and studied the effect of vitexin, isovitexin and quercetin on its protection against hemolysis. As can be seen from Fig. 7, vitexin and isovitexin inhibited the rat erythrocytes hemolysis in a dose-dependent manner. Known antioxidant quercetin was used for comparison. Quercetin showed better inhibition whereas, vitexin and isovitexin were also comparable. The IC50 values for the vitexin, isovitexin and quercetin were 6, 5.7 and 2.37 μg, respectively. Vitexin and isovitexin alone were tested and found that it did not show any harmful effect on erythrocytes. The percentage of hemolysis in presence of vitexin, isovitexin and quercetin alone was found to be less than 3% that is comparable to control which was around 2%.
Fig. 7.
In vitro protective effects of vitexin and isovitexin against H2O2 induced hemolysis of rat erythrocytes.
3.6. Protective effect on erythrocyte structural morphology
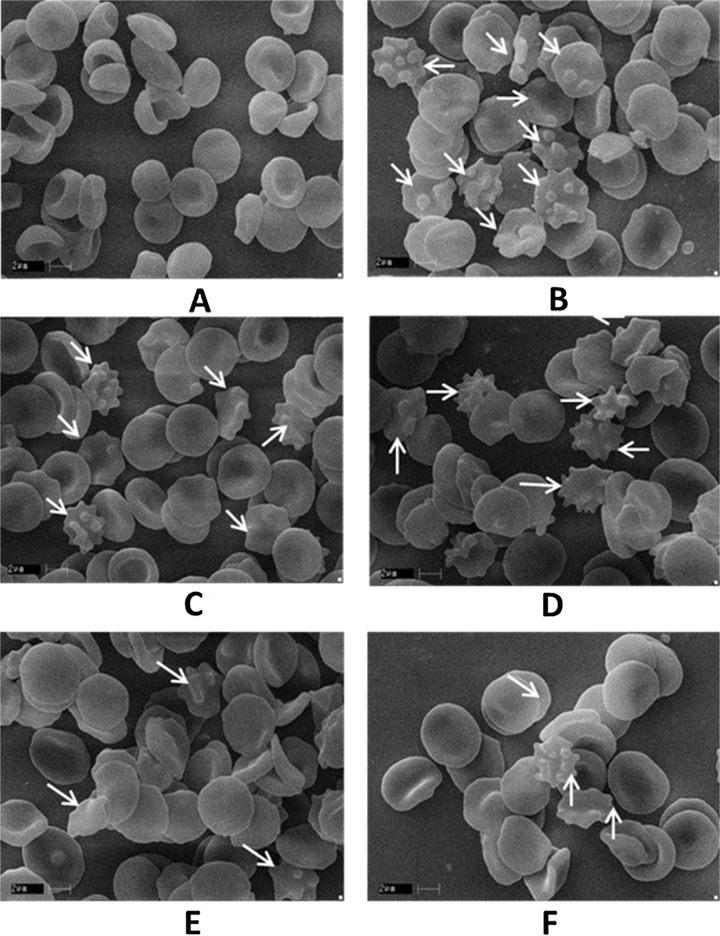
SEM studies were carried out to know the structural alterations of the erythrocytes (RBCs) treated in vitro with H2O2 in presence and absence of vitexin and isovitexin are shown in Fig. 8. RBCs from healthy individuals showed a typical disc-shape (discocyte) when not subjected to external stress, whereas, in pathological conditions oxidative stress exerts significant changes in the structure and function of erythrocytes [6]. Native erythrocytes were clearly showing as typical discocytes while the erythrocytes exposed to H2O2 resulted in a significant change in the morphology and cell shape with distinct echinocyte formation. The morphological changes induced by the oxidative system were prevented when the cells were treated with vitexin, isovitexin and quercetin. The damage to the RBCs ultimately leads to the improper functioning, changes in cell rigidity and shape and results in the formation of echinocytes and stomatocytes [17]. The results of the present study indicate that C-glycosyl flavone compounds prevent the structural and morphological changes induced by fenton’s mediated oxidative stress in erythrocytes.
Fig. 8.
Scanning electron micrograph of normal erythrocytes (RBCs) and protective effect of vitexin and isovitexin against H2O2 induced oxidative damage on RBC. (A) RBC control; (B) RBC+ H2O2; (C) RBC + H2O2 + vitexin, 2.5 μg; (D) RBC + H2O2 + isovitexin, 2.5 μg; (E) RBC + H2O2 + vitexin, 10 μg; (F) RBC + H2O2 + isovitexin, 10 μg.
3.7. Effect of C-glycosyl flavones on cell viability of cervical carcinoma cells
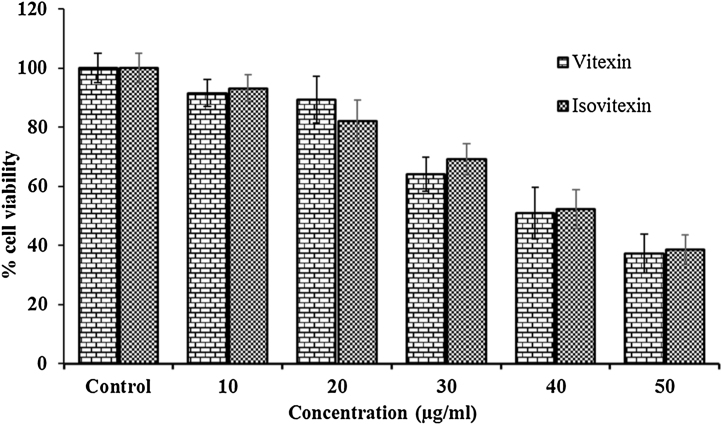
C-Glycosyl flavones tested on HeLa (cervical cancer) cells showed a dose-dependent reduction in the viable cell numbers (Fig. 9). Cell viability was determined by the MTT assay. The absorbance of the untreated control was considered as 100%. The percentage reduction in MTT absorbance reveals apoptotic ability of the C-glycosyl flavones in HeLa cells. Concentration dependent effects of vitexin and isovitexin on cell viability are shown in Fig. 9. The viable cells were significantly reduced in vitexin and isovitexin treated cervical carcinoma cells. At concentrations of 10, 20, 30, 40 and 50 μg/mL, vitexin and isovitexin reduced the viable HeLa cells by 9, 11, 36, 50, 63% and 7, 18, 31, 48 and 62%, respectively (Fig. 9).
Fig. 9.
Effect of C-glycosyl flavones on HeLa cells. Cell viability (%) upon treatment with different concentrations of vitexin and isovitexin. Values are means ± SD of three replicates.
3.8. DAPI staining
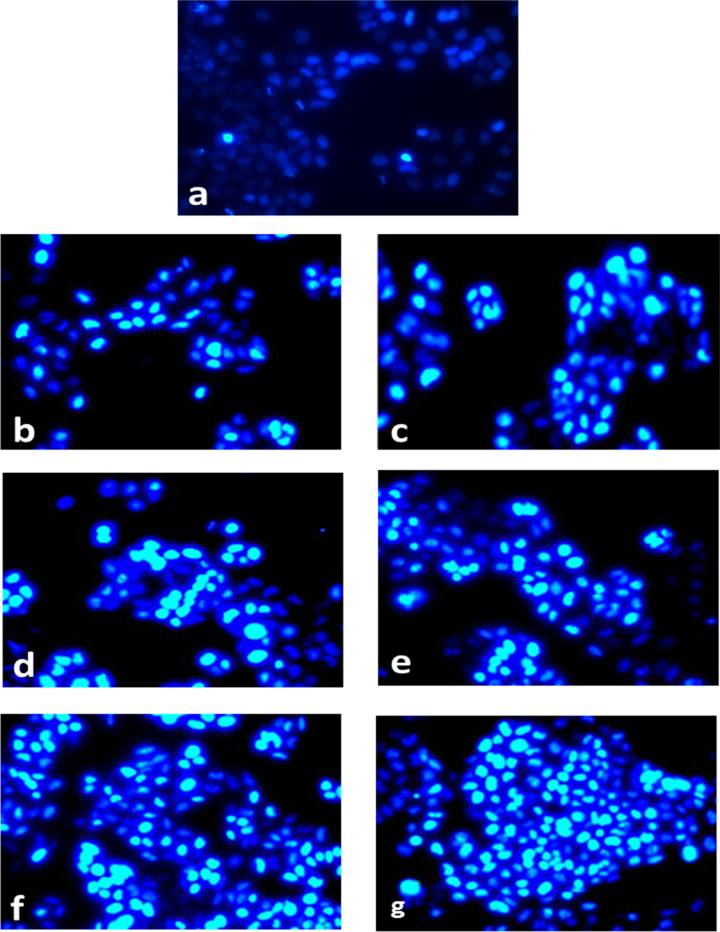
Effect of C-glycosyl flavones such as vitexin and isovitexin were studied for their nuclear fragmentation ability by staining with DAPI using fluorescence microscopy. Cells stained with DAPI were photographed under a fluorescence microscope (Fig. 10a–g). The results indicated that untreated cells had homogeneous nuclei, whereas C- glycosyl flavones treated cells showed condensed nuclei and apoptotic bodies in a dose dependent manner (Fig. 10b–g).
Fig. 10.
Microscopic photographs of control HeLa cells by DAPI staining. Apoptotic HeLa cells resulting from the treatment with 30, 40 and 50 μg of vitexin and isovitexin are represented as (a) control HeLa cells without sample treatment, (b, d and f) (30, 40 and 50 μg of vitexin) and (c, e and g) (30, 40 and 50 μg of isovitexin), respectively.
3.9. Effect of C-glycosyl flavones on the levels of apoptosis-associated protein expression in cervical carcinoma cells
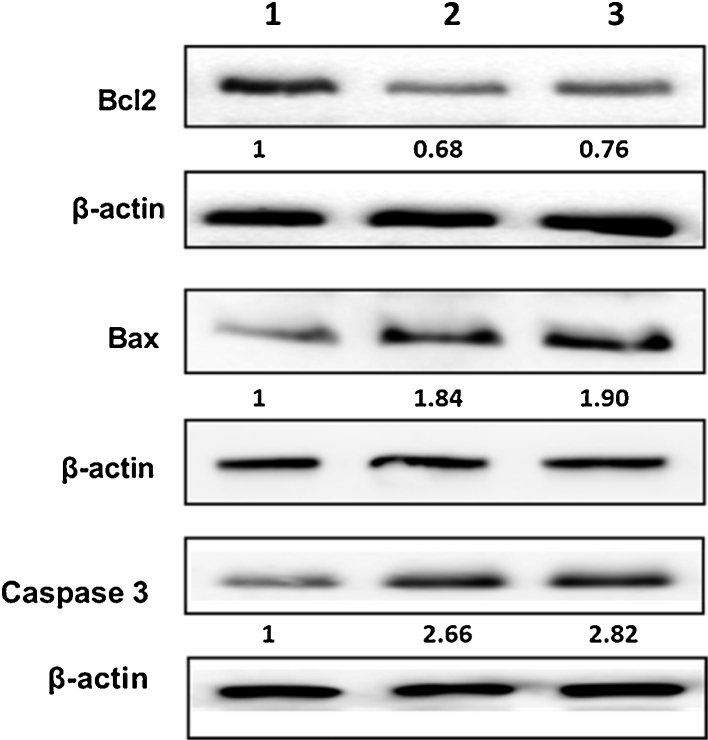
Apoptosis is an ordered cascade of enzymatic events that culminates in cell death and the cleavage of DNA into characteristic nucleosomal fragments [21]. Bax is a proapoptotic protein and it has been shown to induce the activation of caspases in vivo and in vitro [7], [22]. We have examined the apoptosis-associated protein expression levels in HeLa cells treated with vitexin (40 μg/mL) and isovitexin (41 μg/mL). The data observed indicated that vitexin and isovitexin triggered the death of HeLa cells through the oxidative stress mediated signaling pathway. Both the compounds downregulated the protein expression of Bcl2 with the simultaneous upregulation of Bax and caspase 3 (Fig. 11) in cervical carcinoma cells.
Fig. 11.
Effect of vitexin and isovitexin on apoptosis-associated protein expression in HeLa cells. Cells were treated with 40 μg of vitexin and 41 μg of isovitexin for 24 h and then harvested for western blotting to examine the protein levels of Bcl-2, Bax and Caspase-3. Lane 1 Untreated HeLa cells (control); Lane 2 HeLa cells + vitexin (40 μg); Lane 3 HeLa cells + isovitexin (41 μg).
4. Conclusions
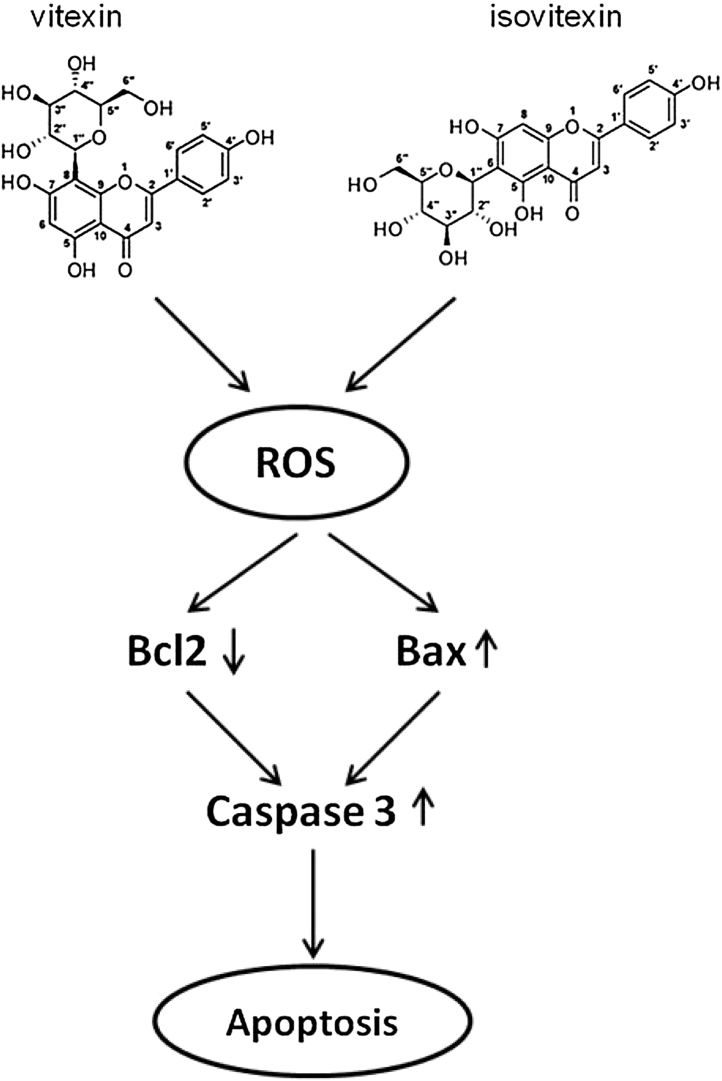
Black gram milled by-products were found to be rich in bioactive constituents. This study reports that black gramhusk C-glycosyl flavones, such as vitexin and isovitexin were effective against radical induced oxidative stress. Both vitexin and isovitexin exhibited antioxidant activities and anticancer activity against HeLa cells. The stimulation of apoptotic cell death by vitexin and isovitexin of cancer cells was due to the activation of Bax and caspase-3 and the proposed mechanism is shown in Fig. 12. As husk is rich in antioxidant compounds such as vitexin and isovitexin, it can be a good source of nutraceuticals.
Fig. 12.
Proposed mechanism of vitexin and isovitexin triggered apoptotic death of HeLa cells. The flow chart shows that vitexin and isovitexin induced apoptosis through the oxidative stress pathway and caspase 3 dependent signaling in cervical carcinoma cells in vitro.
Acknowledgements
Authors are thankful to Prof. Ram Rajasekharan, Director, CFTRI for his interest in this study. T K. Girish thanks Indian Council of Medical Research, New Delhi, India for the award of Senior Research Fellowship.
References
- 1.Ajila C.M., Naidu K.A., Bhat S.G., Rao U.J.S.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007;105:982–988. [Google Scholar]
- 2.Ajila C.M., Prasada Rao U.J.S. Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangifera indica L. peel extract. Food Chem. Toxicol. 2008;46:303–309. doi: 10.1016/j.fct.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee B., Rao G.R. Superhelical density of goat mitochondrial DNA: fluorimetric studies. Indian J. Biochem. Biophys. 1994;31:77–79. [PubMed] [Google Scholar]
- 4.Choi J.S., Islam M.N., Ali M.Y., Kim E.J., Kim Y.M., Jung H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014;64:27–33. doi: 10.1016/j.fct.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Choo C.Y., Sulong N.Y., Man F., Wong T.W. Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J. Ethnopharmacol. 2012;142:776–781. doi: 10.1016/j.jep.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 6.Diez-Silva M., Dao M., Han J., Lim C.-T., Suresh S. Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull. Mater. Res. Soc. 2010;35:382–388. doi: 10.1557/mrs2010.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finucane D.M., Bossy-Wetzel E., Waterhouse N.J., Cotter T.G., Green D.R. Bax-induced caspase activation and apoptosis via cytochromec release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y., Zu Y., Liu W., Zhang L., Tong M., Efferth T., Kong Y., Hou C., Chen L. Determination of vitexin and isovitexin in pigeonpea using ultrasonic extraction followed by LC–MS. J. Sep. Sci. 2008;31:268–275. doi: 10.1002/jssc.200700312. [DOI] [PubMed] [Google Scholar]
- 9.Gee J.M., DuPont M.S., Rhodes M.J., Johnson I.T. Quercetin glucosides interact with the intestinal glucose transport pathway. Free Radic. Biol. Med. 1998;25:19–25. doi: 10.1016/s0891-5849(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 10.Girish T.K., Pratape V.M., Prasada Rao U.J.S. Nutrient distribution, phenolic acid composition, antioxidant and alpha-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and its milled by-products. Food Res. Int. 2012;46:370–377. [Google Scholar]
- 11.Girish T.K., Vasudevaraju P., Prasada Rao U.J.S. Protection of DNA and erythrocytes from free radical induced oxidative damage by black gram (Vigna mungo L.) husk extract. Food Chem. Toxicol. 2012;50:1690–1696. doi: 10.1016/j.fct.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Hooper L., Kroon P.A., Rimm E.B., Cohn J.S., Harvey I., Cornu K.A.L., Ryder J.J., Hall W.L., Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 13.Jayaram S., Kapoor S., Dharmesh S.M. Pectic polysaccharide from corn (Zea mays L.) effectively inhibited multi-step mediated cancer cell growth and metastasis. Chem. Biol. Interact. 2015;235:63–75. doi: 10.1016/j.cbi.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor S., Dharmesh S.M. Physiologically induced changes in bound phenolics and antioxidants, DNA/cytoprotective potentials in pectic poly/oligosaccharides of tomato (Solanum lycopersicum) J. Sci. Food Agric. 2016 doi: 10.1002/jsfa.7696. [DOI] [PubMed] [Google Scholar]
- 15.Kim D.-K., Kim J.-B., Chon S.-U., Lee Y.-S. Antioxidant potentials and quantification of flavonoids in mung bean (Vigna radiata L.) seeds. Plant Resour. 2005;8:122–129. [Google Scholar]
- 16.Kim S.-J., Zaidul I.S.M., Maeda T., Suzuki T., Hashimoto N., Takigawa S., Noda T., Matsuura-Endo C., Yamauchi H. A time-course study of flavonoids in the sprouts of tartary (Fagopyrum tataricum Gaertn.) buckwheats. Sci. Hortic. 2007;115:13–18. [Google Scholar]
- 17.Linderkamp O., Kiau U., Ruef P. Cellular and membrane deformability of red blood cells in preterm infants with and without growth retardation. Clin. Hemorheol. Microcirc. 1997;17:279–283. [PubMed] [Google Scholar]
- 18.Nagababu E., Rifkind J.M. Reaction of hydrogen peroxide with ferrylhemoglobin: superoxide production and heme degradation. Biochemistry. 2000;39:12503–12511. doi: 10.1021/bi992170y. [DOI] [PubMed] [Google Scholar]
- 19.Nagababu E., Chrest F.J., Rifkind J.M. Hydrogen-peroxide-induced heme degradation in red blood cells: the protective roles of catalase and glutathione peroxidase. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2003;1620:211–217. doi: 10.1016/s0304-4165(02)00537-8. [DOI] [PubMed] [Google Scholar]
- 20.Okoko T., Ere D. Antioxidant activities of Solenostemon monostachyus leaf extract using in vitro methods. Sci. Res. Essays. 2012;7:621–626. [Google Scholar]
- 21.Pawlowski J., Kraft A.S. Bax-induced apoptotic cell death. Proc. Natl. Acad. Sci. 2000;97:529–531. doi: 10.1073/pnas.97.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossé T., Olivier R., Monney L., Rager M., Conus S., Fellay I., Jansen B., Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 23.Sancar A., Lindsey-Boltz L.A., Unsal-Kaçmaz K., Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 24.Scatchard G. The attractions of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 1949;51:660–672. [Google Scholar]
- 25.Stevenson D.E., Hurst R.D. Polyphenolic phytochemicals—just antioxidants or much more? Cell. Mol. Life Sci. 2007;64:2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vauzour D., Rodriguez-Mateos A., Corona G., Oruna-Concha M.J., Spencer J.P.E. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walgren R.A., Lin J.T., Kinne R.K.H., Walle T. Cellular uptake of dietary flavonoid quercetin 4′-β-glucoside by sodium-dependent glucose transporter SGLT1. J. Pharm. Expert Ther. 2000;294:837–843. [PubMed] [Google Scholar]
- 28.Yu L. Free radical scavenging properties of conjugated linoleic acids. J. Agric. Food Chem. 2001;49:3452–3456. doi: 10.1021/jf010172v. [DOI] [PubMed] [Google Scholar]
- 29.Yu L., Haley S., Perret J., Harris M., Wilson J., Qian M. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Bao B., Lu B., Ren Y., Tie X., Zhang Y. Determination of flavone C-glucosides in antioxidant of bamboo leaves (AOB) fortified foods by reversed-phase high-performance liquid chromatography with ultraviolet diode array detection. J. Chromatogr. A. 2005;1065:177–185. doi: 10.1016/j.chroma.2004.12.086. [DOI] [PubMed] [Google Scholar]