Abstract
Consumption of repeatedly heated cooking oil (RHCO) has been a regular practice without knowing the harmful effects of use. The present study is based on the hypothesis that, heating of edible oils to their boiling points results in the formation of free radicals that cause oxidative stress and induce damage at the cellular and molecular levels. Peroxide value of heated oil, histopathological alterations, antioxidant enzyme levels and blood biochemistry were determined in Wistar rats treated with the RHCO. RHCO revealed higher peroxide value in comparison to oil that has been unheated or singly heated. Histopathological observation depicted significant damage in jejunum, colon and liver of animals that received oil heated repeatedly for 3 times. The altered antioxidant status reflects an adaptive response to oxidative stress. Alteration in the levels of these enzymes might be due to the formation of reactive oxygen species (ROS) through auto oxidation or enzyme catalyzed oxidation of electrophilic components within RHCO. Analysis of blood samples revealed elevated levels of glucose, creatinine and cholesterol with declined levels of protein and albumin in repeatedly heated cooking oil group. Hematological parameters did not reveal any statistically significant difference between treated and control groups. Results of the present study confirm that the thermal oxidation of cooking oil generates free radicals and dietary consumption of such oil results in detrimental health effects.
Keywords: Repeatedly heated cooking oil, Peroxide value, Oxidative stress, Hematological parameters
1. Introduction
There were about 14.1 million cancer cases around the world in 2012. This number is expected to increase to 24 million by 2035. Colorectal cancer (CRC) is the third most commonly diagnosed and leading cause of cancer deaths in both men and women. Decades of expensive and replicating research has little impact on primary prevention of CRC. Sporadic cancer incidence attributes to 6% rate while environment and lifestyle constitute two third cases of CRC incidence (http://www.wcrf.org). Increased risk is attributed to factors like obesity, physical inactivity, alcohol consumption, long-term smoking, increased consumption of meat and fat rich food and low intake of fruits and vegetables [23]. Though several environmental chemicals have been implicated as the contributing factors to CRC in humans, one group of chemicals, the polycyclic aromatic hydrocarbons (PAHs) have generated the most interest as they are formed during cooking at high temperatures [41]. They are a single large family of compounds that have the potential to contribute significantly to dietary contamination, human intake and development of gastrointestinal tract (GIT) cancers [9]. Epidemiological studies have shown that diet contributes to 80% of the known CRC cases [6]. Foods like vegetables, fruits, oils, dairy products and meat are more prone to contamination with PAH during processing of food, cooking methods, time, temperature, amount of fat/oil added [38]. In this context, understanding the role of chemicals that are generated in food stuffs during its preparation towards the development of GIT cancers is important. Several studies on formation of mutagens during preparation of food have been done earlier but most of them were on items/cooking methods that are most often in practice in developed countries.
In India, consumption of fried foods made in road side eateries, food outlets in markets and restaurants is quite common. Socioeconomic status of people determines their food intake pattern. For example, in India people from low income group subsist on fried foods in roadside stalls. It has been reported in a survey that 48% of people consumed fried food 1–6 times/week [7]. Snacks account for 21% of all meals with the major types of snacks consumed constituting shallow and deep-fried foods [8]. Repeated heating of oils at high temperatures (160–190 °C) over a long period of time predisposes the oil to thermal oxidation, hydrolysis and polymerization with a configuration change of fatty acid from cis to trans isomers and accelerates the formation of oxidized and polymerized lipid species in the frying medium [18]. Repeated heating changes the physical appearance of the oil with increase in its viscosity, darkening in color, foaming and decrease in smoke point making it harmful for human consumption.
Few studies in India evaluated the genotoxic potential of such heated oils [49], [50] but to our knowledge, no attempt has been made till date to evaluate the harmful consequences of the usage of repeatedly heated cooking oil (RHCO) in Hyderabad, in particular. Several investigations that have been carried out in animals demonstrate that consumption of RHCO increases the presence of reactive oxygen species (ROS) and thus a decreased radical scavenging activity and thereby oxidative stress [17]. Use of RHCO is known to induce genotoxicity [10] and there by carcinogenicity [45]. Deleterious health effects of consumption of RHCO like increased blood pressure [11], [40], [18], [19], risk of cardiovascular diseases [24], [26], [27], [28], endothelial dysfunction [21], impaired vasorelaxation responses [33], hypertension [48], increased lipid peroxidation and LDL [12], [46] and atherosclerosis [1] are available in literature. Several investigations in rats also revealed functional changes in blood vessels, changes in serum alkaline phosphatase, aspartate aminotransferase and alanine aminotransferase levels [34]; intestinal damage and impaired function, mal-absorption of glucose [30]; impaired kidney function with increased blood pressure [32]. In contrast few studies reported no significant damage induced by use of RHCO in animals [44]. In vitro cytotoxicity assays in Hep G2 cell lines suggested that extract of fish oil that has been repeatedly boiled and heated for frying has substantial cytotoxic potential [36]. In contrast, samples of six cooking oils with different levels of unsaturation both heated and unheated did not show any mutagenicity with Ames test, with or without metabolic activation [51].
The objective of a study from Kuala Lumpur was to determine the level of knowledge, attitude and practice of night market food outlet operators regarding the usage of RHCO. The data collected from the 100 by face-to-face interview using a questionnaire showed that 67.0% agreed this to be not a good practice, 69.0% agreed that the use of RHCO is detrimental to health and 63% admitted that they had used RHCO [4]. In view of the information from various investigations done earlier, the present study has been designed to investigate the harmful consequences of consumption of RHCO (here vegetable oil) in Wistar rats. Peroxide value is a useful method to determine the quality of oil. It is an index to measure the concentration of hydroperoxide, which is formed during lipid oxidation [13]. Since there is dearth of information on the oxidative stress induced pathogenesis, antioxidant status was assessed in the animals by assessment of levels of radical scavenging enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) and rate of lipid peroxidation (LPO). This gives a measure of exposure induced oxidative stress that results in inflammation and damage to macromolecules including DNA, proteins and lipids [5]. Further, exposure dependent changes (if any) in blood biochemistry was estimated in the present investigation by determination of glucose (GLU), cholesterol (CHOL), creatinine (CRE), protein (PRO) and albumin (ALB). The evaluation of hematological parameters like total red blood cell count (RBC) and white blood cell count (WBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), hemoglobin (Hb) and hematocrit gives the profile of perturbations in blood if any, following consumption of RHCO. The present investigation is thus based on the hypothesis that consumption of RHCO contributes to intestinal tumor development through altered biotransformation and generation of free radicals that in turn induce damage at cellular and molecular level.
2. Materials and methods
2.1. Preparation of oil sample for treatment
The refined vegetable cooking oil (5 L) of a standard food grade was purchased from the local market. An aliquot of oil (1 L) was separated and labeled as unheated cooking oil (UHCO). Another aliquot of oil (4 L) was heated (above 300 °C) above its smoke point for 30 min and then cooled to room temperature. From this, a sample of 1L was separated and labeled as singly heated cooking oil (SHCO). The same process was repeated to obtain oil heated 3 times (3RHCO). This process of heating and cooling of the oil was performed without addition of fresh oil. The viscous dark brown oil sample (approximately 2L) thus obtained was then stored in amber color bottles to prevent photodegradation of PAHs.
2.2. Analysis of constituents of oil
The quality of oil used in this study for treatment was investigated by determination of its peroxide value (PV) using standard titration method by American Oil Chemists' Society (AOCS). This method determines all components, generally assumed to be peroxides or other similar products of fatty acid oxidation. PV is expressed in terms of milliequivalents of peroxide per 1000 g of test sample that oxidizes potassium iodide under test conditions (mEqO2/kg) (Fig. 1).
Fig. 1.
Schematic representation of experimental design.
2.3. Animal treatment
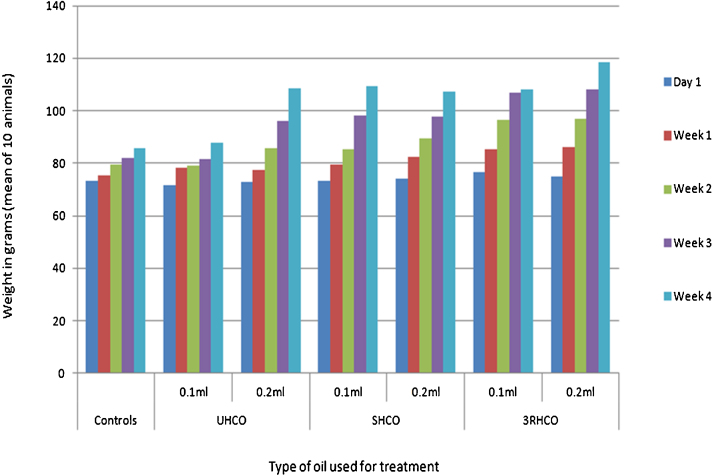
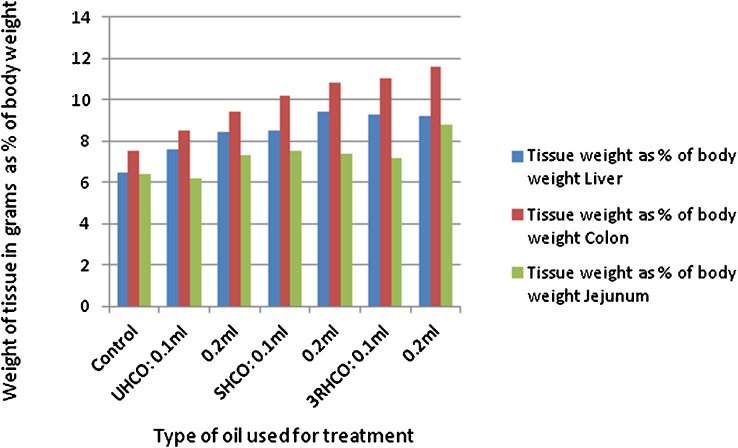
Institutional animal ethical committee approval was taken prior to the beginning of the study. Twenty eight day repeated oral dose toxicity study was performed using male and female rats based on OECD guideline 407 [2008]. Wistar rats of 6–8 week age and weighing approximately 80–120 g were purchased from the research animal suppliers in India (National Institute of Nutrition, Hyderabad). Rats (n = 10; 5 male and 5 female in each treatment group) were then kept in laboratory grade polycarbonate cages and housed in institutional animal care facility to ensure humane care and use of laboratory animals. All animals were allowed a seven-day acclimation period prior to being randomly assigned to the following treatment categories: (i) Unexposed control rats (diet only) (ii) rats treated with unheated cooking oil (UHCO) (iii) rats treated with singly heated cooking oil (SHCO) (iv) rats treated with repeatedly heated cooking oil (3RHCO). Animals were administered 0.10 mL and 0.20 mL of oils of above heated grades via oral gavage for 28 days. The test animals were observed for symptoms and mortality each day during the treatment period. Body weight of animals in control and treated groups were recorded initially at the start of the study and then weekly thereafter. The mean body weight of animals is given in Fig. 2. All treated rats were anesthetized using 3.5% isoflurane in a vapor induction chamber at the end of 28 day treatment. Jejunum, colon and liver were collected from the animals. Organ weights were also recorded (Fig. 3).
Fig. 2.
Effect of treatment on the body weight of animals (Data represented as mean; n = 10; *P < 0.05).
Fig. 3.
Effect of treatment on the organ weight in animals (Data represented as mean; n = 10; *P < 0.05).
2.4. Sample collection
Blood samples were collected from animals in the morning hours just before sacrifice. 3 mL of blood was allowed to clot and centrifuged at 1500g for 10 min for separation of serum for biochemical analysis. The sera were stored at −40 °C until analysis. Tissues of interest such as jejunum, colon and liver were retrieved. The size and number of adenomas in small intestine and colon were recorded. Tissues were then rinsed in ice cold physiological saline and perfused with cold potassium chloride buffer (1.15% KCl and 0.5 mM EDTA) and homogenized in potassium phosphate buffer (KPB, 0.1 M, pH 7.4). The homogenate was then centrifuged at 15000 rpm for 30 min to remove debris. The clear supernatant was collected and stored as aliquots in −80 °C until antioxidant enzyme assays were done. A part of each tissue was stored in 10% formalin for histopathological examinations.
2.5. Histopathological analysis
The tissues (jejunum, colon and liver) obtained from the above-mentioned control and treatment groups were subjected to histopathological examination using H and E staining procedure. With the help of a pathologist, the tissues slides were evaluated for hyperplasias, adenomas and carcinomas. Additionally, they were scored for invasive adenocarcinomas (if any). The intestines were separated from the mesentery with a pair of fine scissors. The large intestine was excised into proximal and distal colon, and rectum portions. The intestine was opened longitudinally and spread in a petri dish and gently rinsed with physiological saline to flush the ingesta. Before the intestines were processed for histopathological studies, the size (>2.5 mm and >5 mm), number (single and multiple) and type (adenoma, carcinoma, degree of dysplasia) of lesions in the small intestine and colon were recorded. The scheme for classification of lesions is as described by Ramesh et al. [42]. The intestines were preserved using the Swiss roll technique for histopathological examination (Boivin et al., 2003). The tissues were processed in a Leica TP 1020 tissue processor and embedded in paraffin blocks using Leica EG 1160 paraffin embedder. The paraffin blocks were cut into ribbons of 4 mm using a Microm HM 360 microtome. The slides were stained in hemotoxylin and eosin using a Microm HMS-70 stainer. The permanent slides were made and evaluated for histopathological changes under Olympus BX51 microscope. The slides were coded to avoid possible bias before analysis.
2.6. Antioxidant enzyme levels
The protein content in the tissue supernatant was estimated using standard protocol Lowry et al., 1951. Bovine serum albumin was used as standard.
2.6.1. Superoxide dismutase
Superoxide dismutase (SOD) activity was estimated in tissue supernatant following the method of Marklund and Marklund [22]. The rate of inhibition of pyrogallol auto-oxidation after the addition of enzyme extract was noted at 420 nm using Spectramax Plus spectrophotometer (Molecular Devices, USA). One unit of enzyme is the amount of enzyme that causes 50% inhibition of pyrogallol auto-oxidation. The enzyme activity was expressed as units per milligram protein (U/mg).
2.6.2. Catalase
The activity of catalase was estimated using the method of Aebi [2] spectrophotometrically. The decrease in absorbance for 1 min at 240 nm was recorded. One unit of enzyme is defined as one μmol H2O2 utilized per minute. The activity was expressed as units per milligram protein (U/mg).
2.6.3. Glutathione peroxidase
The activity of glutathione peroxidase (GPx) was measured similarly as described by Paglia and Valentine [35]. The amount of enzyme that oxidizes one μmol NADPH per min was considered to be one unit (nmol/mim/mL). The GPx activity was expressed in units/mg protein (U/mg).
2.6.4. Lipid peroxidation
Malondialdehyde, an end product of lipid peroxidation in tissue homogenate, was measured according to the method described by Wills (1969) with minor modifications. The absorbance of the pink colored extract in n-butanol was measured at 532 nm. The amount of MDA was expressed as nano moles of MDA formed per gram wet weight of tissue.
2.7. Blood biochemistry
The levels of blood biochemical parameters like GLU, CRE, CHOL, PRO, and ALB were determined using standard kits from Bayer Diagnostics, India. The results were expressed as milligrams per decilitre of serum used.
2.8. Hematological parameters
The following hematological markers were measured: hemoglobin (Hb), haematocrit (HCT), total erythrocyte count (TEC), total leukocyte count (TLC) and mean corpuscular volume (MCV) using auto blood analyzer (Express Plus, Bayer Corporation, USA).
2.9. Statistical analysis
Statistical analysis was performed using Graph Pad Instat Prism 3 Software package for windows (Graph Pad Software, Inc., La Jolla, CA, USA). All results for body weight and organ weight of animals were expressed as mean and standard deviation (mean ± SD) of ten animals. The statistical analysis of data between control and treated groups for levels of antioxidant enzymes, blood biochemistry and hematological parameters was done by one-way ANOVA. All the parameters were estimated in duplicate for each sample and the value was given as mean of each sample and treatment group. Significance was determined at P < 0.05.
3. Results
3.1. Animal observation, food consumption, body weight and organ weight
No mortality, adverse signs and symptoms were observed in animals after 28 days of oral administration with oils of different heating grades. Significant alterations in body weight and relative organ weight (liver) with lowered rate of food consumption were observed in animals receiving 0.1 mL and 0.2 mL of 3RHCO when compared to control and other group of rats. This was not statistically significant. However, the other group of animals receiving 0.1 mL and 0.2 mL UHCO and SHCO did not show any significant changes in feed intake, body and organ weights (Fig. 2, Fig. 3).
3.2. Analysis of constituents of oil
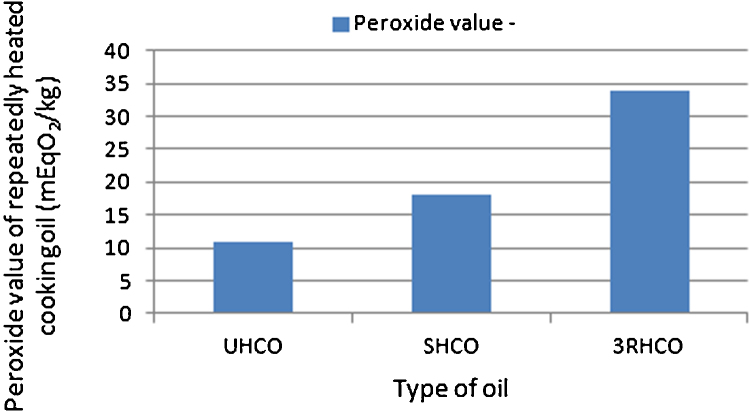
In this study, the quality of 3 individual cooking oil samples of different heating grades (UHCO, SHCO, 3RHCO) used for treatment of animals were analyzed for the PV using the standard titration method by AOCS. The results show that the PV of 3RHCO was 34mEqO2/kg, whereas the PV of SHCO and UHCO was found to be 18mEqO2/kg and 11mEqO2/kg (Fig. 4).
Fig. 4.
Peroxide value of oils of different heating grades (mEqO2/kg; Data represented as mean; n = 10; *P < 0.05).
3.3. Histopathological analysis
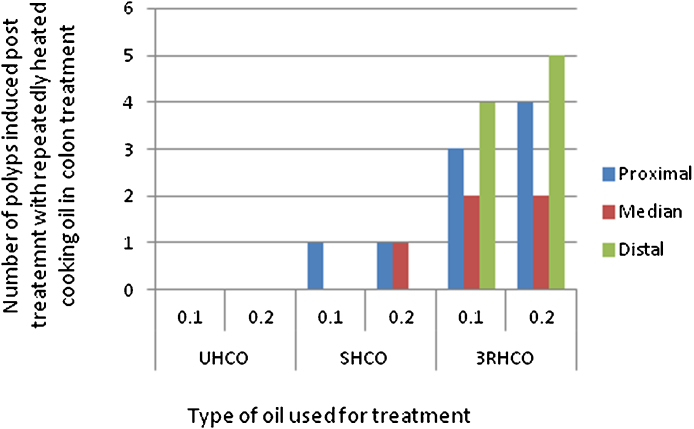
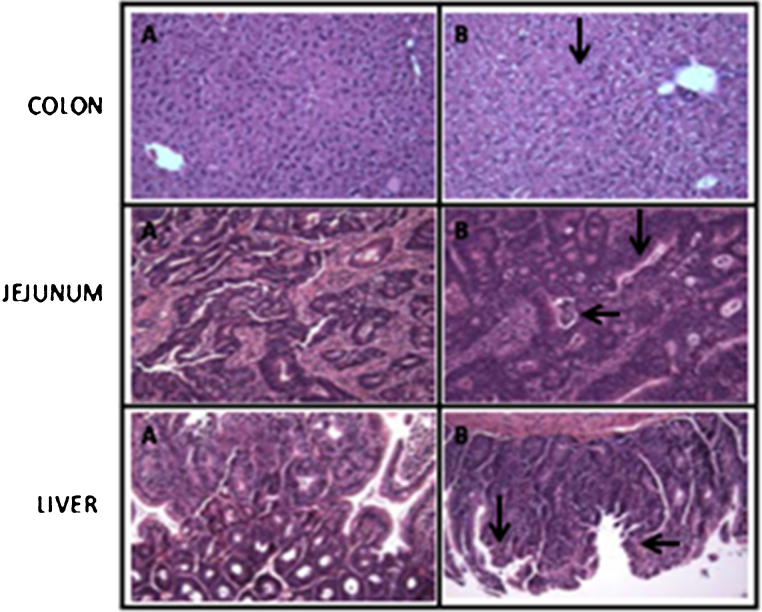
Colon polyps were observed in SHCO and 3RHCO treated rats. Number and distribution of polyps in colon of treated rats in both 0.1 mL and 0.2 ml is summarized in Fig. 5. Polyp type observed in colon of these rats was mostly adenomas. The vacuolated swelling of the cytoplasm of the hepatocytes of the 3RHCO treated rats was observed. Dysplasia and hypertrophy of the sinusoidal Kupffer cells was also observed. This change was more prominent at 3RHCO with dose of 0.2 mL. The routine histologic examination of the colon reveals significant colonic mucosal abnormality like the increased muscle layer thickness of the muscularis mucosa, submucosa and muscularis propria and increased number of aberrant crypt per focus (Fig. 6).
Fig. 5.
Effect of treatment on occurrence and distribution of polyps in the colon of mice (number of polyps).
Fig. 6.
Histopathological representation of tissues, Control (A) and 3RHCO treated (B) colon, jejunum and liver of animals after 28 day treatment (H & E, 400X).
3.4. Antioxidant enzyme levels
The level of antioxidant enzymes like SOD, GPx and MDA were significantly elevated in 3RHCO treated group in comparison to UHCO and SHCO groups. The level of CAT was significantly reduced in these groups in a similar trend. The results were statistically significant in 3RHCO group than in UHCO and SHCO in comparison to controls. The levels of SOD, GPx and MDA were higher and CAT was lowered in 0.2 mL treated animals in 3RHCO, SHCO and UHCO than in 0.1 mL dose group but was not statistically significant (Table 1).
Table 1.
Inter group comparison of antioxidant enzyme levels.
| Parameter | SOD (U/mg) |
GPx (U/mg) |
CAT (U/mg) |
MDA (nmol/g) |
||||
|---|---|---|---|---|---|---|---|---|
| Group | Liver | Colon | Liver | Colon | Liver | Colon | Liver | Colon |
| Control | 4.25 ± 0.47 | 3.23 ± 0.48 | 5.24 ± 0.41 | 2.84 ± 0⋅34 | 205.32 ± 20.80 | 200.31 ± 20⋅15 | 14.45 ± 1.14 | 13.26 ± 1⋅32 |
| UHCO: 0.1 mL 0.2 mL |
7.27 ± 0.64 8.32 ± 0.74 |
5.21 ± 0⋅57 5.84 ± 0⋅42 |
14.17 ± 1.45 14.57 ± 1.42 |
7.86 ± 0⋅71 8.21 ± 0⋅73 |
203.85 ± 18.57 203.67 ± 22.29 |
205.46 ± 19⋅23 207.49 ± 17⋅26 |
15.72 ± 1.32 16.75 ± 1.49 |
14.89 ± 1.41 14.96 ± 1.39 |
| SHCO: 0.1 mL 0.2 mL |
12.25 ± 0.84 12.27 ± 0.18 |
7.84± 0⋅72 8.23 ± 0⋅74 |
17.23 ± 1.69 17.31 ± 1.62 |
11.16 ± 1⋅06 12.37 ± 1⋅17 |
153.68 ± 14.42 163.62 ± 13.13 |
150.24 ± 12⋅34 145.26 ± 13⋅24 |
16.02 ± 1.47 17.05 ± 1.45 |
14.21 ± 1⋅24 14.01 ± 1⋅20 |
| 3RHCO: 0.1 mL 0.2 mL |
23.86 ± 1.34 24.16 ± 1⋅45∗ |
12.30 ± 1⋅15 14.31 ± 1⋅39∗ |
27.18 ± 1.61 27.80 ± 1.72∗ |
15.67 ± 1⋅42∗ 14.39 ± 1⋅37 |
137.67 ± 13.80 139.28 ± 13.05∗ |
126.23 ± 10⋅26 130.26 ± 11⋅23∗ |
28.26 ± 1⋅84 29.27 ± 2⋅01∗ |
23.76 ± 1⋅74∗ 22.71 ± 1⋅94 |
Data represented as mean ± S.D. Significantly different from control at *P < 0.05; n = 10 animals per group.
3.5. Blood biochemistry
GLU levels in rats treated with 0.02 mL of 3RHCO was found to be high in comparison to those treated with 0.2 ml of SHCO, UHCO versus controls. Results were statistically significant (mg/dl: 82.09; 70.97 versus 70.42). Similar trend in results was observed in those that were fed 0.1 mL of 3RHCO (mg/dl: 86.30 ± 2.04; 81.62 ± 1.77; 70.93 ± 0.92 versus 70.42 ± 5.87). However no significant difference was found between those fed 0.2 mL and 0.1 mL of oils. Similar was the result for CHOL (mg/dl, 249.99 ± 13.79; 174.19 ± 13.60; 159.14 ± 14.92: 247.65 ± 14.81; 165.63 ± 12.68; 152.72 ± 13.64 versus 143.06 ± 13.06) and CRE (mg/dl, 1.71 ± 0.91; 1.43 ± 0.26; 1.31 ± 0.12: 1.65 ± 0.62; 1.35 ± 0.28; 1.29 ± 0.17 versus 0.98 ± 0.26) levels. In contrast, the PRO (3.76 ± 0.43; 3.57 ± 0.42); and ALB (1.59 ± 0.70; 1.52 ± 0.64) levels showed statistically significant reduced levels in 0.1 mL and 0.2 mL, 3RHCO in comparison to SHCO (4.83 ± 0.53, 4.77 ± 0.46; 2.95 ± 0.72, 2.63 ± 0.75), UHCO (5.09 ± 1.23, 4.91 ± 1.32; 4.05 ± 1.12, 3.08 ± 0.62) and controls. Though there was no significant difference between the 0.2 mL and 0.1 mL groups (Table 2).
Table 2.
Inter group comparison of blood biochemistry parameters.
| Parameter | Dose (mL) | CONTROL | UHCO | SHCO | 3RHCO |
|---|---|---|---|---|---|
| GLU (mg/dL) | 0.1 | 70.42 ± 5.87 | 70.93 ± 0.92 | 81.62 ± 1.77 | 86.30 ± 2.04 |
| 0.2 | 70.97 ± 0.71 | 82.09 ± 1.46 | 87.75 ± 1⋅71* | ||
| CRE (mg/dL) | 0.1 | 0.98 ± 0.26 | 1.29 ± 0.17 | 1.35 ± 0.28 | 1.65 ± 0.62 |
| 0.2 | 1.31 ± 0.12 | 1.43 ± 0.26 | 1.71 ± 0.91* | ||
| CHOL (mg/dL) | 0.1 | 143.06 ± 13.06 | 152.72 ± 13.64 | 165.63 ± 12.68 | 247.65 ± 14.81 |
| 0.2 | 159.14 ± 14.92 | 174.19 ± 13.60 | 249.99 ± 13.79* | ||
| PRO (mg/dL) | 0.1 | 5.15 ± 0⋅42 | 5.09 ± 1.23 | 4.83 ± 0.53 | 3.76 ± 0.43* |
| 0.2 | 4.91 ± 1.32 | 4.77 ± 0.46 | 3.57 ± 0.42 | ||
| ALB (mg/dL) | 0.1 | 4.21 ± 1⋅15 | 4.05 ± 1.12 | 2.95 ± 0.72 | 1.59 ± 0.70* |
| 0.2 | 3.08 ± 0.62 | 2.63 ± 0.75 | 1.52 ± 0.64 |
Data represented as mean ± S.D. Significantly different from control at *P < 0.05; n = 10 animals per group.
3.6. Hematological parameters
The estimation of hematological parameters in the blood of rats from all the treatment groups in comparison to controls revealed that the levels were significantly reduced in 3RHCO in comparison to SHCO and UHCO. The results were not statistically significant. No significant difference in value was observed between 0.2 mL and 0.1 mL dose groups (Table 3).
Table 3.
Inter group comparison of hematological parameters.
| Parameter | Dose (mL) | Control | UHCO | SHCO | 3RHCO |
|---|---|---|---|---|---|
| Haemoglobin (g/dl) | 0.1 | 12.53 ± 1.21 | 12.25 ± 1.12 | 11.75 ± 1.15 | 9.26 ± 0.94* |
| 0.2 | 11.76 ± 1.16 | 11.25 ± 1.12 | 8.01 ± 0.64 | ||
| Haematocrit (%) | 0.1 | 34.21 ± 2.98 | 33.15 ± 1.34 | 31.26 ± 2.61 | 25.21 ± 1.26* |
| 0.2 | 32.18 ± 1.36 | 30.23 ± 2.75 | 22.85 ± 1.27 | ||
| Total erythrocyte count (x106/μl) | 0.1 | 3.91 ± 1.27 | 3.75 ± 0.37 | 3.21 ± 0.36 | 1.75 ± 0.17* |
| 0.2 | 3.19 ± 0.31 | 3.21 ± 0.35 | 1.55 ± 0.14 | ||
| Total leukocyte count (x103/μL) | 0.1 | 6.97 ± 0.63 | 6.85 ± 0.67 | 6.23 ± 0.53 | 3.01 ± 0.31 |
| 0.2 | 6.45 ± 0.65 | 6.75 ± 0.57 | 3.26 ± 0.36* | ||
| Mean corpuscular volume (pg) | 0.1 | 26.12 ± 0.87 | 25.94 ± 1.24 | 22.23 ± 1.23 | 19.75 ± 1.15* |
| 0.2 | 23.74 ± 1.26 | 21.27 ± 1.21 | 17.52 ± 1.21 |
Data represented as mean ± S.D. Significantly different from control at *P < 0.05; n = 10 animals per group.
4. Discussion
Over the last two decades, the urbanisation and increasing modernisation of India have transformed education, lifestyle, health-care and longevity that contributed to an increased risk profile for chronic diseases such as cancer [43]. Vegetable oil is one of the essential dietary components in daily food consumption. The practice of using RHCO is not uncommon as it reduces the cost of food preparation. However, the benefits of vegetable oil can be deteriorated by repeated heating that leads to lipid oxidation. The quality of dietary oils and fats has been widely recognized to be inextricably linked to the pathogenesis of various deleterious health effects.
Repeated heating of vegetable oils at high temperatures during cooking is a very common practice. Heating or boiling of oils is generally done during meal preparation for cooking or deep frying. Consumption of food cooked in such repeatedly heated oils pose a serious health hazard due to the formation of few nutritionally undesirable products [4]. It was also proven that fat content in the food and cooking at high temperatures significantly contribute to the formation of mutagens and cause cancer in different target sites [53]. Moreover, thermally oxidized lipids enhance peroxidation of membrane macromolecules, contributing to their mutagenicity and genotoxicity which could potentially lead to carcinogenesis. The practice of reusing oil during food preparation processes is widespread. This practice is not only confined to roadside food stalls; established food outlets in large cities also use this method to reduce costs. [47]. Mutagenic activity of heterocyclic amines during cooking of meat and fish by grilling on charcoal and broiling at high temperatures has been long demonstrated [37]. The purpose of this study was to elucidate the possible deleterious health effects of consumption of repeatedly heated vegetable oil in rats.
In the present study, the peroxide content in the oil used for treatment of test animals was determined using the standard titration method by Official Methods and Recommended Practices of the AOCS [31]. This method was chosen since it is highly empirical and any variation in procedure gives altered results. The analysis of PV of oil samples in this study was done to identify samples that have acceptable PV. According to the AOCS the upper limit of PV for edible oil is 10 mEqO2/kg. In Japan, the Food Sanitation Law Guidelines, set PV to not more than 30 mEqO2/kg. Report from Malaysia compared the oxidative quality of twice and four times heated palm and soybean oils, used to fry potato chips. Results revealed significantly increased peroxide values in both oils, with the four-times-heated oils having the highest peroxide values [16]. In accordance to the results from previous studies, the PV of thrice heated vegetable oil in our study was higher than singly or unheated oil. Determination of PV in oils determines that thermally oxidized oil generates ROS that has been implicated in several pathological processes.
Clinical analysis of blood samples revealed that GLU, CHOL and CRE levels were increased in rats when 3RHCO was fed in comparison to those that received SHCO and UHCO. This result is of concern since the enhancement in such parameters is deleterious to health. No studies have been reported that evaluated the blood biochemistry parameters in rats treated with RHCO. The effect of feeding heated vegetable oil on blood biochemical parameters in rats in this study indicates that consumption of heated oils produces indications of altered hematological levels of various blood cells. Similar results were reported by Hageman et al. [14]. However, the hematological status of the animals fed heated corn and peanut oils showed no effect on the proportions of cells comprising the total leukocytes of the blood [3].
Oxidative stress analysis showed a significant (P < 0·05) increase in the levels of antioxidant enzymes such as SOD, GPx and lipid peroxidation and decreased CAT levels. Similar results were reported in a study from India that evaluated the genotoxic and carcinogenic risks associated with the consumption of repeatedly heated coconut oil in Wistar rats in Refs. [49], [50]. It can hence be concluded that, dietary consumption of RHCO can cause genotoxic and pre neoplastic changes [19].
In Hyderabad, no attempt has been made till date to conduct a survey to evaluate the intensity of use of cooking oils that are being continually consumed by heating and reheating. Enzymatic and non-enzymatic antioxidants serve as an important biological defense against oxidative stress that counters the impact of ROS. These endogenously generated ROS react with the lipid content of the cells and result in lipid peroxidation in the tissues which oxidizes the base component of cell membranes and results in membrane disintegrity. Thus this investigation emphasizes the need of the hour with regard to usage of RHCO modification of oxidative injury. Further investigations are needed to affirm if DNA adduct formation or repair are involved in the effects of RHCO in induction of deleterious health effects. In conclusion, this study highlights the need on the level of knowledge of foods made in RHCO and needs to improve and ensure the safety of usage of foods made in such oils.
Declaration of interest
All the authors have read and approved the final manuscript. The authors do not report any declarations of interest and do not have any conflict of interest.
Acknowledgements
The authors thank the authorities at University of Hyderabad for providing facilities to carry out the work. The author (Dr. P.V. Rekha Devi) thanks University Grants Commission for providing financial support for the work through Dr. D.S. Kothari Post-Doctoral Fellowship Scheme (F.4-2/2006 (BSR)/13-1057/2013(BSR).
References
- 1.Adam S.K., Soleiman I.N., Umar N.A., Mokhtar N., Mohamed N., Jaarin K. Effects of repeatedly heated palm oil on serum lipid profile: lipid peroxidation and homocysteine levels in a post-menopausal rat model. Mcgill J. Med. 2008;11:145–1581. [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi H. Catalase. In: Bergmeyer H.U., editor. Vol. 2. Werlag Chemie; Weinheim: 1974. pp. 673–678. (Methods of Enzymatic Analysis). [Google Scholar]
- 3.Alexander J.C. Chemical and biological properties related to toxicity of heated fats. J. Toxicol. Environ. Health. 1987;7:125–138. doi: 10.1080/15287398109529964. [DOI] [PubMed] [Google Scholar]
- 4.Azman A., Mohd Shahrul S., Chan S.X., Noorhazliza A.P., Khairunnisak M., Nur Azlina M.F., Qodriyah H.M., Kamisah Y., Jaarin K. Level of knowledge: attitude and practice of night market food outlet operators in Kuala Lumpur regarding the usage of repeatedly heated cooking oil. Med. J. Mal. 2012;67:91–101. [PubMed] [Google Scholar]
- 5.Bayraktar N.M., Karagözler A.A., Bayraktar M. Investigation of the blood biochemical status of gas station workers. Toxicol. Environ. Chem. 2006;88:587–594. [Google Scholar]
- 6.Bingham S.A. Diet and colorectal cancer prevention. Biochem. Soc. Trans. 2000;28:12–16. doi: 10.1042/bst0280012. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty R., Bose K., Ulijaszek S.J. Income level and food intake patterns among male Bengalee slum dwellers in Kolkata, India. Mal. J. Nutr. 2009;15:19–25. [PubMed] [Google Scholar]
- 8.Dhruv S., Patel S., Iyer U. Snacking pattern of residents of Vadodara: a pilot study. Int. J. Appl.Biol. Pharm. Technol. 2011;2:81–87. [Google Scholar]
- 9.Diggs D.L., Huderson A.C., Harris K.L., Myers J.N., Banks L.D., Rekhadevi P.V., Niaz M.S., Ramesh A. Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. J. Environ. Sci. Health C. 2011;29:1–34. doi: 10.1080/10590501.2011.629974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dung C.H., Wu S.C., Yen G.C. Genotoxicity and oxidative stress of the mutagenic compounds formed in fumes of heated soybean oil, sunflower oil and lard. Toxicol. In Vitro. 2006;20:439–447. doi: 10.1016/j.tiv.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y.J., Lee R.M.K.W. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A2 production. Br. J. Pharmacol. 2001;134:1639–1646. doi: 10.1038/sj.bjp.0704420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrido-Polonio C., García-Linares M.C., García-Arias M.T., López-Varela S., García- Fernández M.C., Terpstra A.H.M. Thermally oxidized sunflower-seed oil increases liver and serum peroxidation and modifies lipoprotein composition in rats. Br. J. Nutr. 2004;92:257–265. doi: 10.1079/BJN20041174. [DOI] [PubMed] [Google Scholar]
- 13.Gotoh N., Wada S. The importance of peroxide value in assessing food quality and food safety. J. Am. Oil Chem. Soc. 2006;83:473–474. [Google Scholar]
- 14.Hageman G., Verhagen H., Schutte B. Biological effects of short-term feeding to rats of repeatedly used deep-frying fats in relation to fat mutagen content. J. Food Chem. Toxicol. 1991;29:689–698. doi: 10.1016/0278-6915(91)90127-s. [DOI] [PubMed] [Google Scholar]
- 16.Kamisah Y., Shamil S., Nabillah M.J., Kong S.Y., Hamizah N.A., Qodriyah H.M., Nur Azlina M.F., Azman A., Jaarin K. Deep-fried keropok lekors Increase Oxidative Instability in Cooking Oils. Mal. J. Med. Sci. 2012;19:57–62. [PMC free article] [PubMed] [Google Scholar]
- 17.Leong X.F., Aishah A., Nor Aini U., Das S., Jaarin K. Heated palm oil causes rise in blood pressure and cardiac changes in heart muscle in experimental rats. Arch. Med. Res. 2008;39:567–572. doi: 10.1016/j.arcmed.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Leong X.F., Najib M.N.M., Das S., Mustafa M.R., Jaarin K. Intake of repeatedly heated palm oil causes elevation in blood pressure with impaired vasorelaxation in rats. Tohoku J. Exp. Med. 2009;219:71–78. doi: 10.1620/tjem.219.71. [DOI] [PubMed] [Google Scholar]
- 19.Leong X.F., Salimon J., Mustafa M.R., Jaarin K. Effect of repeatedly heated palm olein on blood pressure-regulating enzymes activity and lipid peroxidation in rats. Mal. J. Med. Sci. 2012;19:20–29. [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Garcia E., Schulze M.B., Meigs J.B., Manson J.E., Rifai N., Stampfer M.J., Willett W.C., Hu F.B. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J. Nutr. 2005;135:562–566. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 22.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 23.Mohandas K.M. Colorectal cancer in India: Controversies, enigmas and primary prevention. Indian J. Gastroenterol. 2011;30:3–6. doi: 10.1007/s12664-010-0076-2. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D., Katan M.B., Ascherio A., Stampfer M.J., Willet W.C. Trans fatty acids and cardiovascular disease. New Engl. J. Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 26.Ng C.Y., Kamisah Y., Faizah O., Jaarin K. The role of repeatedly heated soybean oil in the development of hypertension in rats: association with vascular inflammation. Int. J. Exp. Pathol. 2012;93:377–387. doi: 10.1111/j.1365-2613.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng C.Y., Leong X.F., Masbah N., Adam S.K., Kamisah Y., Jaarin K. Heated vegetable oils and cardiovascular disease risk factors. Vasc. Pharmacol. 2014;61:1–9. doi: 10.1016/j.vph.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Ng C.Y., Leong X.F., Masbah N., Adam S.K., Kamisah Y., Jaarin K. Reprint of heated vegetable oils and cardiovascular disease risk factors. Vasc. Pharmacol. 2014;62:38–46. doi: 10.1016/j.vph.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Obembe A.O., Owu D.U., Okwari O.O., Antai A.B., Osim E.E. Intestinal Fluid and Glucose Transport in Wistar Rats following Chronic Consumption of Fresh or Oxidised Palm Oil Diet. ISRN Gastroenterol. 2011:972838. doi: 10.5402/2011/972838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Official Methods and Recommended Practices of the AOCS, 6th ed., 3rd printing Fats, Oils and Lipid Related Analytical Methods AOCS Official Method Ja 8-87.
- 32.Osim E.E., Owu D.U., Etta K.M. Arterial pressure and lipid profile in rats following chronic ingestion of palm oil diets. Afr. J. Med. Med. Sci. 1996;25:335–340. [PubMed] [Google Scholar]
- 33.Owu D.U., Orie N.N., Osim E.E. Altered responses of isolated aortic smooth muscle following chronic ingestion of palm oil diets in rats. Afr. J. Med. Med. Sci. 1997;26:83–86. [PubMed] [Google Scholar]
- 34.Owu D.U., Osim E.E., Ebong P.E. Serum liver enzymes profile of Wistar rats following chronic consumption of fresh or oxidized palm oil diets. Acta Trop. 1998;69:65–73. doi: 10.1016/s0001-706x(97)00115-0. [DOI] [PubMed] [Google Scholar]
- 35.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 36.Pandey M.K., Pant A.B., Das M. In vitro cytotoxicity of polycyclic aromatic hydrocarbon residues arising through repeated fish fried oil in human hepatoma Hep G2 cell line. Toxicol. In Vitro. 2006;20:308–316. doi: 10.1016/j.tiv.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Pariza M.W., Ashoor S.H., Chu F.S., Lund D.B. Effects of temperature and time on mutagen formation in pan fried hamburger. Cancer Lett. 1979;7:63–69. doi: 10.1016/s0304-3835(79)80097-x. [DOI] [PubMed] [Google Scholar]
- 38.Perez C., Lopez de Carain A., Bello J. Modulation of mutagenic activity in meat samples after deep-frying in vegetable oils. Mutagenesis. 2002;17:63–66. doi: 10.1093/mutage/17.1.63. [DOI] [PubMed] [Google Scholar]
- 40.Psaltopoulou T., Naska A., Orfanos P., Trichopoulos D., Mountokalakis T., Trichopoulou A. Olive oil, the Mediterranean diet: and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr. 2004;80:1012–1018. doi: 10.1093/ajcn/80.4.1012. [DOI] [PubMed] [Google Scholar]
- 41.Ramesh A., Archibong A.E., Huderson A.C., Diggs D.L., Myers J.N., Hood D.B., Rekhadevi P.V., Niaz M.S. Polycyclic Aromatic Hydrocarbons. In: Gupta R., editor. Veterinary Toxicology. Elsevier Publishers; England: 2012. pp. 797–809. [Google Scholar]
- 42.Ramesh A., Inyang F., Hood D.B., Archibong A.E., Knuckles M.E., Nyanda A.M. Metabolism, bioavailability, and toxicokinetics of benzo[a]pyrene [BaP] in F-344 rats following oral administration. Exp. Toxicol. Pathol. 2001;53:253–270. doi: 10.1078/0940-2993-00192. [DOI] [PubMed] [Google Scholar]
- 43.Reddy K.S. India wakes up to the threat of cardiovascular diseases. J. Am. Coll. Cardiol. 2007;50:1370–1372. doi: 10.1016/j.jacc.2007.04.097. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro R.F., Jr., Fernandes A.A., Meira E.F. Soybean oil increases SERCA2a expression and left ventricular contractility in rats without change in arterial blood pressure. Lipids Health Dis. 2010;9:53. doi: 10.1186/1476-511X-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha R., Chow W.H., Kulldorf M. Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Res. 1999;59:4320–4324. [PubMed] [Google Scholar]
- 46.Siti K.A., Srijit D., Ima N.S., Nor A.U., Kamsiah J. Consumption of Repeatedly heated soy oil increases the serum parameter related to artherosclerosis in overiectomized rats. Tohoku J. Exp. Med. 2008;215:219–226. doi: 10.1620/tjem.215.219. [DOI] [PubMed] [Google Scholar]
- 47.Sivaswamy S.N., Balachandran B., Sivaramakrishnan V.M. Presence of polycyclic aromatic hydrocarbons in some South Indian food components. Indian J. Exp. Biol. 1991;29:611–614. [PubMed] [Google Scholar]
- 48.Soriguer F., Rojo-Martinez G., Dobarganes M.C., García- Almeida J.M., Esteva I., Beltrán M. Hypertension is related to the degradation of dietary frying oils. Am. J. Clin. Nutr. 2003;78:1092–1097. doi: 10.1093/ajcn/78.6.1092. [DOI] [PubMed] [Google Scholar]
- 49.Srivastava S., Singh M., George J., Bhui K., Murari Saxena A., Shukla Y. Genotoxic and carcinogenic risks associated with the dietary consumption of repeatedly heated coconut oil. Br. J. Nutr. 2010;104:1343–1352. doi: 10.1017/S0007114510002229. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava S., Singh M., George J., Bhui K., Shukla Y. Genotoxic and carcinogenic risks associated with the consumption of repeatedly boiled sunflower oil. J. Agric. Food Chem. 2010;58:11179–11186. doi: 10.1021/jf102651n. [DOI] [PubMed] [Google Scholar]
- 51.Van Gastel A., Mathur R., Roy V.V., Rukmini C. Ames mutagenicity tests of repeatedly heated edible oils. Food Chem. Toxicol. 1984;22:403–405. doi: 10.1016/0278-6915(84)90371-5. [DOI] [PubMed] [Google Scholar]
- 53.Woutersen R.A., Appel M.J., van Garderen-Hoetmer A. Dietary fat and carcinogenesis. Mutat. Res. 1999;443:111–127. doi: 10.1016/s1383-5742(99)00014-9. [DOI] [PubMed] [Google Scholar]