Abstract
Cyclophosphamide (CP) is a widely used cytotoxic alkylating agent with antitumor and immunosuppressant properties that is associated with various forms of reproductive toxicity. The significance of natural antioxidants of plant origin should be explored, especially in a world with increasing incidence of patients in need of chemotherapy. The neuro-endocrine effects of aqueous extract of Amaranthus viridis (Linn.) leaf (AEAVL) in Wistar rats with CP-induced reproductive toxicity was determined. Forty rats were used for this study such that graded doses of the extract were administered following CP-induced reproductive toxicity and comparisons were made against control, toxic and standard (vitamin E) groups at p < 0.05. The synthetic drugs (CP, 65 mg/kg i.p. for 5 days; Vitamin E, 100 mg/kg p.o. for 30 days) as well as the extract (100, 200 and 400 mg/kg p.o. for 30 days) were administered to the rats at 0.2 mL/100 g. CP induced reproductive toxicity as evidenced by significantly lowered levels of FSH, LH and testosterone, perturbation of sperm characterization, deleterious disruptions of the antioxidant system as evidenced by decreased levels of GSH as well as elevation of TBARS activity. Histopathological examination showed hemorrhagic lesions with scanty and hypertrophied parenchymal cells in the pituitary while the testis showed ballooned seminiferous tubules with loosed connective tissues and vacuolation of testicular interstitium. These conditions were significantly reversed (p < 0.05) following administration of the graded doses of the extract. It was, therefore, concluded that AEAVL could potentially be a therapeutic choice in patients with CP-induced neuro-endocrine dysfunction and reproductive toxicity.
Keywords: Cyclophosphamide, Neuro-endocrine dysfunction, Reproductive toxicity, Rats, Amaranthus viridis
1. Introduction
Reproductive toxicity is the occurrence of biologically adverse effects on the reproductive system of living organisms that may result from exposure to environmental agents or xenobiotics. The toxicity may be expressed as alterations to the reproductive organs, related endocrine system and or pregnancy outcomes [1]. According to existing literatures, the use of anticancer drugs affects aspects of reproductive function, including fertility, in both human and experimental animals [2], [3], [4]. CP is a widely used cytotoxic alkylating agent with antitumor and immunosuppressant properties [5]. It is used for the treatment of various forms of cancer such as chronic and acute leukemia, multiple myeloma, lymphomas, rheumatic arthritis and systemic lupus erythematosus [5]. It is also used as an immunosuppressive agent for organ transplantation [5]. However, CP treatment is associated with various forms of reproductive toxicity despite its wide spectrum of clinical use [6]. Due to severe toxic and undesirable side effects in multiple organ systems, there is a gradual but increasing limitation to the use of this drug for cancer chemotherapy [6], [7]. It is therefore imperative to develop strategies that are geared towards minimizing the side effects of this apparently health-beneficial drug in a world of increasing incidence of patients in need of chemotherapy.
Vitamin E, a group of compounds comprising both tocotrienols and tocopherols, is a lipid-soluble antioxidant that protects cell membranes from oxidation by reacting with lipid radicals that are produced in the lipid peroxidation chain reaction as well as expresses its antioxidant functions in the glutathione peroxidase pathway [8], [9], [10]. Its most biologically active form is alpha tocopherol [8], [11] and is apparently a conventional drug used in the amelioration of xenobiotics-induced reproductive toxicity in animal models [12], [13], [14].
The pathophysiology of CP has implicated both oxidative stress and reactive oxygen species (ROS) generation [15], [16], [17]. ROS plays a critical role in the pathological mechanism of male reproductive dysfunction [7], [18]. These proposed pathophysiological mechanisms of CP point towards the fact (hypothesis) that treatment with a potent antioxidant can possibly help reverse or ameliorate its toxic effects. This study aimed at engaging ethno-botanical approach to test this hypothesis since plant derived medicines, besides their easy availability and being relatively cheaper for the affordability of a common man, is relatively safer than the synthetic alternatives [19] and has been used as a source of inspiration for the development of novel drugs [20].
Amaranthus viridis (Linn.) is an annual herb of the family Amaranthaceae [21]. In Nigeria, it is commonly called “green leaf” or “tete” amongst the Yoruba tribe. The plant is erect, having stems that are up to about 10–100 cm in length with branched glabrous leaves. In Greek, Amaranthus is translated as “never-fading flower”. In the light of modern research, the various parts of the plant have been explored for health-boosting potentials. In traditional medicine, powdered seeds of the flower have been shown to possess health beneficial effects such as treatment of stomach problems [22] and reduction of labour pain in pregnant women [23]. The stems have potent anti-diuretic potentials while the traditional use of the leaves range from anti-diabetic, anti-rheumatic, laxative, diuretic, analgesic agent as well as an anti-inflammatory agent used in the treatment of asthma, eye and respiratory dysfunctions [24], [25], [26]. Also, the plant experimentally evaluated for antioxidant, anti-hyperlipidemia and anti-diabetic properties [27], [28], anti-nociceptive [29], hepatoprotective [30] as well as antipyretic and analgesic activities [31].
Despite the experimental evidences portraying a wide range of its health-beneficial effects, our literature survey revealed that (till date) there is no experimental evidence of this plant, Amaranthus viridis (Linn.), effects on the neuro-endocrine function in an animal model of drug-induced reproductive toxicity. In order to bridge this gap in knowledge, this study was embarked upon to assess the neuro-endocrine effects of the aqueous extract of Amaranthus viridis (Linn.) leaf (AEAVL) in male Wistar rats with cyclophosphamide-induced reproductive toxicity.
2. Materials and methods
2.1. Plant material, drug and biochemical kits
Fresh leaves of Amaranthus viridis were obtained from the Farm House of the Department of Crop Science and Production, Faculty of Agricultural Science, Obafemi Awolowo University (OAU), Ile Ife, Osun State, and certified by a Taxonomist in the Department of Botany, OAU, Ile-Ife; where a voucher specimen (IFE − 17424) was deposited.
Salt of cyclophosphamide (CP) was purchased from Zuvius Lifesciences Pvt. Ltd, Mumbai, India while th kits for hormone assay were purchased from Monobind Inc., Lake Forest CA 92630, USA (Accu-Bind Elisa Microwells).
2.2. Plant extraction
Fresh leaves of Amaranthus viridis were air-dried, pulverized with an Electric Pulverizer (DIK-2910, Daiki Rika Kogyo Co. Ltd, Tokyo-Japan) and thereafter macerated in water. This mixture was subjected to constant shaking for a period of 24 h with the aid of an Electric Shaker and afterwards filtered under vacuum using Buchner funnel and Whatman number 2 Filter Paper (Whatman PLC, Middlesex, UK). The filtrate was concentrated under vacuum using a Rotary Evaporator (HahnShin Scientific, HS-2005-N) and thereafter freeze-dried in a Lyophilizer (Ilshin Lab. Co. Ltd, Seoul, Republic of Korea). The resulting yield was kept in a desiccator until when needed.
2.3. Detection and quantification of phytochemicals
Alkaloids were qualitatively determined by the method of Halilu and coworkers [32] and quantified as described by Harbone [33]. Also, the presence of flavonoids was determined by the method of Halilu and coworkers [32] and quantified by the method of Obadoni and Ochuko [34]. Tannins was qualitatively determined by the method of Halilu and coworkers [32] and thereafter quantified by the method of Allen and coworkers [35]. Saponins were qualitatively determined using Froth test as described by Benmehdi and coworkers [36] and quantitatively screened by the method of Obadoni and Ochuko [34]. Quantitative determination of cardiac glycosides was by Keller-Kiliani test as described by [37] Anjali and Sheetal. This was thereafter quantified by the method of Harbone [35].
2.4. Stock solutions of cyclophosphamide and aqueous extract of amaranthus viridis (Linn.) leaf
Sixty five (65) mg/kg stock solution of cyclophosphamide (CP) was prepared as follows; 500 mg of CP salt was dissolved in 15 mL of distilled water and administered at 0.2 mL/100 g rat per day via intraperitoneal route. Thus
each 100 g rat received 6.5 mg of CP per day; an equivalent of 65 mg/kg/day of CP.
The extract’s stock solution for 100 mg/kg was prepared by dissolving 1 g of AEAVL in 20 mL of distilled water. Consequently, every 100 g rat received 0.2 mL of the extract to prevent the deleterious effects of fluid overload. Accordingly, stock solutions for 200 mg/kg and 400 mg/kg of AEAVL were prepared by dissolving 2 g and 4 g of the extract, respectively, each in 20 mL of distilled water.
2.5. Animal management and experimental design
All experimental protocols were in strict compliance with the guidelines for animal research, as detailed in the NIH Guidelines for the Care and Use of Laboratory Animals [38] and approved by local Institutional Research Committee. Forty (40) male Wistar rats of about 3 months of age, weighing 150–180 g, were used for this study. They were purchased from the Animal Holdings of the College of Health Sciences, OAU, Ile − Ife, Osun State, Nigeria where the study was carried out. They were housed in plastic cages under natural light and dark cycle and allowed access to standard laboratory rat chow (Caps Feed PLC Osogbo, Nigeria) and water ad libitum.
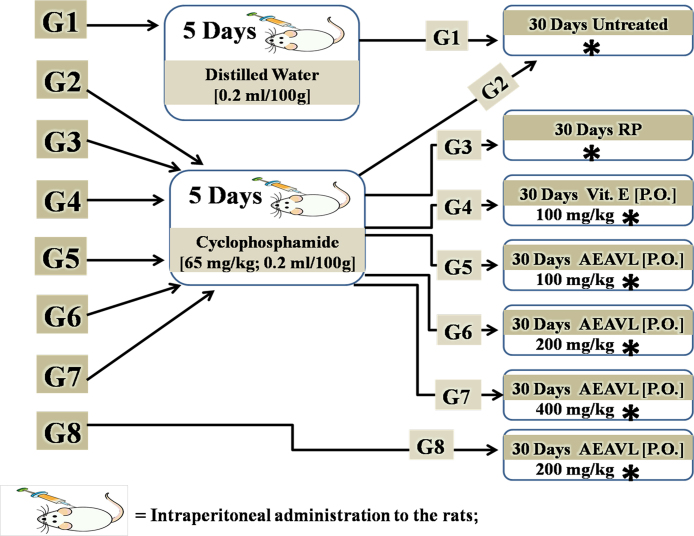
The rats were divided into eight groups of five rats (n = 5) each as follows; Group 1 (Control) received distilled water (0.2 mL/100 g) intraperitoneally for 5 days and thereafter left for a period of 30 days after which they were sacrificed. Group 2 (Toxic) received 65 mg/kg of cyclophosphamide (CP) intraperitoneally for 5 consecutive days after which they were sacrificed. Group 3 (CP + Recovery) were pretreated as group 2 and thereafter left for a recovery period of 30 days after which they were sacrificed. Group 4 (CP + Vitamin E) were pretreated as group 2 and thereafter received 100 mg/kg/day Vitamin E via oral route for 30 days after which they were sacrificed. Groups 5, 6 and 7 were each pretreated as group 2 and thereafter received graded doses of aqueous extract of Amaranthus viridis (Linn.) leaf (AEAVL) at 100, 200 and 400 mg/kg/day respectively via oral route for 30 days after which they were sacrificed. Group 8 received AEAVL (only) at 200 mg/kg/day for 30 days and thereafter sacrificed (Fig. 1). At the end of the study, the rats were euthanized and their blood samples were collected by cardiac puncture into separate EDTA bottles.
Fig. 1.
Experimental Protocol and Dose Regimen.
G1 = Group 1 (5rats); G2 = Group 2 (5rats); G3 = Group 3 (5rats); G4 = Group 4 (5rats); G5 = Group 5 (5rats); G6 = Group 6 (5rats); G7 = Group 7 (5rats); G8 = Group 8 (5rats); RP = Recovery Period; P.O. = Par Oral; Vit. E = Vitamin E; AEAVL = Aqueous Extract of Amaranthus viridis (Linn.) Leaf; * = Point that rats were sacrificed.
The blood samples were centrifuged at 4000 rpm for 15 min at −4 °C using a cold centrifuge (Centurium Scientific, Model 8881). Plasma obtained was decanted into separate plain bottles using sterile syringes. Assessment of reproductive hormones in the plasma obtained was carried out using appropriate kits by Enzyme − Linked Immuno Sorbent Assay (ELISA) technique.
The procedures for hormone assay were as provided in the appropriate kits. The left caudal epididymis of each rat was carefully excised to obtain semen for sperm characterization. While the left testis and 1 g of each rat’s brain were kept in a cooler for homogenate preparation, the right testis and pituitary of each rat were fixed in Bouin’s fluid and 10% formal-saline respectively, for Histopathological examination using Hematoxylin − Eosin (H & E) staining technique.
2.6. Measurement of body and organ weight
Assessment of weekly weight change was carried out using Hanson digital weighing balance (Hanson, China) to measure weekly body weight. On the other hand, organ weights were determined using Camry sensitive weighing balance (Camry, China). The relative testicular weight, relative brain weight and percentage weight change were calculated using the formulae below;
2.7. Sperm characterization
Semen from the caudal epididymis was released onto a microscope slide and sperm counts were carried out using hemocytometer and expressed as million per milliliter of suspension. The epididymal sperm motility was assessed by calculating motile spermatozoa per unit area and expressed as motility in percentage. By the method of Bloom and Fawcett [40], sperm viability was determined after preparing uniform smear spermatozoa on slides using Eosin − Nigrosin stain.
2.8. Hormone assays
The plasma levels of follicle stimulating hormone, luteinizing hormone and testosterone were determined using the standard laboratory protocols that were provided in the appropriate (aforementioned) kits. The adopted technique was Enzyme − Linked Immuno Sorbent Assay (ELISA).
2.9. Assessment of oxidative stress indicators
Using an electric homogenizer (SI601001), 10% homogenate in phosphate buffer (100 mM) was prepared with the tissues at pH of 7.4. The homogenate was centrifuged at 3000 rpm for 20 min and the supernatant was collected for the assessment of the following indicators of oxidative stress;
Reduced glutathione (GSH) level was determined by the method of Beutler and coworkers [41] while the activity of Thiobarbituric Acid Reactive Substances (TBARS) was determined as described by Ohkawa and coworkers [42].
2.10. Histopathological examination
The pituitary and testis of each rat were dehydrated in graded alcohol and embedded in paraffin wax. Sections >4 μm thick were stained with Hematoxylin − Eosin and thereafter viewed under a Leica DM750 camera. Photomicrographs of the testis were taken at magnifications of ×40, ×100 and ×400 while that of the pituitary was taken at ×400 only.
2.11. Statistical analysis
Data obtained were expressed as mean ± Standard Error of Mean using one-way analysis of variance. These were thereafter subjected to Bonferonni’s post-hoc test and level of significance was set at p < 0.05. Graph pad prism 5.03 (Graph Pad Software Inc., CA, USA) statistical package was used for the statistical analysis of the data obtained.
3. Results
3.1. Phytochemical analysis
Except for saponins that were absent, the phytochemical screening of the extract showed the presence of alkaloids, flavonoids, tannins and cardiac glycoside (Table 1).
Table 1.
Qualitative and Quantitative Screening of Aqueous Extract of Amaranthus viridis (Linn.) Leaf.
| Phytochemical Constituents | Status | Percentage Composition (g/100 g) |
|---|---|---|
| Alkaloids | + | 14.25 ± 0.92 |
| Flavonoids | + | 51.0 ± 0.47 |
| Tannins | + | 8.80 ± 0.69 |
| Saponins | − | nil |
| Cardiac Glycoside | + | 63.20 ± 0.92 |
Each value (n = 3) is expressed as mean ± Standard Error of Mean; + = present; − = absent.
3.2. Sperm counts [SC (millions/mL)], sperm motility [SM (%)] and sperm viability [SV (%)]
While group 8 (AEAVL only) showed no significant difference in SC when compared with group 1 (control) (p < 0.05), groups 2–7 were significantly reduced when compared with group 1 (p < 0.05). The AEAVL-treated groups 5, 6 and 7 showed a significantly higher amount of SC when compared with both group 2 (toxic) and group 3 (toxic + recovery) (p < 0.05). There was no significant difference between the AEAVL-treated groups 5, 6 and 7 when compared with group 4 (CP + vitamin E) (p < 0.05). Group 3 never recovered from the significant decrease in SC when compared with groups 1 and 2 (p < 0.05). This trend was shown to be consistent with SV (Table 2).
Table 2.
Effects of AEAVL on the Sperm Characterization of Rats with CP-Induced Reproductive Toxicity.
| Groups (n = 5) | [1] Control | [2] Toxic | [3] CP + Recovery | [4] CP + Vitamin E | [5] CP + 100 mg/kg AEAVL | [6] CP + 200 mg/kg AEAVL | [7] CP + 400 mg/kg AEAVL | [8] 200 mg/kg AEAVL |
|---|---|---|---|---|---|---|---|---|
| Sperm Counts (millions/mL) | 84.80 ± 1.99 | 43.80 ± 2.04* | 40.60 ± 1.75* | 62.80 ± 1.02*αβ | 61.60 ± 0.93*αβ | 59.40 ± 1.17*αβ | 60.20 ± 1.46*αβ | 82.40 ± 1.75αβγ |
| Sperm Motility (%) | 70.00 ± 3.16 | 45.60 ± 1.63* | 38.00 ± 4.90* | 54.00 ± 4.00*β | 55.80 ± 3.11*β | 52.20 ± 2.08* | 54.00 ± 3.03*β | 72.20 ± 2.60αβγ |
| Sperm Viability (%) | 78.60 ± 1.17 | 37.80 ± 1.83* | 35.00 ± 1.64* | 46.20 ± 2.58*αβ | 47.20 ± 2.44*αβ | 50.60 ± 1.33*αβ | 52.40 ± 1.08*αβ | 76.20 ± 1.07αβγ |
Each value represents mean ± Standard Error of Mean; * = significantly different from group 1 (Control) at p < 0.05; α = significantly different from group 2 (Toxic) at p < 0.05; β = significantly different from group 3 (CP + Recovery) at p < 0.05; γ = significantly different from group 4 (CP + Vitamin E) at p < 0.05.
The result for SM showed no significant difference between groups 8 and 1 (p < 0.05). However, while groups 4–7 showed no significant difference in SM when compared with group 2 (p < 0.05), SM was found to be significantly higher in these groups (4–7) when compared with group 3 (p < 0.05). At the end of the study, no significant difference was recorded between the AEAVL-treated groups 5–7 when compared with group 4 (CP + vitamin E) (p < 0.05). Group 3 never recovered from the significant decrease in SM when compared with groups 1 and 2 (p < 0.05) (Table 2).
3.3. Relative testicular weight [RTW (%)], relative brain weight [RBW (%)] and percentage weight change [PWC (%)]
There was no significant difference in RTW of the AEAVL-treated groups 5, 6 and 7 when compared with group 1 (control) (p < 0.05). Also, group 8 (AEAVL only) showed no significant difference in RTW when compared with group 1 (p < 0.05). Groups 2, 3 and 4 showed a significantly higher RTW when compared with group 1 (p < 0.05). At the end of the study, except for group 5 that was significantly lower than group 3 alone, the AEAVL-treated groups 6 and 7 showed a significantly lower RTW when compared with groups 2 and 3 (p < 0.05) (Table 3).
Table 3.
Effects of AEAVL on the Relative Testicular Weight (%), Relative Brain Weight (%) and Percentage Weight Change of Rats Exposed to CP Toxicity.
| Groups (n = 5) | [1] Control | [2] Toxic | [3] CP + Recovery | [4] CP + Vitamin E | [5] CP + 100 mg/kg AEAVL | [6] CP + 200 mg/kg AEAVL | [7] CP + 400 mg/kg AEAVL | [8] 200 mg/kg AEAVL |
|---|---|---|---|---|---|---|---|---|
| RTW (%) | 1.25 ± 0.05 | 1.63 ± 0.09* | 1.68 ± 0.04* | 1.51 ± 0.01* | 1.43 ± 0.02β | 1.42 ± 0.03αβ | 1.38 ± 0.01αβ | 1.23 ± 0.05αβγ |
| RBW (%) | 0.88 ± 0.03 | 0.61 ± 0.02* | 0.47 ± 0.02*α | 0.71 ± 0.02*β | 0.74 ± 0.04*αβ | 0.75 ± 0.04*αβ | 0.79 ± 0.03*αβ | 0.86 ± 0.02αβγ |
| PWC (%) | 22.50 ± 0.70 | 14.88 ± 1.22* | 9.90 ± 0.50*α | 17.61 ± 0.46*β | 18.41 ± 0.85β | 19.21 ± 1.59β | 18.01 ± 1.18β | 24.10 ± 0.58αβγ |
RTW = Relative Testicular Weight; RBW = Relative Brain Weight; PWC = Percentage Weight Change.
Each value represents mean ± Standard Error of Mean; * = significantly different from group 1 (Control) at p < 0.05; α = significantly different from group 2 (Toxic) at p < 0.05; β = significantly different from group 3 (CP + Recovery) at p < 0.05; γ = significantly different from group 4 (CP + Vitamin E) at p < 0.05.
With the exception of group 8, groups 2–7 showed a significantly lower RBW when compared with group 1 (p < 0.05). The AEAVL-treated groups 5–7 showed a significantly higher RBW when compared with groups 3 and 4 (p < 0.05). There was no significant difference in the AEAVL-treated groups 5–7 when compared with group 4 (p < 0.05) (Table 3).
Groups 2–4 showed a significantly lower PWC when compared with group 1 (p < 0.05). However, group 8 showed no significant difference when compared with group 1 (p < 0.05). The AEAVL-treated groups 5–7 showed a significantly higher PWC when compared with group 3 (p < 0.05). Group 3 (CP + Recovery) never recovered from the weight loss when compared with groups 1 and 2 (p < 0.05) (Table 3).
3.4. Plasma levels of follicle stimulating hormone [FSH (mIU/mL)], luteinizing hormone [LH (mIU/mL)] and testosterone (ng/mL)
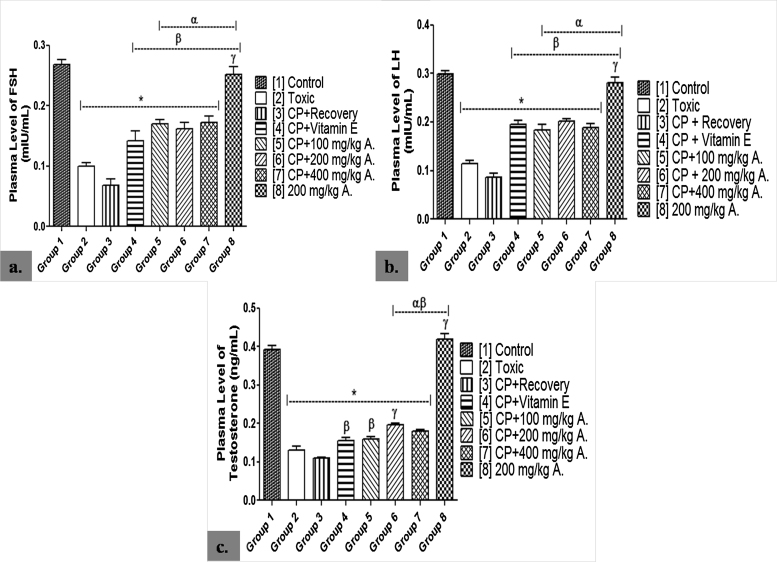
Plasma FSH level was significantly lowered in groups 2–7 when compared with group 1 (p < 0.05). However, group 8 showed no significant difference when compared with group 1 (p < 0.05). There was a significantly higher level of plasma FSH in the AEAVL-treated groups 5–7 when compared with groups 2 and 3 (p < 0.05). Group 3 never recovered from the significantly lowered plasma FSH level when compared with groups 1 and 2 (p < 0.05). This result is consistent with that of LH (Fig. 2).
Fig. 2.
Effects of AEAVL on Plasma [a.] Follicle Stimulating Hormone (mIU/mL), [b.] Luteinizing Hormone (mIU/mL) and [c.] Testosterone (ng/mL) Levels in Male Wistar Rats with Cyclophosphamide-Induced Reproductive Toxicity.
A. = Aqueous Extract of Amaranthus viridis (Linn.) Leaves (AEAVL); FSH = Follicle Stimulating Hormone; LH = Luteinizing Hormone. Each bar represents mean ± Standard Error of Mean (n = 5); * = significantly different from group 1 (Control) at p < 0.05; α = significantly different from group 2 (Toxic) at p < 0.05; β = significantly different from group 3 (CP + Recovery) at p < 0.05; γ = significantly different from group 4 (CP + Vitamin E) at p < 0.05.
The result showed a significantly lowered plasma testosterone level in groups 2–7 when compared with group 1 (p < 0.05). Group 8 recorded no significant difference when compared with group 1 (p < 0.05). AEAVL-treated groups 6 and 7 showed a significantly higher level of plasma testosterone when compared with groups 2 and 3 (p < 0.05) while group 5 was significantly higher than group 3 only (p < 0.05). Group 6 showed a significantly higher level when compared with group 4 (p < 0.05) while group 3 never recovered from the significantly lowered level when compared with groups 1 and 2 (p < 0.05) (Fig. 2).
3.5. Reduced glutathione (GSH) levels in the brain (μg/mg protein) and testis (μg/mg protein)
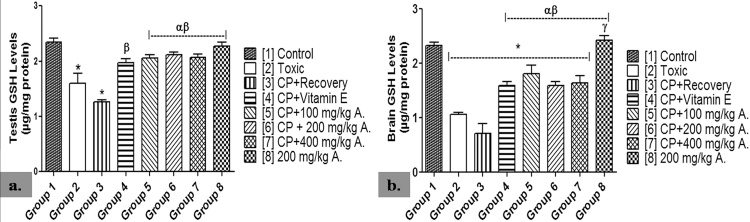
GSH level was significantly lowered in the brain homogenate of groups 2–7 when compared with group 1 (p < 0.05). This was shown to be significantly higher in groups 4–7 when compared with groups 2 and 3 (p < 0.05). Group 3 never recovered from the significantly lowered GSH level in the brain when compared with group 2 (p < 0.05). At the end of the study, group 8 showed no significant difference when compared with group 1 (p < 0.05) (Fig. 3).
Fig. 3.
Effects of AEAVL on [a.] Testis Reduced Glutathione (μg/mg protein) [b.] Brain Reduced Glutathione (μg/mg protein) Levels in Male Wistar Rats with Cyclophosphamide-Induced Reproductive Toxicity.
A. = Aqueous Extract of Amaranthus viridis (Linn.) Leaves (AEAVL); GSH = Reduced Glutathione. Each bar represents mean ± Standard Error of Mean (n = 5); * = significantly different from group 1 (Control) at p < 0.05; α = significantly different from group 2 (Toxic) at p < 0.05; β = significantly different from group 3 (CP + Recovery) at p < 0.05; γ = significantly different from group 4 (CP + Vitamin E) at p < 0.05.
Groups 2 and 3 showed significantly lowered levels of testicular GSH when compared with group 1 (p < 0.05). However, the AEAVL-treated groups 5–7 showed significantly higher levels of testicular GSH when compared with both groups 2 and 3 (p < 0.05). While group 8 showed no significant difference when compared with group 1 (p < 0.05), group 3 never recovered from the significantly lowered testis GSH level when compared with groups 1 and 2 (p < 0.05) (Fig. 3).
3.6. Activity of thiobarbituric acid reactive substances (TBARS) in the brain (nmol/mg protein) and testis (nmol/mg protein)
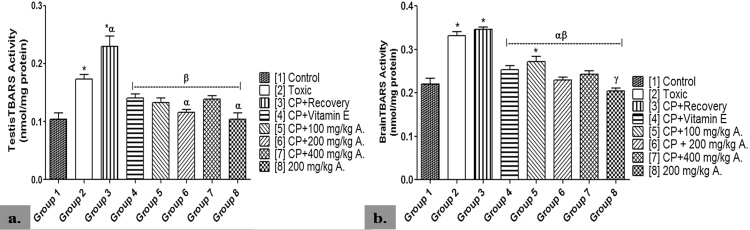
TBARS activity was significantly higher in the brain of groups 2, 3 and 5 when compared with group 1 (p < 0.05). However, the AEAVL-treated groups 5–7 recorded a significantly lower TBARS activity when compared with groups 2 and 3 (p < 0.05). Group 4 also showed a significantly lower TBARS activity when compared with groups 2 and 3 (p < 0.05). Group 3 never recovered from the significantly lowered activity of TBARS when compared with group 2, while group 8 showed no significant difference when compared with group 1 (p < 0.05) (Fig. 4).
Fig. 4.
Effects of AEAVL on [a.] Testes TBARS (nmol/mg protein) [b.] Brain TBARS (nmol/mg protein) Activities in Male Wistar Rats with Cyclophosphamide-Induced Reproductive Toxicity.
A. = Aqueous Extract of Amaranthus viridis (Linn.) Leaves (AEAVL); TBARS = Thiobarbituric Acid Reactive Substances. Each bar represents mean ± Standard Error of Mean (n = 5); * = significantly different from group 1 (Control) at p < 0.05; α = significantly different from group 2 (Toxic) at p < 0.05; β = significantly different from group 3 (CP + Recovery) at p < 0.05; γ = significantly different from group 4 (CP + Vitamin E) at p < 0.05.
TBARS activity in the testis was significantly higher in groups 2 and 3 when compared with group 1 (p < 0.05). Except for group 6 that showed significantly lower activity of TBARS when compared with group 2, the AEAVL-treated groups 5–7 showed a significantly lower level of TBARS activity when compared with group 3 (p < 0.05). At the end of the study, group 8 showed no significant difference in TBARS activity when compared with group 1 (p < 0.05) while group 3 never recovered from the significantly increased TBARS activity when compared with both groups 1 and 2 (p < 0.05) (Fig. 4).
3.7. Histopathological examination
3.7.1. Pituitary
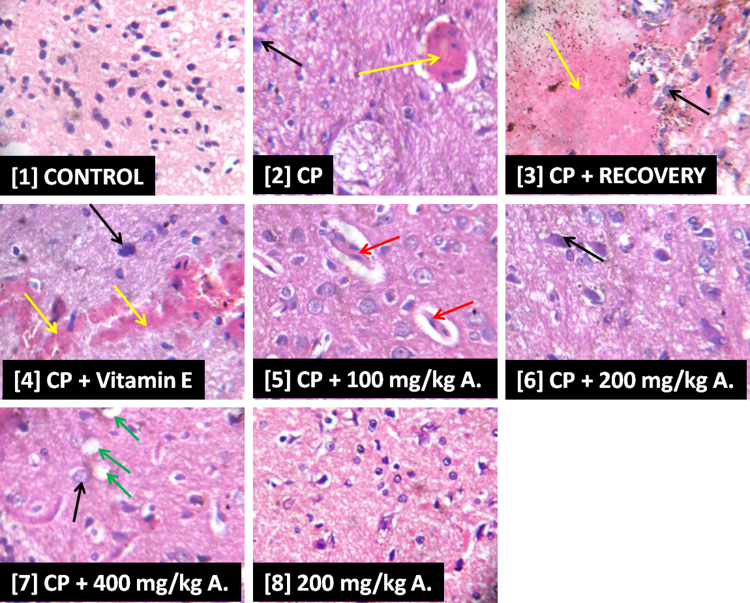
Slide [1] shows normal histoarchitecture of parenchymal cells in the interstitium of rat’s pituitary while slide [2] shows an area of hemorrhagic lesion and congestion of veins and capillaries (yellow arrow). Also, parenchymal cells appear scanty and hypertrophied (black arrow) in slide [2]. There was evidence of severe hemorrhagic lesion (yellow arrow) in slide [3] while slide [4] shows focal area of hemorrhagic lesion (yellow arrow) with apparently hypertrophied parenchymal cells (black arrow). Slides [5]–[7] show evidence of improvement in the histoarchitecture of the pituitary when compared with slides [2] and [3]. However, in slide [5] there was evidence of fat embolism (red arrow) with scanty parenchymal cells while [6] showed evidence of scanty and hypertrophied cells (black arrow). Scanty and hypertrophied cells (black arrow) as well as vacuolation of interstitial spaces in the pituitary (green arrow) were also distinct features in slide [7]. Slide [8] show similarity in features as depicted in [1] (Fig. 5).
Fig. 5.
Photomicrographs Showing the Effects of AEAVL on the Pituitary of Rats Exposed to Cyclophosphamide Toxicity.
Photomicrographs showing the effects of AEAVL administration on the pituitary of rats exposed to Cyclophosphamide toxicity. H & E staining (mg ×400). [1] = Group 1, [2] = Group 2, [3] = Group 3, [4] = Group 4, [5] = Group 5, [6] = Group 6, [7] = Group 7 and [8] = Group 8.
3.7.2. Testis
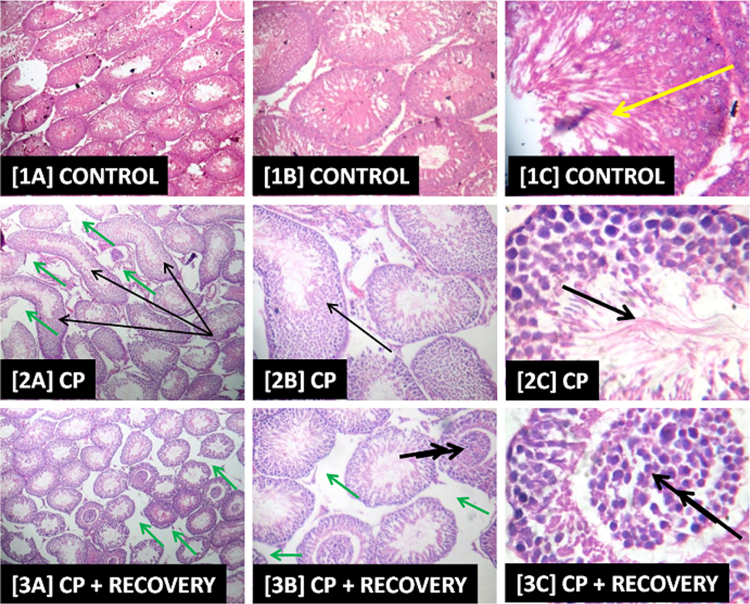
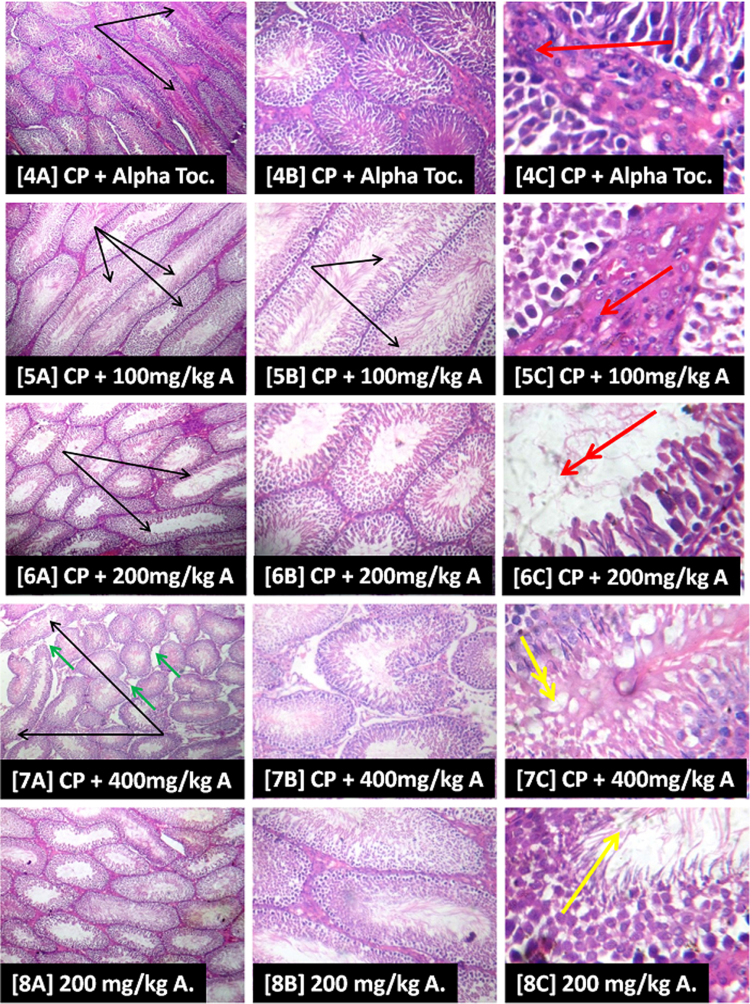
Slide [1] shows evidence of apparently intact seminiferous tubules as well as active cell division and maturation of the germ cells as revealed by the presence of abundance of terminally differentiated cells/spermatozoa (green arrow). However, in slide [2] there is evidence of poorly differentiated spermatozoa (thick black arrow), ballooned and abnormally shaped/elongated seminiferous tubules (thin black arrow) with loosed connective tissues and mild vacuolation of testicular interstitium (green arrow) while in slide [3] there was evidence of sloughing in the seminiferous epithelium into the lumen, almost occluding it (double black arrow) as well as mild vacuolation of testicular interstitium (green arrow). Slide [4] shows hyperplasia of the leydig cells (red arrow) with evidence of abnormally shaped/elongated seminiferous tubules (thin black arrow). Overall, the testicular histoarchitecture of the representative AEAVL-treated groups [5]–[7] shows appreciable improvement when compared with the CP-induced histopathology as depicted in [2] and [3]. Nevertheless, in slide [5] there is evidence of hyperplasia of the leydig cells (red arrow) with ballooned and abnormally shaped/elongated seminiferous tubules (thin black arrow). Slide [6] also has evidence of ballooned and abnormally shaped/elongated seminiferous tubules (thin black arrow) as well as inactive/inhibition of active cell division and maturation of germ cells (double red arrow) while slide [7] has a focal area of mild fatty infiltration of the seminiferous epithelium (double yellow arrow), ballooned and abnormally shaped seminiferous tubules (thin black arrow) and mild vacuolation of testicular interstitium (green arrow). Slide [8] depicts similar testicular histoarchitecture as shown in [1] (Fig. 6).
Fig. 6.
Photomicrographs Showing the Effects of AEAVL on the Testis of Rats Exposed to Cyclophosphamide Toxicity.
Photomicrograph showing the effects of AEAVL administration on the testis of rats exposed to Cyclophosphamide toxicity. H & E staining, Left column [A] = mg ×40; Centre column [B] = mg ×100; Right column [C] = mg ×400; [1] = Group 1, [2] = Group 2, [3] = Group 3, [4] = Group 4, [5] = Group 5, [6] = Group 6, [7] = Group 7 and [8] = Group 8.
4. Discussion
The study investigated the neuro-endocrine effects of thirty days administration of aqueous extract of Amaranthus viridis (Linn.) leaves (AEAVL) in rats with cyclophosphamide-induced reproductive toxicity. There was a comparative study of the effects of the extract (AEAVL) and vitamin E on some reproductive indices of the rats. Also, a dose of the extract (medium dose, only) was administered to a group in order to appraise its effects on the reproductive indices of study.
The significantly lowered plasma FSH level that was associated with CP administration can be attributed to two factors; first is the deleterious alteration of the pituitary histoarchitecture (as shown in the representative photomicrograph) with a consequent decrease in pituitary FSH secretion and secondly, (subject to further verification) a possible decreased responsiveness of the sertoli cells to the available plasma FSH. It is, however, not unlikely that a possible proliferation of the spermatogonia via its active binding with FSH was altered by the decreased plasma level of testosterone (possibly secondary to the reduced plasma LH level) that was associated with CP administration. This was characterized by poorly differentiated spermatozoa, as depicted by the representative photomicrograph. The representative plate also showed evidence of vacuolation in both interstitium and seminiferous tubule. The study therefore suggests that CP-induced reproductive toxicity is expressed through injury inflicted on both the pituitary and testis in rat model, secondary to oxidative stress. A body of literature exists showing the fact that CP induces its deleterious effects in biological systems principally by inducing oxidative stress through generation of reactive oxygen species (ROS) [43], [44], [45], [46]. Although, administration of the extract was associated with reduced level of reproductive hormones in the CP-exposed rat model when compared with the control, the level was found to be significantly higher than that of the toxic group (toxicity model). This demonstrates the fertility-boosting potential of the extract. Phytochemical screening of the extract was found to be rich in flavonoids and tannins. These phytochemicals are reputed to express potent antioxidant, anti-inflammatory and anticancer potentials [47], [48]. The restorative potential of the extract, characterized by improvements in the histoarchitecture of the pituitary and testis as well as increase in the level of reproductive hormones, may be attributed to the presence of tannins and flavonoids in it. It is, therefore, implied that the extract has a promising therapeutic effect in rat model of CP-induced imbalance in the level of reproductive hormones.
Sperm counts provide information on the cumulative result of all stages in sperm production, making it one of the most reliable sensitive tests for spermatogenesis with a high correlation with fertility [49], [50]. The CP-induced decrease in sperm counts demonstrated the impairment of reproductive function by deleterious interference of the critical stages of spermatogenesis. Hence, the sperm counts-boosting potential of the extract in the CP-exposed model suggests that one of its possible mechanisms of fertility-boosting action is protection of the stages of spermatogenesis against the deleterious effects of xenobiotics such as drugs and chemicals that have the potential of inducing reproductive toxicity. This could also have been achieved by the presence of important phytochemicals (alkaloids, tannins and flavonoids) with potent antioxidant and, possibly, membrane-stabilizing potentials that are present in the extract. Considered as an integral part of some reproductive toxicity guidelines, assessment of sperm motility and viability is crucial in sperm characterization [51], [52]. A compromise of reproductive function can be induced by a deleterious change of the microenvironment in the inner aspects of the seminiferous tubules secondary to the permeation of blood-testis barrier by chemical agents [53]. The representative photomicrograph revealed evidence of luminal sloughing of the seminiferous tubules with interstitial vacuolation. This may explain the decrease in sperm motility and viability that was associated with CP administration. The study suggests that CP-induced reproductive toxicity is enhanced by a possible permeation of blood-testis barrier to bring about adverse alterations in the microenvironment of the seminiferous tubules. There was a significant boost of sperm motility and viability in the extract-treated groups when compared with the toxic model, although with values lesser than the control. A possible mechanism by which it achieves this is through its potential to reinstate apparently normal antioxidant status in the testes by blocking CP-induced assault as depicted by the indices of oxidative stress that were assessed in this study. The deleterious alterations in these indices of oxidative stress (GSH and TBARS) are evidences of an assault to the reproductive tissue by the drug. This report on deleterious alterations in sperm characterization following CP administration in animal model is consistent with the findings of Haubitz [54]; Abarikwu et al. [55]; Aroona et al. [46] and Divya et al. [56].
The neurotoxic effects of CP have been reported by quite a body of literature [44], [45], [57]. The representative photomicrograph revealed evidence of severe hemorrhagic lesions as well as scanty parenchymal cells in the pituitary (which could be non exclusive to this region) during the period of CP administration. The significantly lowered relative brain weight (RBW) that was recorded in this study can be attributed to a possible decrease in the total number of cells that constitutes the brain tissue. This is subject to further verification. The extract was able to block this alteration via a, possibly, tissue regenerative mechanism; as the representative photomicrograph revealed restoration of lost parenchymal cells in the pituitary. This could have been secondary to its antioxidant and membrane stabilizing effects, potentiated by the presence of tannins and flavonoids in the extract. Contrariwise, CP administration was associated with a significant increase in the relative testicular weight (RTW) of exposed rats. This can be attributed to the alteration in structure of the seminiferous tubules which appeared to be ballooned and abnormally elongated instead of assuming its regular ovoid shape. Since the availability of testosterone in the circulation is required for the growth, development and maintenance of male reproductive organs [58], the promising but apparently incomplete therapeutic effects of the extract on the RTW can be attributed to its testosterone-boosting potential which may be secondary to its antioxidant effect.
Reduced glutathione (GSH) and Thiobarbituric acid reactive substances (TBARS) are indices of antioxidant status in biological systems [59]. GSH, a measure of non-enzymatic antioxidant status, helps to effectively removes hydrogen peroxide and serves as a cofactor for glutathione transferase, which helps to remove certain drugs, chemicals and other reactive molecules from cells [59], [60]. TBARS is a measure of lipid peroxidation and oxidative stress [59], [60]. The decreased levels of GSH in both the pituitary and testis of CP-exposed rats can be attributed to its excessive use by these tissues to scavenge the free radicals that were generated following CP intoxication and or decreased GSH production by these tissues. On the other hand, TBARS activity was highly increased in the pituitary and testis of exposed rats, indicating high degree of lipid peroxidation and oxidative stress. Administration of the extract showed blocking of the CP-induced deleterious alterations in the antioxidant homeostasis as evidenced by a significantly higher levels of GSH and significantly lower levels of TBARS in the extract-treated groups when compared with the toxicity model. The study, therefore, demonstrated the antioxidant potential of the extract.
The period of CP administration to the rats was characterized by strange behaviors of isolation and depression; an expression of neurotoxic effect. Deleterious alterations in feeding pattern were also observed as feeds and water were almost untouched in the feeding trough and drinkers respectively during this period. CP-induced decrease in percentage weight change (PWC) of the rats can be attributed to its neurotoxic effect. This may have suppressed the feeding center in the lateral hypothalamus [61] with a consequent inhibition of appetite. A balance between dietary intake and expenditure of energy is a determinant of weight gain or loss [61]. Therefore, the significantly lowered PWC can be attributed to the deleterious alterations in feeding pattern that was observed. It is not unlikely that the extract is rich in multivitamins and or contains phytochemical(s) that mimics the appetite restorative capacity of multivitamins as the depressive state of the rats was observed to appreciably reduce following administration of the extract when compared with the toxic model. This is worthy of further exploration. Another possible explanation for the decrease in the PWC is the corresponding decrease in the plasma testosterone level that was recorded during the study period. Plasma level of testosterone is responsible for the growth and development of male sexual organs as well as expression of sexual characters such as the buildup of muscles [62], [63]. Administration of the extract was associated with testosterone-boosting potential in the CP-treated rats.
The group that received the extract alone (without CP) showed similarities in biochemical indices as well as in sperm characterization when compared with the control. The same is true for their histological examinations. This suggests that ingestion of aqueous extract of Amaranthus viridis (Linn.) leaf does no potentiate deleterious effects on the neuro-endocrine or reproductive function of apparently normal rats.
The study showed that administration of Vitamin E alone may be insufficient in the management or treatment of reproductive toxicity in CP-exposed subjects. Apparently, in animal model there seem to be a standard reference of 100 mg/kg of vitamin E administration in the treatment of drug- or chemically-induced reproductive toxicity [13], [14], [15]. This study, however, showed that this dose-reference did not prove much of a standard as shown in some of the reproductive indices such as FSH and lipid peroxidation (TBARS) when compared with both the extract-treated groups and the control. A review of this apparently accepted dose is therefore suggested [64], otherwise its administration at this dose may not be sufficient to produce the desired or anticipated therapeutic effects in rat models of CP-induced reproductive toxicity.
We strongly recommend a study of the prophylactic effects of the extract (AEAVL) in models of CP-induced reproductive toxicity. It is imperative to conduct an advance study, at a molecular level, with a view to having a better understanding of the extract’s mechanism(s) of action. Also, there is need for future study of the extract fractions for the biochemical basis of its therapeutic effects.
5. Conclusion
In conclusion, the administration of aqueous extract of Amaranthus viridis (Linn.) leaf showed promising therapeutic effects in male Wistar rat model of cyclophosphamide-induced neuro-endocrine dysfunction and reproductive toxicity. The extract could potentially be a therapeutic choice in the management of subjects with cyclophosphamide-induced reproductive toxicity. Its therapeutic potential can at least be attributed to both its antioxidant and membrane stabilizing properties.
Transparency document
Acknowledgements
The authors wish to appreciate Prof. O.K. Adekunle of the Faculty of Agricultural Sciences, Obafemi Awolowo University, Ile-Ife for assisting in making the vegetable (Amaranthus viridis) available in sufficient amount (from OAU Farm House) for this study. We also acknowledge the members of staff of DR. Obuotor’s Laboratory, Department of Biochemistry, OAU, Ile-Ife for their kind support and technical assistance.
References
- 1.Guidelines for Reproductive Toxicity Risk Assessment, 1996. https://archive.epa.gov/raf/web/html/guidelines-reproductive-tox-risk-assessment.html (access date: 4.8.16).
- 2.Qureshi M.S., Pennington J.H., Goldsmith H.J., Cox P.E. Cyclophosphamide therapy and sterility. Lancet. 1972;2(7790):1290–1291. doi: 10.1016/s0140-6736(72)92657-8. [DOI] [PubMed] [Google Scholar]
- 3.Elangovan N., Chiou T.J., Tzeng W.F., Chu S.T. Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. Toxicology. 2006;222(1–2):60–70. doi: 10.1016/j.tox.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Rezvanfar M., Sadrkhanlou R., Ahmadi A., Shojaei-Sadee H., Rezvanfar M., Mohammadirad A., Salehnia A., Abdollahi M. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum. Exp. Toxicol. 2008;27(12):901–910. doi: 10.1177/0960327108102046. [DOI] [PubMed] [Google Scholar]
- 5.Dollery C. Churchill Livingstone; Edinburg: 1999. Cyclophosphamide: Therapeutic Drugs; pp. 349–353. [Google Scholar]
- 6.Sahin K., Sahin N., Kucuk O. Lycopene and chemotherapy toxicity. Nutr. Cancer. 2010;62(7):988–995. doi: 10.1080/01635581.2010.509838. [DOI] [PubMed] [Google Scholar]
- 7.Sung-Hwan K., In-Chul L., Hyung-Seon B., Changjong M., Sung-Ho K., Jong-Choo n.K. Protective effects of diallyl disulphide on cyclophosphamide-induced testicular toxicity in rats. Lab. Anim. Res. 2013;29(4):204–211. doi: 10.5625/lar.2013.29.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brigelius-Flohe R., Traber M.G. Vitamin E: function and metabolism. FASEB J. 1999;13(10):1145–1155. [PubMed] [Google Scholar]
- 9.Herrera E., Barbas C. Vitamin E: action, metabolism and perspectives. J. Physiol. Biochem. 2001;57(2):43–56. [PubMed] [Google Scholar]
- 10.Traber M.G., Akinson J. Vitamin E: antioxidant and nothing more. Free Radic. Biol. Med. 2007;43(1):4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reboul E., Richelle M., Perrot E., Desmoulins-Malezet C., Pirisi V., Borel P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006;54(23):8749–8755. doi: 10.1021/jf061818s. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini A., Zare S., Pakdel F.G., Ahmadi A. Effects of vitamin E and Ginseng extract on fertility changes induced by cyclophosphamide in rats. J. Reprod. Fertil. 2010;11:227–237. [Google Scholar]
- 13.Oyeyemi W.A., Shittu S.T., Kolawole T.A., Ubaneche P., Akinola A.O. Protective effect of vitamin E on nicotine-induced reproductive toxicity in male rats. Niger. J. Basic Appl. Sci. 2015;23:7–13. [Google Scholar]
- 14.Santana A.T., Guelfi M., Medeiros H.C.D., Tavares M.A., Bizerra P.F.V., Mingatto F.E. Mechanisms involved in reproductive damage caused by gossypol in rats and protective effects of vitamin E. Biol. Res. 2015;48:43. doi: 10.1186/s40659-015-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manda K., Bhatia A.L. Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol. Toxicol. 2003;19(6):367–372. doi: 10.1023/b:cbto.0000013342.17370.16. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia K., Ahmad F., Rashid H., Raisuddin S. Protective effect of Sallylcysteine against cyclophosphamide-induced bladder hemorrhagic cystitis in mice. Food Chem. Toxicol. 2008;46(11):3368–3374. doi: 10.1016/j.fct.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Motawi T.M., Sadik N.A., Refaat A. Cytoprotective effects of DLalpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: an experimental study on rat myocardium, testicles and urinary bladder. Food Chem. Toxicol. 2010;48(8–9):2326–2336. doi: 10.1016/j.fct.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 18.Kothari S., Thompson A., Agarwal A., du Plessis S.S. Free radicals: their beneficial and detrimental effects on sperm function. Indian J. Exp. Biol. 2010;48(5):425–435. [PubMed] [Google Scholar]
- 19.Iwu M.M., Duncan A.R., Okunji C.O. New an- timicrobials of plant origin. In: Janick J., editor. Prospec- tive on New Crops and New Uses. ASHS, Press; Alexan-dria: 1999. [Google Scholar]
- 20.Robbers J., Speedie M., Tyler V. Williams, Wilkins; Baltimore: 1996. Pharmacognosy and Pharmacobiotechnology. [Google Scholar]
- 21.Ramdas P., Sangameswaran B., Popat M., Shantaram K. Antidiabetic and antihyperlipidaemic potential of Amaranthus viridis (L.) Merr. in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Dis. 2012:S180–S185. [Google Scholar]
- 22.L.P. Sena, Plant Foods for Human Nutrition, 1998. https://scholarsresearchlibrary.com/ABR-vol2-iss4/ABR-2011-2-4-435-438.pdf (access date: 48.16).
- 23.Kirtikar K.R., Basu B.D. In: 2nd ed. Kirtikar K.R., Basu B.D., editors. vol. 3. International book distributors; Dehra Dun, India: 1987. pp. 2061–2062. (Indian Medicinal Plants). [Google Scholar]
- 24.Council of Scientific and Industrial Research (CSIR), 1988. https://www.cbd.int/iyb/doc/celebrations/iyb-India-WealthofIndia.pdf (access date: 4.8.16).
- 25.Agra M.F., Baracho G.S., Nurit K., Basilio I.J.L.D., Coelho V.P.M. Effect of methanolic extract of Amaranthus viridis (MEAV) on hot plate test in mice. Braz. J. Ethnopharmacol. 2007;111(2):283–395. doi: 10.1016/j.jep.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 26.De Fatima Agra M., Silva K.N., Basilio I.J.L.D., De Freitas P.F., Filho J.M.B. Survey of medicinal plants used in the region northeast of Brazil. Braz. J. Pharmacogn. 2008;18(3):472–508. [Google Scholar]
- 27.Ashok Kumara B.S., Lakshmanb K., Jayaveeac K.N., Sheshadri Shekard D., Saleemulla K., Thippeswamy B.S., Veeresh V. Antidiabetic, antihyperlipidemicand antioxidant activities of methanolic extract of Amaranthus viridis Linn. in alloxan induced diabetic rats. Exp. Toxicol. Pathol. 2012;64:75–79. doi: 10.1016/j.etp.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Nisha S., Gupta P.C., Rao C.V. Nutrient content, mineral content and antioxidant activity of Amaranthus viridis leaves. Res. J. Med. Plant. 2012;69(3):253–259. [Google Scholar]
- 29.Ashok Kumar B.S., Lakshman K., Jayaveera K.N., Sheshadri Shekar D., Vivek C. Antinociceptive and antipyretic activities of Amaranthus viridis Linn. in different experimental models. Arch. Biol. Sci. Belgrade. 2010;62(2):397–402. [PMC free article] [PubMed] [Google Scholar]
- 30.Lakshman K. Hepatoprotective and antioxidant activities of Amaranthus viridis. Maced J. Med. Sci. 2011:1–6. [Google Scholar]
- 31.Bagepalli S., Ashok K., Kuruba L., Korala K., Narsimha J., Devangam S.S., Chinna Swamy Vel M., Bachappa M. Antinociceptive and antipyretic activities of Amaranthus viridis Linn. in different experimental models. Avicenna J. Med. Biotechnol. 2009;1(3):167–171. [PMC free article] [PubMed] [Google Scholar]
- 32.Halilu M.E., Abubakar A., Garbar M.K., Isah A.A. Antimicrobial and preliminary phytochemical studies of methanol extract of root bark of Crossopteryx febrifuga (Rubiaceae) J. Appl. Pharm. Sci. 2012;2:066–070. http://dx.doi.org/10.7324/japs.2012.21212 [Google Scholar]
- 33.Harborne J.B. 2nd edition. Chapman and Hall; London: 1980. Phytochemical Methods; pp. 288–293.http://dx.doi.org/10.1007/978-94-009-5921-7 [Google Scholar]
- 34.Obadoni B.O., Ochuko P.O. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Glob. J. Pure Appl. Sci. 2002;8:203–208. http://dx.doi.org/10.4314/gjpas.v8i2.16033 [Google Scholar]
- 35.Allen S.E., Grinshaw H.M., Parkinson J.A., Quarmbay C. 1st edition. Blackwell Scientific Publication; London: 1973. Chemical Analysis of Ecological Materials. [Google Scholar]
- 36.Benmehdi H., Hasnaoui O., Benali O., Salhi F. Phytochemical investigation of leaves and fruit extracts of Chamaerops humilis L. J. Mater. Environ. Sci. 2012;3:320–337. [Google Scholar]
- 37.Anjali S., Sheetal S. Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J. Pharmacogn. Phytochem. 2013;2:22–29. [Google Scholar]
- 38.Guide for the care and use of laboratory animals, 2011. 8th ed. https://grants.nih.gov/grants/.../Guide-for-the-Care-and-use-of-laboratory-animals.pdf (access date: 5.10.15).
- 39.C.E. Imafidon T.R. Olatoye F.S. Bamidele O.E. Ojo K.A. Ademoye rich extract of Vernonia amygdalina (Del.) leaf. Journal of Interdisciplinary Histopathology. http://dx.doi.org/10.5455/jihp.20160618041629.
- 40.Bloom W., Fawcett D.W. Text Book of Histology. 2nd edition. Bloom W. Saunders Company; Philadelphia: 1975. Male reproductive system. [Google Scholar]
- 41.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood Glutathione. J. Lab. Clin. Med. 1963;61:882–888. PMid:13967893. [PubMed] [Google Scholar]
- 42.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. http://dx.doi.org/10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- 43.Tripathi D.N., Jena G.B. Ebselen attenuates cyclophosphamide-induced oxidative stress and DNA damage in mice. Free Radic. Res. 2008;42(11–12):966–977. doi: 10.1080/10715760802566558. [DOI] [PubMed] [Google Scholar]
- 44.Oboh G., Akimolafe T.L., Adetuyi A.O. Inhibition of cyclophosphamide-induced oxidative stress in brain by dietry inclusion of red dye extracts from sorghum (sorghum bicolor) stem. J. Med. Food. 2010;13(5):1075–1080. doi: 10.1089/jmf.2009.0226. [DOI] [PubMed] [Google Scholar]
- 45.Oboh G., Akomolafe T.L., Adefegha S.A., Adetuyi A.O. Inhibition of cyclophosphamide-induced oxidative stress in rat brain by polar and non-polar extracts of Annatto (Bixa orellana) seeds. Exp. Toxicol. Pathol. 2011;63(3):257–262. doi: 10.1016/j.etp.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Aroona C., Shokrzadeh M., Farshad N., Salehi F., Amirhossein A. Melatonin ameliorates oxidative stress and reproductive toxicity induced by cyclophosphamide in male mice. Hum. Amp Exp. Toxicol. 2014;33(2):185–195. doi: 10.1177/0960327113489052. [DOI] [PubMed] [Google Scholar]
- 47.Health benefits of plant tannins, 2016. http://www.medibiztv.com/articles/health-tannins (access date: 3.1.16).
- 48.Health benefits of flavonoids, 2016. http://www.livestrong.com/article/492244-what-are-the-health-benefits-of-flavonoids/ (access date: 5.4.16).
- 49.Meistrich M.L. Quantitative correlation between testicular stem cell survival, sperm production, and fertility in the mouse after treatment with different cytotoxic agents. J. Androl. 1982;3:58–68. [Google Scholar]
- 50.Afaf A.E. Influence of subchronic exposure of profenofos on biochemical markers and microelements in testicular tissue of rats. J. Am. Sci. 2009;5(1):19–28. [Google Scholar]
- 51.Foster P. World Health Organization; 2001. Principles for Evaluating Health Risks to Reproduction Associated with Exposure to Chemicals.https://books.google.com.ng/books?id=v4qW4XgXXrcC&pg=PA62&lpg=PA62&dq=∼Morrissey+1989+%2B+US+EPA+1998&source=bl&ots=V7iABkntTy&sig=DLx35v9VDuPKDWbb82d6Z-971nc&hl=en&sa=X&ved=0ahUKEwjvktD-4OHJAhXBPhQKHQP4Ak8Q6AEIHjAA#v=onepage&q=∼Morrissey%201989%20%2B%20US%20EPA%201998&f=false (Aceess Date 11.10.15.). [Google Scholar]
- 52.Ramesh C.G. Academic Press; 2011. Reproductive and Developmental Toxicology.https://books.google.com.ng/books?id=jGHRR32wz5MC&pg=PA116&lpg=PA116&dq=∼Morrissey+1989+%2B+US+EPA+1998&source=bl&ots=xx45-rvQnv&sig=fD9cE4i9C5ZujIFNj1h4I3xElZs&hl=en&sa=X&ved=0ahUKEwjvktD-4OHJAhXBPhQKHQP4Ak8Q6AEIPDAI#v=onepage&q=∼Morrissey%201989%20%2B%20US%20EPA%201998&f=false (Access Date 11.10.15.). [Google Scholar]
- 53.Raji Y., Salman T.M., Akinsomisoye O.S. Reproductive functions in male rats treated with methanolic extract of Alstonia boonei stem bark. Afr. J. Biomed. Res. 2005;8:105–111. [Google Scholar]
- 54.Haubitz M. Acute and long term toxicity of cyclophosphamide. Transplant. Med. 2007;19:26–31. [Google Scholar]
- 55.Abarikwu S.O., Otuechere C.A., Ekor M., Monwuba K., Osobu D. Rutin ameliorates cyclophosphamide reproductive toxicity in male rats. Toxicol. Int. 2012;19(2):207–214. doi: 10.4103/0971-6580.97224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Divya B., Harish S., Krishna A.P. Ameliorative effect of punica granatum ethanolic extract in cyclophosphamide induced testicular toxicity in male wistar rats. Int. J. Appl. Biol. Pharm. Technol. 2015;6(3):230–236. [Google Scholar]
- 57.Trushrendra S., Kasture S.B., Mohanty P.K., Yusuf J., Manvendra S.K., Abhisek A., Yashraj Y. Cyclophosphamide-induced oxidative stress in brain: protective effect of Garcinia indica fruit extract. Int. J. Pharm. Life Sci. 2011;2(9):1035–1040. [Google Scholar]
- 58.Mooradan A.D., Morley J.E., Koreman S.G. Biological actions of androgens. Endocr. Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 59.Thangarajan S., Chandrasekar S., Varadharajan M., Sureka M., Subathra M., Vishali A., Krishnamoorthi R. Oxidative stress in brains of male rats intoxicated with aluminium and neuromodulating effect of celastrus paniculatus alcoholic seed extract. Asian J. Pharm. Clin. Res. 2013;6(3) [Google Scholar]
- 60.Rahmat A.K. Protective effects of Launaea procumbens on rat testis damage by CCl4. Lipids Health Dis. 2012;11:103. doi: 10.1186/1476-511X-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katherine A.S., Niamh M.M., Steve R.B. Hypothalamic regulation of apetite. Expert Rev. Endocrinol..Metab. 2008;3:577–592. doi: 10.1586/17446651.3.5.577. [DOI] [PubMed] [Google Scholar]
- 62.Vornberger W., Prins G., Musto N.A., Suarez-Quian C.A. Androgen receptor distribution in rat testis: new implications for androgen regulation of spermatogenesis. Endocrinology. 1994;134:2307–2316. doi: 10.1210/endo.134.5.8156934. [DOI] [PubMed] [Google Scholar]
- 63.Ballester J., Carmen M.M., Jorge D., Teresa R., Joan J.G., Joan E.R. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J. Androl. 2004;25(5):706–719. doi: 10.1002/j.1939-4640.2004.tb02845.x. [DOI] [PubMed] [Google Scholar]
- 64.Ayoka O.A., Ademoye K.A., Imafidon C.E., Ojo O.E., Oladele A.A. Aqueous extract of Allium sativum (Linn.) bulbs ameliorated pituitary-testicular injury and dysfunction in Wistar rats with Pb-induced reproductive disturbances. OA Maced J. Med. Sci. 2016 doi: 10.3889/oamjms.2016.039. http://dx.xoi.org/10.3889/oamjms.2016.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.