Abstract
The association between the polymorphic GGN repeat in androgen receptor gene and prostate cancer susceptibility has been studied extensively. But the results of these polymorphisms with prostate cancer risk remain inconclusive. Previous meta-analysis showed short GGN repeats (≤16 repeats) had high risks for prostate cancer compared with longer GGN repeats (>16 repeats). Many studies have been published since the release of the previous meta-analysis. Here, we conducted an updated meta-analysis to demonstrate whether short repeats have higher risks for prostate cancer compared to long repeats. Five databases (PubMed, EMBASE, Cochrane Library, The China National Knowledge Infrastructure, and Web of Science) were last searched until January 1, 2016. Random- or fixed-effects model was performed based on the heterogeneity among studies. The potential publication bias was assessed via Begg funnel plot and Egger regression test. Twelve out of 157 studies were extracted. The result indicated that there was no significant difference between short repeat group and long repeat group in the overall analysis (I 2 = 80.6%, P = .000, odds ratio = 1.31, 95% confidence interval: 0.93-1.83). There was no association between the length of GGN repeats and the occurrence of prostate cancer in both Caucasian and African American (I 2 = 6.7%, P = .359, odds ratio = 1.11, 95% confidence interval: 0.94-1.32; and I 2 = 74.1%, P = .050, odds ratio = 0.963, 95% confidence interval: 0.36-2.58). Our result demonstrated that a shorter GGN repeat polymorphism cannot increase the risk of prostate cancer compared to the longer GGN repeats. That’s different with previous meta-analysis.
Keywords: GGN repeats, androgen receptor, prostate cancer, meta-analysis, gene
Introduction
Prostate cancer (PCa) is the second most common cause of death in male.1 It is the most frequently diagnosed cancer and second most frequent cause of deaths in the US males.2 Otherwise, the morbidity of PCa in Asia is lower than the places like Oceania, Europe, and North America.3 But a recent study showed that there has been a steady increase in the rate of diagnosis and in mortality from PCa in the examined Asian countries.4 Well-established risk factors for PCa include age, race/ethnicity, and family history.5 In addition, genetic and environmental factors, especially the former, have been suggested to be associated with PCa risk.6
One of the most studied genes implicated in PCa susceptibility is the androgen receptor (AR) gene on chromosome Xq11-12. The AR plays a vital role in the growth, proliferation, and differentiation of prostate cells.7 The amino-terminal domain of the AR protein, which is important for transcriptional activity, is encoded by exon 1. This exon contains 2 trinucleotide repeat sequences, CAG and GGN, encoding variable lengths of polyglutamine and polyglycine stretches, respectively.8 It has been reported that a shorter CAG repeat polymorphism may increase the risk of PCa compared with the longer CAG repeat.9 But the relationship between GGN and PCa is controversial. The (GGN)n array is less polymorphic in the general population and encodes a polyglycine tract of 10 ± 29 repeats.10 The functional consequences of variation in the GGN tract are less clear. Early in 2004, Zeegers et al performed a meta-analysis indicating short GGN repeats may be related to PCa.11 However, Lange et al found no evidence for an association between GGN repeat lengths and PCa risk in a population-based sample of African Americans.12 Moreover, a Chinese meta-analysis indicated people with ≤16 GGN polymorphism repeats displayed a higher risk of PCa.13
Due to many studies that have been published since the release of the previous meta-analysis, it seems reasonable to update the original analysis.14 Given the essential impact of the GGN repeats polymorphism on PCa risk, we conducted an updated meta-analysis based on current published evidence to explore the relationship between GGN repeat polymorphism and PCa risk.
Materials and Methods
Search Strategy
Five databases (PubMed, EMBASE, Cochrane Library, The China National Knowledge Infrastructure, and Web of Science) were last searched until January 1, 2016, with the following key words (GGN [All Fields] AND prostatic neoplasms [MeSH Terms] AND “receptors, androgen” [MeSH Terms]). No language restriction was set. To further identify all potentially eligible articles for the meta-analysis, the reference lists of review and retrieved articles were searched by hand at the same time.
Inclusion and Exclusion Criteria
Assessment of eligibility for inclusion into the meta-analysis was performed by 2 reviewers (J.L. and F.X.). The exclusion criteria for the meta-analysis are (1) case reports, conference abstracts, and letters to the editor, (2) not being case–control studies, and (3) duplication of the previous publication. Studies were included in the meta-analysis if they fulfilled the following criteria: (1) case–control studies, research content about PCa and GGN repeats, (2) test method should be rational: analysis of GGN repeats was performed on genomic DNA by polymerase chain reaction (PCR), (3) method of experimental design should be reasonable: all included cases were diagnosed with PCa.
Data Extraction
Each potentially eligible study was abstracted in duplicate and evaluated independently by F.X. and J.L. using a data collection form. The following data were recorded: the first author, year of publication, country, ethnicity, source of control, genotyping method, and the number of each type of GGN repeat polymorphism.
Statistical Analysis
Data were divided into 2 categories, GGN repeats ≤16 and GGN repeats >16, which are based on the previous meta-analysis. The I 2 statistic with 25% <25% to 50%, and >50% indicated low, moderate, and high heterogeneity, respectively.15 The pooled odds ratio [OR] was calculated by a fixed-effects model (I 2 < 50%) or a random-effects model (I 2 ≥ 50%) based on the heterogeneity among studies. The potential publication bias was assessed via Begg funnel plot and Egger regression test, and P < .05 was considered statistically significant in publication bias. All statistical analyses were conducted using Stata version 12.1 software (StataCorp, College Station, Texas)
Results
Study Characteristics
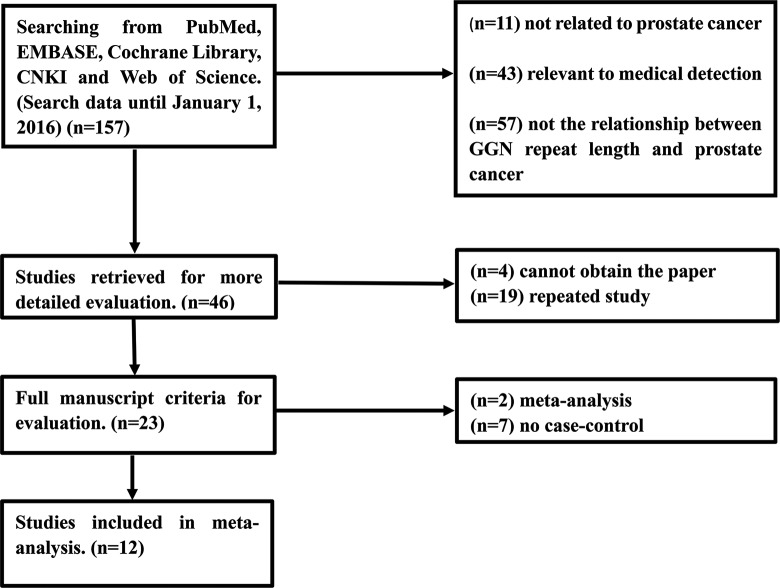
A total of 157 records were yielded by initial search. After reading the abstract of the remaining 46 articles, 23 articles with access to the full manuscript were selected for further evaluation. The screening process is presented in detail in Figure 1, and eventually 12 publications10,12,16–25 with 13 case–control studies were included in our meta-analysis.
Figure 1.
Flowchart showing the selection process for the included studies.
Literature Analysis
The main characteristics of the included studies are summarized in Table 1. All of the 12 studies were published in English. The sample size of each publication varied from 86 to 1367, and there were 3141 cases and 3673 controls in our meta-analysis. Four studies were conducted in Caucasian population and 2 studies were conducted in African American population.
Table 1.
Characteristics of the Included Studies.
| Cases | Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Author | Year | Country | Ethnicity | ≤16 | >16 | ≤16 | >16 | Source of Control | Genotyping Method |
| Rodriguez-Gonzalez | 2009 | Spain | NA | 0 | 72 | 1 | 105 | Hospital based | PCR |
| Edwards | 1999 | UK | Mixed | 98 | 64 | 168 | 116 | Hospital based | PCR |
| Ciceka | 2004 | USA | Caucasian | 108 | 289 | 119 | 315 | Hospital based | PCR |
| African American | 14 | 24 | 12 | 36 | Hospital based | PCR | |||
| Salinas | 2005 | USA | Caucasian | 353 | 146 | 318 | 139 | Hospital based | PCR |
| Platz | 1998 | USA | NA | 3 | 570 | 8 | 786 | Population based | PCR |
| Chang | 2002 | USA | NA | 227 | 100 | 102 | 72 | Hospital based | PCR |
| Chen | 2002 | USA | Caucasian | 29 | 271 | 27 | 273 | Population based | PCR |
| Irvine | 1995 | USA | Mixed | 44 | 23 | 14 | 108 | Hospital based | PCR |
| Stanford | 1997 | USA | Caucasian | 201 | 56 | 175 | 75 | Hospital based | PCR |
| Hsing | 2000 | China | Chinese | 3 | 184 | 3 | 292 | Hospital based | PCR |
| Miller | 2001 | USA | NA | 82 | 51 | 44 | 25 | Hospital based | PCR |
| Lange | 2008 | USA | African American | 73 | 56 | 229 | 111 | Population based | PCR |
Abbreviations: PCR, polymerase chain reaction; NA, not applicable.
aStudy included 2 case–controls.
Meta-Analysis
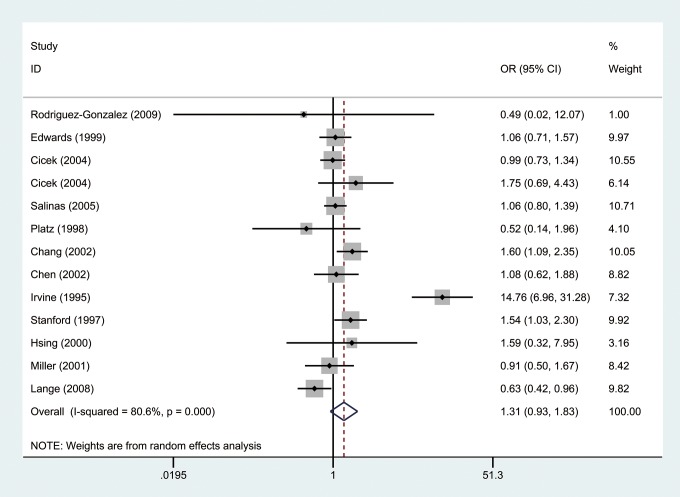
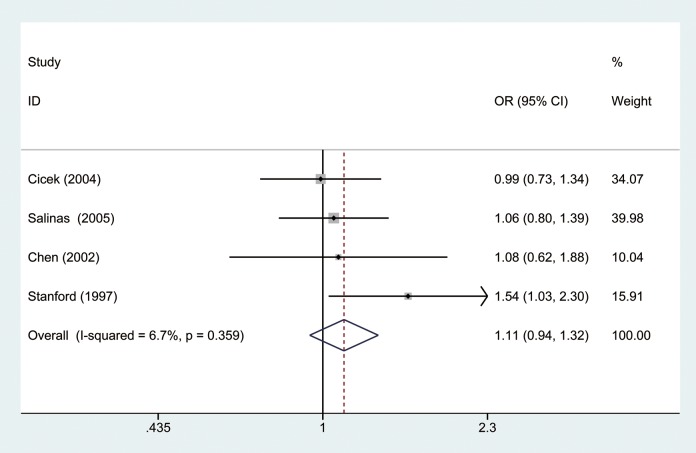
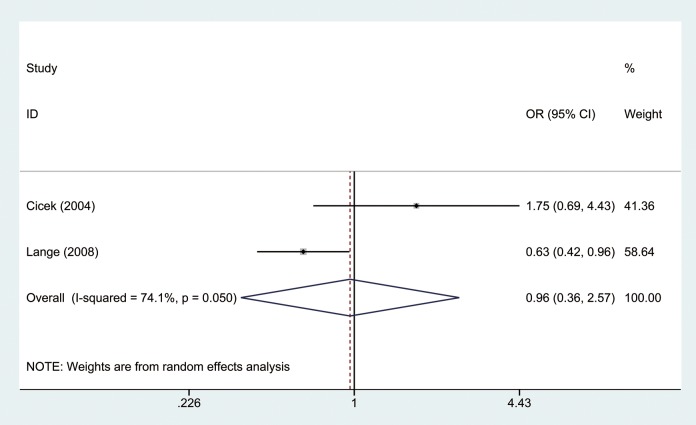
The OR was calculated by random-effects model because there was significant heterogeneity between studies (I2 = 80.6%, P = .000). As shown in Figure 2, our results indicated that there was no significant difference between short repeat group and long repeat group in the overall analysis (OR = 1.31, 95% CI: 0.93-1.83). Furthermore, we divided our study population into Caucasian and African American groups by ethnicity. Results showed there was no significant difference in both Caucasian (I2 = 6.7%, P = .359, OR = 1.11, 95% CI: 0.94-1.32; Figure 3) and African American groups (I2 = 74.1%, P = .050, OR = 0.963, 95% CI: 0.36-2.58; Figure 4).
Figure 2.
Forest plot of odd ratios (ORs) of all studies using a random-effects model.
Figure 3.
Forest plot of odd ratios (ORs) of Caucasian by ethnicity using a fixed-effects model.
Figure 4.
Forest plot of odd ratios (ORs) of African American by ethnicity using a random-effects model.
Sensitivity Analysis
The stability of the study was detected by sensitivity analysis, by excluding 1 individual study in the sequence. Our analysis displayed that the result was not changed.
Assessment of Publication Bias
The publication bias was assessed by Begg funnel plot and Egger test. The results showed statistical evidence of publication bias in the overall analysis and subgroup analysis (Figure 5).
Figure 5.
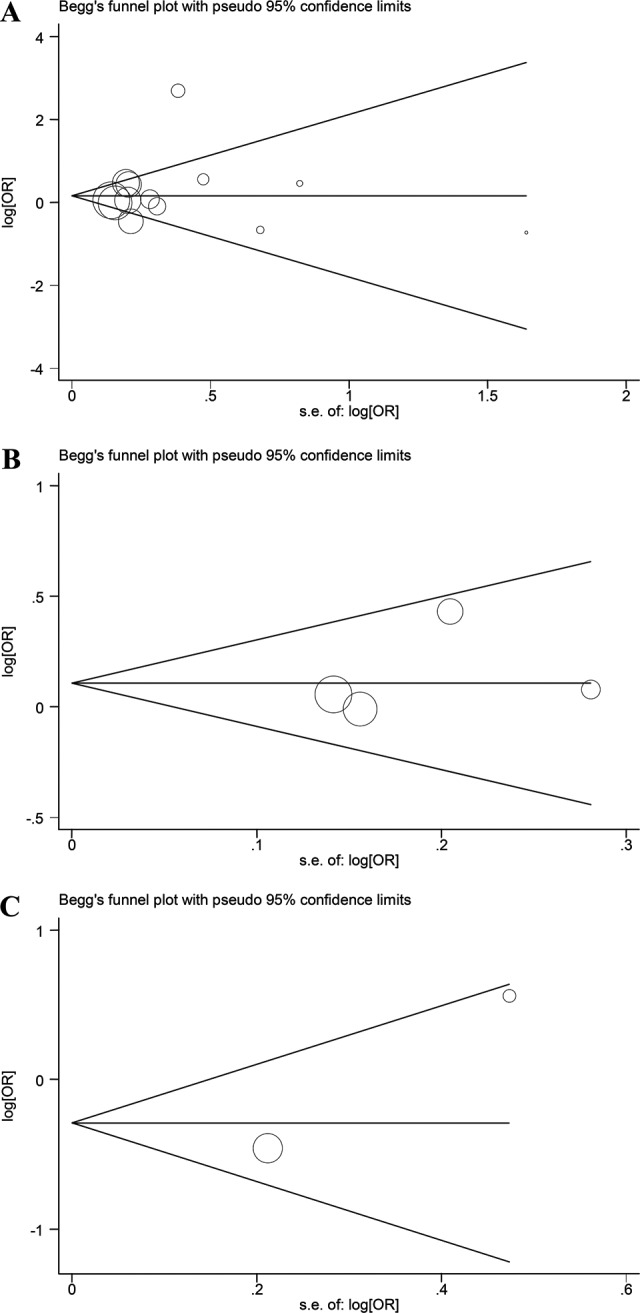
A, Begg funnel plot for publication bias test of overall analysis. B, Begg funnel plot for publication bias test of Caucasian by subgroup. C, Begg funnel plot for publication bias test of African American by subgroup.
Discussion
Nowadays a number of studies have investigated the association between GGN repeat polymorphisms and the risk of cancer. Meng et al didn’t detect any significant association between GGN repeat polymorphisms and ovarian cancer risk.26 But Rodriguez et al suggested shorter GGN repeats in the AR gene were associated with benign endometrial cancer.27 Jiang et al suggested that GGN repeat polymorphism may be involved in the etiology of testicular cancer.28 Thus, we can conclude that GGN repeat polymorphisms may have different impact on different cancers. Rodriguez-Gonzalez et al thought shorter GGN repeats increased the risk for PCa.25 However, Platz et al supported that there was no relation between GGN repeat length and PCa risk.18 More importantly, a previous meta-analysis showed short GGN repeats may be related to PCa.11 In recent years, with more publications on these studies, previous results may be changed. Thus, an updated meta-analysis is essential.
Based on the 6814 participants, this study demonstrated that men with shorter GGN repeats (GGN ≤ 16) had no higher risk of being patients with PCa compared to longer GGN repeats (GGN > 16). Compared with the previous study, we drew a different conclusion. However, considering that our meta-analysis included more cases and controls, and nearly double the sample size, which can strengthen the statistical power, we think our result is more reliable. Otherwise, previous meta-analysis did not investage the influences of ethnicity, we thus analyzed GGN repeat polymorphisms in Caucasian and African Americans. Results showed that shorter GGN repeats did not increase the risk of PCa compared to longer GGN repeats in Caucasian and African American groups.
Since prostatic epithelium is androgen dependent, it is possible that the AR gene may influence an individual’s susceptibility to PCa. It has been reported that the growth of most early forms of PCa requires the normal activity of the AR.29 The AR is necessary for the translation of the well-known effects of androgens on prostate cells.30 The GGN encodes variable lengths polyglycine stretches and may play an important role in AR. However, results from different studies of transient transfection of reporter constructs have shown that deletion of the GGN tract resulted in no alteration in AR transcriptional activities.31,32 It indicated that GGN repeats cannot increase the risk of PCa. Interestingly, Stanford et al indicated that men with ≤16 repeats had higher risk estimates than men with >16 repeats.17 Considering the complex etiology of cancer, PCa risk cannot be only related to a single factor. Demographic patterns, inflammation, Statin drugs, lifestyle, hormonal influences, cholesterol, and genome wide variation play an important role in prostate carcinogenesis.33 Thus, it is not surprising that GGN repeat polymorphism is not associated with PCa.
For subgroup analysis based on ethnicity, same results were obtained. Previously, Lange et al found no evidence for an association between AR GGN repeat lengths and PCa risk in a population-based sample of African Americans12 which consists with our results. Otherwise, Salinas et al detected that shorter GGN repeats would not increase the risk of PCa compared with longer GGN repeats, moreover, this finding was not changed after controlling for body mass index or family history of PCa.24 Notably, Stanford et al 17 and Irvine et al 16 supported that short GGN repeat length had higher risks than long GGN repeat length. A possible reason may be associated with the test methods. The 2 research were conducted in the early year, however, the test methods are more rational and accurate in the recent years, which means more rational results are obtained now.34 Interestingly, Hsing et al used a different dividing method, and their results showed that those with a GGN repeat length shorter than 23 had increased risk of PCa compared to those with >23 GGN repeats.20 Furthermore, Chen et al indicated that men with short GGN repeats (≤17) were not at increased risk of PCa compared to men with >17 GGN repeats.22 Thus, we thought the dividing method also played an important role. Further analysis is needed in the future study.
Heterogeneity is a major concern for meta-analysis. In our overall analysis, the value for I2 was 80.6%, which indicated high heterogeneity. After we excluded Irvine et al’s16 study, the I2 changed to 34.5%, which means moderate heterogeneity but the result did not change, which means the overall results have enough statistical power. However, our results should be illustrated with caution due to the following limitations. First, because the included studies were only written in English, the language bias is unavoidable. Second, because of only 2 publications in African American group, it can result in a potential selection bias in the subgroup meta-analysis. Third, stratified analysis cannot be conducted by age and family history because of the insufficient information. Therefore, further analysis with more studies are required.
In conclusion, our meta-analysis demonstrated that a shorter GGN repeat polymorphism cannot increase the risk of PCa compared with the longer GGN repeats. Otherwise, the effect that the shorter GGN repeats would increase the risk of PCa was not observed in Caucasians and African Americans. Our results suggested that GGN repeat length should not be prescribed as a specific diagnose marker in PCa. Considering the limitations, more well-designed and large-scale studies are required in the future.
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (Grant Numbers: 81160097, 21505025); the Research Fund for the Doctoral Program of Higher Education of China (Grant Number: 20104503120008); the Guangxi Natural Science Foundation (Grant Numbers: 2011GXNSFA018175, 2013GXNSFGA019005); and Students’ platform for innovation and entrepreneurship training program (Grant Number: 201610598112).
Abbreviations
- AR
androgen receptor
- Pca
prostate cancer
Authors’ Note: Jiatong Li and Feifan Xiao contributed equally to this work and should be considered as the first coauthors
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the National Natural Science Foundation of China (Grant Numbers: 81160097, 21505025); the Research Fund for the Doctoral Program of Higher Education of China (Grant Number: 20104503120008); the Guangxi Natural Science Foundation (Grant Numbers: 2011GXNSFA018175, 2013GXNSFGA019005); Students’ platform for innovation and entrepreneurship training program (Grant Number: 201610598112).
References
- 1. De Hong C, Liang Ren L, Qiang W, et al. Comparison of efficacy and safety of conventional laparoscopic radical prostatectomy by the transperitoneal versus extraperitoneal procedure. Sci Rep. 2015;5:14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turay D, Khan S, Diaz Osterman CJ, et al. Proteomic profiling of serum-derived exosomes from ethnically diverse prostate cancer patients. Cancer Invest. 2016;34(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou QX, Tang JQ, Zhao F, Wei FL, Huang Y. The P275A polymorphism in the macrophage scavenger receptor 1 gene and prostate cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2015;16(13):5407–5413. [DOI] [PubMed] [Google Scholar]
- 4. Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079–1092. [DOI] [PubMed] [Google Scholar]
- 5. Bloom JR, Stewart SL, Oakley Girvan I, Banks PJ, Chang S. Family history, perceived risk, and prostate cancer screening among African American men. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2167–2173. [DOI] [PubMed] [Google Scholar]
- 6. Printz C. Hereditary link: scientists seek clues in genetic risks for prostate cancer. Cancer. 2014;120(24):3847–3848. [DOI] [PubMed] [Google Scholar]
- 7. Janne OA, Palvimo JJ, Kallio P, Mehto M. Androgen receptor and mechanism of androgen action. Ann Med. 1993;25(1):83–89. [DOI] [PubMed] [Google Scholar]
- 8. Ding D, Xu L, Menon M, Reddy GP, Barrack ER. Effect of GGC (glycine) repeat length polymorphism in the human androgen receptor on androgen action. Prostate. 2005;62(2):133–139. [DOI] [PubMed] [Google Scholar]
- 9. Sun JH, Lee SA. Association between CAG repeat polymorphisms and the risk of prostate cancer: a meta-analysis by race, study design and the number of (CAG)n repeat polymorphisms. Int J Mol Med. 2013;32(5):1195–1203. [DOI] [PubMed] [Google Scholar]
- 10. Miller EA, Stanford JL, Hsu L, Noonan E, Ostrander EA. Polymorphic repeats in the androgen receptor gene in high-risk sibships. Prostate. 2001;48(3):200–205. [DOI] [PubMed] [Google Scholar]
- 11. Zeegers MP, Kiemeney LA, Nieder AM, Ostrer H. How strong is the association between CAG and GGN repeat length polymorphisms in the androgen receptor gene and prostate cancer risk? Cancer Epidemiol Biomarkers Prev. 2004;13(11 pt 1):1765–1771. [PubMed] [Google Scholar]
- 12. Lange EM, Sarma AV, Ray A, et al. The androgen receptor CAG and GGN repeat polymorphisms and prostate cancer susceptibility in African-American men: results from the Flint Men’s Health Study. J Hum Genet. 2008;53(3):220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu A, Yang X, Hu P, Yan S. Association between the GGN trinucleotide repeat polymorphism in androgen receptor gene and prostate cancer risk: a systematic review and meta-analysis. Int J Lab Med. 2014;35(17):3. [Google Scholar]
- 14. Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med. 2015;4(12):1933–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Li M, Xiao F, et al. Impact of partial DAZ1/2 deletion and partial DAZ3/4 deletion on male infertility. Gene. 2015;571(1):9–16. [DOI] [PubMed] [Google Scholar]
- 16. Irvine RA, Yu MC, Ross RK, Coetzee GA. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer Res. 1995;55(9):1937–1940. [PubMed] [Google Scholar]
- 17. Stanford JL, Just JJ, Gibbs M, et al. Polymorphic repeats in the androgen receptor gene: molecular markers of prostate cancer risk. Cancer Res. 1997;57(6):1194–1198. [PubMed] [Google Scholar]
- 18. Platz EA, Giovannucci E, Dahl DM, et al. The androgen receptor gene GGN microsatellite and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7(5):379–384. [PubMed] [Google Scholar]
- 19. Edwards SM, Badzioch MD, Minter R, et al. Androgen receptor polymorphisms: association with prostate cancer risk, relapse and overall survival. Int J Cancer. 1999;84(5):458–465. [DOI] [PubMed] [Google Scholar]
- 20. Hsing AW, Gao YT, Wu G, et al. Polymorphic CAG and GGN repeat lengths in the androgen receptor gene and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2000;60(18):5111–5116. [PubMed] [Google Scholar]
- 21. Chang BL, Zheng SL, Hawkins GA, et al. Polymorphic GGC repeats in the androgen receptor gene are associated with hereditary and sporadic prostate cancer risk. Hum Genet. 2002;110(2):122–129. [DOI] [PubMed] [Google Scholar]
- 22. Chen C, Lamharzi N, Weiss NS, et al. Androgen receptor polymorphisms and the incidence of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(10 pt 1):1033–1040. [PubMed] [Google Scholar]
- 23. Cicek MS, Conti DV, Curran A, et al. Association of prostate cancer risk and aggressiveness to androgen pathway genes: SRD5A2, CYP17, and the AR. Prostate. 2004;59(1):69–76. [DOI] [PubMed] [Google Scholar]
- 24. Salinas CA, Austin MA, Ostrander EO, Stanford JL. Polymorphisms in the androgen receptor and the prostate-specific antigen genes and prostate cancer risk. Prostate. 2005;65(1):58–65. [DOI] [PubMed] [Google Scholar]
- 25. Rodriguez-Gonzalez G, Cabrera S, Ramirez-Moreno R, et al. Short alleles of both GGN and CAG repeats at the exon-1 of the androgen receptor gene are associated to increased PSA staining and a higher Gleason score in human prostatic cancer. J Steroid Biochem Mol Biol. 2009;113(1-2):85–91. [DOI] [PubMed] [Google Scholar]
- 26. Meng X, Lu P, Chu Z, Fan Q. The androgen receptor cytosine-adenine-guanine repeat length contributes to the development of epithelial ovarian cancer. Oncotarget. 2016;7(2):2105–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez G, Bilbao C, Ramirez R, et al. Alleles with short CAG and GGN repeats in the androgen receptor gene are associated with benign endometrial cancer. Int J Cancer. 2006;118(6):1420–1425. [DOI] [PubMed] [Google Scholar]
- 28. Jiang W, Zhang J, Zhou Q, et al. Predictive value of GGN and CAG repeat polymorphisms of androgen receptors in testicular cancer: a meta-analysis. Oncotarget. 2016;7(12):13754–13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaddipati JP, McLeod DG, Heidenberg HB, et al. Frequent detection of codon 877 mutation in the androgen receptor gene in advanced prostate cancers. Cancer Res. 1994;54(11):2861–2864. [PubMed] [Google Scholar]
- 30. Ross RK, Henderson BE. Do diet and androgens alter prostate cancer risk via a common etiologic pathway? J Natl Cancer Inst. 1994;86(4):252–254. [DOI] [PubMed] [Google Scholar]
- 31. Jenster G, de Ruiter PE, van der Korput HA, Kuiper GG, Trapman J, Brinkmann AO. Changes in the abundance of androgen receptor isotypes: effects of ligand treatment, glutamine-stretch variation, and mutation of putative phosphorylation sites. Biochemistry. 1994;33(47):14064–14072. [DOI] [PubMed] [Google Scholar]
- 32. Gao T, Marcelli M, McPhaul MJ. Transcriptional activation and transient expression of the human androgen receptor. J Steroid Biochem Mol Biol. 1996;59(1):9–20. [DOI] [PubMed] [Google Scholar]
- 33. Zhang CD, Li HT, Liu K, et al. Impact of caspase-8 (CASP8)-652 6N del and D302H polymorphisms on prostate cancer in different ethnic groups. Asian Pac J Cancer Prev. 2014;15(18):7713–7718. [DOI] [PubMed] [Google Scholar]
- 34. Xiao F, Lan A, Lin Z, et al. Impact of CAG repeat length in the androgen receptor gene on male infertility—a meta-analysis. Reprod Biomed Online. 2016;33(1):39–49. [DOI] [PubMed] [Google Scholar]