Abstract
The aim of this study is to determine whether stereotactic body radiotherapy for multiple vertebral metastases treated with a single isocenter results in greater intrafraction errors than stereotactic body radiotherapy for single vertebral metastases and to determine whether the currently used spinal cord planning organ at risk volume and planning target volume margins are appropriate. Intrafraction errors were assessed for 65 stereotactic body radiotherapy treatments for vertebral metastases. Cone beam computed tomography images were acquired before, during, and after treatment for each fraction. Residual translational and rotational errors in patient positioning were recorded and planning organ at risk volume and planning target volume margins were calculated in each direction using this information. The mean translational residual errors were smaller for single (0.4 (0.4) mm) than for multiple vertebral metastases (0.5 (0.7) mm; P = .0019). The mean rotational residual errors were similar for single (0.3° (0.3°) and multiple vertebral metastases (0.3° (0.3°); P = .862). The maximum calculated planning organ at risk volume margin in any direction was 0.83 mm for single and 1.22 for multiple vertebral metastases. The maximum calculated planning target volume margin in any direction was 1.4 mm for single and 1.9 mm for multiple vertebral metastases. Intrafraction errors were small for both single and multiple vertebral metastases, indicating that our strategy for patient immobilization and repositioning is robust. Calculated planning organ at risk volume and planning target volume margins were smaller than our clinically employed margins, indicating that our clinical margins are appropriate.
Keywords: radiotherapy, intensity modulated, radiotherapy, image guided, radiosurgery, spine, cone beam computed tomography
Introduction
Conventional palliative external beam radiotherapy for spinal metastases has been employed for the palliation of pain and spinal cord compression for decades.1,2 Several strategies have been explored in recent years to improve upon historic outcomes, and the most recent development is the use of stereotactic body radiotherapy (SBRT).1 Stereotactic body radiotherapy refers to the precise delivery of highly conformal and image-guided hypofractionated external beam radiotherapy, delivered in a single or few fractions, with doses at least biologically equivalent to a radical curative course.3 Stereotactic body radiotherapy relies on the use of modern radiotherapy technologies such as image guidance, sophisticated immobilization, advanced treatment planning systems, and, most importantly, adaptive patient realignment capabilities that permit millimetric precision.3
Initial experiences of SBRT for spinal metastases have demonstrated a risk of radiation myelopathy that increases substantially as the dose delivered to the spinal cord increases.4,5 Treatment failures have also been noted, particularly within the epidural compartment, where dose may be lowered in order to meet spinal cord dose constraints.6,7 One particular challenge with spinal SBRT is that even when precise radiotherapy is planned such that high doses to the target and low doses to organs at risk (OARs) are achieved, errors in patient setup can result in substantially different delivered radiotherapy doses.8,9 For instance, Wang et al reported that a 2-mm translational error in any direction can result in >5% tumor coverage loss and >25% maximal dose increase to OARs.9 Rotational errors combined with translational errors can also result in large discrepancies in delivered doses.8
We have previously reported our initial experience with spinal SBRT setup, in a cohort of patients primarily with single vertebral metastases (SVM), employing the use of a dual vacuum immobilization device, pretreatment and intrafraction cone beam computed tomography (CBCT), and a 6 degrees of freedom (6-DOF) robotic couch. We were able to maintain the position of a spine SBRT target to within 1.2 mm and 0.9° (95% confidence), reflecting the robustness of this technique.10 We have subsequently treated a large cohort of patients with multiple vertebral metastases (MVM) with long target volumes, treated with a single isocenter, which is potentially susceptible to greater translational and rotational errors than that experienced with SVM.11 In this study, we compared the intrafractional error of SBRT for MVM versus that for SVM to determine the optimal margins for the planning target volumes (PTVs) and planning organ-at-risk volumes (PRVs).
Materials and Methods
This was a retrospective study, approved by the Sunnybrook Health Sciences Center research ethics board, evaluating 65 SBRT treatments for patients with vertebral metastases between January 2009 and October 2012 at the Sunnybrook Odette Cancer Centre. Patients who were treated using 1- to 5-fraction SBRT and immobilized using the BodyFIX (Elekta AB, Stockholm, Sweden) dual vacuum immobilization device were included. Patients who were immobilized using a thermoplastic mask (those with metastases at C1-T2) were excluded from this study. Patients were stratified into 2 groups: those with SVM and those with MVM.
Patients had radiotherapy planning magnetic resonance imaging (MRI) scans generally performed within 7 days of the planning CT scans without immobilization. T1- and T2-weighted volumetric sequences were acquired in the region of the target vertebrae, typically with 1.5- to 2-mm slice thickness. The typical in-plane resolution was 0.4 mm × 0.4 mm for T2-weighted scans and 0.85 mm × 0.85 mm for T1-weighted scans. Planning CT scans were acquired through the region of the target vertebrae, typically with 1-mm slice thickness. The typical in-plane resolution was 1.17 mm × 1.17 mm. The planning MRI and CT scans were imported into Pinnacle v9.2 treatment planning system (Philips, Amsterdam, Netherlands), where the scans were manually, rigidly coregistered.
Clinical target volumes (CTVs) were manually contoured according to the consensus criteria.12 Planning target volumes were generated by 2-mm isotropic expansions of the CTVs. The spinal cord was contoured with a 1.5-mm isotropic expansion to create the PRV. At the level of the cauda equina, the thecal sac was contoured. Other OARs such as esophagus, lungs, and kidneys were contoured as per institutional guidelines, depending on the vertebral level treated.
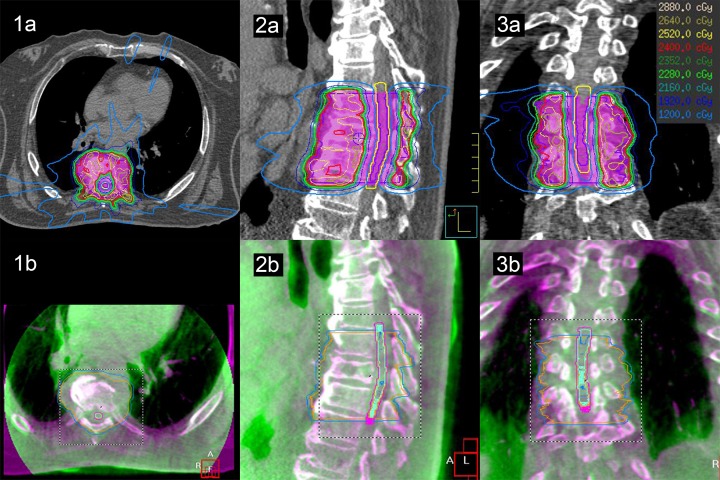
All patients were treated using single-isocenter step-and-shoot intensity-modulated radiotherapy (IMRT) plans. The initial patient setup was based on isocenter tattoos. An initial CBCT was acquired at 1-mm isotropic resolution and coregistered to the initial planning CT scan using X-ray volume imaging software v4.5 (Elekta AB). An initial automated rigid coregistration was performed using the grey-level correlation ratio image-matching algorithm13 (with the region of interest set to encompass the PTV and adjacent vertebrae), followed by manual rigid adjustment if required. Based on the translations and rotations required to coregister the CBCT to the planning CT scan, patient positioning was automatically adjusted using the HexaPOD (Elekta AB) 6-DOF robotic couch. A second CBCT was then acquired for positional verification CBCT scan (Verif). Provided that the Verif CBCT showed the patient target position was within tolerance (1 mm translation, 1° rotation), treatment delivery was initiated. For single-fraction treatments (n = 27), 2 intrafraction CBCTs were acquired (after one-third and two-third of the beams had been delivered, respectively, which are designated Intra1 and Intra2, respectively), whereas for multiple-fraction treatments (n = 38), a single intrafraction CBCT was acquired (after one-half of the beams had been delivered). If the patient was out of tolerance for the Intra1 and/or Intra2 scans, the patient was repositioned, but no Verif CBCT scan was required. A posttreatment CBCT scan was performed (Post) to verify the final position. An example treatment plan with corresponding CBCT is shown in Figure 1.
Figure 1.
A radiotherapy plan with isodose distributions (1A-3A) and CBCT matching (1B-3B) for a multilesion spine SBRT treatment prescribed to 24 Gy in 2 fractions. The images are represented in the axial (1), sagittal (2), and coronal (3) planes. The spinal cord PRV is contoured in yellow and the PTV is represented in pink color wash on the radiotherapy plans. The isodose lines are shown with dose legend on the top right corner of the figure. The CBCT matching is represented in the purple/green color scheme, where purple is the planning CT scan and green is the CBCT. CBCT indicates cone beam computed tomography; PRV, planning organ at risk volume; PTV, planning target volume; SBRT, stereotactic body radiotherapy.
Translational and rotational errors in patient positioning were recorded after each CBCT. Translations were recorded in the x-axis (right–left [RL]), y-axis (superior–inferior [SI]), and z-axis (anterior–posterior [AP]). Rotations were similarly recorded about the x-axis (pitch), y-axis (roll), and z-axis (yaw). The mean, standard deviation, vector, and 95% confidence intervals (CIs) were calculated for these errors, as well as the percentage of shifts exceeding the setup tolerance of 1 mm and 1°. The mean residual translational and rotational errors (defined as errors occurring after the initial CBCT and patient repositioning) were compared between SVM and MVM.
Random and systematic errors were calculated in each direction,14 and the PRV and PTV margins were calculated accordingly. Stroom and Heijmen’s margin recipe (1.6Σ + 0.2σ)15 was used for calculating the spinal cord PRV margin, and Herschtal et al’s SDE2 hypofractionated margin recipe (calculated using code provided by the author)16 was used for calculating the PTV margin. In addition to the random and systematic errors, the following parameters were used for the hypofractionated margin calculation: 3-mm penumbral width, 2 or 5 fractions, and margin covering 90% of patients with at least 95% of the prescribed dose.
Continuous variables such as translational and rotational errors were summarized with means and standard deviations (SD). Analysis of variance (ANOVA) was used to evaluate differences in errors between the differently timed scans. For the calculations that were found to be significant, further post hoc analysis using least significant difference was used to evaluate which pairs contributed to the difference. Independent Student t tests were also used to evaluate differences in patient positioning errors between SVM and MVM treatment groups. All P values were 2 sided, and P < .05 was considered to indicate a significantly different result. Statistical analysis was carried out using Statview 5.0.1 (SAS Institute, Cary, North Carolina).
Results
Of the 65 SBRT treatments included in this study, 44 were for SVM and 21 were for MVM. Two of the patients with MVM had noncontiguous vertebral bodies treated (with a single isocenter) and the rest had contiguous vertebral bodies treated. For the patients with MVM, the number of vertebral bodies included in target volumes were 2 vertebral bodies (11 patients), 3 vertebral bodies (7 patients), 4 vertebral bodies (2 patients), and 5 vertebral bodies (1 patient), whereas the number of vertebral bodies from the highest treated to the lowest treated vertebral body (including the untreated vertebral bodies in the “gap” between the treated vertebral bodies) were 2 vertebral bodies (13 patients), 3 vertebral bodies (5 patients), 4 vertebral bodies (2 patients), and 5 vertebral bodies (1 patient). The length of the PTV in the craniocaudal direction (including the “gap” in between noncontiguously treated vertebrae) ranged from 4.5 to 14 cm. Twenty-seven patients were treated with single-fraction SBRT, whereas 38 patients were treated with multiple-fraction SBRT. In total, 565 CBCTs were acquired and analyzed.
The mean (SD) of the absolute translational residual errors (Verif, Intra1, Intra2, and Post) were 0.5 (0.6) mm, 0.4 (0.4) mm, and 0.5 (0.5) mm for the for x, y, and z directions, respectively. The mean (SD) of the absolute rotational residual errors were 0.3° (0.3°), 0.3° (0.4°), and 0.2° (0.3°) for the x, y, and z directions, respectively. The residual error per CBCT type for each treatment group is summarized in Table 1.
Table 1.
Absolute Residual Setup Error Across CBCT Types for Patients Treated for SVM and MVM.
| CBCT Type | CBCT Sample Size | Translational Deviation (mm), Mean (SD) | Rotational Deviation (degrees), Mean (SD) | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||
| SVM (n = 44) | |||||||
| Verif | 87 | 0.4 (0.4) | 0.3 (0.3) | 0.4 (0.3) | 0.3 (0.3) | 0.3 (0.4) | 0.2 (0.2) |
| Intra1 | 87 | 0.5 (0.5) | 0.4 (0.4) | 0.5 (0.4) | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.3) |
| Intra2 | 24 | 0.4 (0.4) | 0.3 (0.3) | 0.4 (0.4) | 0.3 (0.2) | 0.2 (0.2) | 0.1 (0.2) |
| Post | 76 | 0.4 (0.4) | 0.5 (0.5) | 0.6 (0.7) | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.3) |
| MVM (n = 21) | |||||||
| Verif | 53 | 0.5 (0.4) | 0.4 (0.3) | 0.3 (0.2) | 0.2 (0.3) | 0.4 (0.6) | 0.2 (0.2) |
| Intra1 | 53 | 0.7 (0.8) | 0.6 (0.7) | 0.5 (0.7) | 0.3 (0.4) | 0.5 (0.4) | 0.2 (0.2) |
| Intra2 | 5 | 0.4 (0.4) | 0.4 (0.4) | 0.4 (0.3) | 0.4 (0.3) | 0.4 (0.4) | 0.1 (0.1) |
| Post | 40 | 0.7 (1.3) | 0.5 (0.4) | 0.6 (0.7) | 0.3 (0.3) | 0.4 (0.4) | 0.3 (0.2) |
Abbreviations: CBCT, cone beam computed tomography; Intra1, first intrafraction scan; Intra2, second intrafraction scan; MVM, multiple vertebral metastases; Post, posttreatment CBCT scan; SD, standard deviation; SVM, single vertebral metastases; Verif, verification CBCT.
The overall out-of-tolerance registrations for translations for Verif, Intra1, Intra2, and Post CBCT scans were 13%, 7%, and 6% for x, y, and z directions, respectively. The overall out-of-tolerance registrations for rotations were 2%, 2%, and 1% for x, y, and z directions, respectively. Overall, 21% were out of tolerance (3% of which had translational and rotational failures simultaneously). The frequency of out-of-tolerance registrations for each CBCT type is summarized in Table 2.
Table 2.
The Percentage of Translational and Rotational Shifts That Were Out of Tolerance (Threshold of 1 mm and 1°) for Verification, Intra, and Post CBCT Registrations for Patients With Single and Multiple Spine Metastases.
| CBCT Type | Translation | Rotation | ||||
|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |
| SVM | ||||||
| Verif (%) | 18 | 15 | 10 | 3 | 5 | 0 |
| Intra1 (%) | 20 | 15 | 24 | 5 | 15 | 5 |
| Intra2 (%) | 6 | 0 | 15 | 0 | 0 | 0 |
| Post (%) | 33 | 23 | 34 | 3 | 0 | 8 |
| MVM | ||||||
| Verif (%) | 22 | 13 | 5 | 9 | 13 | 0 |
| Intra1 (%) | 37 | 24 | 8 | 6 | 0 | 5 |
| Intra2 (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Post (%) | 30 | 34 | 27 | 3 | 12 | 8 |
Abbreviations: CBCT, cone beam computed tomography; Intra1, first intrafraction scan; Intra2, second intrafraction scan; MVM, multiple vertebral metastases; Post, posttreatment CBCT scan; SVM, single vertebral metastases; Verif, verification CBCT.
The absolute mean (SD) translational residual errors for the Verif, Intra1, Intra2, and Post CBCT scans were 0.4 (0.4) mm, 0.5 (0.6) mm, 0.4 (0.3) mm, and 0.5 (0.7) mm, respectively. The ANOVA result (F 3,1197 = 6.58, P < .0002) followed by pairwise comparisons revealed a significant difference in intrafraction translation residual error between Intra1 and Intra2 (P = .02), Verif and Post (P = .0003), and Post and both Intra1 and Intra2 (P < .001). The ANOVA (F 3,1197 = 1.59, P = .19) revealed there was no sufficient evidence of a difference for rotations.
The mean translational residual errors were smaller with SVM (0.4 (0.4) mm) than for MVM (0.5 (0.7) mm; P = .0019). The mean rotational residual errors were similar for SVM (0.3° (0.3°)) as for MVM (0.3° (0.3°); P = .862). The 95% CIs using a setup threshold of 1 mm and 1° for SVM were 1.3 mm and 0.8°, 1.0 mm and 0.8°, and 1.4 mm and 0.7° in the x, y, and z directions, respectively. Similarly, for MVM, the 95% CIs in the x, y, and z directions were 1.9 mm and 0.8°, 1.3 mm and 1.1°, and 1.5 mm and 0.6° in the x, y, and z directions, respectively. The overall 95% CIs were 1.2 mm and 0.8° for SVM and 1.6 mm and 0.7° for MVM.
The random and systematic errors in each direction are shown in Table 3. The margin calculations for PRV, PTV with 2 fractions, and PTV with 5 fractions are also shown in Table 3. The maximum PRV in any direction for PRV was 0.8 mm for SVM and 1.2 mm for MVM. The maximum PTV for 2-fraction treatment in any direction was 1.4 mm for SVM and 1.9 mm for MVM. The maximum PTV for 5-fraction treatment in any direction was 1.4 mm for SVM and 1.8 mm for MVM. These margins are smaller than the margins currently used in clinical practice (1.5 mm for PRV and 2 mm for PTV for both SVM and MVM).
Table 3.
Errors and Margins in Each Direction.
| x | y | z | |
|---|---|---|---|
| Systematic error | |||
| SVM (mm) | 0.41 | 0. 38 | 0. 47 |
| MVM (mm) | 0.72 | 0.45 | 0.56 |
| Random error | |||
| SVM (mm) | 0.50 | 0.33 | 0.42 |
| MVM (mm) | 0.34 | 0.35 | 0.23 |
| PRV margin | |||
| SVM (mm) | 0.75 | 0.67 | 0.84 |
| MVM (mm) | 1.22 | 0.79 | 0.94 |
| PTV margin (2 fractions) | |||
| SVM (mm) | 1.4 | 1.2 | 1.4 |
| MVM (mm) | 1.9 | 1.3 | 1.5 |
| PTV margin (5 fractions) | |||
| SVM (mm) | 1.2 | 1.1 | 1.4 |
| MVM (mm) | 1.8 | 1.3 | 1.4 |
Abbreviations: MVM, multiple vertebral metastases; PRV, planning organ at risk volume; PTV, planning target volume; SVM, single vertebral metastases.
Discussion
In this study, we have shown that intrafraction errors were small for both SVM and MVM when treated with a robust spine SBRT technique. Translational errors but not rotational errors were observed to be smaller with SVM than for MVM. In fact, the calculated PRV and PTV margins are smaller than what we use clinically.
Previous studies investigating intrafraction errors specific to spinal SBRT have yielded similar results. Finnigan et al reported on 30 courses of SBRT delivered in 27 patients, 24 of which were SVM and 6 were MVM.11 Patients were immobilized in a thermoplastic mask and/or customized vacuum bag depending on the location of the spinal target. Treatment was delivered using either static-field IMRT or volumetric-modulated arc therapy. Cone beam computed tomography was performed before, during, and after SBRT, and patients were repositioned using a 6-DOF robotic couch. Based on a 2 mm and 2° correction thresholds, minimum PTV/PRV margins of 3 mm were recommended. The number of treated vertebral bodies was not associated with greater translational errors but was associated with greater rotational errors. Increasing treatment duration was also associated with greater rotational errors. However, our results indicate MVM to be associated with greater translational but not rotational errors. Their magnitude of the errors and PTV margins were also greater than that reported in our study. These discrepant results can possibly be explained by differences in repositioning strategies between our study and theirs, our larger sample size of 65 patients evaluated and 565 CBCT analyzed, and our strict repositioning thresholds of 1 mm and 1°. The latter may be an important distinction as shown previously in Hyde et al that stricter repositioning thresholds can have an impact on precision.10
Nishimura et al also performed a similar study on 25 courses of SBRT delivered in 24 patients, 13 of which were for SVM and 12 of which were for MVM.17 Patients were immobilized in a thermoplastic mask or vacuum cushion depending on the location of the spinal target. Treatment was delivered using helical tomotherapy. Megavoltage (MV) CT images were acquired before and during the delivery of SBRT. Translational errors (but not rotational errors) were adjusted for after each MV CT. The required PTV margins were 0.98 mm in the RL direction, 0.69 mm in the SI direction, and 1.26 mm in the AP direction. No comparisons of errors between SVM and MVM were performed. Nevertheless, their results are comparable to what we report.
The main strength of our study is that we have performed formal calculations of PRV and PTV margins required for spinal SBRT, including the use of a formula that takes the small number of fractions into account. This study confirms the appropriateness of our clinically employed margins. The maximum calculated margins (PRV: 0.8 mm for SVM and 1.2 mm for MVM; PTV: 1.4 mm for SVM and 1.9 mm for MVM) were smaller than our clinically employed margins (PRV: 1.5 mm; PTV: 2.0 mm). It is appropriate that the clinically employed margins are slightly larger than the calculated margins because of errors that are unaccounted for as described in the paragraph below. Another strength is our comparison of intrafraction errors between SVM and MVM. Our study confirms the findings of Finnigan et al 11 showing that MVM results in larger intrafraction errors. Our study takes this 1 step further and shows the difference in PRV and PTV margins required for SVM and MVM.
There are a number of limitations to this study. First, random movements in between CBCTs could not be captured, potentially resulting in greater actual intrafraction errors than what was reported in this study. These movements are likely to be small with the use of the near-rigid body immobilization employed in this study, and the fact that past cine MRI studies have shown physiologic spinal cord oscillatory motion to be submillimeter.18 More frequent imaging (eg, with a stereoscopic X-ray system) or real-time imaging (eg, with an integrated MRI-linear accelerator) may be able to overcome this limitation, but these technologies are not routinely applied to our system of delivery. Other sources of uncertainty that were not taken into account include target volume delineation errors, CBCT and MRI registration errors, and machine tolerance errors, which may also lead to an underestimation of the PRV and PTV margins. Additionally, there was variation in the number and timing of CBCTs performed in each patient. For instance, in 17% of the fractions, a posttreatment CBCT was not performed because the patient had already exceeded the manufacturer’s recommended time limit in the BodyFIX immobilization system (60 minutes). Also, the time in between each patient move and the subsequent CBCT was variable. This limits the generalizability of these results, as the CBCTs for each patient are not identical. However, these limitations reflect the difficulty of adhering strictly to study protocols when there are real-world considerations in getting multiple patients through treatment. The margin calculations have a number of limitations. The PRV calculation has been validated for conventionally fractionated treatments and therefore may not be completely applicable to hypofractionated treatments.15 The SDE2 hypofractionated PTV margin does take the number of fractions into account, however assumes a spherical target with a uniform penumbral margin.16 This may not be completely applicable to the irregularly shaped targets in spinal SBRT. Furthermore, neither of these calculations takes rotations into account. Finally, a higher than expected proportion of Post scans (23%-34%) were out of tolerance. Most scans were in fact only slightly out of tolerance, as evidenced by only 3% to 10% of Post scans being out of tolerance if the tolerance for translations was increased from 1 to 1.2 mm. The number of out of tolerance in the Post scans may potentially be reduced by optimizing the timing of the intrafraction scans, however, this requires further study.
It is reassuring that both SVM and MVM have smaller calculated PRV and PTV margins than what we use clinically (1.5 mm for PRV and 2 mm for PTV). These findings support our published clinical results of spinal SBRT, which indicate high local control rates (80%-90%) and low toxicity rates (no cases of myelopathy), consistent with publications from other centers.19,20
This study has established the fact that based on residual errors alone, our clinically employed margins are appropriate. Furthermore, the margins for SVM can potentially be smaller than those for MVM. This leads to the possibility of potentially further reducing the margins in those with SVM. This can potentially improve the therapeutic ratio by allowing better coverage of the PTV, where the PTV comes into contact with the spinal cord PRV, and reducing the dose to surrounding OARs. However, this must be done with extreme care because as described above, not all sources of error have been taken into consideration, and the margin calculations have certain limitations. The next step to determine whether margin reductions are possible is a study on errors that were not accounted for in this study, for example, delineation errors, CBCT registration errors, machine tolerance errors, and the impact of rotations and deformations.
Conclusion
Intrafraction errors were small for both SVM and MVM, with calculated PRV and PTV margins that are smaller than what we use clinically. Single vertebral metastases was observed to have smaller intrafraction translational errors and smaller PRV and PTV margins than MVM. Our currently clinically employed margins of 1.5 mm for PRV and 2 mm for PTV are appropriate, and further studies are required to determine whether the margins can be reduced in SVM.
Abbreviations
- 6-DOF
6 degrees of freedom
- CBCT
cone beam computed tomography
- CTVs
clinical target volumes
- IMRT
intensity-modulated radiotherapy
- Intra1
first intrafraction cone beam CT scan
- Intra2
second intrafraction cone beam CT scan
- MRI
magnetic resonance imaging
- MV
megavoltage
- MVM
multiple vertebral metastases
- OAR
organ at risk
- Post
posttreatment CBCT scan
- PRV
planning organ-at-risk volume
- PTV
planning target volume
- SBRT
stereotactic body radiotherapy
- SVM
single vertebral metastases
- Verif
verification CBCT.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Arjun Sahgal has received honorarium for past educational seminars from Elekta AB and research grants from Elekta AB. Dr Arjun Sahgal has received honorarium in participating on a medical advisory board for Varian Medical Systems.
References
- 1. Ejima Y, Matsuo Y, Sasaki R. The current status and future of radiotherapy for spinal bone metastases. J Orthop Sci. 2015;20(4):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systematic review of the randomised trials. Cochrane Database Syst Rev. 2004;(2):CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sahgal A, Roberge D, Schellenberg D, et al. ; The Canadian Association of Radiation Oncology-Stereotactic Body Radiotherapy Task Force. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol). 2012;24(9):629–639. [DOI] [PubMed] [Google Scholar]
- 4. Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):107–116. [DOI] [PubMed] [Google Scholar]
- 5. Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85(2):341–347. [DOI] [PubMed] [Google Scholar]
- 6. Al-Omair A, Masucci L, Masson-Cote L, et al. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. 2013;15(10):1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7(2):151–160. [DOI] [PubMed] [Google Scholar]
- 8. Guckenberger M, Meyer J, Wilbert J, et al. Precision required for dose-escalated treatment of spinal metastases and implications for image-guided radiation therapy (IGRT). Radiother Oncol. 2007;84(1):56–63. [DOI] [PubMed] [Google Scholar]
- 9. Wang H, Shiu A, Wang C, et al. Dosimetric effect of translational and rotational errors for patients undergoing image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2008;71(4):1261–1271. [DOI] [PubMed] [Google Scholar]
- 10. Hyde D, Lochray F, Korol R, et al. Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: intrafraction motion analysis accounting for all six degrees of freedom. Int J Radiat Oncol Biol Phys. 2012;82(3):e555–e562. [DOI] [PubMed] [Google Scholar]
- 11. Finnigan R, Lamprecht B, Barry T, et al. Inter- and intra-fraction motion in stereotactic body radiotherapy for spinal and paraspinal tumours using cone-beam CT and positional correction in six degrees of freedom. J Med Imaging Radiat Oncol. 2016;60(1):112–118. [DOI] [PubMed] [Google Scholar]
- 12. Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(5):e597–e605. [DOI] [PubMed] [Google Scholar]
- 13. Roche A, Malandain G, Pennec X, Ayache N. The Correlation Ratio as a New Similarity Measure for Multimodal Image Registration. In: Proceedings of the First International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer-Verlag; October 11-13, 1998:1115–1124. [Google Scholar]
- 14. van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14(1):52–64. [DOI] [PubMed] [Google Scholar]
- 15. Stroom JC, Heijmen BJ. Limitations of the planning organ at risk volume (PRV) concept. Int J Radiat Oncol Biol Phys. 2006;66(1):279–286. [DOI] [PubMed] [Google Scholar]
- 16. Herschtal A, Foroudi F, Silva L, Gill S, Kron T. Calculating geometrical margins for hypofractionated radiotherapy. Phys Med Biol. 2013;58(2):319–333. [DOI] [PubMed] [Google Scholar]
- 17. Nishimura T, Yamazaki H, Iwama K, et al. Assessment of planning target volume margin for a small number of vertebral metastatic lesions using image-guided intensity-modulated radiation therapy by helical tomotherapy. Anticancer Res. 2013;33(6):2453–2456. [PubMed] [Google Scholar]
- 18. Tseng CL, Sussman MS, Atenafu EG, et al. Magnetic resonance imaging assessment of spinal cord and cauda equina motion in supine patients with spinal metastases planned for spine stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;91(5):995–1002. [DOI] [PubMed] [Google Scholar]
- 19. Thibault I, Al-Omair A, Masucci GL, et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine. 2014;21(5):711–718. [DOI] [PubMed] [Google Scholar]
- 20. Thibault I, Campbell M, Tseng CL, et al. Salvage stereotactic body radiotherapy (SBRT) following in-field failure of initial SBRT for spinal metastases. Int J Radiat Oncol Biol Phys. 2015;93(2):353–360. [DOI] [PubMed] [Google Scholar]