Abstract
Purpose:
The aim of this study was to compare dosimetric characteristics, monitor unit, and delivery efficiency of 4 different stereotactic body radiotherapy techniques for the treatment of prostate cancer.
Methods:
This study included 8 patients with localized prostate cancer. Dosimetric assets of 4 delivery techniques for stereotactic body radiotherapy were evaluated: robotic CyberKnife, noncoplanar intensity-modulated radiotherapy, and 2 intensity-modulated arc therapy techniques (RapidArc and Elekta volumetric-modulated arc therapy). All the plans had equal treatment margins and a prescription dose of 35 Gy in 5 fractions.
Results:
Statistically significant differences were observed in homogeneity index and mean doses of bladder wall and penile bulb, all of which were highest with CyberKnife. No significant differences were observed in the mean doses of rectum, with values of 15.2 ± 2.6, 13.3 ± 2.6, 13.1 ± 2.8, and 13.8 ± 1.6 Gy with CyberKnife, RapidArc, volumetric-modulated arc therapy, and noncoplanar intensity-modulated radiotherapy, respectively. The highest dose conformity was realized with RapidArc. The dose coverage of the planning target volume was lowest with noncoplanar intensity-modulated radiotherapy. Treatment times and number of monitor units were largest with CyberKnife (on average 34.0 ± 5.0 minutes and 8704 ± 1449 monitor units) and least with intensity-modulated arc therapy techniques (on average 5.1 ± 1.1 minutes and 2270 ± 497 monitor units).
Conclusion:
Compared to CyberKnife, the RapidArc, volumetric-modulated arc therapy, and noncoplanar intensity-modulated radiotherapy produced treatment plans with similar dosimetric quality, with RapidArc achieving the highest dose conformity. Overall, the dosimetric differences between the studied techniques were marginal, and thus, the choice of the technique should rather focus on the delivery accuracies and dose delivery times.
Keywords: prostate cancer, SBRT, IMRT, CyberKnife, RapidArc, VMAT
Introduction
External beam radiotherapy is one of the primary treatment modalities for patients with a localized prostate cancer (PCa). Stereotactic body radiation therapy (SBRT) is one of the noninvasive external beam treatment options. In SBRT, high degree of anatomic precision and localization are coupled with very high doses of radiation delivered in a small number of fractions. Accurate image guidance allows reducing the uncertainty margins and minimizing the radiation-related injury in adjacent normal tissues.
Clinical studies support the efficacy and safety of SBRT in PCa with low- to intermediate-risk disease.1–4 Preliminary clinical results support the hypothesis of low α–β ratio (1.4-3 Gy) in PCa compared to adjacent late-responding tissues, leading to a significant increase in the therapeutic ratio using hypofractionation.5,6 Using fewer fractions in SBRT enables efficient patient throughput and is also more convenient for patients by reducing the treatment course from 7 to 9 weeks to 1 to 2 weeks. The aforementioned issues are among the main reasons for the increased use of SBRT in a localized PCa.
Currently, there are several image-guided techniques available for exact SBRT dose delivery for the PCa. To date, most of the clinical studies related to SBRT have been realized with a CyberKnife (CK) system.2,3 In addition, intensity-modulated techniques have been used and found feasible and tolerated for SBRT of PCa.7–9 So far, the results of comparative studies among CK, RapidArc (RA), and intensity-modulated radiation therapy (IMRT) have been rather conflicting because of different patient groups or dissimilar treatment margins.10–13 On the other hand, the Elekta volumetric-modulated arc therapy (VMAT) technique has never been compared against CK or RA radiation dose delivery techniques with the SBRT of PCa. At present, there is a limited knowledge of how the dosimetric properties of different SBRT treatment modalities compare, although there are several techniques available.
The aim of this multi-institutional study is to evaluate the dosimetric differences between the SBRT delivery techniques of CK, Varian RA, Elekta VMAT, and noncoplanar Brainlab intensity-modulated radiation therapy (ncpIMRT) for PCa. In this work, identical computed tomography (CT) data sets and patient contours were used with every treatment planning system (TPS). This ensured a valid dosimetric comparison of different techniques and treatment plans.
Materials and Methods
Patients and Imaging
This retrospective study included 8 patients with PCa consecutively treated with CK system. The summary of the patient population is given in Table 1. The patients were imaged with CT (Aquilion LB; Toshiba Medical Systems, Japan) and with magnetic resonance imaging (Avanto 1.5T; Siemens AG, Munich, Germany) with a slice thickness of 1 mm. Written instructions were given to the patients in order to ensure that they empty their bowel and retain urine in their bladder before the imaging procedures and the treatments.
Table 1.
Patient Characteristics.
| Patient # | Clinical Stage | Gleason Score | Vprostate, cm3 | VPTV, cm3 | Vrectum, cm3 | Vbladder, cm3 | PTV/Rectum Overlap, cm3 | PTV/Bladder Overlap, cm3 |
|---|---|---|---|---|---|---|---|---|
| 1 | T1c | 3 + 4 | 74 | 122 | 94 | 234 | 0.9 | 9.1 |
| 2 | T2a | 3 + 4 | 29 | 61 | 35 | 330 | 1.2 | 5.3 |
| 3 | T1c | 3 + 3 | 59 | 106 | 41 | 155 | 0.4 | 7.3 |
| 4 | T1c | 3 + 3 | 36 | 71 | 73 | 98 | 0.9 | 3.1 |
| 5 | T3 | 4 + 4 | 66 | 113 | 69 | 164 | 1.4 | 8.4 |
| 6 | T1c | 4 + 4 | 39 | 76 | 40 | 166 | 0.3 | 4.2 |
| 7 | T1c | 3 + 3 | 44 | 85 | 97 | 245 | 2.2 | 10.2 |
| 8 | T2 | 2 + 2 | 39 | 74 | 41 | 91 | 0.4 | 3.9 |
| Mean | 48 ± 16 | 88 ± 22 | 61 ± 25 | 185 ± 80 | 1.0 ± 0.6 | 6.4 ± 2.7 |
Abbreviation: PTV, planning target volume.
Contouring
Target volumes and organs at risk (OARs) were delineated on the CT images with the aid of the coregistered magnetic resonance images. Prostate was delineated without the seminal vesicles as a clinical target volume (CTV). A planning target volume (PTV) was created by expanding the CTV by 3 mm posteriorly and 5 mm in other directions. These margins are clinically used in Kuopio Cancer Center and are also used in a clinical prostate SBRT study (ClinicalTrials.gov identifier NCT01764646). The OARs delineated were bladder, rectum, femoral heads, penile bulb, and normal tissue (defined as body contour—PTV). Rectum was delineated starting from the anal canal and ending up to the sigmoid colon. Bladder wall was assumed to be a 4-mm-thick structure inward from the full bladder.
Treatment Planning Criteria and Constraints
The prescription dose was 35 Gy in 5 fractions. The prescription dose had to cover at least 95% of the volume of the PTV, with a requirement of a maximum dose (Dmax) being less than 120% of the prescribed dose. The dose–volume constraints shown in Table 2 were categorized as minimum and ambition constraints. The minimum constraints were compiled from a review article by Grimm et al.14 The ambition constraints were set as secondary goals to be met in order to decrease the dose to the normal tissues, thus lowering the probability of complications.
Table 2.
Dose Constraints for Treatment Planning.
| Rectum | Bladder Wall | Penile Bulb | Femoral Heads | Normal Tissue | |
|---|---|---|---|---|---|
| Minimum constraints | Dmax < 38 Gy | Dmax < 38 Gy | D3cm3 < 30 Gy | D10cm3 < 30 Gy | CI ≤ 1.25 |
| D1cm3 <36 Gy | D5cm3 <37.5 Gy | ||||
| D20cm3 <25 Gy | D15cm3 <18.3 Gy | ||||
| V18Gy < 50% | |||||
| Ambition constraints | D4cm3 < 32 Gy | D2cm3 < 36.25 Gy | D10cm3 < 18 Gy | D4cm3 < 18 Gy | CI ≤ 1.15 |
| D20cm3 < 16 Gy | D4cm3 < 32.62 Gy | ||||
| D10cm3 < 18.12 Gy |
Abbreviations: CI, conformity index; Dmax, maximum dose.
Treatment Planning and Dose Optimization
To enable a valid comparison between all the systems, the treatment plans were created with a 6-MV photon energy. Each patient was replanned with 4 different techniques, taking into account the dose constraints in Table 2.
CyberKnife (Accuray Inc, Sunnyvale, California) treatments were planned with Multiplan (v.4.6.0; Accuray Inc) with a dose rate of 1000 monitor units (MU)/min using Iris variable aperture collimator. On average, 8 different aperture sizes were used in each treatment plan ranging from 12.5 to 60.0 mm. The dose optimization was performed with a sequential method. The time reduction tool was used in optimization to enhance the directions of treatment fields. The final dose calculation was performed with a high-resolution grid (∼2 mm) together with ray tracing algorithm including the entire 3-dimensional data set. The dose was normalized to the isodose line of 85% ± 2%, which is the standard approach used in Kuopio University Hospital.
The RA plans were delivered with Novalis Tx (Varian Medical Systems Inc, Palo Alto, California, and Brainlab AG, Feldkirchen, Germany) linear accelerator with a Dmax rate of 600 MU/min equipped with a high-definition multileaf collimator with 2.5-mm leaf width in the center. The treatment plans were optimized and calculated with Eclipse 10.0.28 (Varian Medical Systems Inc) TPS using an anisotropic analytical algorithm (AAA; v.10.0.28). The grid sizes for optimization and dose calculation were set to 0.3125 and 2 mm, respectively. Each plan consisted of two 360° coplanar arcs with collimator angle rotations of 30° and 330°. Arc Geometry Tool by Eclipse was used for creation of the arcs.
Volumetric-modulated arc therapy treatment plans were created with Monaco (v.3.20.01; Elekta-CMS Inc, St Louis, Missouri) TPS for Axesse (Elekta Ltd, Crawley, United Kingdom) linear accelerator with 4.0-mm leaves at isocenter (Beam Modulator) and a Dmax rate of 600 MU/min. The VMAT was utilized in a single 360° arc (increments of 30°) with collimator angle rotation of 3°. An optimization was performed in a constrained mode, and the constraints were established based on biological cost functions. The maximum number of control points was set to 200, the minimum segment width to 1.0 cm, and the fluence smoothing to medium. The dose calculation was performed with X-ray Voxel Monte Carlo (v.1.6) algorithm with 1.0% uncertainty per plan and a dose grid of 2.0 mm.
For the prostate SBRT, noncoplanar search spaces have been reported to improve OAR sparing.15 In this study, ncpIMRT treatment planning was realized with Eclipse (v.11.0.31). A 10-field noncoplanar template was applied to all patients (except 12 fields for #7). The plans were created for Novalis (Brainlab AG) treatment unit with central leaf width of 3 mm at the isocenter and a Dmax rate of 800 MU/min (limited to 480 MU/min in this study). The gantry rotation angles (and corresponding couch rotation angles) were 230° (0°), 265° (0°), 330° (0°), 30° (0°), 95° (0°), 130° (0°), 50° (330°), 310° (30°), 290° (10°), and 70° (350°). The optimization objectives were defined with physical dose points for the PTV, rectum, and bladder wall. The smoothing factor was set to 30, and the number of iterations to 70. Both the optimization and dose calculation were performed by AAA (v.11.0.31), with a resolution of 2.5 mm.
Plan Comparison Parameters
The number of MUs and the treatment delivery efficiency were evaluated from the treatment plans. Imaging time was not considered in the radiation delivery efficiency. The dosimetric properties were evaluated using dose–volume histograms (DVHs) and were assessed by dose constraints given in Table 2 and by dose coverage (dose to 99% of the PTV volume, D99), homogeneity, and conformity of the PTV. A homogeneity index (HI) was defined as a difference between D1 and D99 divided by the prescription dose. A conformity index (CI) was expressed as a ratio of volume covered by both the prescription isodose and the PTV volume. The calculations of statistical significance were performed with SPSS (v.19.0.0; IBM, New York) using linear mixed models for testing the patient variance and 1-way analysis of variance to determine the significant differences.
Results
Typical dose distributions of each technique are shown in Figure 1. The cumulative DVHs for the PTV, rectum, and bladder wall of the 8 patients are shown in Figure 2. The average prescription dose coverage of the PTVs was 96.0% ± 1.7%, 96.0% ± 0.0%, 95.3% ± 0.2%, and 95.0% ± 0.3% with CK, RA, VMAT, and ncpIMRT, respectively. The corresponding coverage of the CTVs was 99.5% ± 0.0%, 99.8% ± 0.2%, 99.4% ± 0.4%, and 99.9% ± 0.5%. An average number of MUs and the mean beam-on times with 1 fraction were greatest with CK (data given in Table 3). The mean values for D1cm3, D99, HI, and CI are summarized in Table 3 and other dosimetric quantities in Table 4. Significant pairwise differences were evaluated with the linear mixed models. The mean doses to the prostate and the PTV were significantly higher (P < .001) in CK plans compared to the plans of other techniques. The mean dose to the bladder wall was on average 3.4 Gy (P < .05) higher in the CK plans. Also, the mean dose to the penile bulb (P < .05) and the Dmax to rectum (P < .05) were the highest with CK plans.
Figure 1.
Typical dose distributions for 1 patient (#4) planned with CK (A), RA (B), VMAT (C), and ncpIMRT (D). CK indicates CyberKnife; ncpIMRT, non-coplanar intensity-modulated radiation therapy; RA, RapidArc; VMAT, volumetric-modulated arc therapy.
Figure 2.
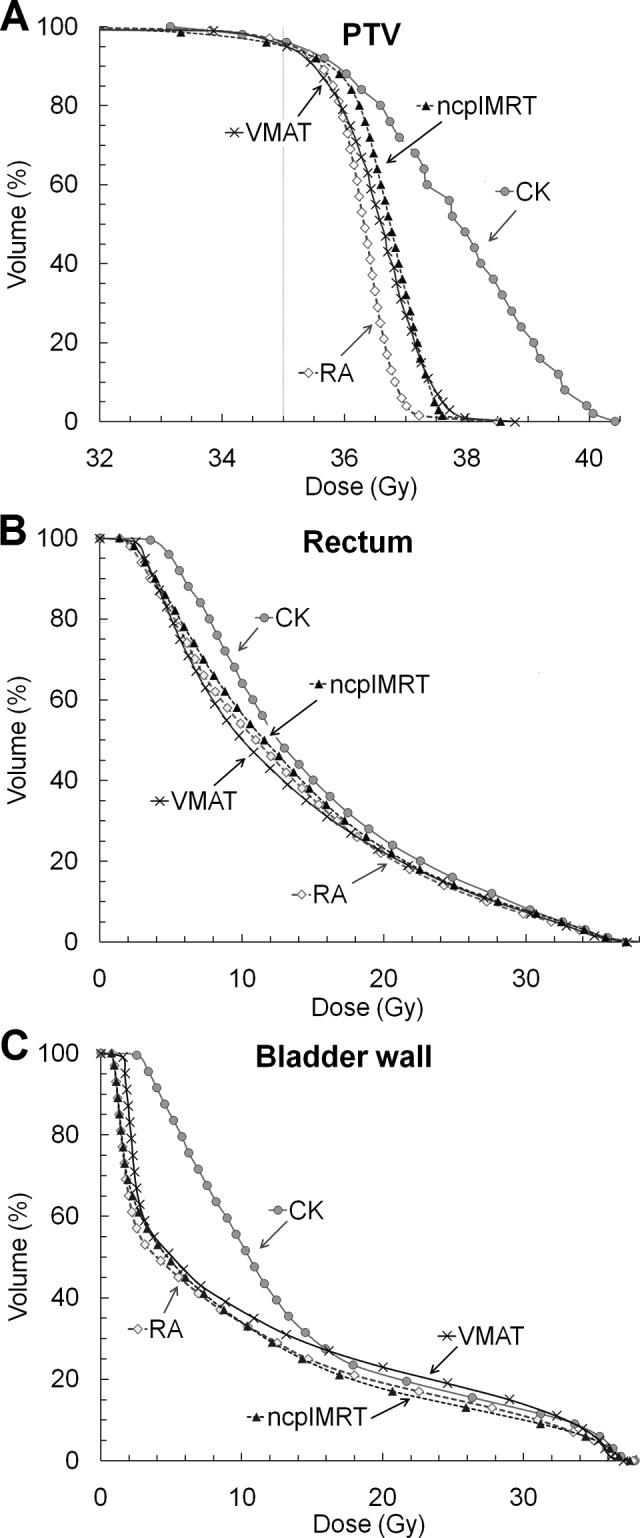
Averaged cumulative DVHs of PTV (A), rectum (B), and bladder wall (C) for CK, RA, VMAT, and ncpIMRT treatment plans (n = 8 patients). CK indicates CyberKnife; DVH, dose–volume histogram; ncpIMRT, non-coplanar intensity-modulated radiation therapy; PTV, planning target volume; RA, RapidArc; VMAT, volumetric modulated arc therapy.
Table 3.
Summary of the Mean Treatment Plan Parameters.
| Dose Delivery Technique | Number of Beams/Arcs | Number of MUs/fr | Treatment Time, minutes | D1cm3, Gy | D99, Gy | CI | HI |
|---|---|---|---|---|---|---|---|
| CyberKnife | 195 ± 34 | 8704 ± 1449a | 34.0 ± 5.0a | 40.3 ± 0.7a | 33.5 ± 0.9 | 1.11 ± 0.02 | 0.19 ± 0.02a |
| RapidArc | 2 ± 0 | 2623 ± 521 | 4.6 ± 0.9 | 37.3 ± 0.8 | 33.8 ± 0.7 | 1.02 ± 0.04a | 0.10 ± 0.04 |
| Elekta VMAT | 1 ± 0 | 2192 ± 498 | 5.5 ± 1.8 | 37.9 ± 0.7 | 33.8 ± 0.5 | 1.10 ± 0.02 | 0.12 ± 0.03 |
| ncpIMRT | 10.3 ± 0.7 | 2953 ± 473 | 6.2 ± 1.0 | 37.6 ± 0.4 | 32.7 ± 0.7a | 1.12 ± 0.05 | 0.14 ± 0.03 |
Abbreviations: CI, conformity index; D99, dose to 99% of the structure volume; HI, homogeneity index; MU, monitor unit; ncpIMRT, noncoplanar intensity-modulated radiation therapy; VMAT, volumetric-modulated arc therapy.
aThe mean value differs significantly (P < .05) from the value of the other techniques.
Table 4.
Average Dosimetric Parameters for 4 Techniques Studied.a
| Structure | CK, Mean ± SD | RA, Mean ± SD | VMAT, Mean ± SD | ncpIMRT, Mean ± SD |
|---|---|---|---|---|
| Mean values | ||||
| Prostate, Gy | 38.3 ± 0.6b | 36.4 ± 0.4 | 36.8 ± 0.5 | 36.9 ± 0.5 |
| PTV, Gy | 37.8 ± 0.4b | 36.2 ± 0.4 | 36.5 ± 0.3 | 36.6 ± 0.4 |
| Rectum, Gy | 15.2 ± 2.6 | 13.3 ± 2.6 | 13.1 ± 2.8 | 13.8 ± 1.6 |
| Bladder wall, Gy | 13.8 ± 2.5b | 9.9 ± 2.3 | 11.3 ± 2.2 | 9.9 ± 2.2 |
| Left femur, Gy | 5.4 ± 2.1 | 5.1 ± 2.3 | 8.5 ± 2.6 | 7.1 ± 2.5 |
| Right femur, Gy | 4.7 ± 1.4 | 5.2 ± 2.2 | 7.9 ± 2.5 | 6.4 ± 2.3 |
| Penile bulb, Gy | 17.6 ± 7.1b | 5.9 ± 4.4 | 7.8 ± 5.8 | 9.8 ± 7.0 |
| Minimum constraints | ||||
| Rectum | ||||
| Dmax < 38 Gy | 38.0 ± 0.9b | 37.1 ± 0.5 | 37.1 ± 0.5 | 37.1 ± 0.4 |
| D1cm3 < 36 Gy | 35.1 ± 1.1 | 35.0 ± 0.7 | 34.5 ± 1.0 | 35.1 ± 0.8 |
| D20cm3 < 25 Gy | 16.1 ± 5.0 | 14.1 ± 4.6 | 14.1 ± 7.2 | 15.0 ± 4.1 |
| D50% < 18 Gy | 12.5 ± 2.9 | 11.0 ± 2.9 | 10.0 ± 3.5 | 11.6 ± 2.2 |
| Bladder wall | ||||
| Dmax < 38 Gy | 37.9 ± 0.8 | 37.8 ± 0.4 | 37.1 ± 0.4 | 37.6 ± 0.6 |
| D5cm3 < 37.5 Gy | 33.9 ± 2.4 | 32.3 ± 3.6 | 33.9 ± 1.7 | 31.4 ± 5.8 |
| D15cm3 < 18.3 Gy | 17.2 ± 2.0 | 14.3 ± 6.1 | 18.7 ± 8.7 | 14.8 ± 6.0 |
| Ambition constraints | ||||
| Rectum | ||||
| D4cm3 < 32 Gy | 31.1 ± 2.5 | 29.8 ± 2.5 | 29.7 ± 3.3 | 30.5 ± 2.2 |
| D20cm3 < 16 Gy | 16.1 ± 5.0 | 14.1 ± 4.6 | 14.1 ± 7.2 | 15.0 ± 4.1 |
| Bladder wall | ||||
| D2cm3 < 36.25 Gy | 36.3 ± 0.8 | 36.0 ± 0.4 | 35.7 ± 0.4 | 35.8 ± 1.3 |
| D4cm3 < 32.62 Gy | 34.9 ± 1.7 | 34.0 ± 2.1 | 34.7 ± 1.1 | 33.1 ± 4.3 |
| D10cm3 < 18.12 Gy | 25.7 ± 5.2 | 23.5 ± 7.4 | 26.5 ± 7.9 | 22.4 ± 7.4 |
Abbreviations: CK, CyberKnife; ncpIMRT, noncoplanar intensity-modulated radiation therapy; RA, RapidArc; SD, standard deviation; VMAT, volumetric-modulated arc therapy.
aN = 8 Patients.
bThe mean value differs significantly (P < .05) from the value of the other techniques.
The minimum dose constraints were exceeded with 4 patients in CK (total of 8 violations) and VMAT (5 violations) and with 3 patients in RA (5 violations) and ncpIMRT (5 violations). The minimum dose constraints that were met with every patient and treatment plan were D50% <18 Gy for rectum, D5cm3 <37.5 Gy for bladder wall, and constraints for penile bulb, femoral heads, and the CI.
Discussion
Previously, extensive dosimetric comparisons between the CK and intensity-modulated techniques in PCa have not been performed. The existing literature comparing different techniques is very limited and to some extent conflicting.10–13 Hossain et al 11 observed no significant differences in rectal and bladder doses between the plans of CK and coplanar IMRT, although CK had a significant advantage over IMRT in V30% and V40% of the rectum. King et al 12 detected significant sparing of bladder and rectum with CK compared to IMRT treatment plan. In contrast, MacDougall et al 13 found no dosimetric advantage of choosing CK over RA, although RA had a small advantage over CK in the lower dose areas of bladder and rectum. In the latter study, the treatment margins were not equal between the 2 techniques compared. All existing studies performed with CK system have also been executed with 2 fixed collimators rather than Iris collimator, which allows more time-efficient use of various apertures and could increase the treatment plan quality, since the plans will be accomplished with optimum aperture sizes.
In this study, the dosimetric properties between the CK, ncpIMRT, RA, and VMAT techniques were investigated. The mean dose to the prostate (P < .001), PTV (P < .001), bladder wall (P < .05), and penile bulb (P < .05) were significantly higher with CK as was the Dmax of the bladder wall (P < .05). The mean bladder wall dose being highest with CK is explained by the noncoplanar nature of the delivery system and due to the system’s mechanical inability to irradiate directly from both lateral directions. The consequence of this can be seen in the DVH of the bladder wall in Figure 2, as the low dose area is larger in volume with CK in comparison to the plans of the other techniques. This effect can also be observed in the mean dose of the penile, which was highest with CK dose delivery. In this study, both the minimum and the ambition dose constraints set for penile bulb were met for every patient with every technique. It should however be noted that the mean dose received by the penile bulb has been found to correlate with erectile dysfunction, and thus, it is suggested to limit the dose below 50 Gy for 90% of the bulb with conventional fractionation.16
The minimum planning requirements were not fulfilled with every treatment plan. With 4 patients (#1, #3, #5, and #7), the base of the prostate was inside the apical bladder (average overlap of 8.8 cm3) making it difficult to achieve the treatment goals. The average values for V18 of the bladder wall in this study (23.7% ± 4.6%, 20.9% ± 5.4%, 26.0% ± 6.8%, and 20.4% ± 5.5% for CK, RA, VMAT, and ncpIMRT, respectively) were on average larger than the reported values of MacDougall et al 13 (13.2% and 10.4% for CK and RA, respectively) or Hossain et al 11 (18% and 21% for CK and IMRT, respectively), which underlines the challenging anatomy of our patient group. In this work, the percentage of the rectum receiving more than 18 Gy (V18) was 31.6% ± 10.0%, 27.2% ± 10.2%, 27.0% ± 9.2%, and 28.1% ± 4.2% on average for CK, RA, VMAT, and ncpIMRT, respectively. The corresponding values of MacDougall et al 13 were 31.9% and 24.8% for CK and RA and 13% and 19% for CK and IMRT, respectively, approximated from DVH data of Hossain et al.11 It should also be underlined that in our study, the treatment margins were kept equal with every technique investigated unlike in the study of MacDougall et al.
The patient-specific and unpredictable prostate motion during SBRT dose delivery should be considered since there are only a few fractions with which the dose is delivered, and the treatment duration is usually greater than 4 to 6 minutes.17 The CK unit is equipped with 2 orthogonal X-ray imaging devices for automated image guidance. The system utilizes gold fiducials to track and account for the prostate motion throughout the treatment delivery. With the CK dose delivery, there is no need to stop the treatment to correct for the intrafraction position changes, since the robotic system will take the movements into account during the dose delivery based on the intrafractional orthogonal X-ray images of the gold fiducials. There are also techniques available for real-time prostate tracking to achieve submillimeter accuracy with the intensity-modulated arc therapy techniques.18,19 As the intrafractional movement of the prostate correlates strongly with time, the RA and VMAT techniques benefit of having the shortest beam-on times (on average 5.1 minutes) when compared to ncpIMRT (8.3 minutes) and CK (34 minutes). The use of a higher dose rate, available in a flattening filter-free (FFF) dose delivery, would further reduce the treatment time. With the FFF techniques, the mean dose delivery duration can be reduced down to 2 to 3 minutes when using a dose rate of 2400 MU/min.9 Thus, the linac-based systems do benefit from being more time efficient.
This study is limited by a relatively small size of the patient group, although the observations were very consistent with every patient, and no statistical random effects were recorded within the patient data. It should also be noted that more clinical data are needed on the safety and efficacy of SBRT among clinical patient cohorts, especially in high-risk patients with PCa, which were also included in our dosimetric study. Another limitation of the study is the fact that the SBRT planning requires advanced skills, and the dosimetric results can be somewhat planner dependent, although all the treatment plans were generated by experienced planners. Likewise, the optimization criteria used with different planning systems can vary greatly. Subsequently, some compromises regarding the typical treatment planning for the different equipment were made in this study to standardize the treatment plans and make them better comparable. Therefore, for example, noncoplanar arc fields or more than 1 photon energy were not allowed in this study, although they might have been used in clinical practice. We also acknowledge that the study did not consider inter- or intrafractional positioning or delivery accuracy of the studied techniques. Nonetheless, like stated earlier, there are techniques available for every dose delivery system to take into account these inaccuracies. It should also be considered that the treatment margins used in this study could need the use of a 6-dimensional robotic couch to correct the rotational deviations of the prostate. Radiobiological aspects of the extended treatment delivery times, dose heterogeneity inside PTV, or heterogeneity of the PCa were not considered in this study.5,20,21 It should, however, be underlined that the high-dose areas inside the prostate and PTV were significantly (P < .001) higher with CK than with the other techniques studied. These higher doses inside the prostate gland could have a positive effect on the tumor control probability. However, the effect of the dose heterogeneity to the biochemical control was not in the scope of this investigation, but it needs further clinical studies to evaluate its possible effects.
Conclusion
In this study, the dosimetric differences between the explored techniques were small. The major differences between the techniques were mainly related to dose delivery characteristics of the CK system, resulting in higher maximum doses in the target and larger low dose volumes in the studied OARs. The dosimetric properties between the studied techniques were relatively similar, and thus, the comparison of the delivery systems in clinical practice should focus on factors such as dose delivery accuracies, beam-on and delivery times, and the compensation of inter- and intrafractional positional errors.
Abbreviations
- AAA
anisotropic analytical algorithm
- CI
conformity index
- CK
CyberKnife
- CT
computed tomography
- CTV
clinical target volume
- D99
dose to 99% of the structure volume
- Dmax
maximum dose
- DVH
dose–volume histogram
- FFF
flattening filter-free
- HI
homogeneity index
- IMRT
intensity-modulated radiation therapy
- MU
monitor unit
- ncpIMRT
noncoplanar intensity-modulated radiation therapy
- OAR
organ at risk
- PCa
prostate cancer
- PTV
planning target volume
- RA
RapidArc
- SBRT
stereotactic body radiation therapy
- TPS
treatment planning system
- VMAT
volumetric-modulated arc therapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Buyyounouski MK, Price RA, Jr, Harris EE, et al. Stereotactic body radiotherapy for primary management of early-stage, low-to intermediate-risk prostate cancer: report of the American Society for Therapeutic Radiology and Oncology Emerging Technology Committee. Int J Radiat Oncol Biol Phys 2010;76(5):1297–304. doi:10.1016/j.ijrobp.2009.09.078. [DOI] [PubMed] [Google Scholar]
- 2. King CR, Brooks JD, Gill H, Presti JC., Jr Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):877–882. doi:10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 3. King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109(2):217–221. doi:10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 4. Rubio C, Morera R, Hernando O, Leroy T, Lartigau SE. Extracranial stereotactic body radiotherapy. Review of main SBRT features and indications in primary tumors. Rep Pract Oncol Radiother. 2013;18(6):387–396. doi:10.1016/j.rpor.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43(5):1095–1101. [DOI] [PubMed] [Google Scholar]
- 6. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17–e24. doi:10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 7. Madsen BL, Hsi RA, Pham HT, Fowler JF, Esagui L, Corman J. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys. 2007;67(4):1099–1105. [DOI] [PubMed] [Google Scholar]
- 8. Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol. 2011;29(15):2020–2026. doi:10.1200/JCO.2010.31.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alongi F, Cozzi L, Arcangeli S, et al. Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: preliminary report of a phase II study. Radiat Oncol. 2013;8:171 doi:10.1186/1748-717X-8-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ceylan C, Kucuk N, Bas Ayata H, Guden M, Engin K. Dosimetric and physical comparison of IMRT and CyberKnife plans in the treatment of localized prostate cancer. Rep Pract Oncol Radiother. 2010;15(6):181–189. doi:10.1016/j.rpor.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hossain S, Xia P, Huang K, et al. Dose gradient near target-normal structure interface for nonisocentric CyberKnife and isocentric intensity-modulated body radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78(1):58–63. doi:10.1016/j.ijrobp.2009.07.1752. [DOI] [PubMed] [Google Scholar]
- 12. King CR, Lehmann J, Adler JR, Hai J. CyberKnife radiotherapy for localized prostate cancer: rationale and technical feasibility. Technol Cancer Res Treat. 2003;2(1):25–30. [DOI] [PubMed] [Google Scholar]
- 13. MacDougall ND, Dean C, Muirhead R. Stereotactic body radiotherapy in prostate cancer: is rapidarc a better solution than CyberKnife? Clin Oncol (R Coll Radiol). 2014;26(1):4–9. doi:10.1016/j.clon.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 14. Grimm J, LaCouture T, Croce R, Yeo I, Zhu Y, Xue J. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys. 2011;12(2):3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rossi L, Breedveld S, Heijmen BJ, Voet PW, Lanconelli N, Aluwini S. On the beam direction search space in computerized non-coplanar beam angle optimization for IMRT-prostate SBRT. Phys Med Biol. 2012;57(17):5441–5458. doi:10.1088/0031-9155/57/17/5441. [DOI] [PubMed] [Google Scholar]
- 16. Mangar SA, Sydes MR, Tucker HL, et al. ; MRC RT01 Trial Management Group. Evaluating the relationship between erectile dysfunction and dose received by the penile bulb: using data from a randomised controlled trial of conformal radiotherapy in prostate cancer (MRC RT01, ISRCTN47772397). Radiother Oncol. 2006;80(3):355–362. http://dx.doi.org/10.1016/j.radonc.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 17. Cramer AK, Haile AG, Ognjenovic S, et al. Real-time prostate motion assessment: image-guidance and the temporal dependence of intra-fraction motion. BMC Med Phys. 2013;13(1):4 doi:10.1186/1756-6649-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cho B, Poulsen PR, Sloutsky A, Sawant A, Keall PJ. First demonstration of combined kV/MV image-guided real-time dynamic multileaf-collimator target tracking. Int J Radiat Oncol Biol Phys. 2009;74(3):859–867. doi:10.1016/j.ijrobp.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poulsen PR, Fledelius W, Cho B, Keall P. Image-based dynamic multileaf collimator tracking of moving targets during intensity-modulated arc therapy. Int J Radiat Oncol Biol Phys. 2012;83(2):e265–e271. doi:10.1016/j.ijrobp.2011.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joiner MC, Mogili N, Marples B, Burmeister J. Significant dose can be lost by extended delivery times in IMRT with x rays but not high-LET radiations. Med Phys. 2010;37(6):2457–2465. [DOI] [PubMed] [Google Scholar]
- 21. Seppälä J, Seppänen M, Arponen E, Lindholm P, Minn H. Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother Oncol. 2009;93(2), 234–240. doi:10.1016/j.radonc.2009.08.010. [DOI] [PubMed] [Google Scholar]



