Abstract
Purpose:
To assess changes in the volumes and spatial locations of tumors and surrounding organs by cone beam computed tomography during treatment for cervical cancer.
Materials and Methods:
Sixteen patients with cervical cancer had intensity-modulated radiotherapy and off-line cone beam computed tomography during chemotherapy and/or radiation therapy. The gross tumor volume (GTV-T) and clinical target volumes (CTVs) were contoured on the planning computed tomography and weekly cone beam computed tomography image, and changes in volumes and spatial locations were evaluated using the volume difference method and Dice similarity coefficients.
Results:
The GTV-T was 79.62 cm3 at prior treatment (0f) and then 20.86 cm3 at the end of external-beam chemoradiation. The clinical target volume changed slightly from 672.59 cm3 to 608.26 cm3, and the uterine volume (CTV-T) changed slightly from 83.72 cm3 to 80.23 cm3. There were significant differences in GTV-T and CTV-T among the different groups (P < .001), but the clinical target volume was not significantly different in volume (P > .05). The mean percent volume changes ranged from 23.05% to 70.85% for GTV-T, 4.71% to 6.78% for CTV-T, and 5.84% to 9.59% for clinical target volume, and the groups were significantly different (P < .05). The Dice similarity coefficient of GTV-T decreased during the course of radiation therapy (P < .001). In addition, there were significant differences in GTV-T among different groups (P < .001), and changes in GTV-T correlated with the radiotherapy (P < .001). There was a negative correlation between volume change rate (DV) and Dice similarity coefficient in the GTV-T and organs at risk (r < 0; P < .05).
Conclusion:
The volume, volume change rate, and Dice similarity coefficient of GTV-T were all correlated with increase in radiation treatment. Significant variations in tumor regression and spatial location occurred during radiotherapy for cervical cancer. Adaptive radiotherapy approaches are needed to improve the treatment accuracy for cervical cancer.
Keywords: cone beam computed tomography, cervical cancer, image-guided radiation therapy, Dice similarity coefficient, volume change rate
Introduction
Cervical cancer is the most common female cancer worldwide. Most patients have locally advanced disease in which the standard treatment is chemotherapy with radiation followed by brachytherapy, and expected cure rates range from 30% to 90%, depending on the cancer stage.1,2 Recent advances in radiotherapy treatment planning and delivery technology have enabled the formulation of highly accurate conformal radiotherapy treatment plans and low damage to organs at risk (OAR). Some studies have found that intensity-modulated radiation therapy (IMRT) is associated with less gastrointestinal and hematologic toxicity, relative to conventional techniques.3
The IMRT has been increasingly accepted as a treatment for gynecologic cancer. The IMRT enables highly conformal uniform dose delivery to target volumes (eg, the cervix, uterus, parametrium, and pelvic lymph nodes) while reducing doses to OAR (bowel, bladder, rectum, and bone marrow) compared to the conventional 4-field box techniques.3,4 A retrospective study showed that, with IMRT, late gastrointestinal toxicity was reduced from 50% to 11% for all grades, with favorable tumor control and survival.5 However, tumor regression and organ motion can affect the dose received regardless of the method of delivery. Some studies have found that the uterus and cervix can move substantially during treatment.6–8 Due to motion of the uterus and cervix, changes in the clinical target volume (CTV) can influence the exposure to radiation of adjacent organs, such as the intestine, bladder, and rectum. Therefore, it is necessary to evaluate these changes during radiotherapy to reduce errors.
Image-guided radiotherapy was developed to detect anatomical motion and adjust radiation therapy accordingly. The motion has 2 major components—volumetric changes in targets (ie, tumors) and positional changes of the targets and surrounding organs. In clinical practice, we usually use center of mass or fiducial markers to evaluate target changes and motions.9 However, this method is not comprehensive enough, so here, we introduce the method of Dice similarity coefficient (DSC) and volume change rate (ΔV) to compare fully the space location of 2 registration image spaces and the volumetric changes for targets.
The purpose of the study was to use cone beam computed tomography (CBCT) to monitor the regression of volumes and changes in spatial location of the GTV-T, uterus (CTV-T), and the CTV in patients with cervical cancer during IMRT.
Methods and Materials
Patients
This prospective study comprised 16 patients with cervical cancers at stages IB2 to IIIB, based on the International Federation of Gynecology and Obstetrics classification.10 Specifically, 3, 5, 2, and 6 patients had stage IB2, IIB, IIIA, and IIIB cervical cancer, respectively. This study was approved by our hospital institutional review board in September 2014.
Patients underwent IMRT with concurrent cisplatin-based chemotherapy, followed by high-dose-rate intracavitary brachytherapy. Each patient was given an external beam radiation therapy dose of 48.6 Gy in 27 fractions of 1.8 Gy using 7 beams and 6 MV photons to the planning target volume. After this course, each patient received high-dose-rate intracavitary implants once per week that weekly delivered 28 Gy in 4 fractions. All patients received concurrent cisplatin (80 mg/m2) every 3 weeks per cycle (total 2-4 cycles).
Distinguishing Tumors From Normal Tissue
All patients were treated with IMRT, in accordance with the guidelines of the International Commission on Radiation Units and Measurements 50 and 62. The CTV consisted of the regional lymph nodes (common, internal and external iliac, obturator, and presacral), upper vagina, parametrium, cervix, and uterus. The CTV extended from the L4-5 interspace superiorly to 3 cm to the midobturator foramen. The CTV was expanded 0.8 to 1.0 cm uniformly to obtain the pelvic planning target volume.
Cervix and fundus were contoured for each patient, as were bladder, rectum, intestine, and sigmoid.11 We also contoured the gross tumor volume (GTV) and uterus (CTV-T) to evaluate the regression of the target and the movement of uterus and CTV. The CTV should comprise GTV, cervix, uterus, upper vagina, parametrium, and pelvic nodes (obturator, common, internal, and external iliac). We mainly used CBCT to contour the tumor target and OAR, and magnetic resonance imaging (MRI) and computed tomography (CT) were both taken as only subsidiary references. In order to measure the volume of GTV more precisely, weekly MRI images were obtained during radiotherapy.12
Imaging and Delivery
Patients in the supine position underwent simulations with a customized vacuum immobilization device using a CT simulator (Big Bore CT, Philips Brilliance Pinnacle) with a 5-mm slice thickness scan. To minimize organ motion, and in accordance with standard practice in our department, patients drank 200 mL of water and emptied their bowels 1 hour before the planning CT scan.
Each patient had a pelvic CT and MRI (Philips Achieva 3.0 T X-Series MRI System) scan before radiotherapy. Each patient also had weekly CBCT scans performed in the treatment position throughout the course of the external-beam radiation treatment.13
The serial images were acquired at the radiotherapy dose of 9 Gy/5f, 18 Gy/10f, 27 Gy/15f, 36 Gy/20f, and 48.6 Gy/27f in which every 5 fractions were followed by CBCT and once-weekly pelvic MRI scans. Therefore, the total serial images were 80 CBCTs and 80 MRIs. Each CBCT image was set at the same window/level (800/1200). In our daily practice, each CBCT data set was reconstructed and transferred electronically to a Pinnacle3 treatment planning workstation. Then image fusion was achieved by using CT–CT mutual information.
Volumetric Analysis
The CTV-T and GTV-T were separately contoured using diagnostic imaging information and descriptions from the physical examination. The cervix, uterus, lymph nodes, and structures were contoured on each CT scan using the Pinnacle software suite.14 We used CT–CT normalized mutual information to proceed with fusion (Pinnacle version 9.2s), and the window/level was 400/800. To ensure that the uterus and cervix were accurately differentiated, the uterus volumes were calculated independently. In addition, cervical volumes were contoured based on T2-weighted MRI images.
Positional Analysis and Evaluation Method
To determine the change in position of the cervix during treatment, DSCs were evaluated between the target and spatial position changes.15 The DSC similarity method has been widely used for deformation in image segmentation and registration assessment and is defined by Equation 1.
| 1 |
Dice similarity coefficients range from 0 to 1, and no overlap indicates agreement. The volumetric changes for targets were calculated using Equation 2.
| 2 |
For Equations 1 and 2, V 0f is the volume of the target before radiotherapy and Vi f is the volume of the nth fraction during IMRT treatment. Greater ΔV values represent greater difference.
Statistics Analysis
Paired t tests were used to compare differences in volumetric changes of organs during treatment. Mixed effects were used to test correlations between bladder and rectal volumes with CTV motion.
Results
Cervical Regression and Evaluation
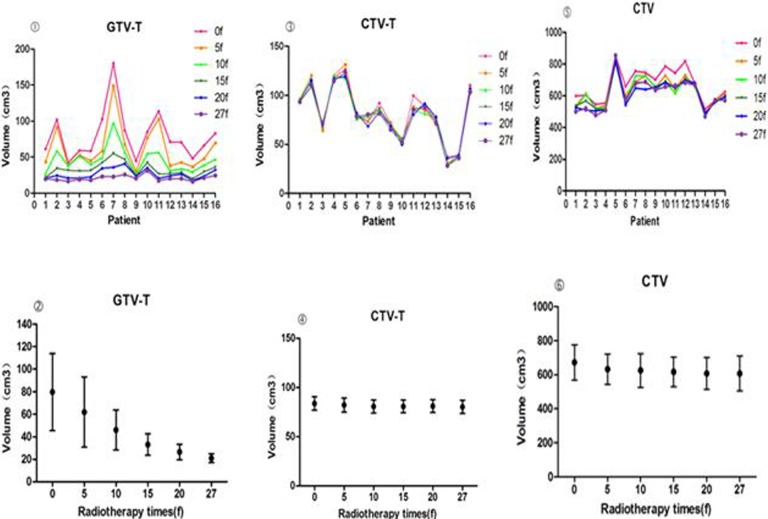
The cervical contours and measurements of interfraction variation showed dramatic tumor regression and significant positional changes during the course of treatment (Figure 1). The mean GTV-T volume of 79.62 cm3 (range: 42.3-180.3 cm3) at the start of treatment (0f) was reduced to 0.86 cm3 (range: 15.9-31.3 cm3) by the end of treatment (Table 1). On average, the cervical volume was significant different in GTV-T at all treatment points (P < .001).
Figure 1.
Volumetric changes in GTV-T, CTV-T, and clinical target volume (CTV) during therapy.
Table 1.
Tumor Volumes (GTV-T, CTV-T, and CTV) During Therapy (cm3).
| Fraction | Mean GTV-T (Range) | Mean CTV-T (Range) | Mean CTV (Range) |
|---|---|---|---|
| 0f | 79.62 (42.3-180.3) | 83.72 (36.9-126.2) | 672.59 (516.3-854.3) |
| 5f | 61.93 (29.9-149.2) | 82.01 (31.3-131.3) | 632.24 (491.4-803.2) |
| 10f | 45.96 (25.1-97.4) | 80.65 (25.5-119.8) | 625.16 (470.3-844.7) |
| 15f | 33.00 (19.9-55.1) | 80.72 (27.5-120.7) | 616.64 (493.6-805.3) |
| 20f | 26.41 (17.7-40.8) | 81.08 (35.6-118.9) | 607.92 (489.5-822.2) |
| 27f | 20.86 (15.9-31.3) | 80.23 (28.4-123.5) | 608.26 (470.9-853.5) |
Abbreviation: CTV, clinical target volume.
The CTV and CTV-T decreased slightly over the course of treatment. The mean CTV went from 672.59 cm3 (range: 516.3-854.3 cm3) to 608.26 cm3 (range: 470.9-853.5 cm3); The CTV-T changed slightly from 83.72 cm3 (range: 36.9-126.2 cm3) to 80.23 cm3 (range: 28.4-123.5 cm3). There were significant differences in the CTV-T volume among the different treatment points (P < .001), but the CTV did not significantly change in volume (P > .05).
Motion of the Cervix and CTV
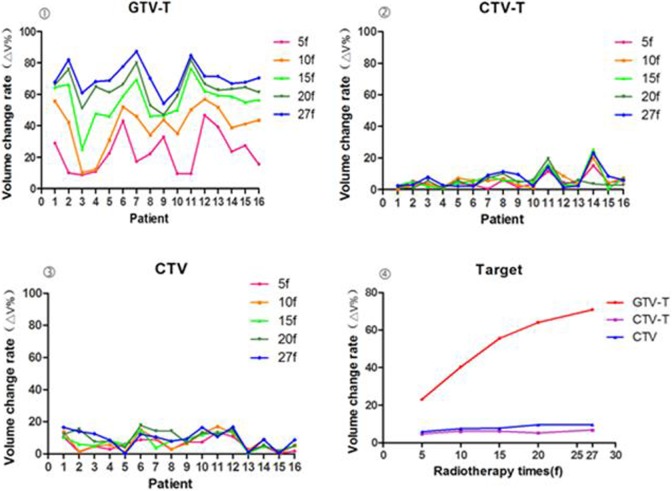
The location of the cervix in the pelvis varied greatly during treatment (Tables 2 and 3). The volume change rates of GTV-T and CTV gradually increased throughout the treatment course. The mean volume change rate (ΔV) ranged from 23.05% to 70.85% for the GTV-T, 4.71% to 6.78% for the CTV-T, and 5.84% to 9.59% for the CTV (Figure 2). There were significant differences between different treatment points (P < .05).
Table 2.
The Volume Change of Target During Therapy.
| Fraction | Mean ΔV GTV-T (Range) | Mean ΔV CTV-T (Range) | Mean ΔV CTV (Range) |
|---|---|---|---|
| 5f | 23.05 (8.8-46.7) | 4.71 (0.4-15.2) | 5.84 (0.3-13.5) |
| 10f | 40.35 (10.3-57.0) | 6.08 (0.4-20.0) | 7.47 (0.1-17.0) |
| 15f | 55.57 (25.2-76.6) | 6.12 (0.6-25.4) | 7.67 (0.4-15.0) |
| 20f | 64.06 (46.9-82.0) | 5.14 (0.9-19.5) | 9.52 (1.0-17.8) |
| 27f | 70.85 (54.2-87.4) | 6.78 (1.5-23.1) | 9.59 (0.1-16.6) |
Abbreviation: CTV, clinical target volume.
Table 3.
DSC Changes of GTV-T, CTV-T, and CTV.
| Fraction | Mean DSC GTV-T (Range) | Mean DSC CTV-T (Range) | Mean DSC CTV (Range) |
|---|---|---|---|
| 5f | 0.65 (0.32-0.86) | 0.48 (0.0-0.92) | 0.86 (0.75-0.96) |
| 10f | 0.59 (0.38-0.73) | 0.59 (0.24-0.85) | 0.87 (0.76-0.94) |
| 15f | 0.54 (0.31-0.67) | 0.56 (0.18-0.89) | 0.86 (0.75-0.95) |
| 20f | 0.48 (0.30-0.66) | 0.50 (0.01-0.88) | 0.85 (0.77-0.96) |
| 27f | 0.37 (0.15-0.58) | 0.46 (0.04-0.77) | 0.84 (0.77-0.94) |
Abbreviations: CTV, clinical target volume; DSC, Dice similarity coefficient.
Figure 2.
The volume change rates of GTV-T, CTV-T and CTV during therapy.
Positional Analyses
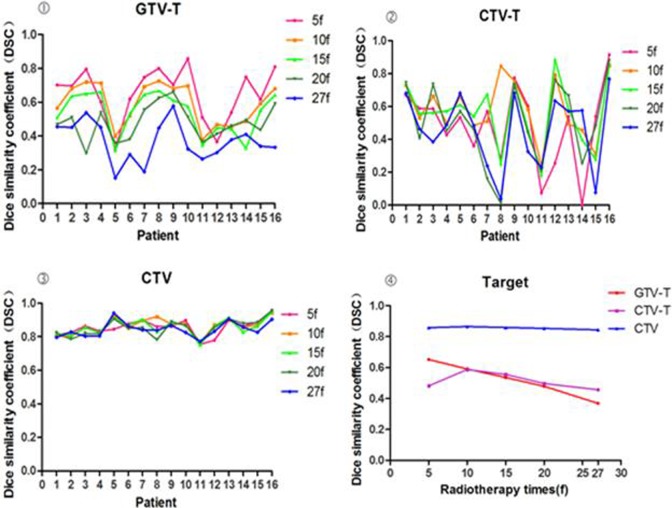
Changes in position of the cervix during treatment, represented by the DSC of the GTV-T, decreased during treatment from 0.65 to 0.37. The mean DSC of the CTV went from 0.46 to 0.59 and the CTV-T went from 0.84 to 0.87. DSC5f was significantly different from DSC15f, DSC20f, and DSC27f (P < .05) but was not significantly different from DSC10f (P > .05; Table 3). The DSC values for CTV-T and CTV did not significantly change during radiotherapy (P > .05).
There was a significant correlation between volume and volume change rate (DV) of the GTV among different time points during the radiotherapy (r < 0; P < .001). The ΔV of the bladder negatively correlated with GTV-T (r = −.349), CTV (−.300), and DSC (−.344; P < .05). The ΔV of the GTV-T negatively correlated with the DSC of both bladder (r = −.295) and rectum (−.231; P < .05). The ΔV of the CTV negatively correlated with the DSC of the bladder (r = −.306), rectum (−.357), and small intestine (−.376; P < .05). There was no significant difference or correlation among CTV-T, CTV, and OAR during radiotherapy (P > .05; Figure 3).
Figure 3.
Dice similarity coefficient (DSC) changes of GTV-T, CTV-T, clinical target volume (CTV) during therapy.
Discussion
The MRI and the integration of CT can accurately determine the tumor and adjacent normal tissues and organs while a clinically automatic fusion method can avoid the error between the subjective factors and the operator. In this study, we show that the volume and location of the cervix changed significantly during the course of treatment. These anatomical changes must be taken into account when planning radiation therapy, particularly when image-based inverse planning (image-guided radiotherapy) is used to design highly conformal radiation therapy.
Tumor volume is an important prognostic factor for local control and survival in cervical cancer. The MRI has been reported to be more precise than any other imaging modality for uterine tumors. It has been previously shown that tumor volume can be measured more precisely using MRI, and tumor regression can be accurately evaluated by sequential MRI obtained during radiotherapy.16 In our study, there was a significant difference in GTV-T volume during the treatment fractions (P < .001). Tumor regression was obvious during the 10 fraction (10f) and 15 fraction (15f) treatments, so the best time to change the radiotherapy plan is between the second and third week. We found that the CTV and uterus volumes decreased slightly over the course of treatment (Figure 2). There were significant differences in the uterus volume among the different treatment points (P < .001), but the CTV did not significantly change in volume (P > .05).
Our study, based on the images of serial CT scans, showed cervical regression and motion in patients during treatment. Our results are consistent with the findings of other studies. Lee et al 17 documented rapid involution of the cervix during chemotherapy/radiation therapy, based on physical examinations. van de Bunt et al 18 observed rapid tumor regression by MRI, after radiation treatment with 30 Gy: a 50% reduction in tumor diameter was achieved after a median of 21 days. We also observed an average 10% regression in CTV, similar to that observed by van de Bunt et al 19 who estimated the average change in CTV to be 18%, based on pre- and posttreatment MRI in 14 patients during radiotherapy.
Tumor regression during radiotherapy can cause increased uterine activity and dramatic changes in tumor position.19,20 Most studies showed that the position of cervix shifted less than uterus. In our study, we showed that the volume change rate of GTV-T and CTV gradually increased with the increase in the fraction of radiotherapy.
The DSC was only used for the evaluation of internal positional change in our study. In addition, ΔV was taken as reference for tumor volume change. Concretely, DSC was used to compare volumes of interest in the reference image to the corresponding volumes in the moving image after deformation. The DSC indicates the overlapping ratio between the 2 volumes of interest. It has been shown that the use of the DSC is appropriate for comparing image registrations that range from 0 to 1, the latter indicating overlap and perfect agreement,21 with MRI studies. This suggests that DSC values >0.70 represent good agreement.22 If the DSC is smaller, the greater the difference in the original target space position, the less the IMRT isodose line surrounding the target. Therefore, a change in the DSC can be used to predict whether IMRT plans need to be modified, and ΔV can be used to predict tumor volume change.
Our results show that the DSC of GTV gradually decreased and correlated with increasing fractions of radiotherapy. There was a significant correlation between the volume and volume change rate (DSC) of the GTV among different time points during the radiotherapy (P < .001). There was no significant difference or correlation among CTV-T, CTV, and OAR during radiotherapy. In fact, DSC was used for the evaluation of spatial motion, and the less of DSC value, the more the change in spatial motion. Thus, the tumor target will be inevitably spared by IMRT.
In this study, we observed that tumor regression and spatial location changes were the most obvious during the second and third weeks. This was also observed in previous studies using MRI, which suggests that the best time to modify radiation treatment planning is in the second or third week,23 and online adaptive radiotherapy might be necessary to manage interfraction motion and volume regression of targets.24
Conclusion
There are large variations in GTV and CTV position in patients with cervical cancer. Dramatic tumor regression and significant positional changes during the course of treatment were observed. The best time to change the radiotherapy plan is at 2-3 weeks. The primary tumor volume, volume change rate, and DSC correlate with greater treatment fractions. Therefore, our study supports the need to develop adaptive therapy approaches to improve therapeutic efficacy and outcomes of radiotherapy for cervical cancer.
Abbreviations
- CBCT
cone beam computed tomography
- CT
computed tomography
- CTV
clinical target volume
- DSC
Dice similarity coefficient
- GTV
gross tumor volume
- IMRT
intensity-modulated radiation therapy
- MRI
magnetic resonance imaging
- OAR
organs at risk
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Clinical Key Specialty Construction Program, China, and Key Clinical Specialty Discipline Construction Program of Fujian, China.
References
- 1. Mell LK, Roeske JC, Mundt AJ. A survey of intensity-modulated radiation therapy use in the United States. Cancer. 2003;98(1):204–211. [DOI] [PubMed] [Google Scholar]
- 2. Georg D, Kirisits C, Hillbrand M, Dimopoulos J, Potter R. Image-guided radiotherapy for cervix cancer: high-tech external beam therapy versus high-tech brachytherapy. Int J Radiat Oncol Biol Phys. 2008;71(4):1272–1278. [DOI] [PubMed] [Google Scholar]
- 3. Chen MF, Tseng CJ, Tseng CC, Kuo YC, Yu CY, Chen WC. Clinical outcome in post-hysterectomy cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy: comparison with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67(5):1438–1444. [DOI] [PubMed] [Google Scholar]
- 4. Hasselle MD, Rose B, Kochanski JD, et al. Clinical outcomes of intensity-modulated pelvic radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2011;80(5):1436–1445. [DOI] [PubMed] [Google Scholar]
- 5. Albuquerque K, Giangreco D, Morrison C, et al. Radiation-related predictors of hematologic toxicity after concurrent chemo-radiation for cervical cancer and implications for bone marrow-sparing pelvic IMRT. Int J Radiat Oncol Biol Phys. 2011;79(4):1043–1047. [DOI] [PubMed] [Google Scholar]
- 6. Tyagi N, Lewis JH, Yashar CM, et al. Daily online cone beam computed tomography to assess inter-fractional motion in patients with intact cervical cancer. Int J Radiat Oncol Biol Phys. 2011;80(1):273–280. [DOI] [PubMed] [Google Scholar]
- 7. Jadon R, Pembroke CA, Hanna CL, et al. A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Oncol (R Coll Radiol). 2014;(26):185–196. [DOI] [PubMed] [Google Scholar]
- 8. Beadle BM, Jhingran A, Salehpour M, Sam M, Iyer RB, Eifel PJ. Cervix regression and motion during the course of external beam chemo-radiation for cervical cancer. Int J Radiat Oncol Biol Phys. 2009;73(1):235–241. [DOI] [PubMed] [Google Scholar]
- 9. Lee CM, Shrieve DC, Gaffney DK. Rapid involution and mobility of carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2002;54(2):576–583. [DOI] [PubMed] [Google Scholar]
- 10. Benedet JL, Bender H, Jones H, III, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancer. Int J Radiat Oncol Biol Phys. 2000;70(2):209–262. [PubMed] [Google Scholar]
- 11. Lim K, Small W, Portelance L, et al. ; Gyn IMRT Consortium. Consensus guideline for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79(2):348–355. [DOI] [PubMed] [Google Scholar]
- 12. Lim K, Kelly V, Stewart J, et al. Pelvic radiotherapy for cancer of the cervix: is what you plan actually what you deliver? Int J Radiat Oncol Biol Phys. 2009;74(1):304–312. [DOI] [PubMed] [Google Scholar]
- 13. Chan P, Dinniwell R, Haider MA, et al. Inter- and intrafractional tumor and organ movement in patients with cervical cancer undergoing radiotherapy: a cinematic-MRI point of-interest study. Int J Radiat Oncol Biol Phys. 2008;70(5):1507–1515. [DOI] [PubMed] [Google Scholar]
- 14. Castadot P, Lee JA, Parraga A, Geets X, Macq B, Grégoire V. Comparison of 12 deformable registration strategies in adaptive radiation therapy for the treatment of head and neck tumors. Radiother Oncol. 2008;89(1):1–12. [DOI] [PubMed] [Google Scholar]
- 15. Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26(3):297–302. [Google Scholar]
- 16. Lim K, Chan P, Dinniwell R, et al. Cervical cancer regression measured using weekly magnetic resonance imaging during fractionated radiotherapy: radiobiologic modeling and correlation with tumor hypoxia. Int J Radiat Oncol Biol Phys. 2008;70(1):126–133. [DOI] [PubMed] [Google Scholar]
- 17. Lee CM, Shrieve DC, Gaffney DK. Rapid involution and mobility of carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2004;58(2):625–630. [DOI] [PubMed] [Google Scholar]
- 18. van de Bunt L, van der Heide UA, Ketelaars M, de Kort GA, Jürgenliemk-Schulz IM. Conventional, conformal, and intensity-modulated radiation therapy treatment planning of external beam radiotherapy for cervical cancer: the impact of tumor regression. Int J Radiat Oncol Biol Phys. 2006;64(1):189–196. [DOI] [PubMed] [Google Scholar]
- 19. van de Bunt L, Jurgenliemk-Schulz IM, de Kort GA, Roesink JM, Tersteeg RJ, van der Heide UA. Motion and deformation of the target volumes during IMRT for cervical cancer: what margins do we need? Radiother Oncol. 2008;88(2):233–240. [DOI] [PubMed] [Google Scholar]
- 20. Herrera FG, Callaway S, Delikgoz-Soykut E, et al. Retrospective feasibility study of simultaneous integrated boost in cervical cancer using tomotherapy: the impact of organ motion and tumor regression. Radiat Oncol. 2013;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zijdenbos AP, Dawant BM, Margolin RA, Palmer AC. Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans Med Imaging. 1994;13(4):716–724. [DOI] [PubMed] [Google Scholar]
- 22. Bharatha A, Hirose M, Hata N, et al. Evaluation of three-dimensional finite element-based deformable registration of pre- and intraoperative prostate imaging. Med Phys. 2001;28(12):2551–2560. [DOI] [PubMed] [Google Scholar]
- 23. Huang Z, Mayr N, Yuh W, et al. Predicting outcomes in cervical cancer: a kinetic model of tumor regression during radiation therapy. Cancer Res. 2010;70(2):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stewart J, Lin K, Kelly V, et al. Automated weekly replanning for intensity-modulated radiotherapy of cervical cancer. Int J Radiat Oncol Biol Phys. 2010;78(2):350–358. [DOI] [PubMed] [Google Scholar]