Abstract
Purpose:
Despite advancements in local and systemic therapy, metastasis remains common in the natural history of sarcomas. Unfortunately, such metastases are the most significant source of morbidity and mortality in this heterogeneous disease. As a classically radioresistant histology, stereotactic radiosurgery has emerged to control spinal sarcomas and provide palliation. However, there is a lack of data regarding pain relief and relapse following stereotactic radiosurgery.
Methods:
We queried a retrospective institutional database of patients who underwent spine stereotactic radiosurgery for primary and metastatic sarcomas. The primary outcome was pain relief following stereotactic radiosurgery. Secondary outcomes included progression of pain, radiographic failure, and development of toxicities following treatment.
Results:
Forty treatment sites were eligible for inclusion; the median prescription dose was 16 Gy in a single fraction. Median time to radiographic failure was 14 months. At 6 and 12 months, radiographic control was 63% and 51%, respectively. Among patients presenting with pain, median time to pain relief was 1 month. Actuarial pain relief at 6 months was 82%. Median time to pain progression was 10 months; at 12 months, actuarial pain progression was 51%. Following multivariate analysis, presence of neurologic deficit at consult (hazard ratio: 2.48, P < .01) and presence of extraspinal bone metastases (hazard ratio: 2.83, P < .01) were associated with pain relief. Greater pain at consult (hazard ratio: 1.92, P < .01), prior radiotherapy (hazard ratio: 4.65, P = .02), and greater number of irradiated vertebral levels were associated with pain progression.
Conclusions:
Local treatment of spinal sarcomas has remained a challenge for decades, with poor rates of local control and limited pain relief following conventional radiotherapy. In this series, pain relief was achieved in 82% of treatments at 6 months, with half of patients experiencing pain progression by 12 months. Given minimal toxicity and suboptimal pain control at 12 months, dose escalation beyond 16 Gy is warranted.
Keywords: radiosurgery, sarcoma, Brief Pain Inventory, vertebral metastasis, stereotactic
Introduction
With more than 100 histologic subtypes, sarcomas are a rare and heterogeneous group of cancers arising from mesenchymal tissue.1,2 In the United States, the 2014 estimated incidence was 12 000, with 5000 deaths attributed to this disease.3 Despite advancements in local therapy, metastasis remains common in the natural history of this disease and confers a 5-year overall survival of 20% to 40%.4–7 In contrast, patients with localized disease may expect a 5-year overall survival of approximately 70%.6
Metastatic disease is generally the most significant source of morbidity and mortality for patients with sarcomas. Although the most common site of metastasis is the lung, extrapulmonary metastases to the spine and paraspinous tissues may also be observed, particularly in liposarcomas.5,7–10 Because the median survival after development of distant metastasis is only 11 to 15 months, it is critical to establish both local control and palliation of pain.4,6–8 For patients with spinal metastases, a combination of surgery and radiotherapy may be utilized to achieve these goals, with the intent of relieving pain and preventing devastating neurologic complications.8,11 Given the radioresistant nature of sarcomas, fractionated doses exceeding 65 Gy are generally required for local control.8,12–15 Although these doses are feasible for extremity sarcomas, primary and metastatic spinal sarcomas pose a significant challenge given proximity to the spinal cord. As such, conventional external beam radiotherapy has historically offered poor durability of local control.8,13,14,16
In contrast to conventional radiotherapy, spine stereotactic radiosurgery (SRS) delivers highly conformal radiotherapy in single- or hypofractionated regimens and may offer superior local control of spinal sarcomas.12–14,17,18 Indeed, 1-year local control of sarcomas following SRS has been reported to range from 70% to 90% with single-fraction doses of 22 to 24 Gy.12,14 However, there is a paucity of data reporting rates of pain relief following SRS for sarcomas. Accordingly, we sought to report rates of pain relief and progression following SRS using the Brief Pain Inventory (BPI).
Methods and Materials
A retrospective institutional review board-approved (#6860) institutional database of patients undergoing spine SRS at a single tertiary care institution was queried. All treatments for either primary or metastatic sarcomas of the spine were eligible for inclusion. Treatments with less than 1-month radiographic or clinical follow-up were excluded. The indications for radiosurgery were either symptomatic (pain, neurologic deficit) or radiographic (control of primary tumor or epidural disease). Patients with rapidly progressive neurologic deficits or emergent spinal instability were not treated with SRS. However, patients were not excluded on the basis of epidural disease or number of involved vertebrae.
Stereotactic radiosurgery planning began with computed tomography (CT) simulation in the supine position. Patients with rostral lesions (T4 and above) were immobilized in a 5-point thermoplastic head mask, and patients with caudal lesions (T5 and below) were immobilized with a BodyFIX system (Medical Intelligence and Elekta, Stockholm, Sweden) and vacuum bag. Simulation CT and high-definition magnetic resonance imaging (MRI) sequences were obtained in 1.5-mm slices and then fused for treatment planning in BrainSCAN or iPlan software (BrainLab, Munich, Germany) or MIM (MIM Software Inc, Cleveland, Ohio). Target volumes were contoured as described previously.19 The spinal cord was contoured 4.5 to 6 mm above and below each target volume.19,20 Patients undergoing single-fraction radiosurgery were prescribed 10 to 16 Gy, whereas patients undergoing hypofractionated regimens were prescribed 24 Gy in 3 fractions or 25 Gy in 5 fractions. For single-session radiosurgery, the spinal cord and cauda equina were constrained to 14 and 16 Gy maximum point doses, and V10Gy < 10% and V12Gy < 10%, respectively. Image guidance was achieved with either planar X-ray images from the ExacTrac system (BrainLab) or cone-beam CT images from the onboard imaging system (Varian Oncology System, Palo Alto, California). Four to 8 weeks following SRS, patients were seen in clinic with follow-up spinal MRI and then followed every 3 months thereafter.
The primary outcome was relief of pain following SRS. To assess this, BPIs were prospectively collected before and after SRS to evaluate symptomatic response.21–23 This validated patient-reported questionnaire is used to assess the severity and impact of pain upon daily functions and is most commonly utilized in populations with chronic disease-related pain, such as osteoarthritis and cancer.22–26 This questionnaire has also been used in the study of SRS-induced vertebral fractures, metastatic bone disease, and sarcoma resections.27–29 Patients rate their worst, current, and average pain at the time of BPI collection (each ranging from 0 to 10) at the treatment site of interest.30 “Current” pain at the time of BPI collection during clinical follow-up was used to evaluate pain relief and symptomatic failure after SRS. Pain relief and progression were both unadjusted and adjusted for narcotic usage per RTOG 0631.19 Adjusted pain relief and progression required a difference of at least 3 points from pre-SRS BPI scores or any increase in narcotic utilization. Worst, current, and average pain were presented in a longitudinal manner to depict the progression of pain during clinical follow-up; a cubic fit was applied to model relief and relapse of pain.
Secondary outcomes included progression of pain, overall survival, radiographic failure, and new or progressed vertebral fracture following SRS. Lesions demonstrating any progression within or immediately adjacent to the treatment site of interest were considered radiographic failures.31, 32 Vertebral bodies with 20% height loss or greater were considered fractures; preexisting fractures demonstrating further height loss following SRS were considered progressed fractures. Kaplan-Meier analysis was used to calculate median survival and failure times after SRS. Independent predictors of primary and secondary outcome variables were identified with univariate and multivariate Cox proportional hazards modeling. Models were adjusted for demographic, clinical, dosimetric, and disease-related characteristics. Analysis was conducted with the JMP 10 software package (2012; SAS Institute Inc, Cary, North Carolina).
Results
Patient and Treatment Site Characteristics
Among 854 SRS treatments (577 patients) in our institutional database, 40 treatments (18 patients) for sarcoma were eligible for this study; 4 treatments for sarcoma were excluded for follow-up less than 1 month. The characteristics of these 18 patients are presented in Table 1.
Table 1.
Patient Characteristics.a
| Characteristic | Statistic |
|---|---|
| No. patients | 18 |
| Age, years | 57 [43-75] |
| Female | 10 (56) |
| KPS | 80 [50-90] |
| NFS | 1 [0-3] |
| Prior smoker | 8 (44) |
| Comorbid disease | |
| Body mass index | 27 ± 5 |
| Hypertension | 9 (50) |
| Hyperlipidemia | 4 (22) |
| Diabetes | 2 (11) |
| Extent of spinal disease | |
| Single level | 4 (22) |
| Multilevel (2-5 VB) | 11 (61) |
| Diffuse | 3 (17) |
| Visceral metastasesb | 10 (56) |
| Brain metastases | 1 (16) |
| Extraspinal bony metastases | 9 (50) |
| Concurrent medical therapies | |
| Concurrent steroids | 4 (22) |
| Chemotherapy | 2 (11) |
| Bisphosphonates | 2 (11) |
| No. prior chemotherapy regimens | 0 [0-5] |
| Histology | |
| Leiomyosarcoma | 6 (32) |
| Chondrosarcoma | 3 (17) |
| Spindle cell | 3 (17) |
| Pleomorphic | 2 (11) |
| Liposarcoma | 2 (11) |
| Osteosarcoma | 1 (6) |
| Myxofibrosarcoma | 1 (6) |
Abbreviations: KPS, Karnofsky Performance Status; NFS, neurologic function scale; No., number; VB, vertebral bodies.
aValues are presented as mean ± standard deviation, number (percentage), or median [range].
bOrgan metastases other than bone or brain.
At the time of first SRS, the median age was 59 and the median Karnofsky Performance Status (KPS) was 80. Four (22%) patients had single-level disease and 11 (61%) had disease in 2 to 5 vertebral levels. Visceral (56%) and extraspinal bone (50%) metastases were also common. The most common histologies were leiomyosarcoma (33%), chondrosarcoma (17%), and spindle cell sarcoma (17%).
Treatment site characteristics are presented in Table 2. Among the 40 sites, 30 (75%) were metastatic lesions, whereas 10 (25%) were primary sites of disease. The most common sites of disease were in the lumbar (37%) and thoracic (33%) spines. Twenty-four (60%) lesions involved a single vertebral body. Epidural disease was present among 30 (75%) lesions. Sixteen (40%) lesions had been previously irradiated, whereas 14 (35%) had been treated with prior surgical decompression for spinal cord compression or mechanical instability. The median prescription dose was 16 Gy (range, 10-25 Gy) in a single fraction (range, 1-5). Two sites underwent 25 Gy in 5 fractions, whereas 1 site underwent 24 Gy in 3 fractions. The median minimum and maximum target doses were 10 Gy (range, 7-15 Gy) and 18 Gy (range, 12-30 Gy). The median planning target volume (PTV) coverage was 92% (range, 89%-98%), whereas the median V10Gy to the spinal cord/cauda equina was 2% (range, 0%-21%).
Table 2.
Treatment Site Characteristics.a
| Characteristic | Statistic |
|---|---|
| Treatments | 40 |
| Role of sSRS | |
| Primary | 21 (52) |
| Salvage | 11 (28) |
| Adjuvant | 8 (20) |
| Indications for sSRS | |
| Pain alone | 18 (45) |
| Pain with neurologic deficit | 13 (32) |
| Asymptomaticb | 8 (20) |
| Neurologic deficit alone | 1 (3) |
| Primary disease controlled | 24 (60) |
| Systemic disease controlled | 16 (40) |
| Location | |
| Cervical | 4 (10) |
| Thoracic | 13 (33) |
| Lumbar | 15 (37) |
| Sacral | 8 (20) |
| Number of vertebral levels treated | 1 [1-5] |
| Treatment site characteristics | |
| Posterior element involvement | 10 (25) |
| Paraspinal extension | 24 (60) |
| Neural foraminal involvement | 25 (63) |
| Epidural disease | 30 (75) |
| Thecal sac compression | 14 (35) |
| Spinal cord compression | 6 (15) |
| Preexisting vertebral fracture | 9 (23) |
| Prior local therapy | |
| Any radiotherapy | 16 (40) |
| EBRT | 11 (28) |
| SRS | 7 (18) |
| Any surgery | 14 (35) |
| Surgery with instrumentation | 11 (28) |
Abbreviations: EBRT, external beam radiotherapy; SRS, stereotactic radiosurgery; sSRS, spine stereotactic radiosurgery.
aValues are presented as mean ± standard deviation, number (percentage), or median [range].
bAsymptomatic radiographic progression or epidural disease.
Clinical and Radiographic Outcomes
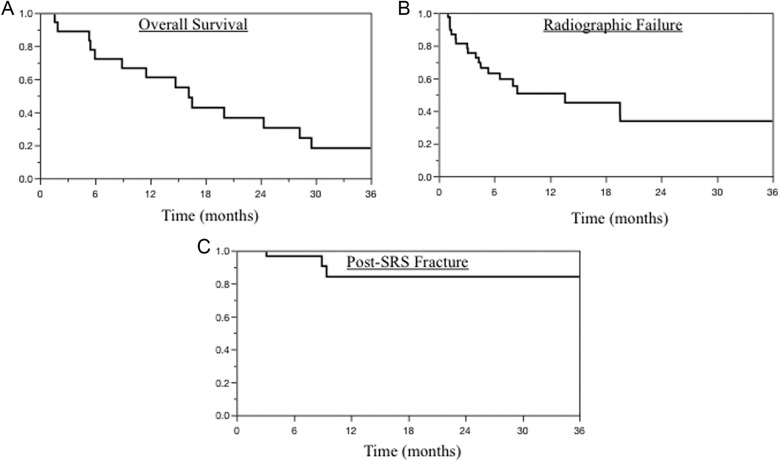
Clinical and radiographic outcomes following SRS are presented in Table 3. Median radiographic and clinical follow-up were 9 and 15 months, respectively. The median overall survival following SRS was 16 months (Figure 1A). Median time to radiographic failure was 14 months (Figure 1B). At 6 and 12 months, radiographic control rates were 63% and 51%, respectively. Radiographic failure was most common in the vertebral body (63%), adjacent segments (53%), and epidural space (47%). Among the 19 total radiographic failures, 10 underwent further local therapy with reirradiation, surgery, or a combination of techniques; accordingly, 75% of treatment sites were free from retreatment following SRS. Among patients with neurologic deficit at consult, 86% achieved relief of deficits.
Table 3.
SRS Outcomes.a,b
| Characteristic | Statistic |
|---|---|
| Radiographic follow-up, months | 9 [1-86] |
| Clinical follow-up, months | 15 [2-95] |
| Death in follow-up | 14 (78) |
| Median overall survival, months | 16 |
| Radiographic failure | 19 (48) |
| Median time to failure, months | 14 |
| Adjusted pain reliefc | 11 (35) |
| Median time to relief, months | NR |
| Unadjusted pain reliefc | 24 (77) |
| Median time to relief, months | 1 |
| Adjusted pain failure | 9 (23) |
| Median time to failure, months | NR |
| Unadjusted pain failure | 18 (45) |
| Median time to failure, months | 10 |
| Neurologic deficit reliefd | 12 (86) |
| Median time to relief, months | 8 |
| Post-SRS complications | |
| Pain flare | 4 (10) |
| Vertebral fracturee | 3 (8) |
| Failure requiring retreatment | 10 (25) |
| SRS | 8 (20) |
| Surgery | 4 (10) |
| EBRT | 1 (3) |
| Characteristics of radiographic failure | |
| In-field and adjacent | 10 (53) |
| In-field only | 9 (47) |
| Site of failure | |
| Vertebral body | 12 (63) |
| Epidural space | 9 (47) |
| Paraspinal | 3 (16) |
| Neural foramen | 3 (16) |
| Posterior elements | 2 (11) |
Abbreviations: EBRT, external beam radiotherapy; NR, not reported; SRS, stereotactic radiosurgery.
aValues are presented as number (percentage) or median [range].
bSubtotals may exceed 100% if treatment sites had, for example, multiple sites of failure.
cAmong patients with pain at consult.
dAmong patients with neurologic deficit(s) at consult.
eNew or progressed fracture.
Figure 1.
Kaplan-Meier survival curves depicting proportion of cohort (A) surviving, (B) free from radiographic failure, and (C) free from post-stereotactic radiosurgery (SRS) fracture.
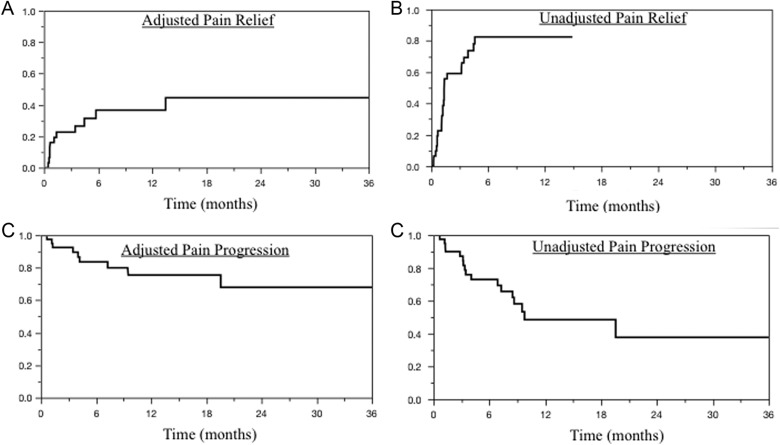
Among patients presenting with pain, median time to unadjusted pain relief was 1 month. Adjusted (Figure 2A) and unadjusted (Figure 2B) actuarial pain relief at 3/6 months were 23%/37% and 59%/82%, respectively. Median time to unadjusted pain progression was 10 months. At 6/12 months, actuarial adjusted (Figure 2C) and unadjusted (Figure 2D) pain progression rates were 16%/24% and 27%/51%, respectively. Unadjusted and adjusted pain progression correlated with radiographic failure (P < .01).
Figure 2.
Kaplan-Meier survival curves depicting proportion of cohort (A) achieving adjusted pain relief, (B) achieving unadjusted pain relief, (C) free from adjusted pain progression, and (D) free from unadjusted pain progression.
Complications following radiosurgery were uncommon. Only 3 (8%) new or progressive vertebral fractures occurred following SRS (Figure 1C). All patients developing such fractures experienced pain progression within 9 months of treatment; however, this observation was not statistically significant (hazard ratio [HR]: 2.70, P = .13). Similarly, surgical intervention for spinal instability following SRS was associated with pain progression (HR: 2.44, P = .16). Pain flare occurred following 4 (10%) treatments. One (2.5%) grade 3 foot drop occurred following 1 treatment. This patient had undergone prior chemotherapy and irradiation (50.4 Gy) for an anal squamous cell carcinoma and subsequently developed an in-field pleomorphic sarcoma 7 years later. She underwent 2 courses of SRS to the sacrum (15 and 13 Gy) and developed a foot drop after the second course of SRS. Her sacral disease has remained stable for 6 years. No other grade ≥3 toxicities were observed.
Cox Proportional Hazards Modeling
Multivariate Cox proportional hazards modeling was utilized to identify independent predictors of outcome variables (Table 4). Karnofsky Performance Status > 70 (HR: 0.15, P < .01) and controlled primary site of disease (HR: 0.18, P < .01) were prognostic for greater overall survival. Factors associated with decreased overall survival included presence of extraspinal bone metastases (HR: 6.90, P < .01), brain metastases (HR: 3.00, P = .04), visceral metastases (HR: 2.49, P = .02), and greater number of irradiated vertebral levels (HR: 5.57, P < .01).
Table 4.
Cox Proportional Hazards Modeling.
| Cox Proportional Hazards | Radiographic Failure | Unadjusted Pain Relief | Unadjusted Pain Progression | Adjusted Pain Progression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | Multivariate | Multivariate | Multivariate | Multivariate | ||||||||
| P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | |
| Age, years | <.01 | 0.81 | 0.70-0.90 | .16 | 0.93 | 0.81-1.03 | ||||||
| Male gender | .06 | 4.63 | 0.94-23.66 | |||||||||
| KPS > 70 | <.01 | 0.07 | 0.01-0.28 | .01 | 0.12 | 0.02-0.62 | <.01 | 0.04 | 0.01-0.22 | |||
| BPI pain at consult | <.01 | 1.92 | 1.18-3.36 | |||||||||
| Indication for SRSa | <.01 | |||||||||||
| Neurologic deficit | ||||||||||||
| Pain and neurologic deficit | <.01 | 2.48 | 1.30-4.76 | |||||||||
| Asymptomatic | ||||||||||||
| No. previous chemo. regimens | <.01 | 12.34 | 2.18->100 | |||||||||
| Bone metastases | <.01 | 2.83 | 1.49-5.98 | |||||||||
| Posterior elements disease | .04 | 2.16 | 1.04-4.65 | |||||||||
| Any prior radiotherapy | .02 | 4.65 | 1.30-20.24 | |||||||||
| PTV coverage, % | .07 | 0.67 | 0.43-1.03 | .04 | 0.44 | 0.19-0.96 | ||||||
| Minimum target dose, Gy | .02 | 0.48 | 0.22-0.89 | |||||||||
| Target volume, mL | .01 | 0.99 | 0.98-1.00 | 0.19 | 1.00 | 0.99-1.00 | ||||||
| No. treated levels | <.01 | .03 | ||||||||||
| 2 versus 1 | <.01 | 58.81 | 5.36->100 | .12 | 0.47 | 0.01-5.72 | ||||||
| 3 versus 1 | .05 | 0.05 | 0.01-0.76 | .80 | 0.04 | 0.01-0.96 | ||||||
| 4 versus 1 | .72 | 2.12 | 0.04->100 | .09 | 1.33 | 0.02-33.41 | ||||||
| 5 versus 1 | .96 | 1.49 | 0.03-85.80 | .01 | >100 | 2.48->100 | ||||||
Abbreviations: BPI, Brief Pain Inventory; chemo, chemotherapy; CI, confidence interval; HR, hazard ratio; KPS, Karnofsky Performance Status; No., number; PTV, planning target volume; SRS, stereotactic radiosurgery.
aReference is pain alone.
Bold values indicate statistical significance
Disease in the posterior elements (HR: 2.16, P = .04) was predictive for developing radiographic failure. In contrast, older age (HR: 0.81, P < .01) and KPS > 70 (HR: 0.07, P < .01) were predictive for freedom from radiographic failure. Greater target volumes were associated with freedom from radiographic failure, but this effect was small (HR: 0.99, P < .01).
No significant independent predictors of adjusted pain relief were identified. However, presence of neurologic deficit at consult (HR: 2.48, P < .01) and presence of extraspinal bone metastases (HR: 2.83, P < .01) were associated with unadjusted pain relief. Finally, several variables were associated with pain progression after SRS. Characteristics associated with unadjusted pain progression included greater pain at consult (HR: 1.92, P < .01), prior radiotherapy (HR: 4.65, P = .02), and greater number of irradiated vertebral levels; in contrast, protective characteristics included KPS > 70 (HR: 0.12, P = .01), greater PTV coverage (HR: 0.44, P = .04), and greater minimum target dose (HR: 0.48, P = .02). Characteristics associated with adjusted pain progression included greater number of prior chemotherapy regimens (HR: 12.34, P < .01) and greater number of irradiated vertebral levels, while KPS > 70 was protective (HR: 0.04, P < .01).
Longitudinal BPI Analysis
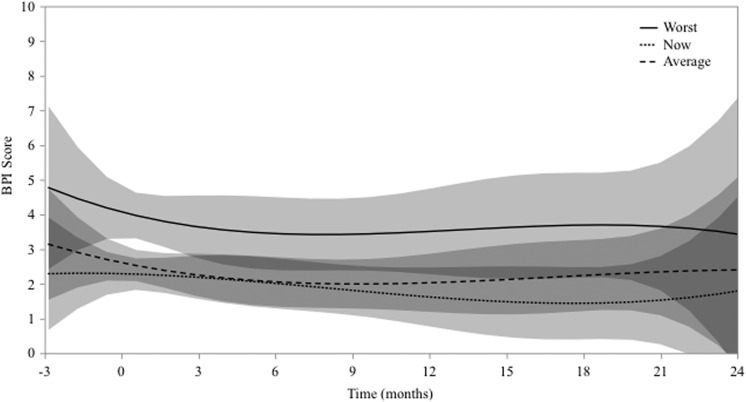
Among the 40 treatment sites, 192 BPIs were longitudinally collected starting at the time of consult. At the time of consultation, the median worst, current, and average pain at treatment sites was 6, 2, and 2, respectively. These pooled data are presented in Figure 3 with 95% confidence intervals and a cubic fit. Although unadjusted pain relief was achieved at many sites (77%), other sites eventually had pain relapse (45%). Accordingly, pooled BPI data demonstrate approximately stable pain scores, with greatest decrease and increase in pain at months 1 and 12, respectively.
Figure 3.
Longitudinal Brief Pain Inventory (BPI) data plotted over time for entire cohort. Curves with 95% confidence intervals represent cubic fit of worst, current, and average pain.
Discussion
In the present investigation, we report clinical and radiographic outcomes following stereotactic irradiation of 40 primary and metastatic spinal sarcomas, with particular attention toward pain relief and relapse. Unadjusted and adjusted for narcotic use, 77% and 35% of patients achieved significant relief of pain, with a median time to relief of 1 month. Pain relief was also durable—less than half of patients eventually experienced pain relapse, with a median time to relapse of 10 months.
Chang et al reported a series of 32 sarcomas (10 primary and 22 metastatic) treated with a median single-fraction dose of 22 Gy. Median follow-up was 22 months, and 60% of these lesions had been previously irradiated.14 Pain was assessed with a visual analog scale (VAS) before and after SRS; pain control was defined as a reduction of at least 1 unit on the VAS scale and was not adjusted for narcotic usage. At 1 year, pain control was 68%. Larger treatment volumes (≥19 mL) were associated with poorer pain control (40% vs 92% at 1 year), although this finding was not evaluated with multivariate analysis.
A similar study was conducted by Levine et al comprising 30 sarcomas among 24 patients.13 The median treatment dose was 30 Gy in 3 fractions. No method of pain assessment was reported, and symptomatic response was only reported for 23 (77%) of the 30 lesions. Of these 23 sites, 96% experienced partial or complete pain relief.
A series of 19 patients with spinal cord compression arising from primary or metastatic spinal sarcomas was reported by Merimsky et al.8 In this series, conventional radiotherapy (30 Gy in 10 fractions) was used to alleviate pain and neurologic deficits. Pain relief was achieved in 83% of patients, but complete relief of neurologic deficits was achieved in only 27% of patients.
In the present study, pain relief and relapse were recorded, both unadjusted and adjusted for narcotic usage. Among all treatment sites, 82% experienced at least partial pain relief at 1 year following SRS, whereas 37% experienced complete or significant relief of at least 3 points on a BPI after adjusting for narcotic usage. The median time to relief was rapid (1 month). Moreover, 86% of treatments resulted in relief of neurologic deficits, and these deficits were associated with achieving relief of pain following multivariate analysis (HR: 2.48, P < .01).
Radiographic Control After Irradiation of Spinal Sarcomas
The largest series of spinal sarcomas treated with SRS was reported by Folkert et al.12 In this study, 88 patients with 120 metastases were treated with either single-fraction (median dose 24 Gy) or hypofractionated (median dose 29 Gy, 3-6 fractions) radiosurgery. No symptomatic outcomes were reported. At 1 year, radiographic control was 88% and was significantly higher in the single-fraction cohort (91% vs 84%). However, the overall rate of grade 3 toxicity was 8% (4%, tracheoesophageal fistulas; 2%, wound complications; 1%, dermatitis; 1%, fatigue). Although the authors note that these cases were complicated by prior chemotherapy and iatrogenic manipulation, an esophageal point dose constraint of 15 Gy has been implemented to prevent future esophageal toxicity. Folkert et al also reported a 40% incidence of post-SRS fracture.33
In the present report, radiographic control at 1 year (51%) was lower compared to the study by Folkert et al. This observation is likely related to our utilization of a lower single-fraction dose (median dose 16 vs 24 Gy) but may also relate to differences in patient populations. This is also reflected in the lower rate of grade ≥3 toxicity (2.5% vs 8%) and post-SRS fracture (8% vs 40%).
Chang et al and Levine et al reported radiographic control rates of 78% and 79% at 1 year, respectively.13,14 One (3%) case of a rectal fistula was observed in the Levine series, requiring diverting colostomy. Of note, Chang et al observed 100% radiographic control at doses ≥22 Gy, compared to just 57% below 22 Gy; no grade 3 or 4 toxicities were reported. Radiographic failures in the present report were most common in the vertebral body (63%), suggesting a role for dose escalation beyond 16 Gy. This is particularly reasonable given current dose constraints. For radioresistant histologies such as sarcoma, dose escalation beyond 16 Gy may be warranted to maximize local control and pain relief. However, one must remain cognizant of normal tissue constraints in order to avoid grade 3 or higher toxicities. Given the results of this study, our standard spine SRS dose has been escalated to 18 Gy.
Prognostic and Predictive Characteristics
Multivariate modeling identified older age and KPS > 70 as prognostic for freedom from radiographic failure, further highlighting the importance of patient selection in SRS. In contrast, treatment sites with disease in the posterior elements failed more frequently. This highlights the challenge of treating the posterior elements, particularly in lesions with bilateral involvement of the pedicles and/or laminae.34
Several characteristics were associated with pain progression following SRS, including greater pain prior to SRS, prior radiotherapy, numerous failed chemotherapy regimens, and greater number of vertebral levels involved. These factors suggest that patients with more advanced and resistant disease may require further dose escalation in an attempt to achieve a more durable response. Moreover, when considering dose escalation in the palliative setting, it is important to weigh the risk of toxicity against expected survival and time to symptomatic failure—indeed, overall survival was just 14 months in this series, with a 10-month median time to symptomatic failure. Additionally, many treatment sites (20%) safely underwent salvage SRS, suggesting that dose escalation beyond 20 to 22 Gy may be unnecessary in light of possible increased toxicity.
Limitations and Strengths
Several limitations should be considered when interpreting the results of this study. As a retrospective series, results are limited by selection bias, measurement bias, and confounding of results. Certainly, few sarcomas have been treated with SRS, and no prospective data exist to date regarding the general efficacy of spine SRS upon pain relief and radiographic control.19,35 Attempts to limit bias included utilization of multivariate modeling to control for confounding associated with outcome variables. We also collected BPIs prospectively and longitudinally. The precision of modeling techniques is certainly limited by sample size. Interpretation of pain data was performed using adjusted time-to-event analysis with polynomial fitting of longitudinal data in an effort to visually demonstrate associations with pain progression. Despite this, this remains one of the largest series of spinal sarcomas treated with SRS and offers detailed rates of pain relief and relapse over clinical follow-up. The strengths of this study lie in the consistency of treatment and follow-up and in the utilization of multivariate methods to identify prognostic and predictive factors associated with radiographic and clinical outcomes.
Conclusions
Local treatment of spinal sarcomas has remained a challenge for decades, with poor rates of local control and limited pain relief following conventional radiotherapy. In this series of 40 sarcomas treated with spine radiosurgery to a median dose of 16 Gy in a single fraction, unadjusted pain relief was achieved in 82% of treatments at 6 months, with approximately half of patients experiencing pain progression by 1 year following SRS. At 12 months, radiographic control was 51%, suggesting that dose escalation beyond 16 Gy may be warranted given low rates of toxicity. A single-fraction dose of 18 Gy is our current institutional standard for the treatment of spinal metastases.
Abbreviations
- BPI
Brief Pain Inventory
- CT
computed tomography
- HR
hazard ratio
- KPS
Karnofsky Performance Status
- MRI
magnetic resonance imaging
- SRS
stereotactic radiosurgery
- PTV
planning target volume
- RTOG
Radiation Therapy Oncology Group
- VAS
visual analog scale
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973-2011). 2014. Accessed December 5, 2015. Web site www.seer.cancer.gov.
- 2. Borden EC, Baker LH, Bell RS, et al. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res. 2003;9(6):1941–1956. [PubMed] [Google Scholar]
- 3. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4. Corey RM, Swett K, Ward WG. Epidemiology and survivorship of soft tissue sarcomas in adults: a national cancer database report. Cancer Med. 2014;3(5):1404–1415. doi:10.1002/cam4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217(1):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang L, Jiang S, Lin Y, et al. Significance of local treatment in patients with metastatic soft tissue sarcoma. Am J Cancer Res. 2015;5(6):2075–2082. [PMC free article] [PubMed] [Google Scholar]
- 7. van Geel AN, Pastorino U, Jauch KW, et al. Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer. 1996;77(4):675–682. doi:10.1002/(SICI)1097-0142(19960215)77:4<675:: AID-CNCR13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8. Merimsky O, Kollender Y, Bokstein F, et al. Radiotherapy for spinal cord compression in patients with soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2004;58(5):1468–1473. doi:10.1016/j.ijrobp.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 9. Huth JF, Eilber FR. Patterns of metastatic spread following resection of extremity soft-tissue sarcomas and strategies for treatment. Semin Surg Oncol. 1988;4(1):20–26. [DOI] [PubMed] [Google Scholar]
- 10. Potter DA, Glenn J, Kinsella T, et al. Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J Clin Oncol. 1985;3(3):353–366. [DOI] [PubMed] [Google Scholar]
- 11. Bilsky MH, Boland PJ, Panageas KS, Woodruff JM, Brennan MF, Healey JH. Intralesional resection of primary and metastatic sarcoma involving the spine: outcome analysis of 59 patients. Neurosurgery. 2001;49(6):1277–1286; discussion 1286-1287. [DOI] [PubMed] [Google Scholar]
- 12. Folkert MR, Bilsky MH, Tom AK, et al. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys. 2014;88(5):1085–1091. doi:10.1016/j.ijrobp.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 13. Levine AM, Coleman C, Horasek S. Stereotactic radiosurgery for the treatment of primary sarcomas and sarcoma metastases of the spine. Neurosurgery. 2009;64(2 suppl):A54–A59. doi:10.1227/01.NEU.0000339131.28485.4A. [DOI] [PubMed] [Google Scholar]
- 14. Chang UK, Cho WI, Lee DH, et al. Stereotactic radiosurgery for primary and metastatic sarcomas involving the spine. J Neurooncol. 2012;107(3):551–557. doi:10.1007/s11060-011-0777-0. [DOI] [PubMed] [Google Scholar]
- 15. Kepka L, DeLaney TF, Suit HD, Goldberg SI. Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2005;63(3):852–859. doi:10.1016/j.ijrobp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16. Boriani S, Saravanja D, Yamada Y, Varga PP, Biagini R, Fisher CG. Challenges of local recurrence and cure in low grade malignant tumors of the spine. Spine. 2009;34(22 suppl):S48–S57. doi:10.1097/BRS.0b013e3181b969ac. [DOI] [PubMed] [Google Scholar]
- 17. Braccini A-L, Bondiau PY, Litrico S, Burel-Vandenbos F, Thyss A. Long term survival of an atlas osteosarcoma treated by surgery, chemotherapy and robotic stereotactic radiotherapy: a case report. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2010;97(3):608–609. doi:10.1016/j.radonc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 18. Rock J, Kole M, Yin F-F, Ryu S, Guttierez J, Rosenblum M. Radiosurgical treatment for Ewing’s sarcoma of the lumbar spine: case report. Spine. 2002;27(21): E471–E475. doi:10.1097/01.BRS.0000030204.96019.90. [DOI] [PubMed] [Google Scholar]
- 19. Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase II/III study of image-guided stereotactic radiosurgery for localized (1-3) spine metastases: phase II results. Int J Radiat Oncol Biol Phys. 2011;81(2):S131–S132. doi:10.1016/j.prro.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 20. Ryu S, Jin J-Y, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109(3):628–636. doi:10.1002/cncr.22442. [DOI] [PubMed] [Google Scholar]
- 21. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 22. Atkinson TM, Rosenfeld BD, Sit L, et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J Pain Symptom Manage. 2011;41(3):558–565. doi:10.1016/j.jpainsymman.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferreira KA, Teixeira MJ, Mendonza TR, Cleeland CS. Validation of brief pain inventory to Brazilian patients with pain. Support Care Cancer. 2011;19(4):505–511. doi:10.1007/s00520-010-0844-7. [DOI] [PubMed] [Google Scholar]
- 24. Mendoza TR, Koyyalagunta D, Burton AW, et al. Changes in pain and other symptoms in patients with painful multiple myeloma-related vertebral fracture treated with kyphoplasty or vertebroplasty. J Pain. 2012;13(6):564–570. doi:10.1016/j.jpain.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cleeland CS. The measurement of pain from metastatic bone disease: capturing the patient’s experience. Clin Cancer Res. 2006;12(20 pt 2):6236s–6242s. doi:10.1158/1078-0432.CCR-06-0988. [DOI] [PubMed] [Google Scholar]
- 26. Kalyadina SA, Ionova TI, Ivanova MO, et al. Russian Brief Pain Inventory: validation and application in cancer pain. J Pain Symptom Manage. 2008;35(1):95–102. doi:10.1016/j.jpainsymman.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 27. Callegaro D, Miceli R, Brunelli C, et al. Long-term morbidity after multivisceral resection for retroperitoneal sarcoma. Br J Surg. 2015;102(9):1079–1087. doi:10.1002/bjs.9829. [DOI] [PubMed] [Google Scholar]
- 28. Boehling NS, Grosshans DR, Allen PK, et al. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J Neurosurg Spine. 2012;16(4):379–386. doi:10.3171/2011.11.SPINE116. [DOI] [PubMed] [Google Scholar]
- 29. Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13(4):395–402. doi:10.1016/S1470-2045(11)70384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zanoli G, Strömqvist B, Jönsson B. Visual analog scales for interpretation of back and leg pain intensity in patients operated for degenerative lumbar spine disorders? Spine (Phila Pa 1976). 2001;26(21):2375–2380. [DOI] [PubMed] [Google Scholar]
- 31. Benzil DL, Saboori M, Mogilner AY, Rocchio R, Moorthy CR. Safety and efficacy of stereotactic radiosurgery for tumors of the spine. J Neurosurg. 2004;101(suppl 3):413–418. doi:10.3171/jns.2004.101.supplement 3.0413. [PubMed] [Google Scholar]
- 32. Gerszten PC, Ozhasoglu C, Burton SA, et al. CyberKnife frameless stereotactic radiosurgery for spinal lesions: clinical experience in 125 cases. Neurosurgery. 2004;55(1):89–98; discussion 98-99. [PubMed] [Google Scholar]
- 33. Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27(30):5075–5079. doi:10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(5):e597–e605. doi:10.1016/j.ijrobp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 35. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32(2):193–199. doi:10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]