Abstract
Aim:
The objective of this study was to evaluate the efficacy of percutaneous radiofrequency ablation of locally advanced pancreatic cancer located in the pancreatic body.
Materials and Methods:
Patients with biopsy-proven locally advanced pancreatic adenocarcinoma were considered for percutaneous radiofrequency ablation. Postprocedural computed tomography studies and Ca19.9 tumor marker evaluation were performed at 24 hours and 1 month. At computed tomography, treatment effect was evaluated by excluding the presence of complications. The technical success of the procedure is defined at computed tomography as the achievement of tumoral ablated area.
Results:
Twenty-three patients have been included in the study. Five of the 23 patients were excluded. At computed tomography, the mean size of the intralesional postablation necrotic area was 32 mm (range: 15-65 mm). Technical success of the procedure has been obtained in 16 (93%) of the 18 cases. None of the patients developed postprocedural complications. Mean Ca19.9 serum levels 1 day before, 1 day after, and 1 month after the procedure were 285.8 U/mL (range: 16.6-942.0 U/mL), 635.2 U/mL (range: 17.9-3368.0 U/mL), and 336.0 U/mL (range: 7.0-1400.0 U/mL), respectively. Follow-up duration was less than 6 months for 11 patients and more than 6 months for 7 patients. At the time of the draft of this article, the mean survival of the patients included in the study was 185 days (range: 62-398 days).
Conclusion:
Percutaneous radiofrequency ablation of locally advanced adenocarcinoma has a high technical success rate and is effective in cytoreduction both at imaging and laboratory controls.
Keywords: pancreatic adenocarcinoma, pancreatic cancer, RFA, RFA percutaneous, RFA pancreas
Introduction
Ductal adenocarcinoma is the most common primary malignancy of the pancreas and represents 80% of malignant pancreatic tumors.1
Radical resection is currently the sole treatment capable of improving long-term survival in pancreatic cancer. Unfortunately, this is possible in only 20% to 30% of the patients. After resection, the 5-year survival rate does not exceed 30% even in high-volume surgical centers and in combination with adjuvant therapy.2 Basically, prognosis and treatment approach depend on the lesion resectability at presentation.3
In almost 95% of the cases, pancreatic ductal adenocarcinoma is diagnosed at an advanced stage,4 with locally advanced or metastatic disease in 40% of the cases.3,5–7
Approximately 40% of newly diagnosed patients present with locally advanced unresectable disease as a result of involvement of major vascular structures. These patients are now defined as having locally advanced pancreatic cancer (LAPC) and generally have a better survival than patients with metastatic disease, but the overall outcome still remains poor.8
Therapies for advanced pancreatic adenocarcinoma consist of chemotherapy and radiotherapy. One-third of the patients with LAPC may show borderline resectable or resectable disease after neoadjuvant therapy. These patients can have comparable survival to those who initially presented with resectable disease. The efficacy of neoadjuvant therapy as a bridge to potential curative resection is broad, ranging from 3% to 79%.9–12
Standard systemic chemotherapy for unresectable pancreatic cancer is usually gemcitabine based.13 Recently, new chemotherapy drugs and combination have shown significantly better response rates and survival than gemcitabine in patients who have a good performance status, and trials of this regimen in the neoadjuvant setting are ongoing.14 Unfortunately, many patients with LAPC will continue to have unresectable disease, despite exhaustion of all standard therapies.
Radiofrequency ablation (RFA) of pancreatic ductal adenocarcinoma is nowadays restricted to locally advanced, nonresectable but nonmetastatic tumors. This ablation treatment is in fact reported to be applied to LAPC and mainly performed during laparotomy using intraoperative ultrasound (US) guidance,15 making in those kind of patients the invasiveness of the approach one of the major points of discussion considering that the ablation, as an intrinsic aspect of the procedure for the pancreatic application, will be incomplete and limited in the inner portions of the tumor preserving the most peripheral ones for safety reasons as previously reported.15
The primary objective of this study was to evaluate the efficacy of US-guided percutaneous RFA of LAPC located in the pancreatic body. Secondary objective was to determine the response to treatment in terms of cytolytic and cytoreductive effects after the procedure. To our knowledge, this is the first study in which the percutaneous approach is considered to guide RFA in locally advanced pancreatic adenocarcinoma in order to reduce invasiveness of the laparotomy.15
Materials and Methods
Patients and Preoperative Imaging
All patients gave written informed consent before undergoing the procedure. Patients with biopsy-proven unresectable locally advanced nonmetastatic pancreatic adenocarcinoma of the pancreatic body were considered as potential candidates for percutaneous RFA. Inclusion criteria were pancreatic tumor still unresectable, based on NCCN16 guidelines definition due to the presence of celiac trunk and/or superior mesenteric artery encasement and/or unreconstructible superior mesenteric vein/portal occlusion, after chemotherapy with mass located at the body of the pancreas and visible at US study of dimensions greater than 2 cm without abundant intralesional fluid component.
Two to 4 weeks after the end of first line, at least 6 months, patients with chemotherapy will be studied by means of contrast-enhanced computed tomography (CT; Brilliance 64; Philips, Eindhoven, the Netherlands) in order to evaluate response to therapy.
Patients with still nonresectable, nonmetastatic cancer are proposed for percutaneous US-guided ablative treatment. Ethics board was obtained for RFA of LAPC in the surgical department. Patients deemed eligible for the procedure underwent pretreatment workup including baseline complete blood count, liver and renal function tests, coagulation profile, serum level of Ca19.9 tumor marker evaluation, and anesthesia evaluation.
Before the procedure, a conventional abdominal US examination is performed (Sequoia 512 US scanner; Acuson, Mountain View, California) in order to assess the possibility to technically perform the procedure in terms of visualization and reachability of the lesion with perfect visualization of a safe needle tract. Otherwise, patients were excluded from the study.
Procedure
The procedure was performed under deep sedation in the interventional radiology suite with constant monitoring of patient’s blood oxygen saturation and cardiac activity. An US and color Doppler evaluation of the route were performed.
Once established the way, under real-time US guidance, the pancreatic mass is approached and the RFA needle (VARI Tip—VCT—needle connected to a Mygen RF generator and cooled by the Cool-Wet Tip pump cooling system; RF Medical Co, Ltd, Seoul, Korea) is positioned taking care to avoid vessels along the tract and the peripancreatic anatomical structures. The electrode, previously opened according to the lesion size, is therefore placed exactly in the middle of the mass. The ablation parameters, power in watt (W) and the time in seconds (s), for the procedure are set according to the lesion size and the tissue impedance recorded by the needle tip. The power (W) is preset at the beginning of the procedure on the RF generator and increases over time, if no change of impedance is recorded by the machine, based on the entity of intralesional gas formation. For safety reasons, it was arbitrarily decided to start the ablation by setting the power at lowest values achievable. The procedure is stopped if change in impedance appears, applying this strategy for each ablation section.
The whole procedure is constantly real time monitored by means of US. During the procedure, the tumor gradually becomes hyperechoic owing to the gas production inside the lesion. This sign can be used to confirm the ablation effect and to monitor the integrity of the surrounding structures.17 At the end of the procedure, the needle is carefully removed.
Postoperative Imaging
The posttreatment US evaluation assesses the presence of free fluid or fluid collections immediately after the procedure. Postprocedural abdominal dynamic CT studies and serum level of Ca19.9 tumor marker evaluation were performed 24 hours and 1 month after the procedure. Computed tomography study was performed acquiring noncontrast, pancreatic, venous, and late phases. The same maximum diameters of the tumor were considered before and after the procedure for evaluating the percentage of intratumoral ablation zone so that the same difficultness in tumor extension evaluation was present in the pre- and postprocedure evaluation. At CT, the radiofrequency treatment effect was evaluated by excluding the presence of complications. The technical success of the procedure is defined at CT as the achievement of tumoral ablated area. If no complications appeared, patient was discharged from hospital. Patients were then followed up.
Results
Patients
Between June 2013 and December 2014, 23 patients with biopsy-proven pancreatic adenocarcinoma unresectable locally advanced nonmetastatic located in the pancreatic body have been included in the study. Four (17%) of the 23 patients have been consequently excluded because of the absence of a safe approach to the pancreatic tumor at percutaneous US evaluation.
One patient has been excluded during follow-up period because the reanalysis of the CT scan obtained before the procedure showed peritoneal spread of the disease, being this case a mistake in the inclusion process.
The present study therefore enrolled 18 patients (10 males and 8 females; mean age: 62.4 years; range: 47-78 years) with 18 pancreatic lesions located in the pancreatic body–tail. The mean size of the lesions was 48.1 mm (range: 25-86 mm).
Regarding the chemotherapy, gemcitabine was administered for 6 months in 7 patients and Folfirinox was administered for 6 months in 5 patients with stable disease at follow-up in all cases. In 1 patient, Folfirinox was administered for 4 months and Abraxane for 4 months with stable disease at follow-up. In 1 patient, Folfirinox was administered for 4 months and Gemcitabina for 6 months, and in 1 patient, Folfirinox for 2 months and Gemcitabina for 4 months with downsizing of the mass at follow-up. In 1 patient, Folfirinox was administered for 4 months and Gemcitabina for 3 months, in 1 patient, Gemcitabina–Abraxane for 6 months, and in 1 patient, Gemox for 6 months and Gemcitabina for 4 months and Folfiri for 6 months with stable disease at follow-up.
Procedure, Technical Success, and Complications
In 16 cases, the procedure has been completed in a single session. In 2 patients, the procedure has been repeated twice over a time interval. In a single session, 4 (25%) of the 16 patients underwent a single ablative procedure, 11 (68.7%) patients underwent 2 passages, and 1 (6.3%) patient underwent 3 passages in the same session.
The mean radiofrequency application time for every single passage was 3 minutes 13 seconds (range: 30 seconds to 10 minutes).
In 2 of the 20 procedures, opening the needle, the conductive portion was set at 5 mm in length; in 17 cases, the conductive portion of the needle was set at 1 cm in length, and in 1 case, the conductive portion of the needle was set at 1.5 cm length.
Table 1 shows the scheme of the technical parameters applied for every procedure. A 20 W power has been applied at the beginning only in the 2 procedures of the first case. In all the other cases, a 30 W was preset to start the procedure. The power of 40 W was reached in 6 cases and the power of 50 W only in 2 cases.
Table 1.
Technical Parameters Applied for Percutaneous Pancreatic RFA Procedures.
| Patient | N Procedures | Watt | Time, seconds | Impedance | Conductive Tip Length, cm |
|---|---|---|---|---|---|
| 1 | First procedure | 20 | 180 | Decrease | 1 |
| Second procedure | 20 | 360 | 1 | ||
| 30 | 240 | Decrease | |||
| 2 | First procedure | 30 | 300 | 1 | |
| 40 | 120 | ||||
| 50 | 120 | Decrease | |||
| Second procedure | 30 (first passage) | 240 | 1 | ||
| 40 | 60 | Decrease | |||
| 30 (second passage) | 210 | Decrease | |||
| 3 | 30 | 300 | 1 | ||
| 40 | 120 | Decrease | |||
| 4 | 30 (first passage) | 300 | Decrease | 1 | |
| 30 (second passage) | 300 | Decrease | 1 | ||
| 5 | 30 (first passage) | 240 | 1 | ||
| 40 | 60 | Decrease | |||
| 30 (second passage) | 240 | Decrease | 1 | ||
| 6 | 30 | 300 | Decrease | 1 | |
| 7 | 30 (first passage) | 180 | Decrease | 1 | |
| 30 (second passage) | 120 | Decrease | 1 | ||
| 8 | 30 (first passage) | 90 | Decrease | 1 | |
| 30 (second passage) | 120 | Decrease | 1 | ||
| 9 | 30 | 60 | Decrease | 0.5 | |
| 10 | 30 (first passage) | 120 | Decrease | 1 | |
| 30 (second passage) | 150 | Decrease | 1 | ||
| 11 | 30 (first passage) | 30 | Decrease | 1 | |
| 30 (second passage) | 60 | Decrease | 1 | ||
| 12 | 30 (first passage) | 60 | Decrease | 1 | |
| 30 (second passage) | 60 | Decrease | 1 | ||
| 13 | 30 (first passage) | 240 | 1 | ||
| 40 | 30 | Decrease | |||
| 30 (second passage) | 150 | Decrease | 1 | ||
| 14 | 30 (first passage) | 60 | Decrease | 1 | |
| 30 (second passage) | 60 | Decrease | 1 | ||
| 15 | 30 (first passage) | 60 | Decrease | 0.5 | |
| 30 (second passage) | 60 | Decrease | 0.5 | ||
| 16 | 30 (first passage) | 120 | Decrease | 1 | |
| 30 (second passage) | 90 | Decrease | 1 | ||
| 30 (third passage) | 90 | Decrease | 1 | ||
| 17 | 30 (first passage) | 180 | 1 | ||
| 40 | 10 | Decrease | 1 | ||
| 30 (second passage) | 180 | 1 | |||
| 40 | 60 | Decrease | 1 | ||
| 18 | 30 | 180 | 1.5 | ||
| 40 | 120 | 1.5 | |||
| 50 | 120 | 1.5 |
Abbreviation: RFA, radiofrequency ablation.
The procedure was stopped after the change in impedance recorded on the machine in 17 of the 18 cases. In 1 case, the procedure was stopped before any changes in impedance because of the abundant intralesional gas formation with the needle opened at 1.5 cm. The hyperechoic gas-filled area was visible, increasing during the procedure and evidently exceeding the length of the conductive portion of the needle at the end of the procedure in all cases.
The CT examination 24 hours after the procedure was performed in all patients. At CT, the mean size of the intralesional postablation necrotic area (Figure 1) was 32 mm (range: 15-65 mm). The mean extension of the ablated area was 70% of the whole pancreatic adenocarcinoma.
Figure 1.
Computed tomography (CT) scan before and after the radiofrequency ablation (RFA) procedure. A and B, Unresectable pancreatic mass hypodense in the pancreatic (A) and venous (B) phase located in the pancreatic body. C and D, Necrotic area appearing markedly hypodense in the pancreatic (A) and venous (B) phase is well visible within the pancreatic body mass.
The procedure was technically successful in all cases, achieving an ablated area as wide as or wider than 50% of the total tumor extension in 16 (93%) of the 18 cases.
None of the patients developed postprocedural complications. This was an unexpected results taking into account the potential risk of the procedure probably related to the site of ablation perfectly intratumoral; in case of larger or extratumoral ablation requested, for sure, the risk of complications will immediately and rapidly increase. Postprocedural serum amylase value was normal in all cases. All patients were discharged from hospital 2 days after the procedure. The 30-day readmission rate and the 30-day postprocedural mortality were 0%.
Laboratory Follow-Up
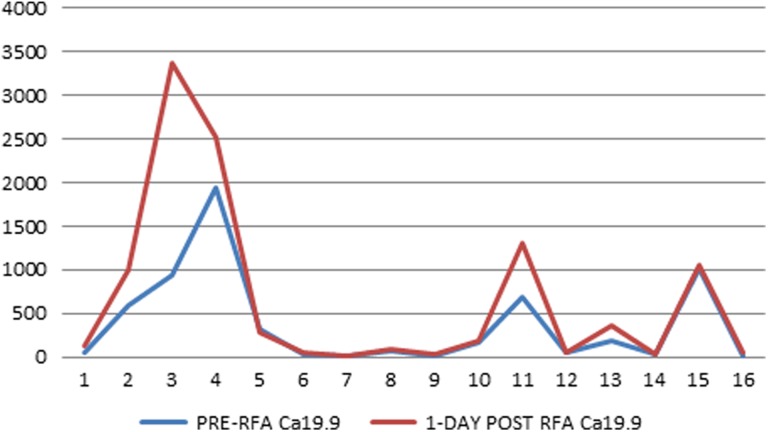
Serum levels of Ca19.9 tumor marker before RFA and after RFA procedure are shown in Table 2. Complete Ca19.9 data were obtained in 10 procedures performed in 9 patients. Mean Ca19.9 serum levels 1 day before, 1 day after, and 1 month after the procedure were 285.8 U/mL (range: 16.6-942.0 U/mL), 635.2 U/mL (range: 17.9-3368.0 U/mL), and 336.0 U/mL (range: 7.0-1400.0 U/mL), respectively. In 7 (70%) of the 10 procedures, Ca19.9 serum levels increased the day after the procedure (Figure 2). One month after the procedure, in 4 (40%) cases, there was a decreased Ca19.9 value, in 4 (40%) cases, there was a stable Ca19.9 value 1 month after the procedure, and in 2 (20%) cases, there was an increased Ca19.9 value (Figure 2). Except for 1 case with clear-cut increase (Table 2 and Figure 3), a substantial stability of Ca19.9 values was found in all patients at 1-month follow-up.
Table 2.
Serum Levels of Ca19.9 Before and After the Procedure.
| Patient | N Procedures | Pre-RFA Ca19.9 | 1-Day Post-RFA Ca19.9 | 30-Day Post-RFA Ca19.9 | ||
|---|---|---|---|---|---|---|
| Value (U/mL) | Value (U/mL) | Delta (U/mL) | Value (U/mL) | Delta (U/mL) | ||
| 1 | First procedure | – | – | – | – | – |
| Second procedure | 47.9 | 122.1 | 74.2 | – | – | |
| 2 | First procedure | 586.3 | 1004.0 | 417.7 | 367.0 | −219.3 |
| Second procedure | 942.0 | 3368.0 | 2426.0 | 947.0 | 5.0 | |
| 3 | 1941.0 | 2522.0 | 581.0 | – | ||
| 4 | 312.0 | 282.3 | −29.7 | 363.6 | 51.6 | |
| 5 | 22.6 | 42.2 | 19.6 | 26.8 | 4.2 | |
| 6 | 19.6 | 17.9 | −1.7 | 23.9 | 4.3 | |
| 7 | 73.6 | 89.3 | 15.7 | 47.0 | −26.6 | |
| 8 | 16.6 | 22.7 | 6.1 | 7.0 | −9.6 | |
| 9 | 164.7 | 184.6 | 19.9 | 170.0 | 5.3 | |
| 10 | 688.0 | 1310.0 | 622.0 | 1400.0 | 712.0 | |
| 11 | 57.3 | 59.2 | 1.9 | – | – | |
| 12 | 178.8 | 365.6 | 186.8 | – | – | |
| 13 | 32.8 | 30.9 | −1.9 | 8.0 | −24.8 | |
| 14 | – | 179.7 | – | – | – | |
| 15 | 1019.0 | 1053.0 | – | – | – | |
| 16 | 4.9 | 48.6 | – | – | – | |
| 17 | 3.65 | – | – | – | – | |
| 18 | – | 2.59 | – | – | – | |
Abbreviation: RFA, radiofrequency ablation.
Figure 2.
Serum levels of Ca19.9 tumor marker before (blue line) and 1-day after (red line) the RFA procedure [Color version of the figure is available online].
Figure 3.
Serum levels of Ca19.9 tumor marker before (blue line) and 30-days after (red line) the RFA procedure [Color version of the figure is available online].
Imaging Follow-Up
An abdominal CT scan 1 month after the procedure has been performed in 18 of the 20 procedures. In 8 (44.4%) of the 18 patients, it showed an increase in lesion size, whereas in 10 (55.6%) of the 18 patients, the lesion size was stable disease, but based on RECIST criteria in all cases, the disease was stable at 1 month after the procedure. However, considering the reduction in the tumor tissue due to the ablation performed, it can be argued that based on RECIST criteria, a partial response was obtained in all cases.
Clinical Follow-Up
The follow-up duration was less than 6 months for 11 patients and more than 6 months for 7 patients. Table 3 shows patients’ survival after the procedure. At the time of the draft of this article, the mean survival of the patients included in the study was 185 days (range: 62-398 days), and all patients alive in the group with follow-up of longer than 6 months show progressive disease.
Table 3.
Patients Survival After the Procedure.
| Patient | Date of Procedure | Status | Survival, days | |
|---|---|---|---|---|
| Follow-up <6 months | 1 | June 17, 2013 | Dead | 129 |
| July 08, 2013 | ||||
| 2 | August 26, 2013 | Dead | 81 | |
| February 24, 2014 | ||||
| 3 | November 25, 2013 | Dead | 93 | |
| 4 | December 02, 2013 | Alive | – | |
| 8 | February 24, 2014 | Alive | – | |
| 10 | March 24, 2014 | Dead | 144 | |
| 11 | May 05, 2014 | Alive | – | |
| 12 | May 12, 2014 | Alive | – | |
| 15 | June 23, 2014 | Dead | 132 | |
| 17 | November 24, 2014 | Alive | 90 | |
| 18 | December 22, 2014 | Alive | 62 | |
| Follow-up >6 months | 5 | January 20, 2014 | Alive | 398 |
| 6 | February 03, 2014 | Dead | 254 | |
| 7 | February 17, 2014 | Dead | 225 | |
| 9 | March 03, 2014 | Dead | 201 | |
| 13 | May 19, 2014 | Alive | 279 | |
| 14 | May 26, 2014 | Alive | 272 | |
| 16 | July 07, 2014 | Alive | 230 |
Discussion
Pancreatic adenocarcinoma is the third most common malignancy and the fourth leading cause of cancer-related mortality. The overall 5-year survival rate for this disease is less than 5%. In almost 95% of the cases, pancreatic ductal adenocarcinoma is diagnosed at an advanced stage,4 with locally advanced or metastatic disease in 40% of the cases.3,5–7 Therapies for advanced pancreatic adenocarcinoma consist of chemotherapy and radiotherapy. Recently, the FOLFIRINOX combination (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) has shown significantly better response rates and survival than gemcitabine in patients who have a good performance status, and trials of this regimen in the neoadjuvant setting are ongoing.14 Unfortunately, many patients with LAPC will continue to have unresectable disease, despite exhaustion of all standard therapies.
Radiofrequency ablation is a relatively new treatment option. Radiofrequency ablation works on the principle that alternating current operated within the radiofrequency range can produce focal thermal injury in tissues.
This ablation treatment is reported to be applied to LAPC15 and based on partial intratumoral necrosis for cytoreduction. This ablation treatment is mainly performed during laparotomy using intraoperative US guidance.15,18,19
The first application of RFA in the normal porcine pancreas was described in 1999. The technical feasibility and effect of RFA were studied in normal porcine pancreatic tissues, and the results were encouraging because discrete zones of coagulation necrosis (8-12 mm) were noticed and no major complications were identified.18 Although this application was performed under EUS guidance, RFA has nearly always been delivered intraoperatively as an US-guided procedure performed during laparotomy in combination with palliative bypass surgery.19,20 In particular, Siriwardena21 recommended that no patient should undergo laparotomy simply for ablation, but the procedure should be used only in patients in whom palliative bypass is required, therefore essentially patients with pancreatic head cancer, or unresectable disease is found at surgery.
In 2000, Matsui et al22 first reported 20 patients with unresectable pancreatic adenocarcinoma treated with RFA. Date et al23 demonstrated the safety and efficacy of RFA in the normal pancreas of a porcine model. Elias et al24 reported 2 cases of multiple pancreatic metastases from renal tumors treated with RFA; unfortunately, both patients developed severe necrotizing pancreatitis and died. The mortality in their series was probably due to multiple ablations that led to severe pancreatitis. Spiliotis et al25 recently reported good results using RFA in 5 patients with inoperable pancreatic cancer. Varshney and coworkers26 showed that RFA was feasible and safe in 3 patients with LAPC. The applications of RF-induced tissue coagulation for tumor treatment are wide. It has been used in early clinical trials for the treatment of hepatocellular and cholangiocellular carcinoma, lung, breast, kidney, prostate, brain, spleen, hepatic and cerebral metastases, and benign bony tumors such as osteoid osteomas.27–34
From the technical point of view, it is reported the importance of the US guidance of the ablation procedure, mainly during the needle positioning into the lesion. During treatment, the tip of the needle must be kept at almost 5 mm from the sensitive structures such as the duodenum and peripancreatic vessels.35–37
Regarding temperature, since protein denaturation begins at 50°C to 60°C, the higher temperatures used during the procedure achieve homogeneous necrosis. On the other hand, the use of too high temperatures (105°C) is proved to increase the risk of complications without a favorable effect.18 Hence, during the ablation procedure of a pancreatic mass, middle-range temperatures are usually applied (90°C).19 Moreover, RF application to internally cooled electrodes enables increased energy delivery to tissues with a resultant increase in the volume of induced coagulation necrosis.
As a result of the internal cooling of the needle, electrodes reduce heat deposition adjacent to the electrode, thereby minimizing tissue vaporization and carbonization, phenomena causing impedance increase and a reduced necrosis extent. Needle cooling therefore permits increased energy deposition that in turn results in larger volumes of coagulation necrosis.38,39
However, because of the anatomical complexity of the pancreatic and peripancreatic regions and the risks of damaging nearby anatomical structures, the procedure of RFA differs from that in other regions, and complete eradication of the pancreatic mass is likely to be either impossible or unsafe and is not indicated. In fact, independent from tumor size, the necrotic area must not overcome the lesion owing to the required safety margins in respect to the contiguous main vascular and digestive structures. It is therefore inevitable that residual tumor tissue infiltrating mesenteric vessels or the duodenal wall is left. This is why it is reasonable to complete cytoreductive treatment by offering patients chemotherapy and/or radiotherapy.
Immune modulation is reported to be the other possible strong motivation to propose RFA40 in a combination treatment to patients with pancreatic adenocarcinoma: an increasing number of studies have been published regarding the role of thermal ablation in stimulating and modulating the immune system and the immune response against the tumor.41–44 Dromi et al45 demonstrate in animals an increase in dendritic cell infiltration, which is the most powerful antigen-presenting cells, following the ablation; subtotal RFA treatment results in systemic antitumor T-cell immune responses and tumor regression. Given that RF ablation generates large amounts of cellular debris, it stimulates necrotic cell death and creates a proimmune environment both locally and systemically, which may be ideally suited for activation of DCs in vivo.46 The natural lack of dendritic cells in pancreatic carcinoma, as compared to other solid neoplasm,47 makes pancreatic adenocarcinoma the natural candidate for thermal ablation to obtain a systemic effect from a local treatment. One possible explanation relies on the peripheral zone just outside the coagulation necrosis volume, which is exposed to slightly elevated temperatures, but not one high enough or for a long enough time to kill the tissue. This zone is known to contain increased amounts of immunostimulatory and inflammatory factors after thermal stress48 and could contribute to the enhanced systemic immune response.
It has also been demonstrated that RFA can provide a reduction in back pain and analgesia requirement.18,34 Postoperative observation (clinical surveillance, laboratory tests, and imaging studies) is mandatory because of the potential for major or minor and early or later complications. After intraoperative pancreatic cancer RFA, the most frequent complications encountered in the earlier postoperative period (within 1 week) are fluid collection, pancreatic fistula, duodenal perforation, and vascular damage; at later times, digestive or abdominal bleeding, infections, or abscesses are more common, whereas severe acute pancreatitis is a rare complication49; in the study by Girelli et al, there was only 1 case and none was reported in the study by Wu et al.19,35
At present, this is the only study that focuses on the percutaneous approach of RFA of LAPC. Quite all pancreatic studies have focused on the feasibility and complications of intraoperative RFA.19,21,35,49 The present study demonstrated the efficacy of the percutaneous ablation procedure with no complications reported in the 18 patients. Technical success, in fact, was obtained in every treated patient with a complete necrosis of an ablated area that covered more than 50% of the lesion extension in 93% of the cases and with no immediate but also delayed complications. Our results open a new possible approach for RFA of pancreatic ductal adenocarcinoma located in the body of the pancreas stated that, at present, this procedure is reported to be mostly applied to LAPC during laparotomy by using intraoperative ultrasonographic guidance, making in those patients the invasiveness of the approach one of the major point of discussion. Moreover, in a surgical patient, the immune response is usually impaired due to laparotomy, whereas in a percutaneously treated patient, it is, on the contrary, proved to be enhanced because of the stimulation and modulation of the immune system and the immune response against the tumor.41–44
From the technical point of view, it is important to stress that inclusion criteria of the present study were pancreatic tumor still unresectable after chemotherapy with mass located at the body of the pancreas and visible at US study of dimensions greater than 2 cm. The presence of vascular involvement does not make the procedure impossible because the dimensions greater than 2 cm allow to insert the needle in the vascular free portion of the tumor, and the intravascular flow, especially arterial, safeguards the structures from heating damages.
Some studies have observed a decrease in Ca19.9 blood levels following an effective ablation.19,50,51 In our series, however, an increase in Ca19.9 blood values has been observed in the majority of patients 1 day after the procedure, most likely due to the cytolytic effect of the procedure itself; nevertheless, a decreased blood value in Ca19.9 marker has been observed in 40% of treated patients 1 month after the procedure.
Regarding the possible impact of survival, when compared to patients at the same disease stage who received standard therapy, patients who received combination therapy (chemotherapy together with RFA) showed a higher survival rate.25 The study by Spiliotis et al25 also evaluated overall survival after RFA according to tumor stage: patients with stage III disease had a significant improvement in survival after RFA compared to patients with the same disease stage receiving best supportive cares (P = .0032). Frigerio et al52 reported an overall survival and disease-specific survival rate of 19 months, without differences between patients who were treated with chemotherapy before the RFA procedure and patients who received RFA as a first-line treatment. In the present study, patients underwent RFA after receiving chemotherapy: in the majority of cases (55.6%), CT examination performed 1 month after the procedure showed a stable disease; at present, 6 patients are still alive with a maximum survival time of 398 days.
Our study presents the following limitations: a small cohort of patients, a short follow-up period, and incomplete data because some patients received their postprocedural evaluation in other hospitals far away from our center. Moreover, given the short-time interval in analysis and the combination of treatments, it is still not possible to deduce any conclusion regarding the improvement in the survival rate after RFA procedure. Therefore proved the efficacy of percutaneous RFA of LAPC, the preliminary results of the present study must be confirmed and supported by a prospective randomized trial.
Conclusion
Percutaneous RFA can produce necrosis in locally advanced unresectable ductal adenocarcinoma of the pancreatic body with high technical success rate. The technique has been demonstrated to be well tolerated without complications in the present series. The procedure seems to be active in local disease control giving the technique possible palliative therapeutic role particularly in combination with chemotherapy.
Abbreviations
- CT
computed tomography
- DC
dendritic cell
- EUS
endoscopic ultrasound
- NCCN
national comprehensive cancer network
- RECIST
response evaluation criteria in solid tumors
- LAPC
locally advanced pancreatic cancer
- RFA
Radiofrequency ablation
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Schima W, Ba-Ssalamah A, Kölblinger C, Kulinna-Cosentini C, Puespoek A, Götzinger P. Pancreatic adenocarcinoma. Eur Radiol. 2007;17(3):638–649. [DOI] [PubMed] [Google Scholar]
- 2. Neoptolemos JP, Stocken DD, Friess H, et al. ; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. [DOI] [PubMed] [Google Scholar]
- 3. Hermanek P. Staging of exocrine pancreatic carcinoma. Eur J Surg Oncol. 1991;17(2):167–172. [PubMed] [Google Scholar]
- 4. D’Onofrio M, Zamboni GA, Malago R, et al. Resectable pancreatic adenocarcinoma: is the enhancement pattern at contrast-enhanced ultrasonography a pre-operative prognostic factor? Ultrasound Med Biol. 2009;35(12):1929–1937. [DOI] [PubMed] [Google Scholar]
- 5. Cubilla Al, Fitzgerald PJ. Tumors of the exocrine pancreas In: Atlas of Tumor Pathology. 2nd series, fascicle 19. Washington, DC: Armed Forces Institute of Pathology; 1984:98–108. [Google Scholar]
- 6. O’Connor TP, Wade TP, Sunwoo YC, et al. Small cell undifferentiated carcinoma of the pancreas. Report of a patient with tumor marker studies. Cancer. 1992;70(6):1514–1519. [DOI] [PubMed] [Google Scholar]
- 7. Sahani DV, Shah ZK, Catalano OA, Boland GW, Brugge WR. Radiology of pancreatic adenocarcinoma: current status of imaging. J Gastroenterol Hepatol. 2008;23(1):23–33. [DOI] [PubMed] [Google Scholar]
- 8. Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–1733. [DOI] [PubMed] [Google Scholar]
- 9. Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coia L, Hoffman J, Scher R, et al. Preoperative chemoradiation for adenocarcinoma of the pancreas and duodenum. Int J Radiat Oncol Biol Phys. 1994;30(1):161–167. [DOI] [PubMed] [Google Scholar]
- 11. Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg. 2002;6(5):763–769. [DOI] [PubMed] [Google Scholar]
- 12. Landry J, Catalano PJ, Staley C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol. 2010;101(7):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore MJ, Goldstein D, Hamm J, et al. ; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. [DOI] [PubMed] [Google Scholar]
- 14. Conroy T, Desseigne F, Ychou M, et al. ; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup; FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. [DOI] [PubMed] [Google Scholar]
- 15. D’Onofrio M, Barbi E, Girelli R, et al. Radiofrequency ablation of locally advanced pancreatic adenocarcinoma: an overview. World J Gastroenterol. 2010;16(28):3478–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2014;12(8):1083–1093. [DOI] [PubMed] [Google Scholar]
- 17. Goldberg SN, Grassi CJ, Cardella JF, et al. ; Society of Interventional Radiology Technology Assessment Committee and the International Working Group on Image-guided Tumor Ablation. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 suppl):s377–s390. [DOI] [PubMed] [Google Scholar]
- 18. Date RS, Siriwardena AK. Radiofrequency ablation of the pancreas. II: Intra-operative ablation of non-resectable pancreatic cancer. A description of technique and initial outcome. JOP. 2005;6(6):588–592. [PubMed] [Google Scholar]
- 19. Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97(2):220–225. [DOI] [PubMed] [Google Scholar]
- 20. Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50(3):392–3401. [DOI] [PubMed] [Google Scholar]
- 21. Siriwardena AK. Radiofrequency ablation for locally advanced cancer of the pancreas. JOP. 2006;7(1):1–4. [PubMed] [Google Scholar]
- 22. Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20(1):14–20. [DOI] [PubMed] [Google Scholar]
- 23. Date RS, Biggins J, Paterson I, Denton J, McMahon RF, Siriwardena AK. Development and validation of an experimental model for the assessment of radiofrequency ablation of pancreatic parenchyma. Pancreas. 2005;30(3):266–271. [DOI] [PubMed] [Google Scholar]
- 24. Elias D, Baton O, Sideris L, Lasser P, Pocard M. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol. 2004;30(1):85–87. [DOI] [PubMed] [Google Scholar]
- 25. Spiliotis JD, Datsis AC, Michalopoulos NV, et al. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392(1):55–60. [DOI] [PubMed] [Google Scholar]
- 26. Varshney S, Sewkani A, Sharma S, et al. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP. 2006;7(1):74–78. [PubMed] [Google Scholar]
- 27. Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170(4):1015–1022. [DOI] [PubMed] [Google Scholar]
- 28. Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livraghi T, Goldberg SN, Monti F, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202(1):205–210. [DOI] [PubMed] [Google Scholar]
- 30. Solbiati L, Ierace T, Goldberg SN, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997;202(1):195–203. [DOI] [PubMed] [Google Scholar]
- 31. Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205(2):367–373. [DOI] [PubMed] [Google Scholar]
- 32. Goldberg SN, Solbiati L, Hahn PF, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209(2):371–379. [DOI] [PubMed] [Google Scholar]
- 33. Anzai Y, Lufkin R, DeSalles A, Hamilton DR, Farahani K, Black KL. Preliminary experience with MR-guided thermal ablation of brain tumors. AJNR Am J Neuroradiol. 1995;16(1):39–48; discussion 49-52. [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815–821. [DOI] [PubMed] [Google Scholar]
- 35. Wu Y, Tang Z, Fang H, et al. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94(5):392–395. [DOI] [PubMed] [Google Scholar]
- 36. Ng KK, Lam CM, Poon RT, Fan ST. Portal vein thrombosis after radiofrequency ablation for recurrent hepatocellular carcinoma. Asian J Surg. 2003;26(1):50–35; discussion 54. [DOI] [PubMed] [Google Scholar]
- 37. Ng KK, Lam CM, Poon RT, et al. Delayed portal vein thrombosis after experimental radiofrequency ablation near the main portal vein. Br J Surg. 2004;91(5):632–639. [DOI] [PubMed] [Google Scholar]
- 38. Lorentzen T. A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol. 1996;3(7):556–563. [DOI] [PubMed] [Google Scholar]
- 39. Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3(8):636–644. [DOI] [PubMed] [Google Scholar]
- 40. Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waitz R, Solomon SB. Can local radiofrequency ablation of tumors generate systemic immunity against metastatic disease? Radiology. 2009;251(1):1–2. [DOI] [PubMed] [Google Scholar]
- 42. Dallal RM, Christakos P, Lee K, Egawa S, Son YI, Lotze MT. Paucity of dendritic cells in pancreatic cancer. Surgery. 2002;131(2):135–138. [DOI] [PubMed] [Google Scholar]
- 43. Teng LS, Jin KT, Han N, Cao J. Radiofrequency ablation, heat shock protein 70 and potential anti-tumor immunity in hepatic and pancreatic cancers: a mini review. Hepatobiliary Pancreat Dis Int. 2010;9(4):361–365. [PubMed] [Google Scholar]
- 44. Muerkoster S, Wegehenkel K, Arlt A, et al. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res. 2004;64(4):1331–1337. [DOI] [PubMed] [Google Scholar]
- 45. Dromi SA, Walsh MP, Herby S, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. den Brok MH, Sutmuller RP, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64(11):4024–4029. [DOI] [PubMed] [Google Scholar]
- 47. Rovere-Querini P, Manfredi AA. Tumor destruction and in situ delivery of antigen presenting cells promote anti-neoplastic immune responses: implications for the immunotherapy of pancreatic cancer. JOP. 2004;5(4):308–314. [PubMed] [Google Scholar]
- 48. Hansler J, Neureiter D, Strobel D, et al. Cellular and vascular reactions in the liver to radio-frequency thermo-ablation with wet needle applicators. Study on juvenile domestic pigs. Eur Surg Res. 2002;34(5):357–363. [DOI] [PubMed] [Google Scholar]
- 49. Hadjicostas P, Malakounides N, Varianos C, Kitiris E, Lerni F, Symeonides P. Radiofrequency ablation in pancreatic cancer. HPB (Oxford). 2006;8(1):61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang Z, Wu YL, Fang HQ, et al. Treatment of unresectable pancreatic carcinoma by radiofrequency ablation with ‘cool-tip needle’: report of 18 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2008;88(6):391–394. [PubMed] [Google Scholar]
- 51. D’Onofrio M, Barbi E, Girelli R, et al. Variation of tumoral marker after radiofrequency ablation of pancreatic adenocarcinoma. J Gastrointest Oncol. 2016;7(2):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frigerio I, Girelli R, Giardino A, Regi P, Salvia R, Bassi C. Short term chemotherapy followed by radiofrequency ablation in stage III pancreatic cancer: results from a single center. J Hepatobiliary Pancreat Sci. 2013;20(6):574–577. [DOI] [PubMed] [Google Scholar]