Abstract
Purpose:
Chordoma is a radioresistant tumor that presents a therapeutic challenge with spine involvement, as high doses of radiation are needed for local control while limiting dose to the spinal cord. The purpose of this study is to determine the efficacy and safety of single-fraction spine stereotactic body radiation therapy for the treatment of spine chordoma.
Methods:
A retrospective review of our institutional database from 2006 to 2013 identified 8 patients (12 cases) with chordoma of the spine who were treated with spine stereotactic body radiation therapy. Surgical resection was performed in 7 of the 12 cases. The treatment volume was defined by the bony vertebral level of the tumor along with soft tissue extension appreciated on magnetic resonance imaging fusion. Medical records and imaging were assessed for pain relief and local control. Treatment toxicity was evaluated using Common Terminology Criteria for Adverse Events version 4.0.
Results:
Median age was 59 years (range, 17-91). Median target volume was 48 cm3 (1-304), and median prescription dose was 16 Gy (11-16). Median conformality index was 1.44 (1.14-3.21), and homogeneity index was 1.12 (1.05-1.19). With a median follow-up time of 9.7 months (.5-84), local control was achieved in 75% of the cases treated. One patient developed limited grade 2 spinal cord myelopathy that resolved with steroids. There were no other treatment toxicities from spine stereotactic body radiation therapy.
Conclusion:
Single-fraction spine stereotactic body radiation therapy can be safely delivered to treat chordoma of the spine with the potential to improve pain symptoms. Although the early data are suggestive, long-term follow-up with more patients is necessary to determine the efficacy of spine stereotactic body radiation therapy in the treatment of chordoma of the spine.
Keywords: chordoma, SBRT, SRS, spine, radiosurgery
Introduction
Chordoma is a slow-growing, locally aggressive bone tumor that arises from embryologic notochord. It is a rare disease, comprising 2% to 4% of bone tumors, with an incidence of less than 300 cases per year in the United States.1 Because chordoma develops from undifferentiated remnants of notochord, it can present anywhere along the craniospinal axis from the base of the skull (clivus) superiorly to the coccyx inferiorly. Typically, these lesions present most frequently at the base of the skull followed by the sacrococcygeal spine. Approximately 15% of cases present in the mobile spine.2,3 Chordomas of the mobile spine have distinct treatment considerations compared with tumors of the clivus and sacrum. A soft tissue component is often present with spine involvement and may invade the paravertebral musculature. Although commonly described as benign, chordoma has malignant potential; nodal metastases and hematogenous dissemination may develop in more than one-third of patients.4 One of the challenges of treating chordoma is that it tends to recur after treatment, with recurrence rates as high as 60% to 70% at 10-year follow-up after treatment.5
The recommended treatment for chordoma is surgery followed by adjuvant radiation therapy. Chordoma can be staged according to the TNM system for primary malignant bone tumors, but this has limited prognostic value. Instead, the extent of surgical resection is most prognostic for tumor control.6–13 However, obtaining a gross total resection with negative margins can be difficult to achieve based on the anatomic location.14 In cases where a tumor is unresectable, definitive management with radiation therapy is the standard of care.4 Since chordoma is radioresistant, high doses of radiation are needed for tumor control.15 Proximity to the spinal cord or cauda equina limits the dose that can be safely delivered, and proton therapy is often considered because of this.
Advances in technology have allowed radiation oncologists to administer high doses of radiation therapy with precise conformity and steep dose falloff outside the target. The ability to deliver high doses becomes even more important for unresectable tumors.16 Stereotactic body radiation therapy (SBRT) is a modality available at many radiation oncology facilities that enables physicians to administer high doses of radiation while sparing normal tissues. Spine SBRT (sSBRT) delivered in a single fraction has been shown to provide excellent local control for metastatic spine lesions of radioresistant histology, such as renal cell carcinoma and melanoma.17–19 The purpose of this study is to assess the effectiveness and safety of single-fraction sSBRT in the treatment of spine chordoma.
Methods and Materials
A retrospective review of our institutional sSBRT database was conducted to identify patients with chordoma of the spine treated with single-fraction sSBRT. The database includes patients treated with sSBRT at the Cleveland Clinic from 2006 to 2013. All patients were treated on a Novalis Radiosurgery unit. A thermoplastic head mask or a vacuum-form body immobilization device BodyFix (Medical Intelligence; Elekta, Stockholm, Sweden) was used for immobilization during computed tomography (CT) simulation depending on the location of the chordoma. The CT simulation was performed with images acquired at 3-mm interval axial slices. Each patient underwent magnetic resonance imaging (MRI; 1.5-mm slice thickness) that was fused with the CT simulation to help define the tumor and the spinal cord.
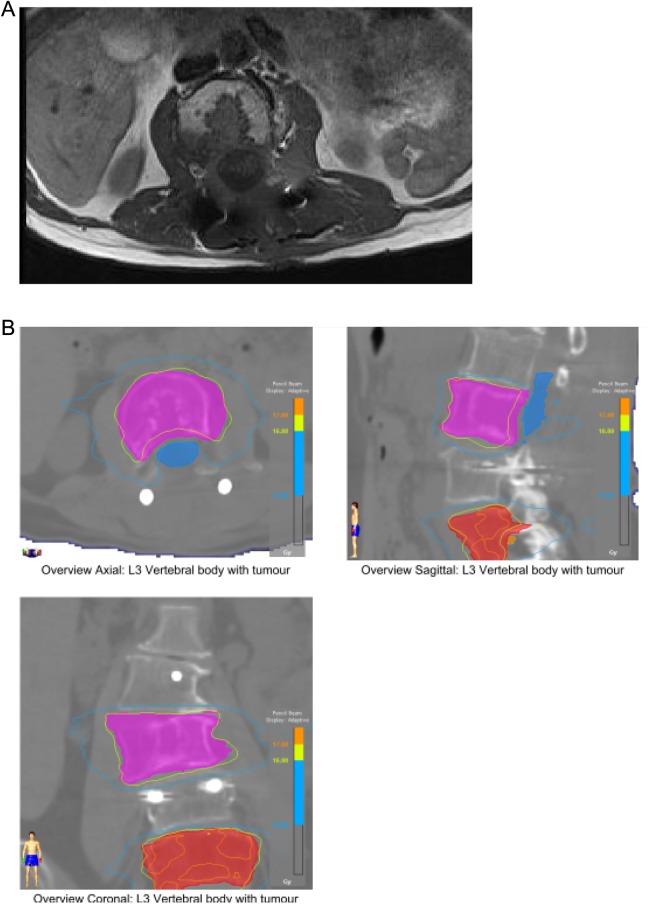
The clinical target volume (CTV) was defined by the bony vertebral level of the tumor along with soft tissue extension appreciated on MRI fusion consistent with Radiation Therapy Oncology Group 0631 guidelines (Figure 1A). The CTV encompasses the entire vertebral body level(s) involved, including the adjacent pedicles and posterior elements depending on the location of tumor involvement. Surgical hardware was not included in the CTV for postoperative cases. No additional margin was placed around the CTV. The spinal cord was contoured as an organ at risk (OAR) 5 mm superior and inferior to the vertebral body of interest (Figure 1B). For lesions below L2 (cauda equina), the spinal canal was contoured with the same margins. A planning organ at risk volume (PRV) expansion was not created for the OAR.
Figure 1.
A, Postoperative MRI is fused with the CT simulation scan for sSBRT treatment planning purposes. B, L3 CTV (pink) and L5 CTV (red) contoured on CT simulation scan with MRI fusion. Cauda equina (blue) or spinal cord is contoured 5 mm superior and inferior to the vertebral level being treated. CT indicates computed tomography; CTV, clinical target volume; MRI, magnetic resonance imaging; sSBRT, spine stereotactic body radiation therapy.
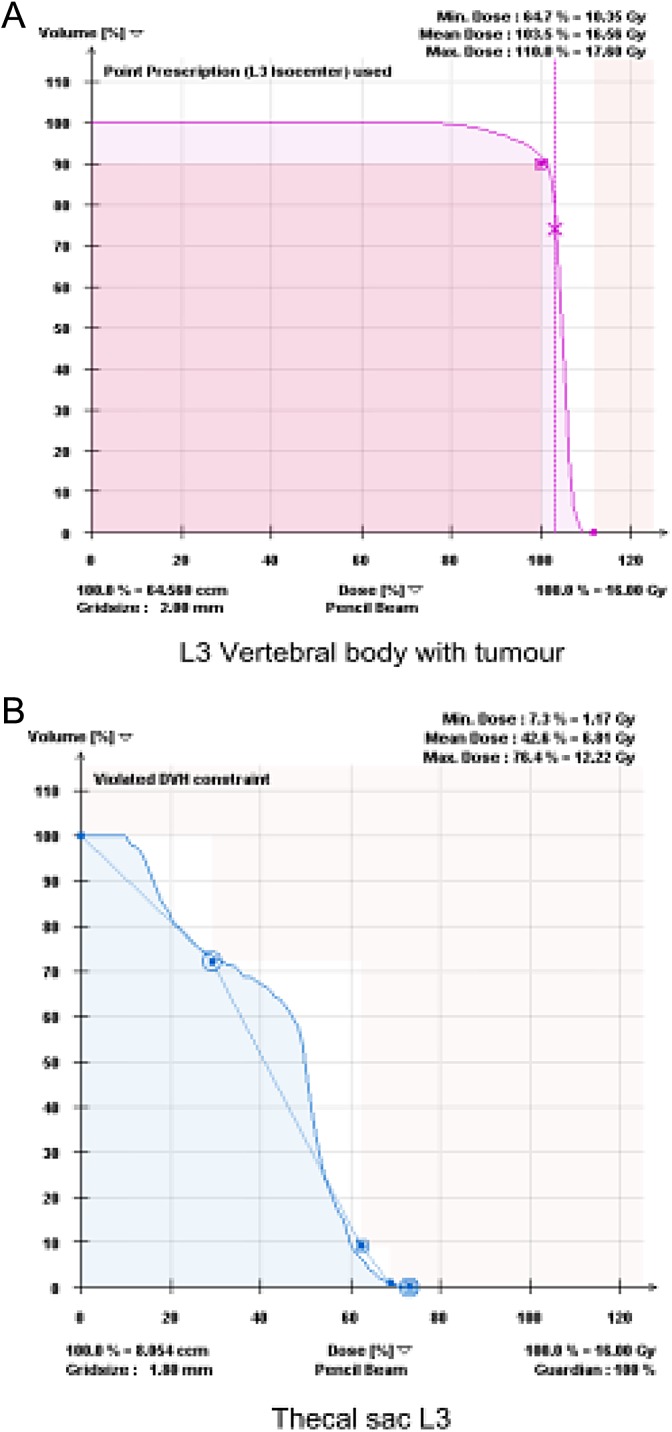
The prescription dose was 16 Gy delivered in 1 fraction to the CTV. Modifications of the prescription dose were due to concerns of spinal cord toxicity. The treatment planning goal was D90 ≥ 100, a minimum of 90% of the CTV receiving 100% of the prescription dose (Figure 2A). Treatment planning consisted of step-and-shoot intensity-modulated radiation therapy (IMRT) with coplanar beams. Dose constraints for the spinal cord volume were a maximum dose of 14 Gy and V10 ≤ 10%. For the cauda equina, which is considered more tolerant to radiation, the maximum dose was 16 Gy with V12 ≤ 10% (Figure 2B). Cone beam CT and ExacTrac (Novalis, Palo Alto, CA) positioning were used for registration and localization prior to the treatment. Conformality index was defined as prescription isodose volume/tumor volume, and homogeneity index was defined as maximum dose/prescription dose.
Figure 2.
Dose–volume histogram of the case treated in Figure 1. A, Dose–volume histogram of L3 CTV treated with sSBRT to 16 Gy in 1 fraction. Ninety percent of the volume is receiving 100% of the prescription dose, D90 ≥ 100. B, Dose–volume histogram of cauda equina. Volume receiving 12 Gy is less than or equal to 10% V12 ≤ 10. Maximum dose is less than or equal to 16 Gy Dmax ≤ 16. CTV indicates clinical target volume; sSBRT, spine stereotactic body radiation therapy.
Posttreatment MRI was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) criteria for local control and disease progression. Local failure was defined as imaging findings that met RECIST criteria for progressive disease, which is 20% increase in the largest diameter of the tumor. Visual analogue scale (VAS) was used for pain assessment. Individual patient records were reviewed to assess for symptomatic pain relief. Pain improvement was defined as a decrease in VAS score by 2 or more points. Treatment toxicity was evaluated using the Common Terminology Criteria for Adverse Events version 4.0.
Results
Patient and Treatment Characteristics
A total of 8 patients with chordoma of the spine were identified with 12 separate sSBRT treatment sites. All patients presented with epidural disease and spine pain. Surgical resection was performed in 7 of the 12 cases. Median age was 59 years (range, 17-91). Median Karnofsky performance status was 70 (range, 40-90). Distribution of tumor locations within the spine is as follows: 4 cervical, 6 lumbar, and 2 sacral spine lesions.
The median prescription dose was 16 Gy (range, 11-16). The median target volume was 48 cm3 (range, 1-304). The median conformality index was 1.44 (range, 1.14-3.21). The median homogeneity index was 1.12 (range, 1.05-1.19).
Treatment Outcomes
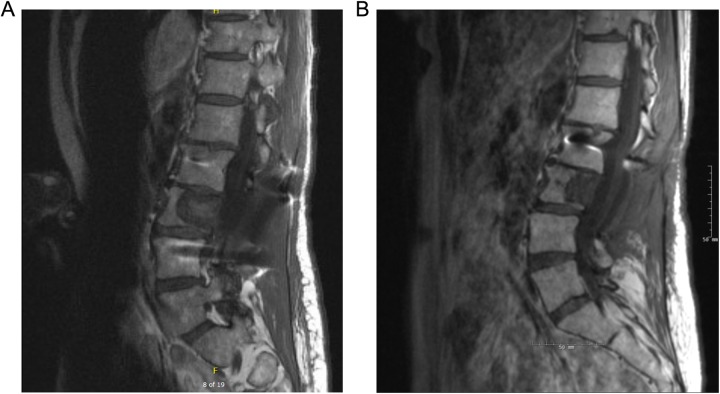
Median follow-up time after treatment with sSBRT was 9.7 months (range, .5-84). Pain improvement was seen in 3 of the 8 patients (Table 1). Local control was achieved in 9 (75%) of the 12 cases. Figure 3 illustrates an example of local control after sSBRT. One patient developed grade 2 spinal cord myelopathy that resolved with dexamethasone steroid treatment. There were no other treatment toxicities from sSBRT. Treatment data and outcomes are summarized in Table 2.
Table 1.
Patient Characteristics and Pain Improvement.
| Patient | Gender | Age | KPS | Spine Levels Treated | Prior EBRT | Pain Improvement After sSBRT | Time to Pain Improvement |
|---|---|---|---|---|---|---|---|
| 1 | Male | 91 | 70 | L2 | No | No | |
| 2 | Female | 78 | 80 | C4, C5, C6 | No | Yes | 17 days |
| 3 | Male | 58 | 80 | C3 | No | Yes | 35 days |
| 4 | Male | 59 | 70 | C2, C3 | No | No | |
| 5 | Female | 59 | 80 | L3, L5 | Yes | No | |
| 6 | Male | 55 | 90 | C4 | No | Yes | 15 days |
| 7 | Female | 53 | 70 | Sacrum | Yes | No | |
| 8 | Male | 17 | 40 | L1, L2, L3, L4, L5, sacrum | No | No |
Abbreviations: EBRT, external beam radiation therapy; KPS, Karnofsky performance status; sSBRT, spine stereotactic body radiation therapy.
Figure 3.
A, Pretreatment sagittal T1 postcontrast MRI of chordoma lesion at L3 vertebra. B, Posttreatment MRI of treated L3 lesion at 6-month follow-up. Local control achieved according to RECIST criteria utilizing the longest anterior–posterior diameter of the lesion with overall reduction from 30 mm pretreatment to 21 mm status post sSBRT (scale on image). MRI indicates magnetic resonance imaging; RECIST, Response Evaluation Criteria in Solid Tumors; sSBRT, spine stereotactic body radiation therapy.
Table 2.
Treatment Data and Radiographic Local Control.
| Patient | Dose, Gy | Target Volume, cm3 | CTV Coverage, % | Homogeneity Index | Conformality Index | Spine Level | Prior Surgery | Follow-Up, months | Local Failure | Time Interval to Radiographic Failure |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 70.8 | 92 | 1.11 | 1.14 | L2 | Yes | 9.2 | No | |
| 2 | 16 | 41.6 | 97 | 1.1 | 1.66 | C4, C5, C6 | Yes | 36.5 | Yes | 31 months |
| 3 | 16 | 11.4 | 97 | 1.1 | 1.41 | C3 | Yes | 84 | Yes | 84 months |
| 4 | 16 | 89.6 | 93 | 1.09 | 1.18 | C2, C3 | Yes | 6.5 | No | |
| 5 | 16 | 64.5 | 100 | 1.1 | 1.61 | L3 | Yes | 5.4 | No | |
| 5 | 16 | 53 | 100 | 1.17 | 1.53 | L5 | No | 5.4 | No | |
| 6 | 16 | 19.1 | 94 | 1.05 | 1.15 | C4 | Yes | 0.5 | No | |
| 7 | 14 | 303.8 | 91 | 1.12 | 1.47 | Sacrum | Yes | 4.8 | Yes | 2 months |
| 8 | 13 | 7.2 | 93 | 1.19 | 1.26 | Sacrum | No | 10.1 | No | |
| 8 | 11 | 1 | 93 | 1.12 | 1.35 | L1 | No | 12.6 | No | |
| 8 | 16 | 146.6 | 100 | 1.15 | 1.26 | L3, L4, L5 | No | 25 | No | |
| 8 | 16 | 17.7 | 100 | 1.19 | 3.21 | L1, L2 | No | 25 | No |
Abbreviation: CTV, clinical target volume.
Illustrative Case of a Patient Who Developed Spinal Cord Myelopathy
A 58-year-old man presented with cervical neck pain along with numbness and weakness of the left arm. Magnetic resonance imaging of the cervical spine was performed, which demonstrated spinal cord compression at C3. The patient underwent an anterior corpectomy with C2 to C4 anterior fusion with polyetheretherketone (PEEK) cage. Postoperative MRI demonstrated a residual anterior epidural soft tissue mass that continued to compress the spinal cord. Postoperatively, the patient’s neck pain resolved, but he had residual peripheral numbness of his left fingers. The C3 vertebra was treated to a dose of 16 Gy delivered in a single fraction. Intensity-modulated radiation therapy was performed using dynamic Multileaf Collimator (MLC) with 8 coplanar beams utilizing 6-MV photons prescribed to the 100% isodose line. Posttreatment MRI of the cervical spine at 1 month demonstrated resolution of mass effect on the spinal cord. Approximately 2 months after completing sSBRT, the patient began to experience right upper extremity pain and hand weakness. He also complained of difficulty with walking. He underwent an urgent MRI, which demonstrated focal abnormal T2 signal on the right side of the spinal cord dorsally centered at C3 to C4, suggestive of radiation myelopathy (Figure 4). He was administered high-dose dexamethasone with taper, which improved these symptoms. A 3-month follow-up MRI of the cervical spine showed decrease in the focal T2 signal. At 7-year follow-up after sSBRT, the patient was found to have local tumor recurrence and underwent repeat surgery with tumor removal of the right side of C2 to C4 along with revision of instrumentation.
Figure 4.
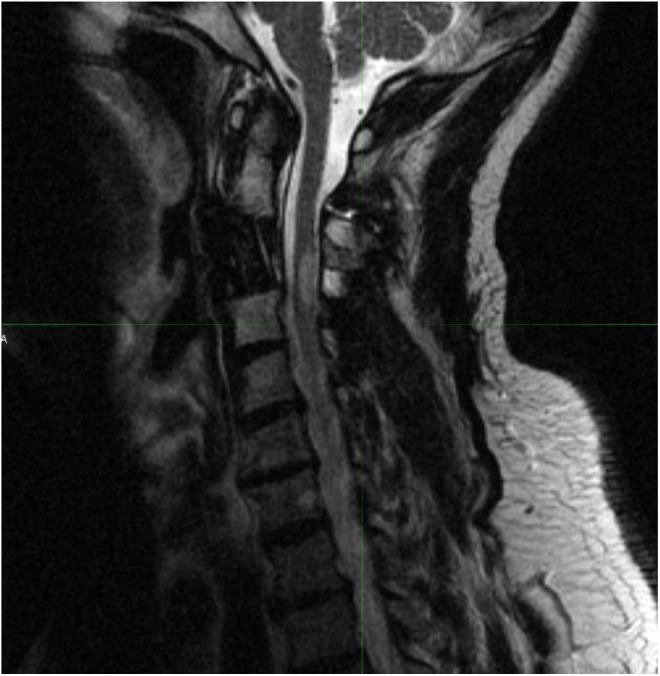
T2-weighted magnetic resonance imaging demonstrating radiation-induced myelopathy after treatment.
Discussion
Chordoma of the spine is a therapeutic challenge. Historically, radiation therapy for chordoma with fractionated external beam radiation therapy (EBRT) was associated with high rates of local recurrence. This is evidenced by a retrospective study of patients treated to a median dose of 50 Gy in 25 fractions, with a local control rate of 27% and median survival of 62 months.20 A major limitation with older techniques utilizing 2-dimensional or 3-dimensional radiation therapy is that a large radiation dose could not be administered due to safety concerns for the brainstem or spinal cord.
With technological improvements in radiation therapy, higher doses can now be safely delivered to the target with greater conformity to decrease the dose to OARs. A number of modalities including proton therapy, SBRT, and heavy-particle irradiation are available. However, there are no randomized clinical trials or studies comparing these modalities for chordoma.
Heavy particle carbon ion therapy has been used in certain disease sites and tumors as a method of delivering high linear energy transfer (LET) radiation which confers a greater relative biologic effectiveness (RBE) than fractionated photon EBRT. A retrospective study from Japan analyzed 38 patients with unresectable sacral chordomas treated with carbon ion therapy.21 Carbon ion dose ranged from 52.8 to 73.6 GyE in 16 fractions administered over 4 weeks. Median CTV volume was 523 cm3. The 5-year local control rate was 89%, with a median follow-up time of 80 months. Two patients developed grade 4 skin toxicity requiring skin grafts. Five-year overall survival was 86%.
Proton therapy is another modality of delivering high doses to tumors that are in proximity to critical structures and is currently being utilized and investigated for many central nervous system diseases. The advantage of this technology lies in the physical properties of proton particles. Protons exhibit a Bragg peak phenomenon, which allows these particles to deposit a large dose at a specific depth, followed by a sharp dose falloff. This enables treatment to high doses while minimizing collateral damage to adjacent structures. A recent retrospective review of 24 patients with spine chordomas treated exclusively with proton therapy or a mixture of proton/photon therapy with proton predominance was conducted.22 Median tumor volume was 198.3 cm3. Median total dose was 77.4 GyE. With a median follow-up of 56 months, local progression-free survival of 90.4% at 3 years and 79.8% at 5 years was achieved. Toxicities included 8 sacral fractures, 1 secondary malignancy, 1 neuropathy causing foot drop, 1 erectile dysfunction, 1 perineal numbness, 2 urinary/fecal incontinence, and 4 grade 2 rectal bleeding toxicities.
Initial results with proton and carbon ion therapy have been promising. Although these treatment modalities may confer some physical and radiobiological advantages over photon therapy, access and cost are significant barriers. There are only 8 operational carbon ion facilities in the world, none of which are in the United States. Currently, there are 20 proton centers in the United States. The costs of proton therapy can be twice the costs of photon therapy, and the costs of carbon ion therapy are even higher.23 Given the trend for increased focus on health-care costs, alternative strategies aside from particle therapy have been explored. Stereotactic body radiation therapy is a technique using hypofractionated photon therapy to deliver conformal high dose to tumors while minimizing dose to critical structures. High doses delivered in one or few fractions yield radiobiological effects via the acid sphingomyelinase pathway leading to vascular endothelial cell damage and overcome issues of tumor reoxygenation, cell cycle dependency, and radioresistance of tumors.24
Fractionated sSBRT for chordoma has been reported in the literature. A retrospective study of 30 patients by Eid et al demonstrated that sSBRT offered superior local control compared to fractionated EBRT for chordoma.25 Tumor progression occurred in all patients treated with fractionated EBRT. Among the patients receiving sSBRT, 38% of the patients developed tumor progression. A study from Georgetown University (Washington, DC) examined the efficacy and safety of CyberKnife radiosurgery in 18 patients.26 Treatment was delivered in 5 fractions to a median dose of 35 Gy (range, 24-40). Mean tumor volume was 128 cm3. The local control rate at 65 months was 59.1%.
Although studies on fractionated sSBRT have been published in the past, there is currently only one other published article in the literature reporting on single-fraction sSBRT for chordoma.27 With a median follow-up time of 24 months for 24 patients, local control was achieved in 95% of cases treated with single-fraction sSBRT. Long-term side effects from this treatment included 1 patient who developed sciatic neuropathy and 1 with vocal cord paralysis. The higher rate of local control with single-fraction sSBRT compared with fractionated sSBRT in prior studies may also be due to shorter follow-up time with single-fraction sSBRT in this study. Regardless, single-fraction sSBRT may be a more effective treatment than fractionated sSBRT. A recent study comparing single fraction versus multifraction sSBRT for renal cell carcinoma—another radioresistant tumor—demonstrates improved 1- and 2-year actuarial local control with single fraction versus multifraction sSBRT (95% vs 71% at 1 year and 86% vs 55% at 2 years, respectively; P = .009).28 In this study, single-fraction sSBRT was delivered to a dose of 24 Gy, and multifraction sSBRT was delivered to a dose of 27 Gy in 3 fractions.
Our results yielded a reasonable local control rate of 75%. This falls short of the rate reported by Yamada et al27 and may be due to the lower dose prescribed in our series. As such, we feel higher doses may be necessary to improve local control for chordoma. This may be achieved using a higher single-fraction dose—such as 24 Gy used in Yamada et al—which we are currently investigating. Our results with single-fraction sSBRT for chordoma, however, are congruous with a previously published report from our institution on the utilization of sSBRT in renal cell carcinoma. In that study, median prescription dose was 15 Gy delivered in 1 fraction with a radiographic progression-free survival of 78.1% at 9 months with similar follow-up as our current study.18
Other strategies to treat chordoma of the spine include escalating the dose using high-dose, image-guided IMRT. Sahgal et al recently published their series of 42 patients to upward of 76 Gy at 2 Gy per fraction. They achieved a 5-year local control rate of 65%, comparable to proton therapy.29
Limitations of this study include relatively short follow-up and the acknowledgment that this is a retrospective review with limited patient numbers due to the scarcity of this disease. Given that chordomas are inherently radioresistant, higher doses may improve outcomes. A future comparison of variations in treatment planning dose and technique correlated with clinical outcomes is an area that can be explored. Our institution has subsequently transitioned to volumetric-modulated arc therapy planning with 2 coplanar arcs for faster sSBRT delivery. We are also now implementing multifraction radiosurgery in an attempt to further escalate the dose safely.
Conclusion
Our results confirm that single-fraction sSBRT can safely be used to treat chordoma of the spine. Further long-term follow-up with more patients is necessary to validate the effectiveness of sSBRT in the management of chordoma. Higher doses may be necessary to achieve control rates similar to proton therapy. Single-fraction versus multifraction radiosurgery will also need to be evaluated, as multifraction radiosurgery may help increase the dose without compromising safety. Regardless, sSBRT can be considered for the management of chordoma of the spine at centers without access to proton therapy or carbon ion therapy.
Abbreviations
- CT
computed tomography
- CTV
clinical target volume
- EBRT
external beam radiation therapy
- IMRT
intensity-modulated radiation therapy
- MRI
magnetic resonance imaging
- OAR
organ at risk
- RECIST
Response Evaluation Criteria in Solid Tumors
- SBRT
stereotactic body radiation therapy
- sSBRT
spine stereotactic body radiation therapy
- VAS
visual analogue scale
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: John Suh—Varian Medical Systems (consultant), Elekta (Travel and Lodging); and Samuel Chao—Varian Medical Systems (Honorarium).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12(1):1–11. [DOI] [PubMed] [Google Scholar]
- 2. Sciubba DM, Chi JH, Rhines LD, Gokaslan ZL. Chordoma of the spinal column. Neurosurg Clin N Am. 2008;19(1):5–15. [DOI] [PubMed] [Google Scholar]
- 3. Heffelfinger MJ, Dahlin DC, MacCarty CS, Beabout JW. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973;32(2):410–420. [DOI] [PubMed] [Google Scholar]
- 4. Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–e76. [DOI] [PubMed] [Google Scholar]
- 5. Tzortzidis F, Elahi F, Wright D, Natarajan SK, Sekhar LN. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery. 2006;59(2):230–237; discussion 230-237. [DOI] [PubMed] [Google Scholar]
- 6. Baratti D, Gronchi A, Pennacchioli E, et al. Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol. 2003;10(3):291–296. [DOI] [PubMed] [Google Scholar]
- 7. Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88(9):2122–2134. [DOI] [PubMed] [Google Scholar]
- 8. Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine (Phila Pa 1976). 2006;31(4):493–503. [DOI] [PubMed] [Google Scholar]
- 9. Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87(10):2211–2216. [DOI] [PubMed] [Google Scholar]
- 10. Hanna SA, Aston WJ, Briggs TW, Cannon SR, Saifuddin A. Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008;466(9):2217–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hulen CA, Temple HT, Fox WP, Sama AA, Green BA, Eismont FJ. Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am. 2006;88(7):1532–1539. [DOI] [PubMed] [Google Scholar]
- 12. Schwab JH, Healey JH, Rose P, Casas-Ganem J, Boland PJ. The surgical management of sacral chordomas. Spine (Phila Pa 1976). 2009;34(24):2700–2704. [DOI] [PubMed] [Google Scholar]
- 13. York JE, Kaczaraj A, Abi-Said D, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44(1):74–79; discussion 9-80. [DOI] [PubMed] [Google Scholar]
- 14. Stacchiotti S, Casali PG, Lo Vullo S, et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol. 2010;17(1):211–219. [DOI] [PubMed] [Google Scholar]
- 15. Yamada Y, Gounder M, Laufer I. Multidisciplinary management of recurrent chordomas. Curr Treat Options Oncol. 2013;14(3):442–453. [DOI] [PubMed] [Google Scholar]
- 16. Staab A, Rutz HP, Ares C, et al. Spot-scanning-based proton therapy for extracranial chordoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e489–e496. [DOI] [PubMed] [Google Scholar]
- 17. Garg AK, Shiu AS, Yang J, et al. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer. 2012;118(20):5069–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balagamwala EH, Angelov L, Koyfman SA, et al. Single-fraction stereotactic body radiotherapy for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2012;17(6):556–564. [DOI] [PubMed] [Google Scholar]
- 19. Joaquim AF, Ghizoni E, Tedeschi H, Pereira EB, Giacomini LA. Stereotactic radiosurgery for spinal metastases: a literature review. Einstein (Sao Paulo). 2013;11(2):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Catton C, O’Sullivan B, Bell R, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41(1):67–72. [DOI] [PubMed] [Google Scholar]
- 21. Imai R, Kamada T, Tsuji H, et al. Effect of carbon ion radiotherapy for sacral chordoma: results of phase I-II and phase II clinical trials. Int J Radiat Oncol Biol Phys. 2010;77(5):1470–1476. [DOI] [PubMed] [Google Scholar]
- 22. Chen YL, Liebsch N, Kobayashi W, et al. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine (Phila Pa 1976). 2013;38(15):e930–e936. [DOI] [PubMed] [Google Scholar]
- 23. Goitein M, Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol (R Coll Radiol). 2003;15(1):s37–s50. [DOI] [PubMed] [Google Scholar]
- 24. Balagamwala EH, Chao ST, Suh JH. Principles of radiobiology of stereotactic radiosurgery and clinical applications in the central nervous system. Technol Cancer Res Treat. 2012;11(1):3–13. [DOI] [PubMed] [Google Scholar]
- 25. Eid AS, Chang UK, Lee SY, Jeon DG. The treatment outcome depending on the extent of resection in skull base and spinal chordomas. Acta Neurochir (Wien). 2011;153(3):509–516. [DOI] [PubMed] [Google Scholar]
- 26. Henderson FC, McCool K, Seigle J, Jean W, Harter W, Gagnon GJ. Treatment of chordomas with CyberKnife: Georgetown University experience and treatment recommendations. Neurosurgery. 2009;64(2 suppl):a44–a53. [DOI] [PubMed] [Google Scholar]
- 27. Yamada Y, Laufer I, Cox BW, et al. Preliminary results of high-dose single-fraction radiotherapy for the management of chordomas of the spine and sacrum. Neurosurgery. 2013;73(4):673–680; discussion 680. [DOI] [PubMed] [Google Scholar]
- 28. Ghia AJ, Chang EL, Bishop AJ, et al. Single-fraction versus multifraction spinal stereotactic radiosurgery for spinal metastases from renal cell carcinoma: secondary analysis of phase I/II trials. J Neurosurg Spine. 2016;24(5):829–836. [DOI] [PubMed] [Google Scholar]
- 29. Sahgal A, Chan MW, Atenafu EG, et al. Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: preliminary outcomes. Neuro Oncol. 2015;17(6):889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]