Abstract
Purpose:
The study was aimed to compare online 6 degree-of-freedom image registrations of TrueBeam cone-beam computed tomography and BrainLab ExacTrac X-ray imaging systems for intracranial radiosurgery.
Methods:
Phantom and patient studies were performed on a Varian TrueBeam STx linear accelerator (version 2.5), which is integrated with a BrainLab ExacTrac imaging system (version 6.1.1). The phantom study was based on a Rando head phantom and was designed to evaluate isocenter location dependence of the image registrations. Ten isocenters at various locations representing clinical treatment sites were selected in the phantom. Cone-beam computed tomography and ExacTrac X-ray images were taken when the phantom was located at each isocenter. The patient study included 34 patients. Cone-beam computed tomography and ExacTrac X-ray images were taken at each patient’s treatment position. The 6 degree-of-freedom image registrations were performed on cone-beam computed tomography and ExacTrac, and residual errors calculated from cone-beam computed tomography and ExacTrac were compared.
Results:
In the phantom study, the average residual error differences (absolute values) between cone-beam computed tomography and ExacTrac image registrations were 0.17 ± 0.11 mm, 0.36 ± 0.20 mm, and 0.25 ± 0.11 mm in the vertical, longitudinal, and lateral directions, respectively. The average residual error differences in the rotation, roll, and pitch were 0.34° ± 0.08°, 0.13° ± 0.09°, and 0.12° ± 0.10°, respectively. In the patient study, the average residual error differences in the vertical, longitudinal, and lateral directions were 0.20 ± 0.16 mm, 0.30 ± 0.18 mm, 0.21 ± 0.18 mm, respectively. The average residual error differences in the rotation, roll, and pitch were 0.40°± 0.16°, 0.17° ± 0.13°, and 0.20° ± 0.14°, respectively. Overall, the average residual error differences were <0.4 mm in the translational directions and <0.5° in the rotational directions. ExacTrac X-ray image registration is comparable to TrueBeam cone-beam computed tomography image registration in intracranial treatments.
Keywords: cone-beam computed tomography, TrueBeam, ExacTrac X-ray imaging, 6 degrees of freedom, image registration, intracranial, radiosurgery
Introduction
Image guidance is widely used in radiation therapy for patient setup corrections. In linear accelerator–based stereotactic radiotherapy (SRT) and stereotactic radiosurgery (SRS), usually cone-beam computed tomography (CBCT) or planar X-ray imaging, for example, ExacTrac X-ray imaging system (BrainLab, Feldkirchen, Germany), is used for image guidance.1–12 Compared to planar imaging, CBCT provides better visualization of anatomy and soft tissue. ExacTrac X-ray imaging system that uses 2 orthogonal X-rays, with 2 X-ray sources located on the floor and 2 detectors mounted on the ceiling, is free of couch collision. Compared to CBCT, it has the advantage of providing image guidance for noncoplanar treatments and allowing faster setup. ExacTrac X-ray imaging, however, is a 2-dimensional planar X-ray imaging and uses less information for image registration, in comparison with CBCT, which is a 3-dimensional volumetric imaging. It is of interest to compare image registrations of ExacTrac X-ray imaging and CBCT. Most of the publications of ExacTrac X-ray imaging and CBCT were focused on evaluating setup accuracy under image guidance.1–13 Ma et al had conducted a study on a hybrid system, Varian Novalis Tx treatment unit (Varian Medical Systems, California), to compare image registrations of ExacTrac X-ray and CBCT.14 Because 6 degree-of-freedom (6DOF) online CBCT registration was unavailable at the time of study, Ma et al were unable to perform online comparison of 6DOF image registrations of CBCT with ExacTrac X-ray. Instead, they performed online 3DOF image registration comparison and offline 6DOF image registration comparison by use of Eclipse treatment planning system (Varian Medical Systems, CA, USA).
A newer hybrid system, TrueBeam STx (Varian Medical Systems, CA, USA), which incorporates current CBCT and ExacTrac X-ray imaging techniques, has been used in clinics. It would be interesting to compare image registrations, especially, online 6DOF image registrations, of the 2 current imaging systems.
In our institution, brain multiple metastases are treated on a TrueBeam STx, with single isocenter treatment plans using dynamic arcs, which are generated on a recently emerged treatment planning system, automatic brain metastases planning (ABMP; BrainLab). In our practice, to ensure patient setup accuracy, both CBCT and ExacTrac X-ray imaging are used in the metastasis radiosurgery. It is important to know whether image registrations agree between ExacTrac and CBCT.
This study aimed to compare 6DOF online image registration of current BrainLab ExacTrac X-ray imaging and CBCT of TrueBeam STx linear accelerator for intracranial radiosurgery. Phantom study and patient study based on brain multiple metastasis radiosurgery were performed.
Materials and Methods
Figure 1 shows the TrueBeam STx linear accelerator system (version 2.5) used in the study, which was equipped with a BrainLab ExacTrac system (version 6.1.1). The coordinate system used in the study is indicated in the figure.
Figure 1.
Picture of the TrueBeam STx linear accelerator, which is equipped with CBCT and BrainLab X-ray imaging systems. Rando head phantom was immobilized with a BrainLab mask on the treatment couch. The coordinate system used in the study is shown. CBCT indicates cone-beam computed tomography.
Phantom Study
A Rando head phantom (The Phantom Laboratory, NY, USA) was used (Figure 1). The phantom was scanned with a GE LightSpeed CT scanner (General Electric Company, Fairfield, Connecticut), with a slice thickness of 1.25 mm. Treatment plans were generated on the CT images. The phantom study was designed to evaluate isocenter location dependence of the image registrations. To include various situations that isocenters are located at various locations, treatment plans were generated with an iPlan treatment planning system (BrainLab, version 4.5) instead of ABMP treatment planning system because iPlan allows a user to select isocenter locations, whereas ABMP does not. In an ABMP system, an isocenter is automatically determined by the system. In planning with the iPlan, tumors (or targets) were assumed to be located at various locations and each isocenter was selected at the geometric center of the individual tumor: isocenters were located in the regions of brain stem, left cerebellum, right cerebellum, left temporal lobe, right temporal lobe, left frontal lobe, right frontal lobe, thalamus, and left and right cerebellopontine angles where acoustic neuroma occurs. Table 1 lists the isocenter locations. The CT images of the phantom were transferred from iPlan to ExacTrac and TrueBeam CBCT, which were used as reference images in the image registrations.
Table 1.
Isocenter Locations in the Head Phantom.
| Isocenters | Isocenter Location |
|---|---|
| 1 | Brain stem |
| 2 | Left cerebellopontine angle |
| 3 | Right cerebellopontine angle |
| 4 | Left cerebellum |
| 5 | Right cerebellum |
| 6 | Left temporal lobe |
| 7 | Right temporal lobe |
| 8 | Left frontal lobe |
| 9 | Right frontal lobe |
| 10 | Thalamus |
In the treatment unit, the phantom was immobilized with a BrainLab mask (BrainLab) on the treatment couch. After the phantom was moved to isocenter with the ExacTrac 6DOF couch (BrainLab), 2 orthogonal ExacTrac X-ray images were taken. The phantom was then shifted using the 6DOF couch according to the image registration results. After shift, ExacTrac X-ray images and TrueBeam CBCT images were taken, and X-ray image registrations and CBCT image registrations were performed and the results were compared. The study was conducted for each of the 10 isocenters.
Patient Study
Thirty-four patients were studied. The patients were CT scanned with the GE LightSpeed CT scanner, with a slice thickness of 1.25 mm. Treatment plans for the multimetastasis patients were generated on an ABMP treatment planning system using single isocenter dynamic arcs. The isocenters were automatically determined by the treatment planning system, which were located at the geometric center of multiple tumors. The study procedure was the same as that of the phantom study: the patient was immobilized with a BrainLab mask on the treatment couch. After initial setup using the ExacTrac infrared photogrammetry guidance system, X-ray images were taken and the patient position was corrected with the X-ray imaging registrations. After correction, the patient was imaged with TrueBeam CBCT and ExacTrac X-ray imaging, respectively, and the image registrations of the 2 imaging modalities were compared.
In both phantom and patient studies, 6DOF online image registrations were performed and residual errors in the 3 translational directions (vertical, longitudinal, and lateral) and in the 3 rotational directions (rotation, pitch, and roll) were evaluated. In CBCT, the head protocol was used in the scan and bone window was used in the image registration. In ExacTrac imaging, 80 kV and 8 mAs were applied to the X-ray generator tubes and bony match was used in the image registration.
Results
Figure 2 shows the results of the phantom study: absolute differences in the calculated couch residual errors between ExacTrac X-ray imaging registration and TrueBeam CBCT imaging registration (difference = ExacTrac − CBCT) of the 10 isocenter studies in translational (vertical, longitudinal, and lateral) and rotational (rotation, roll, and pitch) directions, respectively. Table 2 lists the summary of the absolute differences. The average residual error differences in the vertical, longitudinal, and lateral directions were 0.17 ± 0.11 mm, 0.36 ± 0.20 mm, and 0.25 ± 0.11 mm, respectively. The average residual error differences in the rotation, roll, and pitch were 0.34° ± 0.08°, 0.13° ± 0.09°, and 0.12° ± 0.10°, respectively. It was noticeable that the longitudinal residual error differences at isocenters 8, 9, and 10 were larger than those at the other isocenters.
Figure 2.
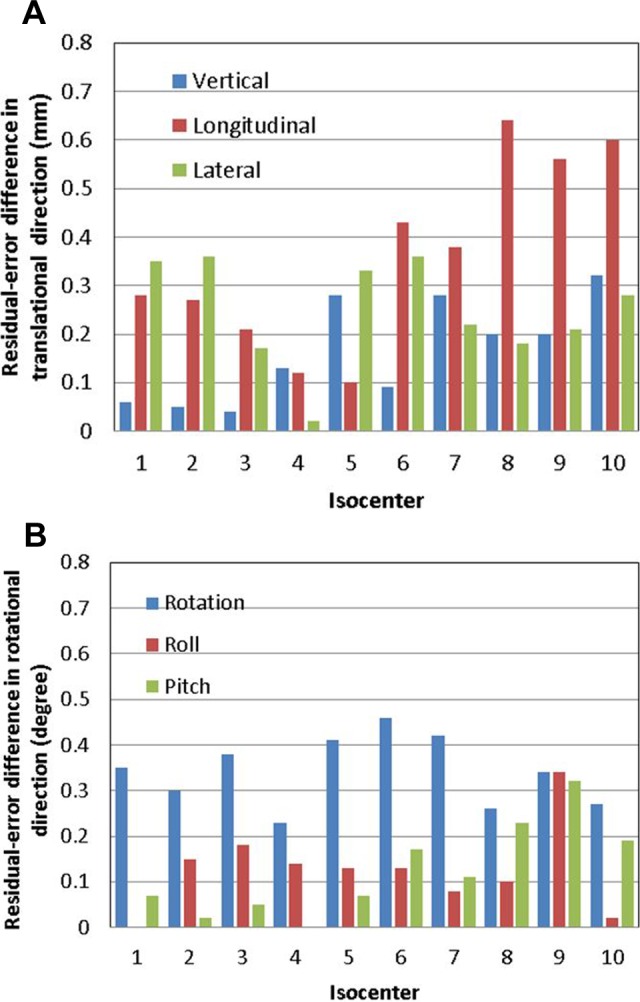
Results of the phantom study of the 10 isocenters: absolute differences in calculated residual errors between ExacTrac X-ray imaging registration and TrueBeam CBCT imaging registration (difference = ExacTrac − CBCT) in (A) translational (vertical, longitudinal, and lateral) and (B) rotational (rotation, roll, and pitch) directions, respectively. CBCT indicates cone-beam computed tomography.
Table 2.
Results of the Phantom Study.a
| Difference | Vertical (mm) | Longitudinal (mm) | Lateral (mm) | Rotation (°) | Roll (°) | Pitch (°) |
|---|---|---|---|---|---|---|
| Minimum | 0.04 | 0.10 | 0.02 | 0.23 | 0.00 | 0.00 |
| Maximum | 0.32 | 0.64 | 0.36 | 0.46 | 0.34 | 0.32 |
| Mean | 0.17 | 0.36 | 0.25 | 0.34 | 0.13 | 0.12 |
| Standard deviation | 0.11 | 0.20 | 0.11 | 0.08 | 0.09 | 0.10 |
Abbreviation: CBCT, cone-beam computed tomography.
aResidual error differences (absolute values) in translational and rotational directions, between ExacTrac X-ray imaging registration and TrueBeam CBCT registration among 10 isocenter studies.
Figure 3 shows the absolute differences in the calculated couch residual errors between ExacTrac X-ray imaging registration and TrueBeam CBCT imaging registration in the patient study. Table 3 lists the summary of the absolute differences. The average residual error differences in the vertical, longitudinal, and lateral directions were 0.20 ± 0.16 mm, 0.30 ± 0.18 mm, 0.21 ± 0.18 mm, respectively. The average residual error differences in the rotation, roll, and pitch were 0.40° ± 0.16°, 0.17° ± 0.13°, and 0.20° ± 0.14°, respectively.
Figure 3.
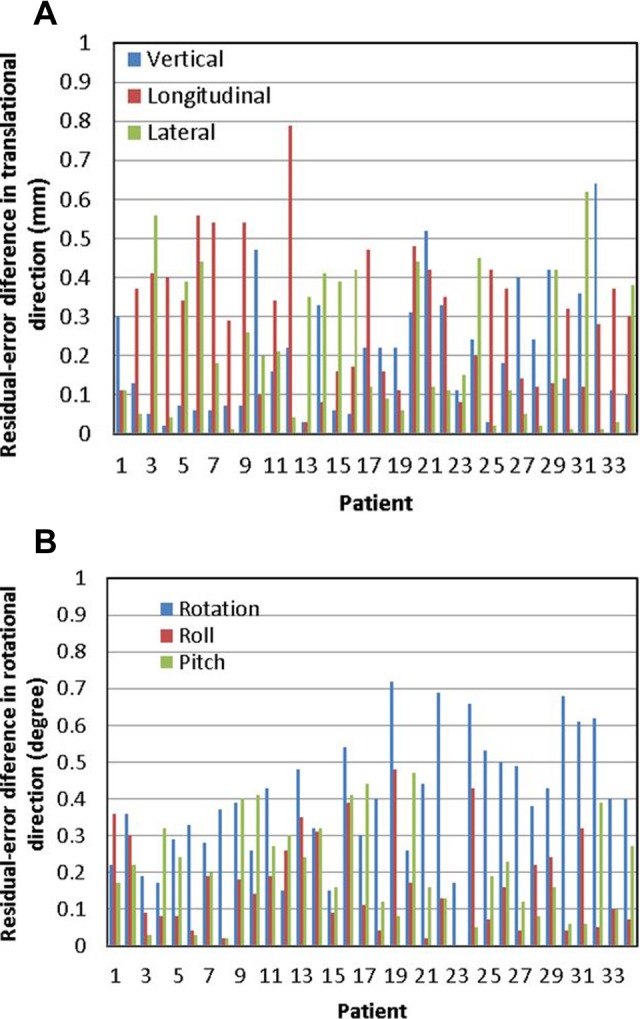
Results of the patient study: absolute differences in calculated residual errors between ExacTrac X-ray imaging registration and TrueBeam CBCT imaging registration (difference = ExacTrac − CBCT) in (A) translational (vertical, longitudinal, and lateral) and (B) rotational (rotation, roll, and pitch) directions, respectively. CBCT indicates cone-beam computed tomography.
Table 3.
Results of the Patient Study.a
| Difference | Vertical (mm) | Longitudinal (mm) | Lateral (mm) | Rotation (°) | Roll (°) | Pitch (°) |
|---|---|---|---|---|---|---|
| Minimum | 0.02 | 0.03 | 0.01 | 0.15 | 0 | 0 |
| Maximum | 0.64 | 0.79 | 0.62 | 0.72 | 0.48 | 0.47 |
| Mean | 0.20 | 0.30 | 0.21 | 0.40 | 0.17 | 0.20 |
| Standard deviation | 0.16 | 0.18 | 0.18 | 0.16 | 0.13 | 0.14 |
Abbreviation: CBCT, cone-beam computed tomography.
aResidual error differences (absolute values) in translational and rotational directions, between ExacTrac X-ray imaging registration and TrueBeam CBCT registration among 34 patient studies.
The average residual error differences in the phantom study had similar magnitudes as those in the patient study. The phantom and patient studies showed that among the results in the 3 translational directions, larger differences occurred in the longitudinal direction, and among the results in the 3 rotational directions, larger differences occurred in the rotation direction.
Discussion
The phantom study, which was designed to evaluate the image registrations for various isocenter locations, demonstrated that longitudinal residual error differences showed isocenter location dependence: the longitudinal residual error differences at isocenters 8, 9, and 10 were larger than the residual error differences at other isocenters. The isocenters 8, 9, and 10 were located in the regions of the left frontal lobe, right frontal lobe, and thalamus, respectively, that is, in the frontal lobe or close to the frontal lobe. In the patient study, the longitudinal residual errors in patients 6, 7, 9, and 12 were larger than those in other patients. The isocenters of these 4 patients were all located in the frontal lobes. The results showed that in general, if isocenters were located in or close to the frontal lobes, that is, located more superior in the head, the longitudinal residual error differences could be larger. Residual error difference up to 0.79 mm was observed in the longitudinal direction in patient 12. It was noticed that when isocenters were located superficially, less patient anatomy information was captured in the images. The reduced anatomy information might result in larger uncertainties in image registrations and as a consequence larger differences between the 2 image registrations in those cases. Based on this assumption, we used “virtual isocenter” functionality in ExacTrac for superiorly located isocenters in patients 13–34, and the longitudinal difference was reduced, as can be seen from Figure 3A. This function allows the user to select a “setup” isocenter other than the treatment isocenter at the correction X-ray imaging step, so that more bony structures can be included in the X-ray imaging receptors’ field of view and more accurate registration can be obtained. The difference between setup isocenter and treatment isocenter locations is applied in addition to the calculated shifts when couch correction is made so that patient is positioned at final treatment isocenter.
In general, the residual error differences in the longitudinal direction were larger than those in the lateral and vertical directions, which were observed in both the phantom and patient studies. The phenomenon could be related to CT slice thickness. The reference CT images in the study had a slice thickness of 1.25 mm and a pixel size of 0.9 mm. That is, the CT image resolution was 1.25 mm in the longitudinal direction and 0.9 mm in the vertical and lateral directions. The image registration thus had larger uncertainty in the longitudinal direction compared to the vertical and lateral directions.
Isocenter location dependence was not observed in rotational residual error differences. Further investigation on the cause of residual error differences between ExacTrac X-ray and CBCT is expected in the future study.
In Ma et al’s study on a Novalis Tx system,14 average residual error differences were found to be <0.5 mm for phantom and <1.5 mm for patients, which are larger than our results. In our study, similar differences were observed in phantom and patients, and the average differences in phantom and patients were <0.4 mm. Compared to Ma et al’s study, the smaller differences observed in our study could be attributed to the improvement in CBCT and ExacTrac X-ray techniques (a new generation of X-ray imaging receptor was used in ExacTrac version 6 and above, and the X-ray image quality has been improved visually), and the fact that the differences were similar in our phantom and patient studies might imply improvement in immobilization.
Conclusion
The phantom and patient studies showed that average residual error differences between ExacTrac X-ray and TrueBeam CBCT registrations were <0.4 mm in the translational directions and <0.5° in the rotational directions. Compared to the previous publication that was based on earlier versions of the imaging systems, better agreement between ExacTrac X-ray and CBCT image registrations was found in our study. The result indicates that image registrations of current ExacTrac X-ray and TrueBeam CBCT are comparable in intracranial treatments. The study provides confidence for using ExacTrac X-ray for image guidance of brain multiple metastasis radiosurgery.
Abbreviations
- 6DOF
6 degrees of freedom
- ABMP
automatic brain metastases planning
- CBCT
cone-beam computed tomography
- SRS
stereotactic radiosurgery
- SRT
stereotactic radiotherapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Infusino E, Trodella L, Ramella S, et al. Estimation of patient setup uncertainty using BrainLab Exatrac X-Ray 6D system in image-guided radiotherapy. J Appl Clin Med Phys. 2015;16(2):5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang Y, Zhao B, Chetty IJ, Brown S, Gordon J, Wen N. Targeting accuracy of image-guided radiosurgery for intracranial lesions: a comparison across multiple linear accelerator platforms. Technol Cancer Res Treat. 2016;15(2):243–248. doi:10.1177/1533034615574385. [DOI] [PubMed] [Google Scholar]
- 3. Dhabaan A, Schreibmann E, Siddiqi A, et al. Six degrees of freedom CBCT-based positioning for intracranial targets treated with frameless stereotactic radiosurgery. J Appl Clin Med Phys. 2012;13(6):3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ali I, Tubbs J, Hibbitts K, et al. Evaluation of the setup accuracy of a stereotactic radiotherapy head immobilization mask system using kV on-board imaging. J Appl Clin Med Phys. 2010;11(3):3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramakrishna N, Rosca F, Friesen S, Tezcanli E, Zygmanszki P, Hacker F. A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Radiother Oncol. 2010;95(1):109–115. doi:10.1016/j.radonc.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 6. Chang HH, Lee HF, Sung CC, Liao TI, Huang YJ. A phantom study of the immobilization and the indications for using virtual isocenter in stereoscopic X-ray image guidance system referring to position localizer in frameless radiosurgery. J Appl Clin Med Phys. 2013;14(4):4133 doi:10.1120/jacmp.v14i4.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gevaert T, Verellen D, Tournel K, et al. Setup accuracy of the Novalis ExacTrac 6DOF system for frameless radiosurgery. Int J Radiat Oncol Biol Phys. 2012;82(5):1627–1635. [DOI] [PubMed] [Google Scholar]
- 8. Ramakrishna N, Rosca F, Friesen S, Tezcanli E, Zyqmanszki P, Hacker F. A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Radiother Oncol. 2010;95(1):109–115. [DOI] [PubMed] [Google Scholar]
- 9. Lamba M, Breneman J, Warnick R. Evaluation of image guided positioning for frameless intracranial radiosurgery. Int J Radiat Oncol Biol Phys. 2009;74(3):913–919. [DOI] [PubMed] [Google Scholar]
- 10. Jin JY, Ryu S, Faber K, et al. 2D/3D image fusion for accurate target localization and evaluation of a mask based stereotactic system in fractionated stereotactic radiotherapy of cranial lesions. Med Phys. 2006;33(12):4557–4566. [DOI] [PubMed] [Google Scholar]
- 11. Ackerly T, Lancaster CM, Geso M, Roxby KJ. Clinical accuracy of ExacTrac intracranial frameless stereotactic system. Med Phys. 2011;38(9):5040–5048. [DOI] [PubMed] [Google Scholar]
- 12. Gevaert T, Verellen D, Engels B, et al. Clinical evaluation of a robotic 6-degree of freedom treatment couch for frameless radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(1):467–474. [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Jin JY, Walls N, et al. Image-guided localization accuracy of stereoscopic planar and volumetric imaging methods for stereotactic radiation surgery and stereotactic body radiation therapy: a phantom study. Int J Radiat Oncol Biol Phys. 2011;79(5):1588–1596. [DOI] [PubMed] [Google Scholar]
- 14. Ma J, Chang Z, Wang Z, Jackie Wu Q, Kirkpatrick JP, Yin FF. ExacTrac X-ray 6 degree-of-freedom image-guidance for intracranial non-invasive stereotactic radiotherapy: comparison with kilo-voltage cone-beam CT. Radiother Oncol. 2009;93(3):602–608. [DOI] [PubMed] [Google Scholar]


