Abstract
Purpose/Objective(s):
To establish a dose–volume response relationship for brain metastases treated with single-fraction robotic stereotactic radiosurgery and identify predictors of local control.
Materials/Methods:
We reviewed a prospective institutional database of all patients treated for intact brain metastases with stereotactic radiosurgery alone using the CyberKnife robotic radiosurgery system from 2012 to 2015. Tumor response was determined based on Response Evaluation Criteria In Solid Tumors version 1.1. Survival was estimated using the Kaplan-Meier method. Logistic regression modeling was used to identify predictors of outcome and establish a dose–volume response relationship. Receiver operating characteristic curves were constructed to evaluate the predictive capability of the relationship.
Results:
There were 357 metastases evaluated in 111 patients with a median diameter of 8.14 mm (2.00-40.77 mm). At 6 and 12 months, local control was 86.9% and 82.2%, respectively. For lesions of similar volumes, higher maximum dose, mean dose, and minimum dose (all P values <.05) predicted for better local control. Tumor volume and diameter were strongly correlated, and a dose–volume response relationship was constructed using mean dose per lesion diameter (Gy/mm) that was predictive of local control (odds ratio: 1.34, 95% confidence interval: 1.06-1.70). Area under the receiver operating characteristic curve for local control and mean dose by volume was 0.6199 with a threshold of 2.05 Gy/mm (local failure 7.6% above and 17.3% below 2.05 Gy/mm).
Conclusion:
A dose–volume response relationship exists for brain metastases treated with robotic stereotactic radiosurgery. Mean dose per volume is strongly predictive of local control and can be potentially useful during stereotactic radiosurgery plan evaluation while respecting previously established dose constraints.
Keywords: SRS, radiosurgery, brain metastases, dose–volume response, robotic
Introduction
Metastatic spread of disease to the brain is common in a range of primary cancers, occurring in an estimated 20% to 40% of all patients.1 Brain metastases are prevalent in patients with breast cancer, melanoma, and especially non–small cell lung cancer (NSCLC). An estimated 30% to 50% of all patients with NSCLC will develop metastatic spread to the brain.2
The identification of targetable receptor mutations in many cancers has led to a proliferation in the development of new systemic therapies, with the promise of achieving meaningful disease control for at least select subsets of patients.3,4 With the advent of these novel agents, local control (LC) of intracranial disease will likely continue to be critical to optimizing patient quality of life and survival. Although several large series have demonstrated stereotactic radiosurgery (SRS) is an effective modality for the treatment of patients with limited brain metastases,5–8 prognosis for these patients remains suboptimal.9,10
Brain metastases are a marker of poor survival; however, brain metastases occurring in eloquent locations or near critical structures can additionally compromise language and sensorimotor functions. Identifying parametric factors that modulate LC for brain metastases while balancing toxicity is critical to prolonging survival in these patients and protecting neurocognitive quality of life. Radiation Therapy Oncology Group (RTOG) 90-05 sought to establish maximum tolerated radiosurgery dose for brain metastases treated stratified by tumor diameter.11 These parameters were widely adopted by subsequent investigations including RTOG 95-08.12,13 It is important to note that these investigations were not designed to determine the optimal dosing to achieve lesion control but targeted defining the maximal safe dose with respect to rates of neurotoxicity. Stereotactic radiosurgery dose selection must balance effective lesion control with acceptable rates of central nervous system (CNS) toxicity. Although RTOG 90-05 and 95-08 demonstrated that proper dose selection can achieve excellent CNS toxicity profiles, there remains debate regarding optimal SRS dose selection balancing the risk of CNS toxicity with effective tumor control.14
In this investigation, we examined patients with brain metastases from a single institution treated with single-fraction robotic radiosurgery using the CyberKnife (CK) linear accelerator (Accuray, Sunnyvale, California). We sought to investigate trends in patients treated with empiric SRS dose selection in order to identify a dose–volume relationship for lesion control. Better understanding of this relationship would be of potential use in plan evaluation by estimating lesion control for selected dose parameters, which should still respect previously established dose constraints.
Methods
For this institutional review board–approved study, we examined the records of all patients treated with single-fraction SRS using the CK linear accelerator (Accuray) at a single institution from August 2012 to December 2015. Of 179 patients receiving single-fraction intracranial SRS, 68 patients were excluded because they received treatment for either vascular lesions or primary CNS tumors. Three hundred fifty-seven individual metastatic lesions were treated in the remaining 111 patients.
Radiosurgery Technique
All SRS procedures were performed using the CK linear accelerator (Accuray). Before treatment planning, patients were simulated supine with a thermoplastic mask for immobilization. Treatment planning computed tomography (CT) was fused with treatment planning magnetic resonance (MR) with 1-mm axial slices. No margin was added to the gross tumor, and planning target volume was defined as the contrast-enhancing tumor. Segmentation of the planning target volume was performed by both a radiation oncologist and a neurosurgeon.
Plans were developed using the CK Multiplan software (version 4.5, Accuray) using either isocentric or nonisocentric sequential optimization on a lesion-by-lesion basis. The sequential optimization was performed based on the maximum dose constraints and the dose objectives setup for the lesions and the critical structures. Dose was prescribed to the margin of the planning target volume by the attending radiation oncologist based primarily on lesion size. Marginal prescribed dose never exceeded safe levels established by RTOG 90-05.11 Percentage isodose prescription line was also chosen at the discretion of the attending radiation oncologist at the time of plan evaluation. An example SRS plan is shown in Figure 1.
Figure 1.
Example CyberKnife radiosurgery plan treating a small 11 mm metastasis in the left thalamus. A, Dose distribution. B, Lesion regressing to 7 mm at 3 months of follow-up. C, Continued resolution of the lesion 2 years following radiosurgery.
Intrafraction motion management was accomplished by 6-dimensional skull tracking using 2 orthogonal diagnostic kV X-ray sources mounted on the ceiling of the treatment room at a 45° angle to the perpendicular axis (Accuray). Real-time images were generated and then compared against the digitally reconstructed radiographs generated from the treatment planning CT. The CK robot adjusted the treatment position based on the comparison of both prior to and during the actual treatment session. For lesions located in the posterior fossa, oral ondansetron was given immediately after SRS. Prophylactic steroids or antiepileptics were not routinely used. Patients were surveyed using MR of the brain every 3 months for the first year following SRS as long as there was no clinical suspicion of recurrence. Following the first year, MR imaging was performed every 4 to 6 months.
Statistical Analysis
Age, sex, performance status, presence of extracranial disease, primary tumor site, histology, and other patient characteristics were collected. Maximum lesion diameter, volume, history of other radiotherapy, dose, and other tumor and treatment-related factors were collected. Statistical analysis was performed using R (R-Project 3.2.5).
The primary outcome of interest was local failure (LF). A LF was defined using a modified Response Evaluation Criteria In Solid Tumors (RECIST version 1.1).15 Lesions that met the criteria for complete response, partial response, or stable disease were considered locally controlled. Lesions that met the criteria for progressive disease based on imaging or received additional SRS or surgical resection with pathologic evidence of active disease were considered LFs. For small lesions less than 10 mm, the additional restriction was made to require growth on at least 2 consecutive imaging studies (while meeting the other criteria).
Secondary outcomes of interest were overall survival (OS), toxicity, development of radionecrosis, and development of radionecrosis requiring craniotomy. Overall survival was defined as the time from the first SRS procedure until death from any cause. Radionecrosis was defined using a combination of imaging and clinical data and determined by a multidisciplinary neuro-oncology clinic including radiation oncologists, surgeons, neuro-oncologists, and neuroradiologists. Imaging techniques including MR spectroscopy, MR perfusion, and diffusion-weighted sequences were used to aid in differentiating radiation necrosis from possible disease progression. Toxicity was coded according to the Common Terminology Criteria for Adverse Events version 4.03.
Descriptive statistics were used to summarize the patient cohort. Kaplan-Meier methods were used to estimate OS and time to LF. Logistic regression modeling was used to identify predictive factors for LC. All analyses were 2 sided, and a significance level was set as α = .05.
Dose–volume response curves were constructed using the logarithm of mean planning target volume dose and volume (Gy/mm3) plotted against predicted LF using a logistic regression model; a response curve for dose by diameter (Gy/mm) was also considered. Testing for differences in outcomes including the effect of mean dose per volume between radioresistant histologies (melanoma and renal cell carcinoma) and radiosensitive histologies was performed. Receiver operating characteristic curves were constructed to better categorize the relationship between dose per volume and LF.
Results
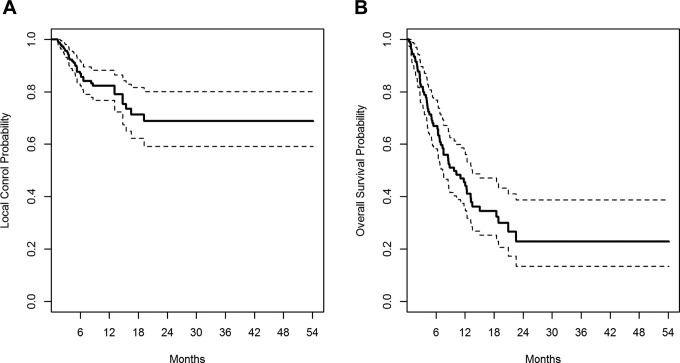
Three hundred fifty-seven metastases were evaluated in 111 patients. Characteristics of patients and tumors are shown in Tables 1 and 2, respectively. At the time of analysis, 61 patients had died. Actuarial survival at 6 months and 1 year were 66.9% (95% confidence interval [CI]: 58%-77%) and 45.4% (95% CI: 36%-57%), respectively (Figure 2). Patients receiving SRS were a well-selected cohort with good performance status (median 80% Karnofsky) and with a median disease-specific GPA of 2.5. Median survival for the cohort was 9.7 months. Most patients treated with radiosurgery had NSCLC (44.1%), melanoma (21.6%), or breast cancer (16.2%). Overall, 26.1% of treated patients had radioresistant tumors.
Table 1.
Patient Characteristics.a,b
| Characteristic | Number (%) |
|---|---|
| Age | |
| <40 years | 5 (4.5) |
| 40-50 years | 12 (10.8) |
| 51-60 years | 39 (35.2) |
| 61-70 years | 33 (29.7) |
| >71 years | 22 (19.8) |
| Karnofsky performance status | |
| 90-100% | 45 (44.6) |
| 70-80% | 51 (45.9) |
| <70% | 5 (4.5) |
| GPA | |
| 0 | 0 (0.0) |
| 0.5 | 2 (1.8) |
| 1.0 | 14 (12.6) |
| 1.5 | 11 (9.9) |
| 2.0 | 21 (18.9) |
| 2.5 | 17 (15.3) |
| 3.0 | 19 (17.1) |
| 3.5 | 4 (3.6) |
| 4.0 | 6 (5.4) |
| Unknown | 16 (14.4) |
| RPA | |
| I | 22 (19.8) |
| II | 74 (66.7) |
| III | 3 (2.7) |
| Unknown | 12 (10.8) |
| Primary histology | |
| Non–small cell lung | 49 (44.2) |
| Melanoma | 24 (39.6) |
| Breast | 18 (16.2) |
| Small-cell lung | 3 (2.7) |
| Other | 17 (15.3) |
| Extracranial disease | |
| Controlled | 46 (41.4) |
| Uncontrolled | 65 (58.6) |
Abbreviations: GPA, graded prognostic assessment; RPA, recursive partition analysis.
aN = 111.
bKarnofsky performance status (KPS) does not equal 100% and 10 patients did not have known KPS.
Table 2.
Radiosurgery Characteristics.a,b
| Characteristic | Number (%) |
|---|---|
| Lesion diameter | |
| <5 mm | 81 (22.7) |
| 5-10 mm | 156 (43.7) |
| 11-20 mm | 87 (2.4) |
| 21-30 mm | 24 (6.7) |
| >30 mm | 9 (2.5) |
| Lesion volume | |
| <20 mm3 | 18 (5.0) |
| 20-100 mm3 | 99 (27.8) |
| 101-500 mm3 | 120 (33.6) |
| 501-1000 mm3 | 37 (10.4) |
| 1001-5000 mm3 | 63 (17.6) |
| >5000 mm3 | 18 (5.0) |
| Brain location | |
| Frontal lobe | 99 (27.7) |
| Temporal lobe | 45 (12.6) |
| Parietal lobe | 93 (26.1) |
| Occipital lobe | 35 (9.8) |
| Cerebellum | 54 (15.1) |
| Midbrain | 1 (0.3) |
| Brain stem | 3 (0.8) |
| Central brain structures | 7 (2.0) |
| Other | 19 (5.3) |
| Peripheral dose | |
| <16 Gy | 7 (2.0) |
| 16-20 Gy | 343 (96.0) |
| >20 Gy | 7 (2.0) |
| Mean dose | |
| <16 Gy | 6 (1.7) |
| 16-20 Gy | 133 (37.3) |
| >20 Gy | 216 (60.5) |
| Prescription isodose | |
| <70% | 38 (10.6) |
| 71%-80% | 218 (61.1) |
| 81%-90% | 98 (27.5) |
| >90% | 3 (0.8) |
| Maximum dose per size | |
| <2.5 Gy/mm | 148 (41.4) |
| 2.5-5 Gy/mm | 147 (41.2) |
| 5-7.5 Gy/mm | 51 (14.3) |
| 7.5-10 Gy/mm | 10 (2.8) |
| >10 Gy/mm | 1 (0.3) |
aN = 357
bLesion volume and mean dose do not equal 100% and 2 patients were missing respective data.
Figure 2.
Kaplan-Meier plot of local failure (A) and overall survival (B) for all 111 patients.
A median of 2 brain metastases were treated per patient (range: 1-13) over 1 to 4 SRS sessions. Treated brain metastases ranged from 2 to 40 mm in diameter (median: 8 mm) and measured 3.15 to 28 079.38 mm3 (median: 210.64 mm3) in volume. Metastases were treated with a median peripheral dose of 18.0 Gy (range: 12.0-24.0 Gy), with a median prescription isodose of 79% (range: 55%-95%). This translated to a median maximum dose of 22.6 Gy (range: 12.4-32.8 Gy) and a median mean dose of 20.4 Gy (range: 10.9-27.6 Gy). The majority of lesions were treated with definitive SRS alone; however, 8 tumors (2.2%) were treated as a boost to whole brain radiotherapy (30 Gy given over 10 fractions).
Over the study period, 41 (11.5%) lesions met the criteria for LF. Actuarial LF at 6 months and 1 year was 13.1% and 17.8%, respectively (Figure 2). Tumor volume (P = .0157), histology (P = .0437), and the number of metastases (P = 0.0019) were predictive of LF using a univariate logistic regression model. Local failure was more common with greater tumor volume for individual lesions and radioresistant histology (odds ratio [OR]: 2.429, 95% CI: 1.26-4.70) and less likely for patients with a greater number of metastases (OR: 0.815, 95% CI: 0.72-0.93). No significant correlation was seen for prescription isodose (P = .1870) or planning target volume coverage (P = .1677). For lesions of similar volumes, higher maximum dose (P = .0167), mean dose (P = .0154), and minimum dose (P = .0188) each predicted for lower risk of LF.
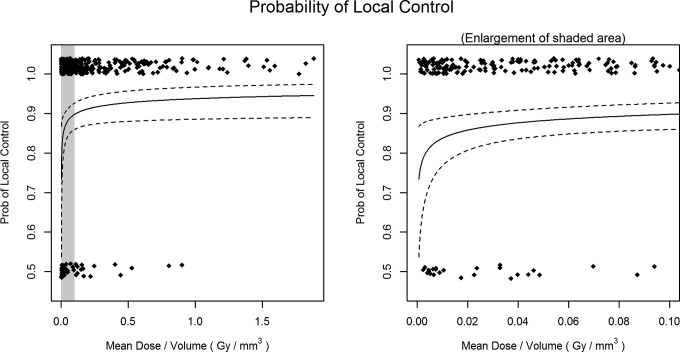
Mean dose per volume was calculated for all lesions because it incorporates margin dose, prescription isodose, and lesion size into a single variable. A dose–volume response relationship was established, which was predictive of LC (P = .0100). Since tumor volume and diameter were strongly correlated in our population (Spearman ρ = 0.97), a dose–volume response relationship was constructed substituting lesion size because of its greater clinical availability. This new dose–size parameter (Gy/mm) was predictive of LC (OR: 1.340, 95% CI: 1.06-1.70; Table 3). Figure 3 shows dose–volume response for all treated brain metastases. No difference in this dose–volume response was seen between radioresistant and radiosensitive histologies testing for difference in OR between the histology groupings (P = .5690). No difference in the mean dose per volume treated was found between the groupings (P = 0.4757). A receiver operating characteristic curve analysis was performed to better characterize this relationship (Figure 4). Area under the curve for LC and mean dose per size (Gy/mm) was 0.62 and found to be somewhat predictive. A threshold value of 2.05 Gy/mm was established. Local failure was 7.6% above this threshold and 17.3% below.
Table 3.
Predicted Probability of Local Control for a Given Level of Mean Dose per Lesion Diameter.
| Dose (Gy/mm) | Probability of LC (95%CI) |
|---|---|
| 0.5 | 81.0% (71.1-88.0) |
| 1.0 | 83.1% (75.7-88.6) |
| 2.0 | 86.8% (82.4-90.3) |
| 3.0 | 89.9% (86.0-92.8) |
| 4.0 | 92.2% (87.6-95.2) |
| 5.0 | 94.1% (88.6-97.0) |
| 6.0 | 95.5% (89.4-98.2) |
| 7.0 | 96.6% (90.0-98.9) |
| 8.0 | 97.5% (90.6-99.4) |
Abbreviations: CI, confidence interval; LC, local control.
Figure 3.
Dose–volume local control relationship for all treated metastases shown across the entire range (left panel) and a magnified view of the steep part of the curve (right panel).
Figure 4.
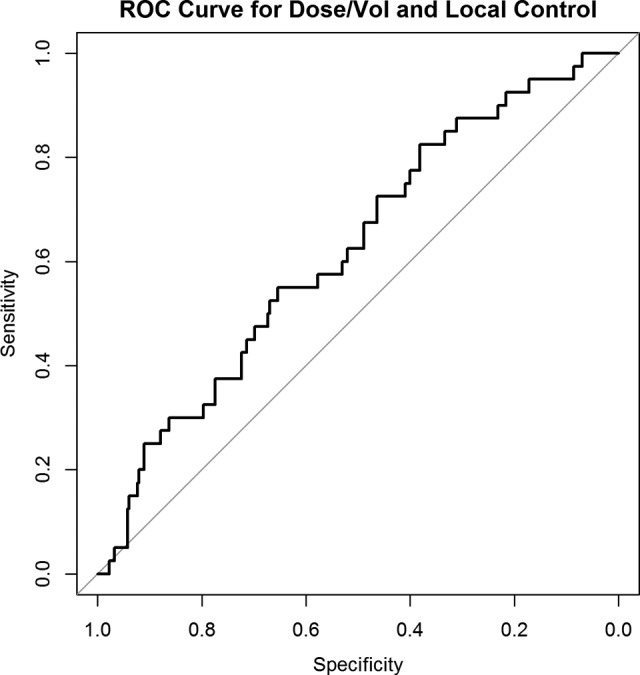
Receiver operating characteristic curve characterizing the relationship between mean dose per lesion size (Gy/mm) and local control.
Treatment was well tolerated overall, with 4.2% (15) of lesions developing radionecrosis. Of these lesions, only 3 required craniotomy (0.8% of the overall cohort). Rates of post-SRS seizure were low, with 14 lesions (3.9%) treated resulted in at least 1 post-SRS seizure. Lesions that resulted in post-SRS toxicity were larger on average than our overall lesion sample (median diameter of 11.1 mm and volume of 430.38 mm3 vs median diameter of 8 mm and volume of 210.64 mm3).
Discussion
Stereotactic radiosurgery has been demonstrated to be an effective method for achieving intracranial tumor control in patients with limited brain metastases.5,6,16,17 Therefore, identifying optimal dose parameters that balance lesion control with acceptable toxicity profiles is vital to providing the highest standard of care. Previous studies such as RTOG 90-05 and 95-08 demonstrated appropriately selected SRS dosing can result in excellent rates of CNS toxicity but were not designed with the intention of identifying optimal dose selection for lesion control.11–13 In this study, we identified a dose–volume response relationship that predicted for LC of brain metastases treated with single-fraction radiosurgery.
In our population, tumor volume and maximal axial diameter were highly correlated. After establishing a dose–volume response, tumor diameter was substituted for volume in constructing our dose–size relationship due to greater clinical applicability. This relationship also predicted LC for lesions. We have previously demonstrated a dose–size response relationship in small brain metastases treated with a linear accelerator,18 but analysis was limited by the lack of tumor volume data and complex dosimetric data. The current study builds on this previous work by using robust statistical analyses to identify factors predictive of LC and to characterize the relationship between dose per volume and LF.
There is a great clinical utility for a parameter that can aid in predicting LC of brain metastases. We sought to integrate dose–volume response into a clinically useful parameter. Mean dose per lesion size (Gy/mm) incorporated margin dose, prescription dose, and lesion size into an inclusive parameter and was found to be predictive of lesion control in our study. Our results indicate that this parameter may be more helpful in predicting treatment outcome than size, which has been shown to be a strong predictor of outcome in other studies,19–21 as our data showed that for lesions of the same size, dose influences outcome.
We sought to further characterize our new parameter by identifying the value at which LC began to rapidly decline. A threshold value of 2.05 Gy/mm was established, with lesions treated below this marker having a failure rate of 17.3% and lesions treated above having a failure rate of 7.6%. An increase of 1 Gy in mean dose per mm was correlated with a 34% increase in the odds of LC for our population. This model is designed to potentially assist in plan evaluation by providing estimates of LC for selected dose parameters, which should respect previously established dose constraints. It is important to note, however, that the dose–volume response reaches a plateau that indicates only marginal benefit above higher doses per volume.
Observed toxicity was low in our study, consistent with previous reports.6,16,17,19,20 Prior investigations identified both lesion size and volume of brain receiving a dose of 12 Gy or greater as predictive for developing radionecrosis.22,23 Though lesions treated in our population resulting in late toxicities tended to be greater in diameter and volume than our overall lesion sample, we maintained acceptable toxicity by strictly adhering to constraints defined by RTOG 90-05.11 Differentiating between treatment effect and disease progression can be challenging. Substantial heterogeneity exists in the definition of LF utilized by previous investigations. In this study, we used robust methods including both a modified RECIST v1.1 criteria and new imaging techniques to define failure.
Radiosurgery procedures were delivered using the CK linear accelerator (Accuray). Studies examining outcomes in this technology are a minority in comparison with those investigating SRS delivered by non-CK linear accelerators and Gamma Knife (GK; Elekta, Stockholm, Sweden).24–27 Tamari et al analyzed a sample of 109 lesions treated with CK and demonstrated both lesion control at 1 and 2 years (83.3% and 78.5%, respectively) and rates of radionecrosis (7%) comparable to studies using GK and non-CK linear accelerator–based SRS.27 Tumor volume (P = .020) and prescription dose (P = .023) were associated with LC. Our results demonstrated for lesions of similar volumes, maximum, median, and minimum dose delivered were predictive of lesion control. This supports the findings of prior investigations, which found both marginal dose28 and maximal dose delivered to the tumor isocenter29 were significantly associated with LC. Although these studies examined the relationship between dose and LC, a parameter incorporating both dose and size was not explored.
Limitations of our study include biases inherent to retrospective analyses. Tumors larger than 30 to 40 mm in diameter are typically treated with fractionated stereotactic radiation therapy at our institution, and our findings may not be applicable to lesions greater than this size. Identification of factors contributing to lesion control for brain metastases is difficult, given heterogeneous treatment techniques and dose selection used to treat lesions with SRS. The influence of newer immunotherapy on LC and rates of toxicity for brain metastases treated with SRS has not been fully described and may have influenced outcomes and toxicities for patients receiving such therapy.30 Prospective dose escalation trials are needed to verify the clinical applicability of median dose per lesion size as a parameter predictive of LC in patients with metastatic brain lesions treated with single-fraction SRS.
Conclusion
Our study examined a large population of brain metastases treated with robotic radiosurgery and identified tumor volume and dose parameters including mean dose, minimum dose, and maximum dose as predictive factors for LC among tumors of similar volume. A dose–response relationship exists and is predictive of LC. Furthermore, we demonstrated using mean dose per lesion size (Gy/mm) may be predictive of LC in patients with small brain lesions treated with single-fraction robotic SRS.
Abbreviations
- CI
confidence interval
- CK
CyberKnife
- GK
Gamma Knife
- LC
local control
- LF
local failure
- NSCLC
non–small cell lung cancer
- OR
odds ratio
- OS
overall survival
- RECIST
Response Evaluation Criteria In Solid Tumors
- SRS
stereotactic radiosurgery
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533–540. [DOI] [PubMed] [Google Scholar]
- 2. Sorensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6(9):1474–1480. [DOI] [PubMed] [Google Scholar]
- 3. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet.2016;387(10030):1837–1846. [DOI] [PubMed] [Google Scholar]
- 5. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 6. Lutterbach J, Cyron D, Henne K, Ostertag CB. Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery. 2003;52(5):1066–1073; discussion 1073-1074. [PubMed] [Google Scholar]
- 7. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 9. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. [DOI] [PubMed] [Google Scholar]
- 10. Jacot W, Quantin X, Boher JM, et al. Brain metastases at the time of presentation of non-small cell lung cancer: a multi-centric AERIO analysis of prognostic factors. Br J Cancer. 2001;84(7):903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05.Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. [DOI] [PubMed] [Google Scholar]
- 12. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. [DOI] [PubMed] [Google Scholar]
- 13. Sperduto PW, Shanley R, Luo X, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys. 2014;90(3):526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Azevedo Santos TR, Tundisi CF, Ramos H, et al. Local control after radiosurgery for brain metastases: predictive factors and implications for clinical decision. Radiat Oncol.2015;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 16. Chang EL, Selek U, Hassenbusch SJ, III, et al. Outcome variation among “radioresistant” brain metastases treated with stereotactic radiosurgery. Neurosurgery. 2005;56(5):936–945; discussion 936-945. [PubMed] [Google Scholar]
- 17. Molenaar R, Wiggenraad R, Verbeek-de Kanter A, Walchenbach R, Vecht C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg. 2009;23(2):170–178. [DOI] [PubMed] [Google Scholar]
- 18. Amsbaugh M, Pan J, Yusuf MB, et al. Dose-volume response relationship for brain metastases treated with frameless single-fraction linear accelerator-based stereotactic radiosurgery. Cureus. 2016;8(4):e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang EL, Hassenbusch SJ, III, Shiu AS, et al. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery. 2003;53(2):272–280; discussion 280-281. [DOI] [PubMed] [Google Scholar]
- 20. Chao ST, Barnett GH, Vogelbaum MA, et al. Salvage stereotactic radiosurgery effectively treats recurrences from whole-brain radiation therapy. Cancer. 2008;113(8):2198–2204. [DOI] [PubMed] [Google Scholar]
- 21. Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg. 2002;97(6):1276–1281. [DOI] [PubMed] [Google Scholar]
- 22. Korytko T, Radivoyevitch T, Colussi V, et al. 12Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64(2):419–424. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura JL, Verhey LJ, Smith V, et al. Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys. 2001;51(5):1313–1319. [DOI] [PubMed] [Google Scholar]
- 24. Liu SH, Murovic J, Wallach J, et al. CyberKnife radiosurgery for brainstem metastases: management and outcomes and a review of the literature. J Clin Neurosci. 2016;25:105–110. [DOI] [PubMed] [Google Scholar]
- 25. Vlachopoulou V, Antypas C, Delis H, et al. Peripheral doses in patients undergoing Cyberknife treatment for intracranial lesions. A single centre experience. Radiat Oncol. 2011;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Floriano A, Santa-Olalla I, Sanchez-Reyes A. Experience with the CyberKnife for intracranial stereotactic radiosurgery: analysis of dosimetry indices. Med Dosim. 2014;39(1):1–6. [DOI] [PubMed] [Google Scholar]
- 27. Tamari K, Suzuki O, Hashimoto N, et al. Treatment outcomes using CyberKnife for brain metastases from lung cancer. J Radiat Res. 2015;56(1):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vogelbaum MA, Angelov L, Lee SY, Li L, Barnett GH, Suh JH. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006;104(6):907–912. [DOI] [PubMed] [Google Scholar]
- 29. Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer.2003;41(3):333–343. [DOI] [PubMed] [Google Scholar]
- 30. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol.2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]