Abstract
Gelsolin is an actin-binding protein and acts as an important regulator of cell survival. This study aimed to determine the function of gelsolin in the radioresistance of non–small cell lung cancer cells. We examined the expression of gelsolin in radioresistant A549 and H460 cells and their parental cells. The effects of gelsolin overexpression and knockdown on the clonogenic survival and apoptosis of non–small cell lung cancer cells after irradiation were studied. The involvement of phosphoinositide 3-kinase/Akt signaling in the action of gelsolin was checked. We found that gelsolin was significantly upregulated in radioresistant A549 and H460 cells. Overexpression of gelsolin significantly (P < .05) increased the number of colonies from irradiated A549 and H460 cells compared to transfection of empty vector. In contrast, knockdown of gelsolin significantly (P < .05) suppressed colony formation after irradiation. Gelsolin-overexpressing cells displayed reduced apoptosis in response to irradiation, which was coupled with decreased levels of cleaved caspase-3 and poly adenosine diphosphate-ribose polymerase. Ectopic expression of gelsolin significantly (P < .05) enhanced the phosphorylation of Akt compared to nontransfected cells. Pretreatment with the phosphoinositide 3-kinase inhibitor LY294002 (20 μmol/L) significantly decreased clonogenic survival and enhanced apoptosis in gelsolin-overexpressing A549 and H460 cells after irradiation. Taken together, gelsolin upregulation promotes radioresistance in non–small cell lung cancer cells, at least partially, through activation of phosphoinositide 3-kinase/Akt signaling.
Keywords: gelsolin, lung cancer, radiotherapy, resistance, signaling pathway
Introduction
Non–small cell lung cancer (NSCLC) is the most common type of lung cancer and remains the leading cause of cancer-related death globally.1 Radiotherapy is an effective therapeutic modality for the management of patients with NSCLC, especially at early stages.2,3 Radioresistance is the key factor limiting the therapeutic outcome of radiotherapy.4 Several molecular mechanisms have been suggested to be linked to the development of radioresistance in NSCLC cells, such as alteration of DNA repair proteins, deregulation of survival proteins,5 and activation of epidermal growth factor receptor (EGFR).6 Ligation of EGFR leads to activation of multiple prosurvival signaling pathways including the phosphoinositide 3-kinase (PI3K)/Akt pathway.7 Inhibition of the PI3K/Akt pathway has been reported to overcome radioresistance in NSCLC cells.8 Despite these advances, the exact mechanisms governing the radiation responses of NSCLC cells are still elusive.
Gelsolin is an actin-binding protein involved in the remodeling of cellular actin cytoskeleton, affecting cell morphology and motility.9 Accumulating evidence indicates a link between gelsolin and tumor progression. For instance, Deng et al10 reported that gelsolin is upregulated in human hepatocellular carcinoma (HCC) tissues, and overexpression of gelsolin promotes the proliferation and invasion of HCC cells. An adverse correlation between gelsolin expression and patient survival was noted in NSCLC,11–13 implying an important role for gelsolin in tumor progression. Gelsolin has been found to regulate the survival of cancer cells.14,15 Klampfer et al14 reported that knockdown of gelsolin sensitizes colon cancer cells to the short-chain fatty acid butyrate through activation of caspase-9 and caspase-7 and induction of apoptosis. Wang et al16 found that gelsolin subserves a prosurvival role in human head and neck cancer (HNC) cells, and its silencing results in an increased apoptotic response to cisplatin. Using a quantitative proteomic approach, Kim et al17 identified gelsolin as an upregulated protein in γ-radiation-exposed MDA-MB-231 breast cancer cells, suggesting its involvement in the regulation of radiosensitivity. However, the role of gelsolin in the radioresistance of NSCLC has not been determined yet.
In this study, we sought to explore the function of gelsolin in the radiosensitivity of NSCLC cells and to evaluate the impact of gelsolin on irradiation-induced apoptosis. In addition, the involvement of PI3K/Akt signaling in the action of gelsolin was checked.
Materials and Methods
Cell Culture
Human NSCLC cell lines (A549 and H460) were purchased from American Type Culture Collection (Rockville, Maryland). The former is derived from lung carcinoma and the latter from large-cell lung cancer. They were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, California) supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% fetal bovine serum in a 5% CO2 humidified incubator.
Establishment of Irradiation-Resistant Cell Lines
Irradiation-resistant cell lines were established as described previously.18 In brief, A549 and H460 cells were grown to a confluency of ∼80% and irradiated using the Faxitron Cabinet X-ray system (Faxitron, Wheeling, Illinois) at a dose of 2 Gy per fraction. Cells were trypsinized and subcultured immediately after each radiation. Reirradiation with 2 Gy of X-rays was given 40 times within a 5-month period. The cells did not receive next irradiation until they reached ∼80% confluence. Parental cells were treated in the same way except that they were not exposed to X-ray irradiation.
Chemical and Irradiation Treatment
A549/R and H460/R cells and their parental cells were plated in triplicate into 6-well plates (800 cells/well) and incubated overnight. Cells were irradiated at single doses (2-8 Gy) with a dose rate of ∼1.8 Gy/min. For inhibitor experiments, cells were preincubated with a PI3K inhibitor LY294002 (20 μmol/L; Sigma-Aldrich, St Louis, Missouri) for 30 minutes before transfection with a gelsolin-expressing plasmid as described below.
Clonogenic Survival Assay
After irradiation, cells were plated in triplicate into 6-well plates (2000 cells/well) and incubated at 37 C for 10 days. Colonies were fixed and stained with 0.1% crystal violet and examined by microscopy. The number of colonies consisting of more than 50 cells was counted. Results are expressed as percentage of the control (nonirradiated cells).
Plasmids, Short Hairpin RNA, and Transfection
Human full-length gelsolin complementary DNA (cDNA) was purchased from OriGene Technologies Inc (Rockville, Maryland) and subcloned into pcDNA3.1(+) expression vector (Invitrogen). The cDNA insertions were confirmed by DNA sequencing. Gelsolin-targeting short hairpin RNA (shRNA) and negative control shRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, California).
Cell transfection was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. In brief, cells were plated onto 12-well plates and grown to ∼80% confluence. They were transfected with the pcDNA3.1-gelsolin plasmid (1 μg), gelsolin shRNA (50 nmol/L), or corresponding control constructs. At 24 hours after transfection, cells were transferred to selection medium containing G418 (800 μg; Sigma-Aldrich). The stable clones were picked up and expanded. The gelsolin levels in these clones were examined by Western blot analysis.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of the messenger RNA (mRNA) level of gelsolin was performed as described previously.19 In brief, total RNA was extracted from cells with TRIzol reagent (Invitrogen) and subjected to reverse transcription using the SuperScript First-Strand Synthesis System (Invitrogen). Polymerase chain reaction conditions were as follows: 95°C for 5 minutes, followed by 38 cycles of 95°C for 15 seconds and 60°C for 40 seconds. The gelsolin primer sequences were used as follows: 5′-CACTGAGCCCGAGGCGATGC-3′ (forward) and 5′-TCAGCCACGAGGGAGACGGAC-3′ (reverse). As an internal control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified with the following primers: forward, 5′-ATTCCACCCATGGCAAATTC-3′; reverse, 5′-GCATCGCCCCACTTGATT-3′. The relative gelsolin mRNA level was determined with the 2−ΔΔCt method20 after normalization against the level of GAPDH.
Western Blot Analysis
Cell lysates were prepared using ice-cold radioimmunoprecipitation assay buffer (Cell Signaling Technology, Danvers, Massachusetts) supplemented with a mixture of protease inhibitors (Roche Diagnostics, Mannheim, Germany). Protein concentrations were measured using a protein assay kit (Bio-Rad Laboratories, Hercules, California). Protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were probed with primary antibodies at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 hour at room temperature. Immune complexes were visualized using an enhanced chemiluminescence kit (Amersham Biosciences, Buckinghamshire, United Kingdom). Protein bands were quantified by densitometry using the Quantity One software (Bio-Rad Laboratories). The primary antibodies used for Western blotting are shown below: rabbit antigelsolin polyclonal antibody (ab76418; Abcam, Cambridge, Massachusetts), rabbit anticleaved caspase-3 polyclonal antibody (#9661), rabbit anticleaved poly adenosine diphosphate-ribose polymerase (PARP) monoclonal antibody (#5625), mouse antiphospho-Akt (Ser473) monoclonal antibody (#12694), mouse anti-Akt monoclonal antibody (#2966; Cell Signaling Technology), and mouse anti-β-actin monoclonal antibody (sc-47778; Santa Cruz Biotechnology).
Apoptosis Detected by Flow Cytometry
Cells were collected at 48 hours after irradiation, and apoptosis was measured using a commercially available annexin V detection kit, according to the manufacturer’s instructions (PharMingen, San Jose, California). In brief, cells were harvested by trypsin treatment and suspended in a binding buffer. Fluorescein isothiocyanate-conjugated annexin V and propidium iodide were added to the cell suspension and incubated for 15 minutes at room temperature in a dark chamber. Stained cells were quantified using a flow cytometer (FACSCalibur; Becton Dickinson Biosciences, San Jose, California).
Statistical Analysis
Data are expressed as mean ± standard deviation. All statistical differences were analyzed using 1-way analysis of variance combined with Tukey post hoc test. A P value of <.05 was considered statistically significant.
Results
Gelsolin Is Upregulated in Radioresistant NSCLC Cells
To confirm the radioresistant phenotype of A549/R and H460/R cells, we examined cell survival after single doses of irradiation ranging from 0 to 8 Gy using clonogenic assays. As shown in Figure 1A, the number of colonies from A549/R cells at 4 to 8 Gy was significantly (P <.05) higher than that from parental A549 cells. Similar findings were seen with H460/R and parental cells (Figure 1A). Therefore, A549/R and H460/R cells were more radioresistant than their parental cells.
Figure 1.
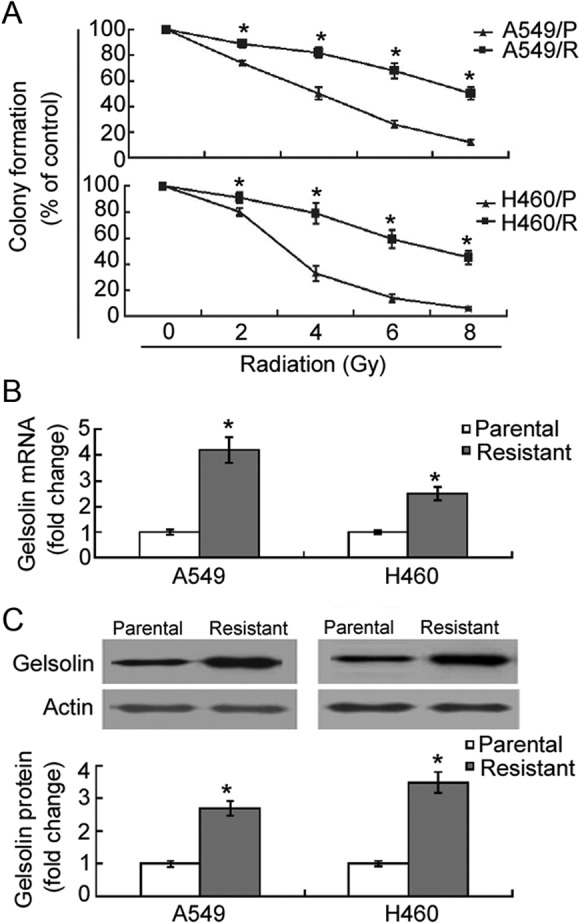
Gelsolin is upregulated in radioresistant non–small cell lung cancer (NSCLC) cells. A, Clonogenic assays in assessing the sensitivity of radioresistant cells (A549/R and H460/R) and their parental cells to X-ray radiation. After radiation, cells were incubated for 10 days, and the number of colonies consisting of >50 cells was counted. Results are expressed as percentage of the control (nonirradiated cells). Quantitative real-time polymerase chain reaction (A; qRT-PCR) and Western blot analysis (C) of gelsolin expression levels in A549/R, H460/R, and their parental cells. Bar graphs represent means ± standard deviation (SD) from 3 independent experiments. *P < .05 between radioresistant and parental cells.
To examine the potential correlation of gelsolin with cancer radiosensitivity, we investigated its expression in radioresistant and parental NSCLC cells. The qRT-PCR analysis revealed a significant (P <.05) increase in gelsolin expression in A549/R and H460/R cells as compared to their parental cells (Figure 1B). Western blot analysis confirmed the upregulation of gelsolin in radioresistant A549 and H460 cells (Figure 1C).
Gelsolin Promotes Radioresistance of NSCLC Cells
Next, we checked whether regulation of gelsolin expression affects the radiosensitivity of NSCLC cells. To this end, we overexpressed or knocked down gelsolin in A549 and H460 parental and resistant cells (Figure 2A and B). Clonogenic survival assay demonstrated that enforced expression of gelsolin significantly (P <.05) increased the number of colonies from irradiated A549 and H460 cells compared to transfection of empty vector (Figure 2C). In contrast, transfection with gelsolin-targeting shRNA significantly (P <.05) suppressed colony formation in A549/R and H460/R cells after irradiation (Figure 2D).
Figure 2.
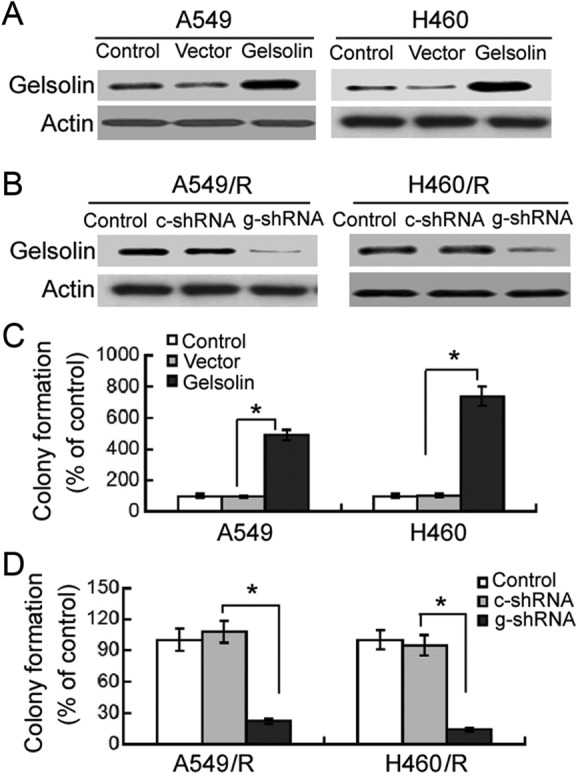
Gelsolin promotes radioresistance of NSCLC cells. A and B, Western blot analysis of gelsolin protein levels in A549 and H460 cells transfected with indicated constructs. Representative blots of 3 independent experiments are shown. C and D, Cells transfected with indicated constructs were exposed to 8-Gy X-ray and incubated for 10 days. The number of colonies consisting of >50 cells was counted. Results are expressed as percentage of the control (nonirradiated cells). *P < .05. c-shRNA indicates control shRNA; g-shRNA, gelsolin shRNA; NSCLC, non–small cell lung cancer.
Gelsolin Confers Resistance to Irradiation-Induced Apoptosis
Next, we examined the effect of gelsolin on irradiation exposure-induced apoptosis. Flow cytometric analysis showed that 8 Gy of X-ray irradiation caused a significant increase in the percentage of annexin V-positive apoptotic cells compared to nonirradiated control cells (Figure 3A). However, the proapoptotic effect of irradiation exposure was significantly (P <.05) compromised in gelsolin-overexpressing A549 and H460 cells. In line with these results, gelsolin overexpression significantly (P <.05) prevented the increase in cleaved caspase-3 and PARP in response to irradiation (Figure 3B and C).
Figure 3.
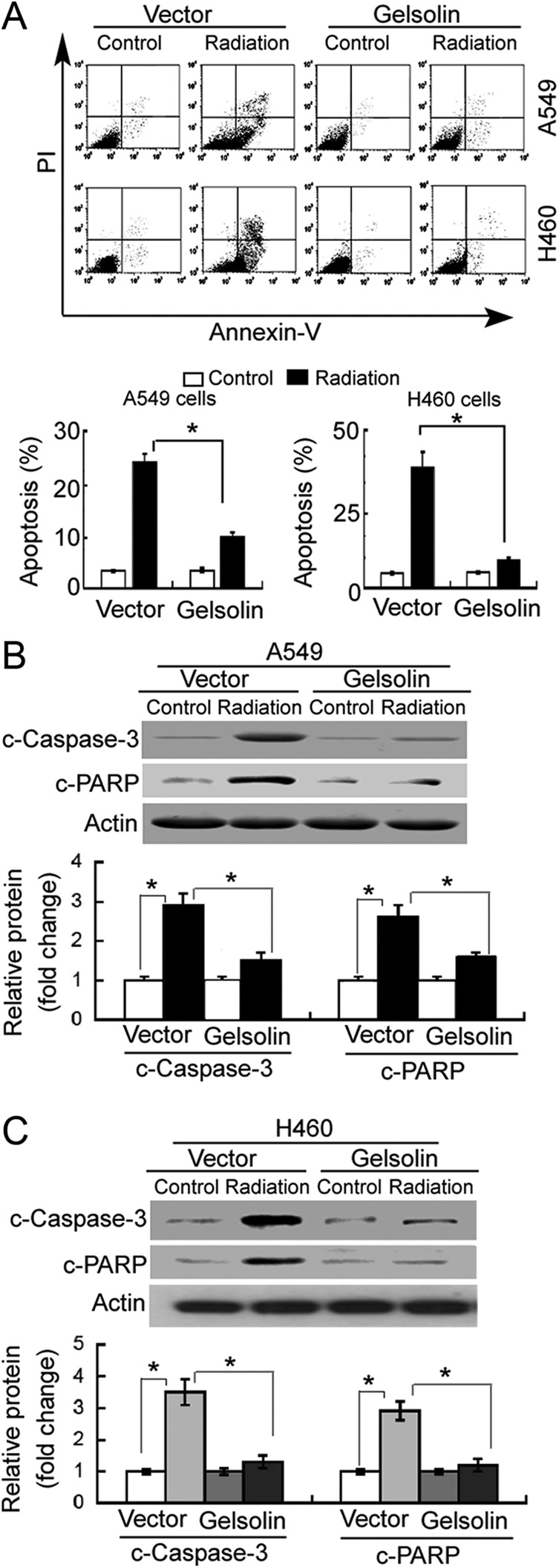
Gelsolin confers resistance to irradiation-induced apoptosis. A549 and H460 cells transfected with empty vector or gelsolin-expressing plasmid were nonirradiated (control) or exposed to 8-Gy X-ray. A, Apoptosis detected by annexin-V/propidium iodide (PI) staining and flow cytometry analysis. Representative flow cytometric dot plots showing apoptotic cells (top panels). Bar graphs (bottom panels) represent quantification of total apoptotic cells (annexin-V+/PI− or annexin-V+/PI+) from 3 independent experiments. Western blot analysis of cleaved caspase-3 (c-caspase-3) and cleaved poly adenosine diphosphate-ribose polymerase(c-PARP) proteins in (B) A549 and (C) H460 cells with indicated treatments. Bar graphs (bottom panels) represent means ± standard deviation (SD) from 3 independent experiments. *P < .05.
Activation of PI3K/Akt Signaling Is Involved in Gelsolin-Mediated Radioresistance
Finally, we tested whether gelsolin-mediated radioresistance is associated with the activation of PI3K/Akt signaling. Western blot analysis revealed that compared to nontransfected cells, ectopic expression of gelsolin enhanced the phosphorylation of Akt, without affecting the total level of Akt (Figure 4A). In contrast, delivery of gelsolin shRNA markedly reduced the phosphorylation of Akt in A549/R and H460/R cells (Figure 4A). Most interestingly, pretreatment with the PI3K inhibitor LY294002 (20 μmol/L) significantly decreased the survival fraction (Figure 4B) and promoted apoptosis (Figure 4C) in gelsolin-overexpressing A549 and H460 cells after irradiation exposure. LY294002-induced inhibition of Akt activation was confirmed in gelsolin-overexpressing A549 and H460 cells by Western blot analysis (Figure 4D).
Figure 4.
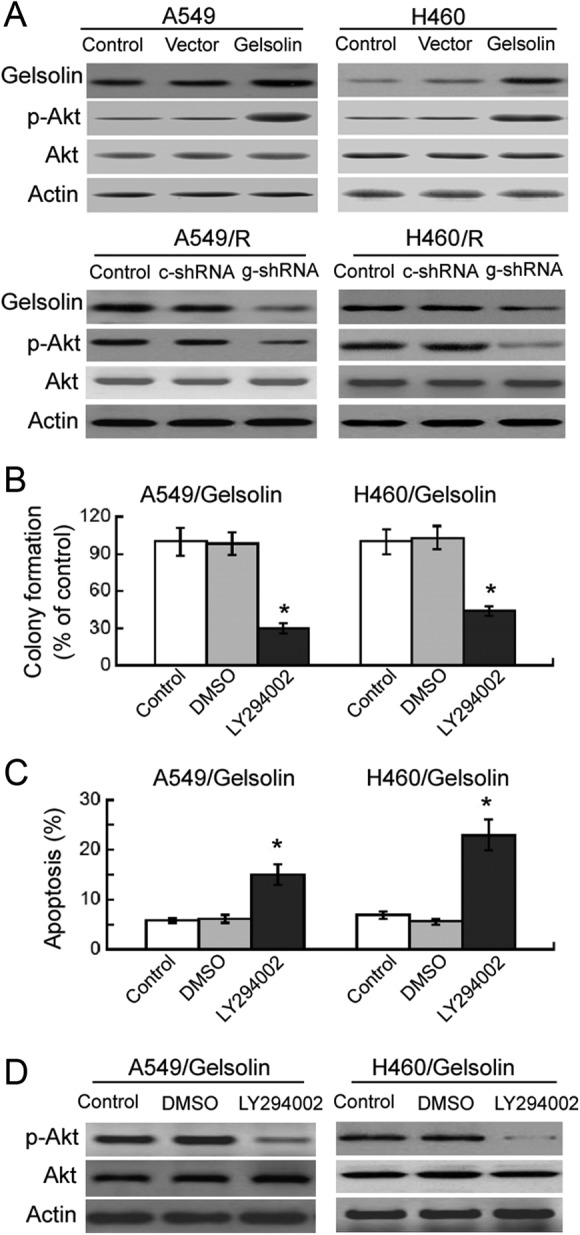
Activation of PI3K/Akt signaling is involved in gelsolin-mediated radioresistance. A, Western blot analysis of indicated proteins in resistant and parental A549 and H460 cells transfected with indicated constructs. Representative blots from 3 independent experiments are shown. B and C, Gelsolin-overexpressing A549 and H460 cells were pretreated with or without LY294002 (20 μmol/L) before radiation exposure. Colony formation assay (B) and apoptosis analysis (C) were performed as described in Materials and Methods. Bar graphs represent means ± standard deviation (SD) from 3 independent experiments. *P < .05 versus control. D, Western blot analysis of Akt phosphorylation in gelsolin-overexpressing A549 and H460 cells with or without LY294002 (20 μmol/L) treatment. Representative blots from 3 independent experiments are shown.
Discussion
Radiation therapy is one of the most important modalities for NSCLC management. However, the emergence of radioresistant tumor cells is a major obstacle to successful treatment. Identification of the key determinants of radioresistance is of significance for improving the efficacy of radiotherapy in NSCLC. Although gelsolin shows the ability to modulate the chemosensitivity of several types of cancer cells, the role of gelsolin in tumor radioresistance has been poorly understood. Our data showed that gelsolin was upregulated in radioresistant A549 and H460 cells compared to their parental cells. Radiation-induced gelsolin expression has also been reported in human breast cancer MDA-MB-231 cells17 and in human intestinal epithelial cells.21 The upregulation of gelsolin may contribute to cell survival after radiation. In support of this hypothesis, Li et al21 demonstrated that administration of exogenous gelsolin into mice confers protection against tissue damage induced by high-dose radiation. Gelsolin has been shown to act as a protective protein in several other pathologies such as stroke22 and acute hyperoxic lung injury.23 However, in some specific cases, gelsolin plays a negative role in cell survival. For instance, Li et al24 reported that gelsolin deficiency reduces apoptotic death in cardiomyocytes of mice after myocardial infarction. To provide direct evidence for the role of gelsolin in radioresistance of NSCLC cells, we overexpressed or knocked down gelsolin and examined the clonogenic response of NSCLC cells to radiation. Notably, we found that gelsolin-overexpressing A549 and H460 cells showed significantly greater fractions of surviving clones after radiation compared to empty vector-transfected cells. In contrast, silencing of gelsolin significantly restored the sensitivity of A549/R and H460/R cells to radiation. These findings suggest the importance of gelsolin in the radioresistance of NSCLC cells.
Inactivation of apoptosis pathways is a key mechanism leading to radioresistance in cancer cells. Previous studies have shown that intact gelsolin has the ability to prevent chemotherapeutic drug-induced apoptosis in HNC cells16 and gynecologic cancers.25 In contrast, gelsolin downregulation was found to augment drug-induced apoptosis in chemoresistant cancer cells.16,25 In agreement with these studies, we found that radiation-induced apoptosis was significantly attenuated in gelsolin-overexpressing A549 and H460 cells, underscoring the antiapoptotic activity of gelsolin. Caspase-3 is a well-defined apoptosis effector and plays an important role in the apoptotic response of NSCLC cells after radiation treatment.26 Therefore, we further determine whether gelsolin affected the activation of caspase-3. Indeed, we found that gelsolin overexpression interfered with the activation of caspase-3 in irradiated A549 and H460 cells, as evidenced by reduced levels of cleaved caspase-3 and its substrate PARP. Taken together, overexpression of gelsolin protects NSCLC cells from radiation-induced apoptosis, likely via inhibition of caspase-3-dependent apoptotic signaling.
Compelling evidence indicates that the PI3K/Akt signaling pathway is an important regulator of the response of cancer cells to radiotherapy.8,27 Inhibition of this pathway has been shown to enhance radiosensitivity in different types of cancer cells including NSCLC cells.8,28 To further clarify the mechanism for gelsolin-mediated radioresistance, we examined the effect of gelsolin overexpression on activation of Akt signaling in NSCLC cells. We found that enforced expression of gelsolin significantly increased the phosphorylation of Akt at Ser473, suggesting the activation of Akt signaling. Pharmacological inhibition of Akt by LY294002 restored the sensitivity of gelsolin-overexpressing cells to radiation, as evidenced by less clonogenic survival and enhanced apoptotic response. These results collectively suggest that gelsolin-mediated radioresistance in NSCLC cells is largely ascribed to activation of PI3K/Akt signaling.
However, some limitations of this study should be noted. First, there is no in vivo evidence for the role of gelsolin in radioresistance of NSCLC. Second, a mediator for gelsolin-induced activation of Akt signaling remains to be identified. It has been reported that Rac activity is required for gelsolin-induced epithelial cell invasion29 and gelsolin-mediated collagen phagocytosis in fibroblasts.30 In some settings, for example, cigarette smoke-exposed pulmonary epithelial cells,31 Rac1 inhibition, or knockdown disrupted the activation of Akt. These studies suggest a possibility that Rac signaling may mediate the link between gelsolin and Akt activation. Finally, no information is available on the relationship between gelsolin levels and radiation response in patients with NSCLC.
Conclusion
In conclusion, this is the first report showing the key role of gelsolin in the radioresistance of NSCLC cells. Gelsolin acts as a protective protein against radiation-induced apoptosis in NSCLC cells, which is mediated through inactivation of PI3K/Akt signaling. Further studies are warranted to investigate the role of gelsolin in the regulation of radiosensitivity of NSCLC in animal models.
Abbreviations
- Akt
Protein kinase B (PKB)
- cDNA
complementary DNA
- EGFR
epidermal growth factor receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HCC
hepatocellular carcinoma
- HNC
head and neck cancer
- mRNA
messenger RNA
- NSCLC
non–small cell lung cancer
- PARP
poly adenosine diphosphate-ribose polymerase
- PI3K
phosphoinositide 3-kinase
- qRT-PCR
quantitative real-time polymerase chain reaction
- shRNA
short hairpin RNA.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 2. Chen H, Louie AV. Stereotactic ablative radiotherapy and surgery: two gold standards for early-stage non-small cell lung cancer? Ann Transl Med. 2015;3(9):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelley KD, Benninghoff DL, Stein JS, et al. Medically inoperable peripheral lung cancer treated with stereotactic body radiation therapy. Radiat Oncol. 2015;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data. Oncologist. 2011;16(5):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desai S, Kumar A, Laskar S, Pandey BN. Differential roles of ATF-2 in survival and DNA repair contributing to radioresistance induced by autocrine soluble factors in A549 lung cancer cells. Cell Signal. 2014;26(11):2424–2435. [DOI] [PubMed] [Google Scholar]
- 6. Javvadi P, Makino H, Das AK, et al. Threonine 2609 phosphorylation of the DNA-dependent protein kinase is a critical prerequisite for epidermal growth factor receptor-mediated radiation resistance. Mol Cancer Res. 2012;10(10):1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–225. [DOI] [PubMed] [Google Scholar]
- 8. Zhang T, Cui GB, Zhang J, et al. Inhibition of PI3 kinases enhances the sensitivity of non-small cell lung cancer cells to ionizing radiation. Oncol Rep. 2010;24(6):1683–1689. [DOI] [PubMed] [Google Scholar]
- 9. Li GH, Arora PD, Chen Y, McCulloch CA, Liu P. Multifunctional roles of gelsolin in health and diseases. Med Res Rev. 2012;32(5):999–1025. [DOI] [PubMed] [Google Scholar]
- 10. Deng B, Fang J, Zhang X, Qu L, Cao Z, Wang B. Role of gelsolin in cell proliferation and invasion of human hepatocellular carcinoma cells. Gene. 2015;571(2):292–297. [DOI] [PubMed] [Google Scholar]
- 11. Zhu WY, Hunag YY, Liu XG, et al. Prognostic evaluation of CapG, gelsolin, P-gp, GSTP1, and Topo-II proteins in non-small cell lung cancer. Anat Rec (Hoboken). 2012;295(5):208–214. [DOI] [PubMed] [Google Scholar]
- 12. Yang J, Ramnath N, Moysich KB, et al. Prognostic significance of MCM2, Ki-67 and gelsolin in non-small cell lung cancer. BMC Cancer. 2006;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Tan D, Asch HL, et al. Prognostic significance of gelsolin expression level and variability in non-small cell lung cancer. Lung Cancer. 2004;46(1):29–42. [DOI] [PubMed] [Google Scholar]
- 14. Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Oncogenic Ras promotes butyrate-induced apoptosis through inhibition of gelsolin expression. J Biol Chem. 2004;279(35):36680–36688. [DOI] [PubMed] [Google Scholar]
- 15. Nowak JM, Klimaszewska-Wiśniewska A, Izdebska M, Gagat M, Grzanka A. Gelsolin is a potential cellular target for cotinine to regulate the migration and apoptosis of A549 and T24 cancer cells. Tissue Cell. 2015;47(1):105–114. [DOI] [PubMed] [Google Scholar]
- 16. Wang PW, Abedini MR, Yang LX, et al. Gelsolin regulates cisplatin sensitivity in human head-and-neck cancer. Int J Cancer. 2014;135(12):2760–2769. [DOI] [PubMed] [Google Scholar]
- 17. Kim MH, Jung SY, Ahn J, et al. Quantitative proteomic analysis of single or fractionated radiation-induced proteins in human breast cancer MDA-MB-231 cells. Cell Biosci. 2015;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. You S, Li R, Park D, et al. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol Cancer Ther. 2014;13(3):606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan X, Yu L, Li J, et al. ATF3 suppresses metastasis of bladder cancer by regulating gelsolin-mediated remodeling of the actin cytoskeleton. Cancer Res. 2013;73(12):3625–3637. [DOI] [PubMed] [Google Scholar]
- 20. Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25(4):386–401. [DOI] [PubMed] [Google Scholar]
- 21. Li M, Cui F, Cheng Y, et al. Gelsolin: role of a functional protein in mitigating radiation injury. Cell Biochem Biophys. 2015;71(1):389–396. [DOI] [PubMed] [Google Scholar]
- 22. Endres M, Fink K, Zhu J, Stagliano NE, et al. Neuroprotective effects of gelsolin during murine stroke. J Clin Invest. 1999;103(3):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Christofidou-Solomidou M, Scherpereel A, Solomides CC, et al. Recombinant plasma gelsolin diminishes the acute inflammatory response to hyperoxia in mice. J Investig Med. 2002;50(1):54–60. [DOI] [PubMed] [Google Scholar]
- 24. Li GH, Shi Y, Chen Y, et al. Gelsolin regulates cardiac remodeling after myocardial infarction through DNase I-mediated apoptosis. Circ Res. 2009;104(7):896–904. [DOI] [PubMed] [Google Scholar]
- 25. Abedini MR, Wang PW, Huang YF, et al. Cell fate regulation by gelsolin in human gynecologic cancers. Proc Natl Acad Sci U S A. 2014;111(40):14442–14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li B, Blanc JM, Sun Y, et al. Assessment of M867, a selective caspase-3 inhibitor, in an orthotopic mouse model for non-small cell lung carcinoma. Am J Cancer Res. 2014;4(2):161–171. [PMC free article] [PubMed] [Google Scholar]
- 27. Chang L, Graham PH, Hao J, et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta M, Khan A, Danish S, Haffty BG, Sabaawy HE. Radiosensitization of primary human glioblastoma stem-like cells with low-dose AKT inhibition. Mol Cancer Ther. 2015;14(5):1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Corte V, Bruyneel E, Boucherie C, Mareel M, Vandekerckhove J, Gettemans J. Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. EMBO J. 2002;21(24):6781–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arora PD, Glogauer M, Kapus A, Kwiatkowski DJ, McCulloch CA. Gelsolin mediates collagen phagocytosis through a rac-dependent step. Mol Biol Cell. 2004;15(2):588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen HJ, Sun YH, Zhang SJ, et al. Cigarette smoke-induced alveolar epithelial-mesenchymal transition is mediated by Rac1 activation. Biochim Biophys Acta. 2014;1840(6):1838–1849. [DOI] [PubMed] [Google Scholar]


