Abstract
Background:
Hilar cholangiocarcinomas are malignant tumors with a poor prognosis. An early prediction of prognosis for patients may help us determine treatment strategies. Aquaporin 1 is a cell membrane channel involved in water transport, cell motility, and proliferation. Increasing evidences showed that aquaporin 1 played a role in tumor prognosis and diagnosis. The purpose of this study is to evaluate the role of aquaporin 1 in hilar cholangiocarcinoma.
Methods:
Here, we analyzed messenger RNA expression data of genes function as bile secretion in a data set of 169 samples using the R2 bioinformatic platform (http://r2.amc.nl). Quantitative polymerase chain reaction was performed to verify the gene expression in 17 hilar cholangiocarcinoma samples. Immunohistochemistry was also performed in a series of specimens from 62 hilar cholangiocarcinoma tissues, and its clinical significance was assessed by clinical correlation and Kaplan-Meier analyses.
Results:
All data were analyzed using the R2 web application, aquaporin 1 was selected for further analysis. The significant expression variation of aquaporin 1 among 17 cases with cholangiocarcinoma was also found using quantitative polymerase chain reaction. The expression level of aquaporin 1 protein significantly correlated with tumor-node-metastasis stage (P = .002) and overall survival time (P = .010). Higher aquaporin 1 expression indicated poor prognostic outcomes (P <.05, log-rank test). Multivariate analysis also showed strong aquaporin 1 protein expression was an independent adverse prognosticator in hilar cholangiocarcinoma (P = .002).
Conclusion:
This study highlighted the prognostic value of aquaporin 1 in hilar cholangiocarcinoma. Strong aquaporin 1 expression predicts poor survival, regardless of pathological features. Immunohistochemical detection of aquaporin 1, as a prognostic marker, may contribute to predicting clinical outcome for patients with hilar cholangiocarcinoma.
Keywords: AQP1, hilar cholangiocarcinoma, prognosis
Introduction
Cholangiocarcinoma is a progressively fatal disease that generally occurs due to malignant transformation of hepatic biliary cholangiocytes.1 It is most frequently found in the confluence of the hepatic ducts, where it is called hilar cholangiocarcinoma (hilCC). Hilar cholangiocarcinoma was first described by Altemeier and Klatskin approximately 50 years ago and comprises over 60% of all cholangiocarcinomas.2–4 The prognosis for hilCC is generally poor, with long-term as well as short-term survival almost exclusively depending on the feasibility of surgical resection.1 Except for principal prognostic factors such as tumor stage and histological grade, new prognostic parameters, including clinical, laboratory, and biomolecular factors, have long been desired to provide additional predictive value in clinical practice.5
The aquaporins (AQPs) are small, integral membrane proteins that selectively transport water and other compounds including ions, gases, and small organic compounds across cell plasma membranes. Discoveries of AQPs involvement in cell migration and proliferation suggested that they play key roles in tumor biology.6 The AQP1, an important member of AQP family, is shown to be strongly expressed in tumor cells of different human origins, such as brain tumors,7 lung adenocarcinoma,8 laryngeal cancer,9 and cholangiocarcinoma.10 Aquaporin 1 plays a role in tumor angiogenesis,11,12 cell migration,13,14 extravasation, and metastasis.14,15 Recent evidence indicated that AQP1 was an independent prognostic factor in advanced colon cancer16 and pleural malignant mesothelioma.17 Moreover, clinical diagnosis value in renal cell carcinoma was shown.18
The goal of this study is to assess the clinical significance of AQP1 in human hilCC, which assists in early identification of a high-risk subset of the patients.
Material and Methods
Patients and Tissue Specimens
After receiving approval from the institutional review board, we searched the patient data bank at The 1st Hospital of Harbin Medical University (Harbin, China) to identify all consecutive patients who underwent operative treatment for hilCC between January 1987 and May 2002. The patients who died within 1 month of the initial surgery were excluded. To eliminate treatment bias, the patients who underwent chemotherapy or radiotherapy both at pre- and postoperation were excluded.
All specimens examined were taken from vital cores of histopathologically confirmed cancers at primary surgery. Tumor samples were reviewed by at least 2 experienced pathologists, and tumor stage was assigned on the basis of system of the International Union Against Cancer.
For the survival analysis, overall survival (OS) was defined as the time from the date of diagnosis of liver malignant to the date of patient death or last follow-up.
Bioinformatics
All data were analyzed using the R2 web application, which is publicly available at http://r2.amc.nl. In R2, a large amount of data sets are available for analysis. We performed a search (last search: March 22, 2015) to identify data sets related to the identification of gene expression pertaining to cholangiocarcinoma.
Messenger RNA Analysis by Quantitative Polymerase Chain Reaction
Total RNA was extracted from frozen tissues using an RNeasy Mini Kit (Qiagen GmbH, Germany) according to the manufacturer’s protocol. The first strand of complementary DNA was synthesized from total RNA using oligo-dT primer and Avian Myeloblastosis Virus (AMV) reverse transcriptase according to the manufacturer’s instructions (Takara, Otsu, Shiga, Japan).
The specific primers to AQP1 were designed as follows: 5′-TGCCATCGGCCTCTCTGTA-3′ (forward primer), 5′-CAGGGTTAATCCCACAGCCA-3′ (reverse primer). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. GAPDH primers were as follows: 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward primer), 5′-GAAGATGGTGATGGGATTTC-3′ (reverse primer). The method of quantitative polymerase chain reaction (qPCR) was described previously.19
Immunohistochemistry
Archival hematoxylin and eosin-stained slides were reviewed by 2 experienced pathologists in accordance with the 2000 World Health Organization classification. A 3-tiered histologic grading system was applied. The tumor-node-metastasis (TNM) stage was evaluated according to the 2002 International Union Against Cancer classification.
A monoclonal mouse antihuman AQP1 antibody was obtained from Santa Cruz Biotechnology, Santa Cruz, California. Sections from archival paraffin blocks were deparaffinized and heated twice for 10 minutes each before exposure to the first antibodies. Immunoperoxidase staining was carried out using the 2-step Envision Method (DAKO, Glostrup, Denmark) according to the manufacturer’s instructions and visualized with 3,3 9-diaminobenzidine tetrachloride (Sigma, St Louis, Missouri).
Cytoplasm and membrane staining were measured for this antibody. Positive cells were counted by 2 pathologists who were blind to clinical outcome. For clinicopathological correlation, we used a 4-tiered scoring system (negative to 3 +), which took into account the percentage of positive cells and staining intensity as described previously.20
Statistical Analysis
We used the SPSS version13.0 for statistical analysis. The differences between groups were determined using the Mann-Whitney U test or the Kruskal-Wallis H test. The association among categorical data was analyzed using the χ2 test. Survival curves were generated by the Kaplan-Meier method, and univariate survival distributions were compared with the use of the log-rank test. The multivariate Cox proportional hazards model was used for detection of independent prognosticator. The 2-tailed P value for significance was established at .05.
Results
Characteristics of the Study Population
A total of 62 hilCC specimens underwent operation for hilCC were collected. Of them, both fresh and paraffin-embedded samples could be obtained from 17 patients. The mean patient age was 66.2 years (range, 28-76 years). Of the patients, 26 were female and 36 were male. All patients underwent curative resection with tumor-free margins. Pathological, demographic, and survival data of the patients were retrieved from the files of the pathology department in our hospital. All tumors were histologically diagnosed, graded, and grouped as lowly and moderately differentiated (n = 35) or highly differentiated (n = 27). The follow-up interval ranged from 1 to 60 months from the date of surgery. Informed consent was obtained for all patients.
AQP1 Was Selected for Further Analysis as a Candidate Gene
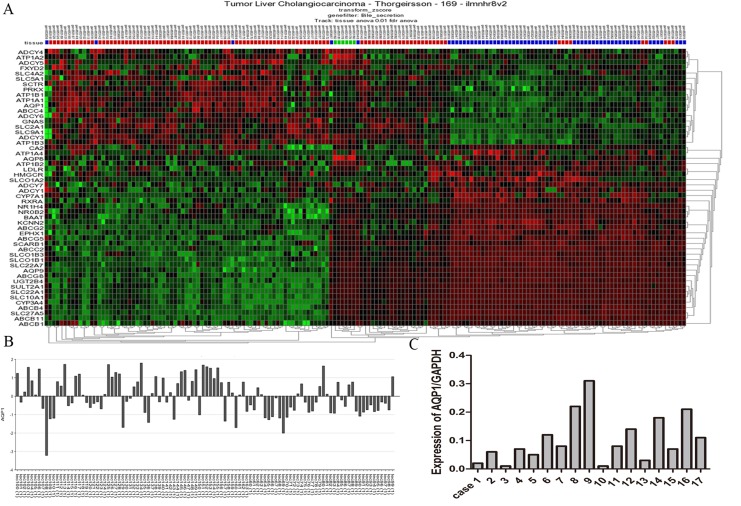
R2 is a web-based collection of bioinformatic applications designed for the analysis of gene expression and genomic data derived from various microarray platforms.21 It is possible to view the differential expression of genes in a user-defined panel of data set. We selected the GEO ID: GSE26566 data set, which aimed to construct molecular classification of cholangiocarcinoma, to identify the prognostic markers for hilCC patients. Considering the normal physiological dysfunction due to cancer, we investigated the expression aberrations of genes involved in the bile secretion. Fifty-two aberrant genes were distinguishable from matched noncancerous tissues (Figure 1A). Among these genes, we found that the variation of AQP1 expression in different cholangiocarcinoma samples was significant (Figure 1B). We speculated that it may be related to the clinical and pathological features of patients. To validate this finding, qPCR was performed in 17 fresh tissues, and the conclusion was confirmed (Figure 1C).
Figure 1.
Aquaporin 1 (AQP1) was selected for further analysis as a candidate gene. A, Heat map of consistent K means clustered z-scores of the differential expression genes regulating bile secretion between cholangiocarcinoma and matched noncancerous tissues based on R2 applications. B, Significant expression variation of AQP1 among cholangiocarcinoma samples. C, The AQP1 expression variation in 17 fresh tissues, which confirmed the finding.
Correlation Between AQP1 Protein Expression and Clinicopathological Features
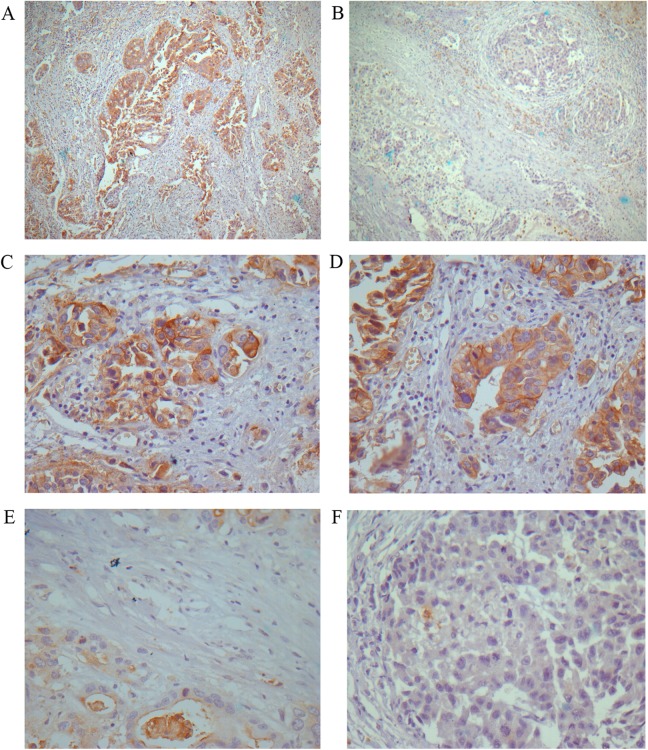
Positive staining was observed primarily in the cytoplasm and membrane of the cancer cells. Immunostaining of AQP1 protein was low (− and 1+; Figure 2B, E, and F) in some samples but strong (2+ and 3+; Figure 2A, C, and D) in others. Analysis of AQP1 expression in the 62 hilCC tissues revealed that 71% of samples demonstrating strong intensities and 29% low intensities.
Figure 2.
Expression of aquaporin 1 (AQP1) in hilar cholangiocarcinoma (hilCC). A and B, The histological appearance of strong and low AQP1 expression, respectively (3,3′-diaminobenzidine [DAB]; magnification, ×10). C and D, The strong AQP1 expresser group (DAB; magnification, ×40). E and F, The low AQP1 expresser group (DAB; magnification, ×40).
No significant differences were observed regarding age, sex, histologic grading, lymphovascular, and perineural invasion. However, the OS rate was significantly different in patients with strong AQP1 protein expression than in those with low expression (P = .010), and AQP1 expression was significantly associated with TNM stage (P = .002). Table 1 showed the relations between clinicopathological features and AQP1 protein expression in 62 samples.
Table 1.
Relationships Between Clinicopathologic Features and AQP1 Expression in Hilar Cholangiocarcinoma.
| Parameters | N, % | AQP1 Expression | P a Value | |
|---|---|---|---|---|
| Strong (n = 44) | Low (n = 18) | |||
| Sex | .785 | |||
| Male | 26 (42%) | 19 (43%) | 7 (39%) | |
| Female | 36 (58%) | 25 (57%) | 11 (61%) | |
| Age, years | .236 | |||
| ≥65 | 20 (32%) | 12 (27%) | 8 (44%) | |
| <65 | 42 (68%) | 32 (73%) | 10 (56%) | |
| Overall 5-year survival | .010b | |||
| Yes | 22 (35%) | 11 (25%) | 11 (61%) | |
| No | 40 (65%) | 33 (75%) | 7 (39%) | |
| Histologic grading | .095 | |||
| Low grade, moderate grade | 35 (56%) | 28 (64%) | 7 (39%) | |
| High grade | 27 (44%) | 16 (36%) | 11 (61%) | |
| Lymph node involvement | .783 | |||
| Yes | 32 (52%) | 22 (50%) | 10 (56%) | |
| No | 30 (48%) | 22 (50%) | 8 (44%) | |
| Vascular invasion | .756 | |||
| Yes | 17 (27%) | 13 (30%) | 4 (22%) | |
| No | 45 (73%) | 31 (70%) | 14 (78%) | |
| Perineural invasion | 1 | |||
| Yes | 12 (19%) | 9 (20%) | 3 (17%) | |
| No | 50 (81%) | 35 (80%) | 15 (83%) | |
| TNM stage | .002 | |||
| I/II | 23 (37%) | 11 (25%) | 12 (67%) | |
| III/IV | 39 (63%) | 33 (75%) | 6 (33%) | |
Abbreviations: AQP1, aquaporin 1; TNM, tumor-node-metastasis.
a P value is obtained from χ2 test.
bStatistically significant, P < .05.
AQP1 Protein Expression and the OS Time
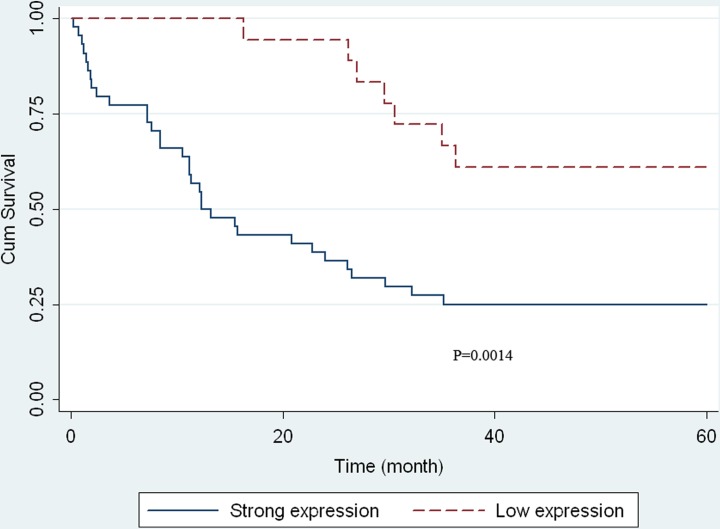
Univariate analysis by Kaplan-Meier plots revealed that strong AQP1 expression was significantly associated with unfavorable OS (P = .0014, log-rank test). Kaplan-Meier curves did not show any survival differences in hilCC indicated by other parameters, including sex, age, and tumor grading. However, survival distributions were significantly different in patients with hilCC of different TNM stage. A characteristic plot AQP1 expression is shown in Figure 3. Multivariate Cox analysis indicated that TNM stage (P < .001) and AQP1 expression (P = .002) were independent variables. Tumor-node-metastasis stage III or IV and strong AQP1 expression were associated with adverse OS (Table 2).
Figure 3.
Kaplan-Meier curve of patients in the strong aquaporin 1 (AQP1) expresser and the low ones. The significance of the Kaplan-Meier curve is shown in the figure and was calculated using a log-rank test.
Table 2.
Multivariate Cox Analysis of Survival in Hilar Cholangiocarcinoma.
| Variables | HR | 95% CI | P Value |
|---|---|---|---|
| Sex: male vs female | 0.897 | 0.525-1.533 | .691 |
| Age: ≥65 vs <65 | 1.305 | 0.757-2.250 | .339 |
| Histologic grading: low, moderate vs high | 1.508 | 0.872-2.611 | .141 |
| Lymph node involvement: yes vs no | 1.461 | 0.879-2.427 | .143 |
| Perineural invasion: yes vs no | 1.230 | 0.709-2.133 | .461 |
| AQP1 expression: strong vs low | 2.288 | 1.364-3.831 | .002 |
| TNM stage: III/IV vs I/II | 12.105 | 5.289-27.706 | <.001 |
Abbreviations: AQP1, aquaporin 1; CI, confidence interval; HR, hazard ratio; TNM, tumor-node-metastasis.
Discussion
In the present study, we investigated the R2 bioinformatic platform to screen the aberrant genes related to hilCC. Aquaporin 1 was selected for further analysis as our target because of the obvious expression variation in cancer samples. To the best of our knowledge, the clinicopathological significance of AQP1 has not been fully studied in hilCC. We examined the messenger RNA expression of AQP1 in hilCC samples using qPCR to verify the results. Subsequently, we measured AQP1 expression in a total of 62 hilCC by immunohistochemistry and examined the detailed relationships between the protein expression levels and clinicopathological factors as well as patient outcomes. The prognosis of patients with hilCC is poor, and the survival rate reported so far describes a very limited life expectancy. The prognosis of hilCC has not been improved significantly during the past several decades. The TNM staging is an admitted parameter to predict the prognosis of malignant tumors. However, it is not sufficient to differentiate the likelihood of survival in the same stage cases. Therefore, it is important to identify molecular biomarkers for prognosis or novel targets for intervention. Although the P27, laminin-γ2, and MTSS1 have been reported as prognostic markers for cholangiocarcinoma,22–25 most of the proposed biological prognostic parameters have not proven reliable enough to be systematically adopted in clinical practice.10,26–30 Aquaporin 1 has been reported as a highly selective marker for the diagnosis of cholangiocarcinoma in previous studies,10,31 and we evaluated its significance as a potential prognostic marker for hilCC in this study.
Aquaporin 1 is a membrane transport protein that assembles in membranes act primarily as a water-selective pore, facilitating osmotically driven water transport across cell plasma membranes.26,30 The level of AQP1 expression has also been found to have significance in tumors. High AQP1 expression has been shown as an independent predictive value for poor outcome.16,17,24 Aquaporin 1-expressing tumor cells were also showed increased plasma membrane osmotic water permeability and accelerated migration.13 In our series of 62 hilCC, proportional hazard regression analysis (Table 2) showed that AQP1 expression was an independent significant predictor of survival (P < .01). Pathological parameters, such as vascular, perineural invasion, lymph node involvement, and tumor grade all failed to correlate with outcome, whereas TNM stage do have prognostic value in hilCCs. We suggested that AQP1 should be a marker in prognosis of hilCC. However, AQP1 is also involved in tumor angiogenesis,11,12 the tumor has not been microdissected in our study, so we must make this conclusion with caution.
In our study, we showed that high AQP1 expresser with hilCC have poor survivals. Experimental results from AQP1-null mice also greatly showed reduced tumor growth and improved survival (compared with wild-type mice) when implanted subcutaneously with tumor cells,9 and in vitro studies indicated the same results.16,17,24 A possible reason is that AQP1 in tumor cells allow water to rapidly penetrate into the growing tumor mass and tumor AQP1 expression may thus cause tumor expansion by exacerbating tumor-associated edema that might be correlated with poor prognosis. In this clinical survey on AQP1 expression in vitro, we found strong AQP1 expression is an independent indicator for an adverse prognosis in hilCC. These results imply that AQP1 could also be a potential therapeutic target in hilCC. However, more studies on this gene will need to be done in future to assess long-term oncological outcomes for hilCC treatment.
Supplementary Material
Abbreviations
- AQP1
aquaporin 1
- DAB
3,3′-diaminobenzidine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- hilCC
hilar cholangiocarcinoma
- OS
overall survival
- qPCR
quantitative polymerase chain reaction
- TNM
tumor-node-metastasis
Footnotes
Authors’ Note: Chunxiang Li and Xiaofu Li contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Scientific Research Foundation for PhD faculty (BS2012-08), Harbin Medical University, China.
Supplemental Material: The online Supplemental Figure is available at http://journals.sagepub.com/doi/suppl/10.1177/1533034616646288.
References
- 1. de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341(18):1368–1378.doi:10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 2. Altemeier WA, Gall EA, Zinninger MM, Hoxworth PI. Sclerosing carcinoma of the major intrahepatic bile ducts. AMA Arch Surg. 1957;75(3):450–460; discussion 460-461. [DOI] [PubMed] [Google Scholar]
- 3. Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241–256. [DOI] [PubMed] [Google Scholar]
- 4. Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463–473; discussion 473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briggs CD, Neal CP, Mann CD, Steward WP, Manson MM, Berry DP. Prognostic molecular markers in cholangiocarcinoma: a systematic review. Eur J Cancer. 2009;45(1):33–47. doi:10.1016/j.ejca.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 6. Verkman AS, Hara-Chikuma M, Papadopoulos MC. Aquaporins—new players in cancer biology. J Mol Med (Berl). 2008;86(5):523–529. doi:10.1007/s00109-008-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87(6):621–623. doi:10.1038/sj.bjc.6600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoque MO, Soria JC, Woo J, et al. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol. 2006;168(4):1345–1353. doi:10.2353/ajpath.2006.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan B, Zhu D, Dong Z, Yang Z. Expression and distribution of aquaporin 1 in laryngeal carcinoma [in Chinese]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;21(6):269–272. [PubMed] [Google Scholar]
- 10. Mazal PR, Susani M, Wrba F, Haitel A. Diagnostic significance of aquaporin-1 in liver tumors. Hum Pathol. 2005;36(11):1226–1231. doi:10.1016/j.humpath.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11. Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434(7034):786–792. doi:10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 12. El Hindy N, Bankfalvi A, Herring A, et al. Correlation of aquaporin-1 water channel protein expression with tumor angiogenesis in human astrocytoma. Anticancer Res. 2013;33(2):609–613. [PubMed] [Google Scholar]
- 13. Hara-Chikuma M, Verkman AS. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J Am Soc Nephrol. 2006;17(1):39–45.doi:10.1681/ASN.2005080846. [DOI] [PubMed] [Google Scholar]
- 14. Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20(11):1892–1894. doi:10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- 15. Kang BW, Kim JG, Lee SJ, et al. Expression of aquaporin-1, aquaporin-3, and aquaporin-5 correlates with nodal metastasis in colon cancer. Oncology. 2015;88(6):369–376. doi:10.1159/000369073. [DOI] [PubMed] [Google Scholar]
- 16. Yoshida T, Hojo S, Sekine S, et al. Expression of aquaporin-1 is a poor prognostic factor for stage II and III colon cancer. Mol Clin Oncol. 2013;1(6):953–958. doi:10.3892/mco.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kao SC, Armstrong N, Condon B, et al. Aquaporin 1 is an independent prognostic factor in pleural malignant mesothelioma. Cancer. 2012;118(11):2952–2961. doi:10.1002/cncr.26497. [DOI] [PubMed] [Google Scholar]
- 18. Morrissey JJ, Kharasch ED. The specificity of urinary aquaporin 1 and perilipin 2 to screen for renal cell carcinoma. J Urol. 2013;189(5):1913–1920. doi:10.1016/j.juro.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C, Cai S, Wang X, Jiang Z. Hypomethylation-associated up-regulation of TCF3 expression and recurrence in stage II and III colorectal cancer. PLoS One. 2014;9(11):e112005 doi:10.1371/journal.pone.0112005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang Z, Xu Y, Cai S. Down-regulated GAS1 expression correlates with recurrence in stage II and III colorectal cancer. Hum Pathol. 2011;42(3):361–368. doi:10.1016/j.humpath.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 21. Santo EE, Ebus ME, Koster J, et al. Oncogenic activation of FOXR1 by 11q23 intrachromosomal deletion-fusions in neuroblastoma. Oncogene. 2012;31(12):1571–1581. doi:10.1038/onc.2011.344. [DOI] [PubMed] [Google Scholar]
- 22. Hui AM, Cui X, Makuuchi M, Li X, Shi YZ, Takayama T. Decreased p27(Kip1) expression and cyclin D1 overexpression, alone and in combination, influence recurrence and survival of patients with resectable extrahepatic bile duct carcinoma. Hepatology. 1999;30(5):1167–1173. doi:10.1002/hep.510300506. [DOI] [PubMed] [Google Scholar]
- 23. Fiorentino M, Altimari A, D’Errico A, et al. Low p27 expression is an independent predictor of survival for patients with either hilar or peripheral intrahepatic cholangiocarcinoma. Clin Cancer Res. 2001;7(12):3994–3999. [PubMed] [Google Scholar]
- 24. Taguchi K, Aishima S, Asayama Y, et al. The role of p27kip1 protein expression on the biological behavior of intrahepatic cholangiocarcinoma. Hepatology. 2001;33(5):1118–1123. doi:10.1053/jhep.2001.24028. [DOI] [PubMed] [Google Scholar]
- 25. Wang F, Liu Y, Zhang H. Loss of MTSS1 expression is an independent prognostic factor for hilar cholangiocarcinoma. Pathol Oncol Res. 2013;19(4):815–820. doi:10.1007/s12253-013-9649-6. [DOI] [PubMed] [Google Scholar]
- 26. Tannapfel A, Engeland K, Weinans L, et al. Expression of p73, a novel protein related to the p53 tumour suppressor p53, and apoptosis in cholangiocellular carcinoma of the liver. Br J Cancer. 1999;80(7):1069–1074. doi:10.1038/sj.bjc.6690465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47(5):721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Argani P, Shaukat A, Kaushal M, et al. Differing rates of loss of DPC4 expression and of p53 overexpression among carcinomas of the proximal and distal bile ducts. Cancer. 2001;91(7):1332–1341. [PubMed] [Google Scholar]
- 29. Isa T, Tomita S, Nakachi A, et al. Analysis of microsatellite instability, K-ras gene mutation and p53 protein overexpression in intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2002;49(45):604–608. [PubMed] [Google Scholar]
- 30. Tannapfel A, Sommerer F, Benicke M, et al. Genetic and epigenetic alterations of the INK4a-ARF pathway in cholangiocarcinoma. J Pathol. 2002;197(5):624–631. doi:10.1002/path.1139. [DOI] [PubMed] [Google Scholar]
- 31. Dong H, Cong WL, Zhu ZZ, Wang B, Xian ZH, Yu H. Evaluation of immunohistochemical markers for differential diagnosis of hepatocellular carcinoma from intrahepatic cholangiocarcinoma [in Chinese]. Zhonghua Zhong Liu Za Zhi. 2008;30(9):702–705. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials