Abstract
One of the most lethal carcinomas is pancreatic cancer. As standard treatment using chemotherapy and radiation has shown limited success, thermal regimens (cryotherapy or heat ablation) are emerging as viable alternatives. Although promising, our understanding of pancreatic cancer response to thermal ablation remains limited. In this study, we investigated the thermal responses of 2 pancreatic cancer cell lines in an effort to identify the minimum lethal temperature needed for complete cell death to provide guidance for in vivo applications. PANC-1 and BxPC-3 were frozen (−10°C to −25°C) or heated (45°C-50°C) in single and repeated exposure regimes. Posttreatment survival and recovery were analyzed using alamarBlue assay over a 7-day interval. Modes of cell death were assessed using fluorescence microscopy (calcein acetoxymethyl ester/propidium iodide) and flow cytometry (YO-PRO-1/propidium iodide). Freezing to −10°C resulted in minimal cell death. Exposure to −15°C had a mild impact on PANC-1 survival (93%), whereas BxPC-3 was more severely damaged (33%). Exposure to −20°C caused a significant reduction in viability (PANC-1 = 23%; BxPC-3 = 2%) whereas −25°C yielded complete death. Double freezing exposure was more effective than single exposure. Repeat exposure to −15°C resulted in complete death of BxPC-3, whereas −20°C severely impacted PANC-1 (7%). Heating to 45°C resulted in minimum cell death. Exposure to 48°C yielded a slight increase in cell loss (PANC-1 = 85%; BxPC-3 = 98%). Exposure to 50°C caused a significant decline (PANC-1 = 70%; BxPC-3 = 9%) with continued deterioration to 0%. Double heating to 45°C resulted in similar effects observed in single exposures, whereas repeated 48°C resulted in significant increases in cell death (PANC-1 = 68%; BxPC-3 = 29%). In conclusion, we observed that pancreatic cancer cells were completely destroyed at temperatures <−25°C or >50°C using single thermal exposures. Repeated exposures resulted in increased cell death at less extreme temperatures. Our data suggest that thermal ablation strategies (heat or cryoablation) may represent a viable technique for the treatment of pancreatic cancer.
Keywords: pancreatic cancer, cryoablation, hyperthermia, thermal therapy, apoptosis, lethal temperature
Introduction
Pancreatic cancer (PaCa) is currently the fourth leading cause of cancer-related deaths in the United States and the eighth worldwide. It is projected that over 48 000 individuals will be diagnosed and over 40 000 will succumb to PaCa in 2015.1 The high lethality rates of PaCa are due to a lack of effective treatment options.2 The most common treatment of PaCa is a combination of chemotherapy (eg, gemcitabine, 5-fluorouracil, etc) and radiation.3–6 This combinatorial regimen is insufficient to provide a cure to PaCa and often is merely palliative for patients.2,7–9 The reasons why many cancer cells, including PaCa, are resilient to conventional therapies can be attributed to “hallmarks of cancer” as well as the presence of cancer stem cells.10 Specifically, these hallmarks are resisting cell death, sustaining proliferative signaling, evading growth suppressors, activation of metastasis and invading cells, inducing angiogenesis, and enabling immortalized replication and proliferation.10 One of these maxims, resisting cell death, directly relates to the normal cell death functions of apoptosis and necrosis. Apoptosis, programmed cell death, is often suppressed in most cancer types.10–14 Necrosis, an inflammation-based cell death, results in the circulation of intracellular contents around the site of cell death.10,11,13,15 It is hypothesized that in some cases, the activation of necrosis leads to further inflammation and possibly the spread of cancer-promoting chemokines.10,11,13 With an improved understanding of cell death mechanisms in solid cancers, researchers have become better able to treat these diseases by accounting for the aforementioned hallmarks. As modern research has improved, the survival rates of individuals treated for many cancers have improved as well. Pancreatic cancer stands as a notable exception with 5-year survival rates remaining in the single digits and an average lifespan of 6 months postdiagnosis.1 As conventional options have not provided an effective treatment of PaCa, there is a need for the development of new treatments to extend patient lives and work toward a cure.
Thermal therapies, such as cryotherapy and hyperthermia, represent an area of continued growth as a cancer treatment over the last decade.5 In many cases, cancer cells are found to be more susceptible to thermal exposure than drug-related therapies. This is due to a cell being physically stressed to induce cell death, bypassing many other genetic-based cancer defense mechanisms. Freezing tissues causes cell rupture, hypoxia, ischemia, reperfusion, and extreme hypothermia.16–21 Heating cells results in protein denaturation, hyperthermia, cell membrane failure, scarring, and destruction of tissue.22,23 As the technologies used to apply a thermal injury have improved so has the effectiveness of the respective therapies. Thermal techniques possess a unique ability in that, unlike surgical resection, they are less invasive and require shorter recovery times.8,20,23 This is a critical factor as the location of the pancreas is a major challenge in the treatment of PaCa.24
Cryotherapy is a minimally invasive technique that uses a cryogen, such as liquid nitrogen (LN2) or argon gas, to freeze diseased tissue to ultracold temperatures.16–21 Cryoablation subjects tissues to multiple cellular stresses beyond freezing, including hypoxia, osmotic imbalance, ischemia, and other forms of molecular stress.16,21,25–27 One of the main uses of cryotherapy is the treatment of prostate cancer. In 2008, the American Urological Association (AUA) published a best practice statement on cryotherapy for the treatment of prostate cancer.28 The AUA and others also recognize cryotherapy as an effective method for the treatment of renal cancer.3,28,29 Although cryotherapy of the pancreas has been limited within the United States and reserved primarily for pancreatitis, cryotherapy offers great potential for the treatment of PaCa.30–33
Heat ablation, such as radiofrequency (RFA) and high-frequency ultrasound, is also a minimally invasive technique that induces a state of hyperthermia (>37°C) in cells and tissues. Radiofrequency has been utilized for the treatments of liver, kidney, and other solid tumors but has not been used to a great extent in the treatment of PaCa.22,34 Although this technique is relatively untested in PaCa, given the reported success in other cancers, the use of heat ablation may also provide for an effective course of treatment for PaCa.
Given the ability of cancer to evade many molecular-based therapeutic strategies coupled with the multifaceted insult (physical and molecular) provided by thermal therapies, we investigated the response of PaCa cells to both cryoablation and hyperthermia. We hypothesized that understanding the characteristics of PaCa cell response to thermal ablation would result in the identification of an improved therapeutic guidance protocol for the treatment of PaCa. Given that the response of PaCa cells to thermal ablation has not been characterized, we investigated PaCa cell response to freezing and heating in an effort to determine the minimum thermal exposures necessary to cause complete cell destruction. The results herein suggest that thermal therapies may be a viable treatment option for the treatment of PaCa.
Materials and Methods
Cell Culture
PANC-1 (CRL-1469) and BxPC-3 (CRL-1687) cell lines were obtained from the American Type Culture Collection (Rockville, Maryland). Dubecco modified Eagle medium was used to culture PANC-1 cells, whereas RPMI-1640 medium was used for BxPC-3 (Caisson Laboratories Inc, Logan, Utah). Cell culture media were supplemented using 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, Georgia) and 1% penicillin/streptomycin (Corning Inc, Corning, New York). Cells were seeded onto Costar 96-stripwell plates (Corning Inc) at a cell density of approximately 3.75 × 104 cells/cm2 with appropriate medium 24 hours prior to experimentation.
Thermal Exposures
Culture medium was exchanged with 75 µL/well fresh media 30 minutes prior to experimentation. Eight-well strips were placed into aluminum blocks in a temperature controlled bath set to achieve and maintain target temperatures. For freezing experiments, samples were exposed to −10°C, −15°C, −20°C, and −25°C for 5 minutes. When sample temperature approached −2°C, ice nucleation was initiated with LN2 vapor to prevent supercooling of samples. Following freezing, samples were placed at room temperature (RT) for 10 minutes to passively thaw before being returned to standard culture conditions. For heating experiments, samples were exposed to 45°C, 48°C, and 50°C for 5 minutes. After the heat exposure, samples were removed and placed at RT for 1 minute before returning to culture conditions. Thermal profiles of samples under each condition were recorded using a T-type thermocouple (Omega Engineering, Stamford, Connecticut).
Double Thermal Exposure
Temperatures of −10°C, −15°C, or −20°C were utilized for double freezing exposure. As in the single-exposure experiments, Costar strips were placed into precooled aluminum blocks, and samples were seeded at −2°C to prevent supercooling. Following the initial 5-minute freeze, samples were removed from the bath, passively thawed at RT for 5 minutes, and then were subjected to a second 5-minute exposure to the same temperature. After the second freeze, samples were passively thawed at RT for 10 minutes and then returned to 37°C culture for recovery. For double heating, temperature exposures of 45°C, 48°C, and 50°C were utilized. Samples were placed into preheated aluminum blocks for 5 minutes, placed at RT for 1 minute, and then exposed to the same temperature for an additional 5 minutes. After the second heat exposure was completed, samples were given 1 minute at RT to recover before returning to culture conditions. Thermal profiles of each sample condition were recorded using a T-type thermocouple (Omega Engineering).
Cell Viability Assay
Viability of PANC-1 and BxPC-3 cells was assessed using the alamarBlue metabolic activity assay. AlamarBlue solution (Invitrogen, Carlsbad, California) constituted in a 1:20 dilution in Hanks balanced salt solution with Ca and Mg (Mediatech, Inc, Herndon, Virginia). Culture medium was removed, and cells were subjected to 100 µL/well alamarBlue solution at 37°C for 60 minutes. Immediately following incubation, samples were analyzed using a Tecan SPECTRAFluor Plus (Tecan, Austria) at excitation/emission of 530 nm and 590 nm. Sample raw fluorescence units (RFUs) were converted to percentage viability based on the pretreatment control RFUs. Viability assessment was conducted for 7 days on days 1, 3, 5, and 7 following thermal exposure.
Apoptosis/Necrosis Assay
Relative levels of apoptosis and necrosis were determined using microfluidic flow cytometry. At 1, 4, 8, and 24 hours following thermal exposures, samples were incubated with propidium iodide (3.75 µmol/L; Molecular Probes, Eugene, Oregon) and YO-PRO-1 (0.35 µmol/L; Invitrogen) for 15 minutes at 37°C. Cells were then lifted from the Costar plates and analyzed using a Guava EasyCyte Plus flow cytometer (Millipore, Billerica, Massachusetts) using a 488 nm excitation laser. Gates were set using an unstained, nonfluorescence control and a positively treated control exposed to Camptothecin (0.2 mmol/L) to induce apoptosis. Flow cytometer collected 5000 events per condition, in triplicate, and the populations of live, apoptotic, necrotic, and secondary necrotic cells were quantified as a percentage of the analyzed populations.
Fluorescence Microscopy
PANC-1 and BxPC-3 cells were probed with propidium iodide (3.75 µmol/L) and calcein acetoxymethyl ester (2.5 µmol/L; EMD Chemicals; Millipore) for 30 minutes at 37°C at 1, 4, 8, and 24 hours after thermal exposures. Following incubation, cells were visualized using a Zeiss Axiovert 200 fluorescence microscope (Carl Zeiss, Oberkochen, Germany) at 20× magnification. Fluorescence micrographs were complemented with phase microscopy to permit visualization of changes in cell morphology. Fluorescence images were correlated with flow cytometry data to verify the levels of cell death.
Statistical Analysis
Statistical significance was determined using single factor analysis of variance and t test. Standard error was used to represent experimental variability. All experiments were repeated a minimum of 3 times (N = 3) with an interexperimental replicate of n = 7. Statistical significance is denoted by P < .05 unless stated otherwise.
Results
Assessment of PaCa to Freezing Injury
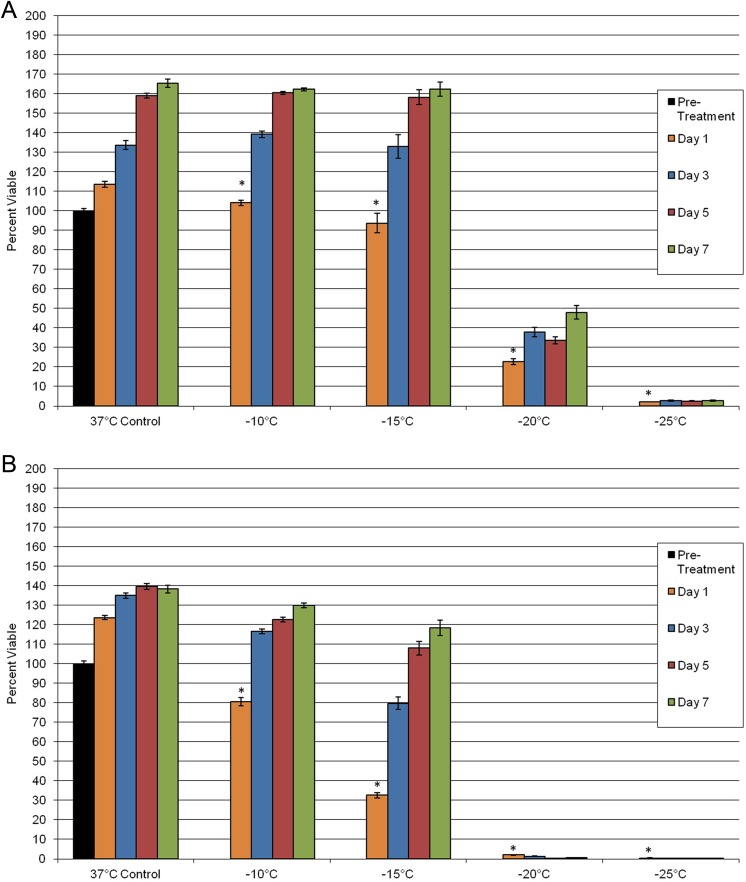
To determine the impact of freezing on PaCa viability, PANC-1 and BxPC-3 cultures were exposed to −10°C, −15°C, −20°C, and −25°C, and then sample viability and repopulation were assessed. Exposure to −10°C resulted in minimal cell death in both PANC-1 and BxPC-3 samples (Figure 1). When samples were exposed to −15°C, a decrease in postfreeze viability was observed in both cell lines. PANC-1 exposure to −15°C yielded a slight decrease in overall viability compared to prefreeze controls, 93% (±5) versus 100% (±1), respectively. Interestingly, when BxPC-3 cells were exposed to −15°C, a marked reduction in viability was observed, 33% (±1), compared to time-matched controls, 100% (±1). As the exposure temperature was decreased to −20°C, cell death was found to increase in both the PANC-1 and the BxPC-3 samples compared to their nonfrozen controls, 23% (±2) and 2% (±0.1) survival, respectively. Following exposure to −25°C, both cell lines yielded minimal survival (<2%), which was consistent with complete ablation. Assessment of cell recovery following freezing revealed that both PANC-1 and BxPC-3 cells were able to repopulate in culture following exposure to −10°C and −15°C, whereas exposure to −20°C and −25°C resulted in stunted to no recovery in both cell systems over the 7-day postfreeze assessment period.
Figure 1.
Assessment of posttreatment viability and recovery of PaCa cells following exposure to a mild freezing insult. PANC-1 (A) and BxPC-3 (B) cells were subjected to freezing, and survival was assessed over 7 days posttreatment. Viability assessment indicated complete cell death was achieved following exposure to temperatures below −25°C for both cell types. (*P < .05). PaCa indicates pancreatic cancer.
Assessment of Heating Injury on PaCa Cells
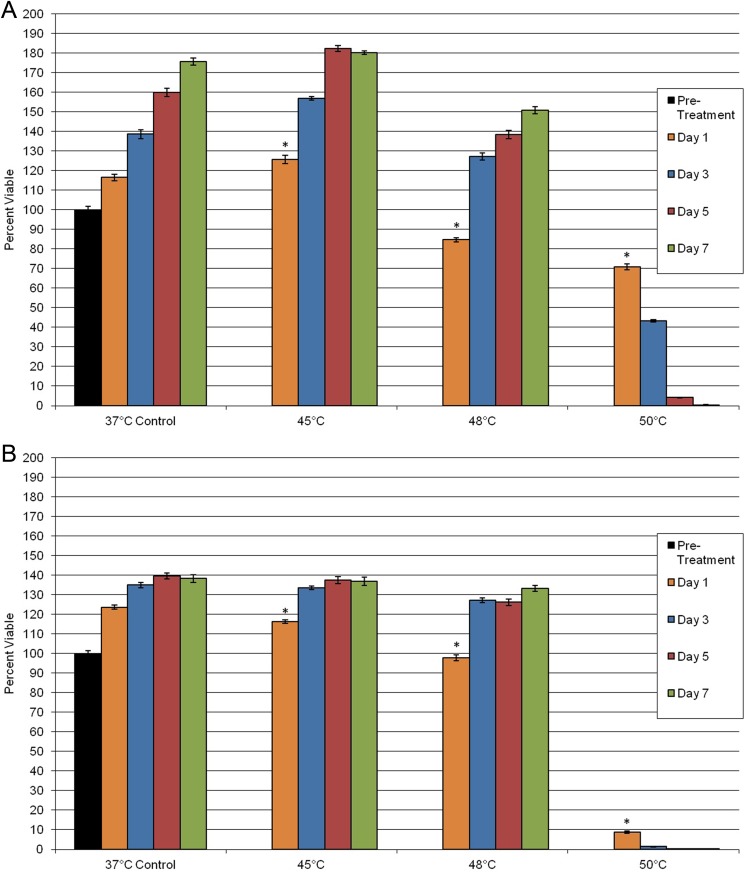
To determine the effect of heating on PaCa cell viability, samples were exposed to mild hyperthermic temperatures of 45°C, 48°C, and 50°C (Figure 2). Following exposure to 45°C, no significant impact on cell death was observed in both the PANC-1 and the BxPC-3 samples compared to nontreated controls. Exposure to 45°C also yielded no long-term impact on sample repopulation over the 7-day assessment interval. Following exposure to 48°C, PANC-1 samples yielded a 15% decrease in viability compared to pretreatment controls, 85% (±1) versus 100% (±2), respectively. BxPC-3 cells after 48°C exposure revealed minimal impact on survival, 98% (±1), compared to pretreatment control samples, 100% (±1). Despite the initial day 1 decrease in viability, both PANC-1 and BxPC-3 samples were able to repopulate to near control levels within the 7-day assessment period. In contrast to 48°C, exposure to 50°C resulted in a significant decline in sample viability on day 1 following exposure for both cell lines. PANC-1 day 1 survival was 70% (±2) and continued to decline to 0% by day 7. BxPC-3 samples were found to have a day 1 viability of 8% (±1), which declined to 0% by day 5. In both cell lines, it was observed that cells subjected to 50°C became rounded in appearance, with a small population of those cells losing adherence to the culture plate (Figure 3). These cells did not regain their normal morphology at any point after heat exposure to 50°C.
Figure 2.
Assessment of PaCa cell viability following exposure to mild hyperthermic treatment. Impact on PANC-1 (A) or BxPC-3 (B) cell proliferation was assessed over a 7-day interval following mild hyperthermia. The results suggest that following exposure to 50°C or warmer complete cell death was attained in both cell models. (*P < .05). PaCa indicates pancreatic cancer.
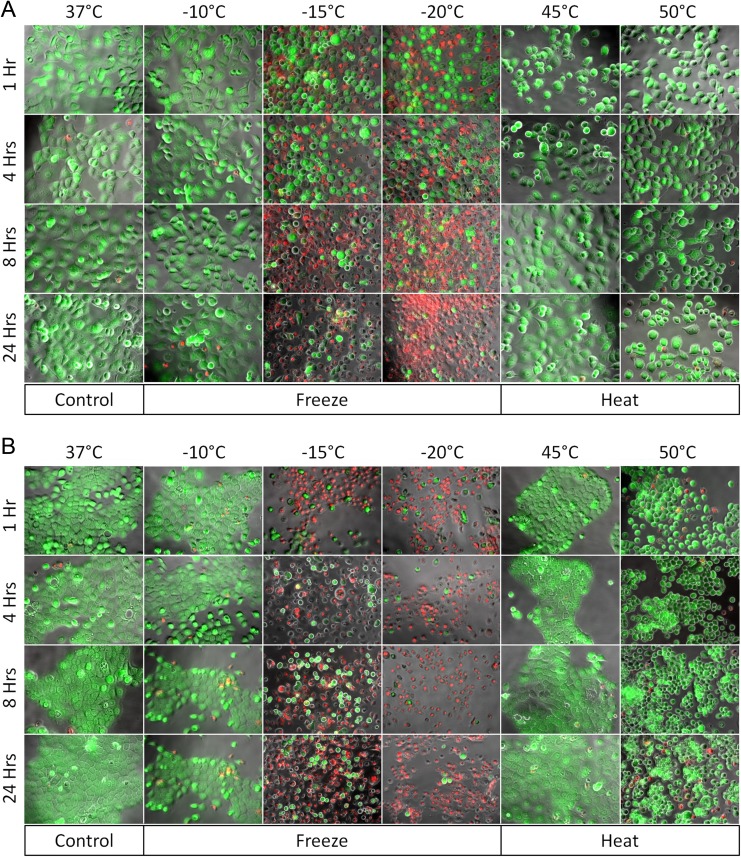
Figure 3.
Fluorescence micrographs of PaCa cells following thermal exposures. Following thermal exposures, PANC-1 (A) or BxPC-3 (B) cells were stained with propidium iodide (red) and Calcein-AM (green) at 1, 4, 8, and 24 hours and visualized under ×20 magnification. Green fluorescence indicates live, whereas red fluorescence indicates dead cells. Fluorescence images illustrate an increase in cell death at temperatures below −15°C and above 50°C at 24 hours posttreatment. These findings appear to correlate with postthaw viability results obtained from flow cytometry and alamarBlue analysis. AM indicates acetoxymethyl ester; PaCa, pancreatic cancer.
Comparison of Thermal Sensitivity of PaCa Cells
In addition to determining PaCa cell response to both freezing and heating injury, investigation of 2 distinct molecular variants of PaCa was conducted to compare their individual response to thermal ablation. To this end, the BxPC-3 cells were found to be more sensitive to thermal injury than PANC-1 cells. Exposure of BxPC-3 samples to −15°C resulted in a marked decrease in viability on day 1, whereas in PANC-1 samples, minimal cell death was observed, 33% (±1) versus 93% (±5) survival, respectively (Figure 1). Exposure to −20°C yielded complete cell destruction in BxPC-3 samples, whereas a significant population of PANC-1 cells survived −20°C, 2% (±0.1) versus 23% (±2) survival, respectively. A similar but less marked trend was observed in response to heating exposure. In the case of heat, exposure to 50°C resulted in a differential initial day 1 viability between the PANC-1 and the BxPC-3 samples, 70% (±2) versus 8% (±1), respectively. Despite the initial response, by day 7, both BxPC-3 and PANC-1 cells were completely dead (Figure 2). Fluorescence microscopy indicated that the different cells subjected to the same exposures experienced varying levels of cell death at different points within the initial 24 hours postexposure (Figure 3). These results indicate that, as with other cancers,25–26,35 PaCa molecular variants exhibit differential responses to mild thermal treatment. However, these data suggest that when a critical temperature threshold is surpassed (<−25°C or >50°C), complete cell death results regardless of the molecular disposition.
Investigation of the Modes of Cell Death Following Thermal Exposure
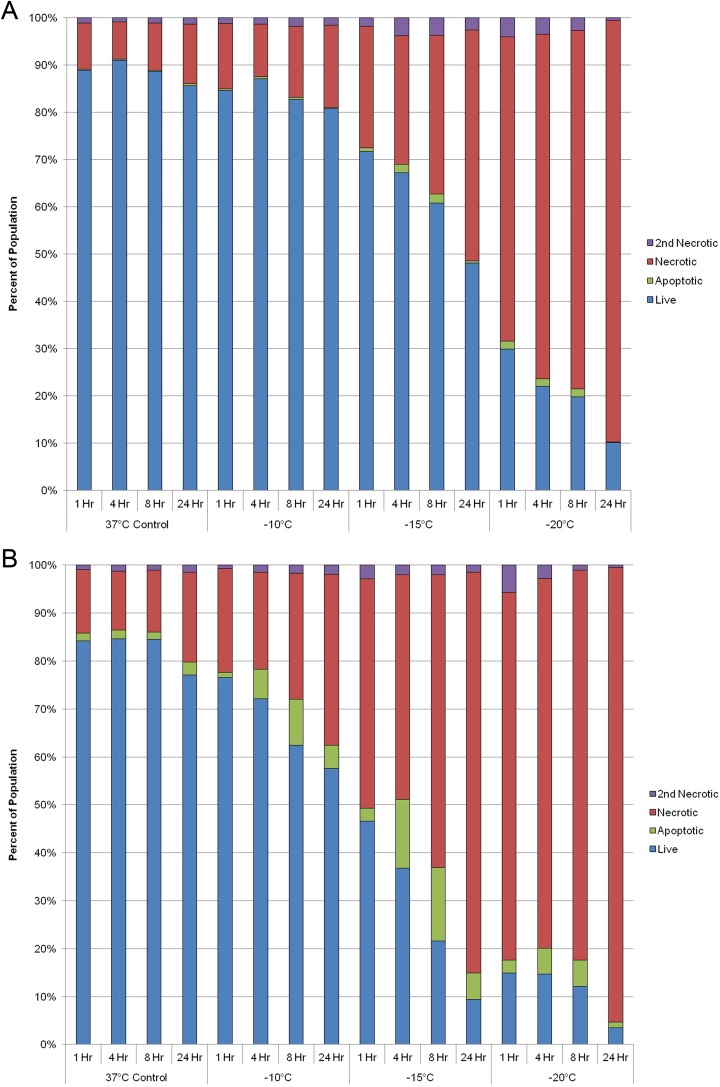
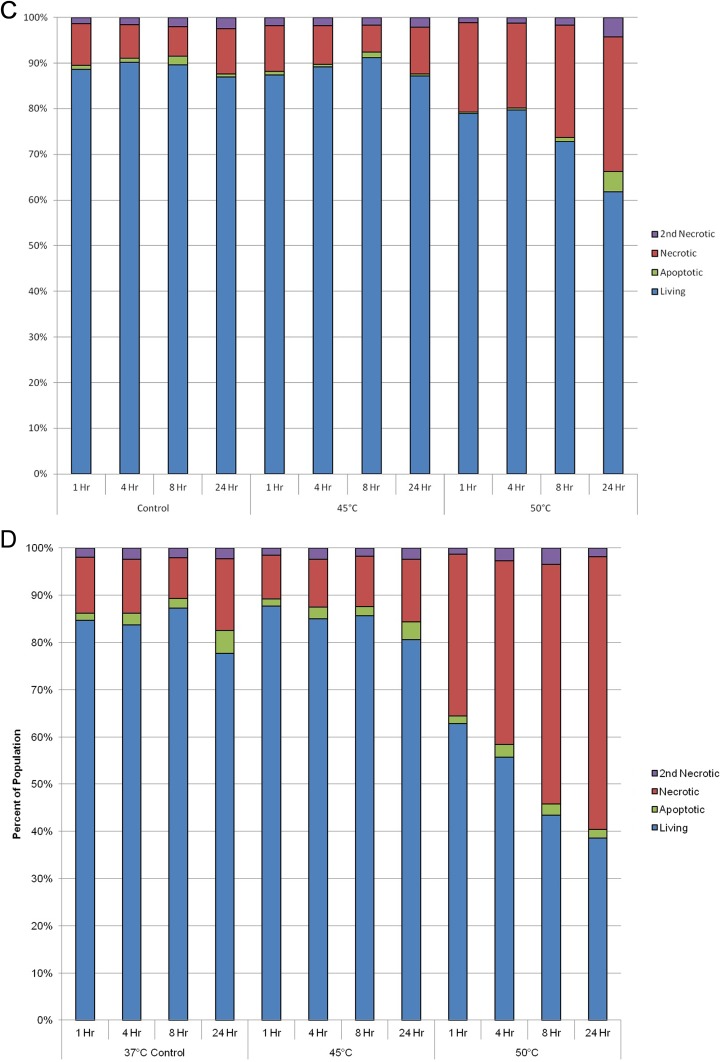
With the identification of a differential response of PaCa cells to thermal exposure, we investigated the relative contributions of apoptosis and necrosis to overall cell death. To this end, samples were analyzed at 1, 4, 8, and 24 hours posttreatment using microfluidic flow cytometry to detect the relative levels of apoptosis, necrosis, and live cells within the assessed populations. Temporal analysis posttreatment revealed necrosis to be the predominant mode of cell death following freezing and heating in both PANC-1 and BxPC-3 samples (Figure 4). Furthermore, the amount of necrotic cell death was found to increase in all samples over the 24-hour assessment period. Interestingly, in the BxPC-3 samples exposed to −15°C, a significant level of apoptotic cell death was observed at 4 and 8 hours postfreeze (8% and 15%, respectively; Figure 4B). While to a lesser extent, the 4- and 8-hour increases in apoptosis in BxPC-3 samples were also observed following exposure to −10°C and −20°C (Figure 4B). This was in contrast to PANC-1 samples where a minimal level of apoptosis was observed at any time point postfreeze (Figure 4A). As with freezing, an increased amount of apoptosis was found in BxPC-3 samples following heating compared to PANC-1 samples (Figure 4D and C, respectively). While increased, the overall levels were much lower in heat-treated BxPC-3 samples than freeze exposure samples. From this analysis it was found that in addition to physical rupture, both molecular based apoptotic and necrotic processes played a significant role in PaCa cell death following a mild thermal insult.
Figure 4.
Assessment of the modes of cell death following thermal treatment. PANC-1 (A and C) and BxPC-3 (B and D) cells were subjected to selected temperatures and assessed using microfluidic flow cytometry. Samples were stained with propidium iodide and YO-PRO-1 to detect levels of necrosis and apoptosis at 1, 4, 8, and 24 hours postthermal exposure. Data illustrate necrosis is the predominant mode of cell death following both mild freezing and heat treatment of PaCa cells, whereas apoptosis contributes to a lesser degree. PaCa indicates pancreatic cancer.
Impact of Repeated Thermal Exposure
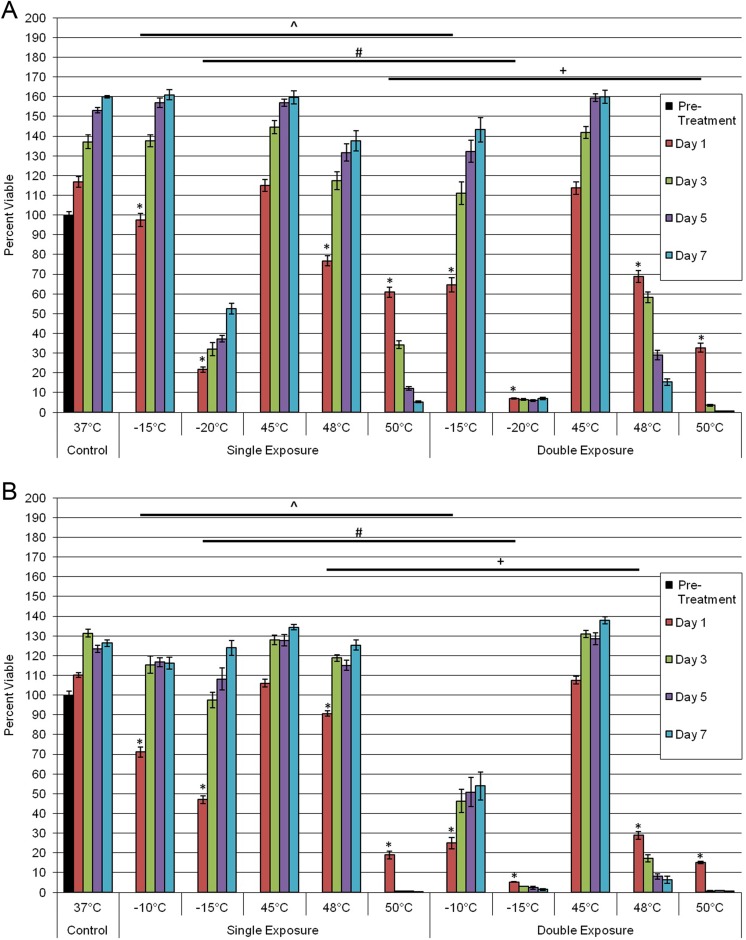
Previous reports have indicated that repeated exposure of cancer cells to a thermal insult results in increased cell death.18–19,25–27 To this end, we investigated the impact of a double freeze (F/T/F) or a double heat (H/T/H) protocol on PaCa survival. In PANC-1 samples, a double freeze exposure to −15°C resulted in an initial decrease in day 1 viability compared to a single freeze, 65% (±4) versus 97% (±3), respectively. However, cells were able to repopulate to near controls within the 7-day assessment period (Figure 5A). Repeat exposure to −20°C resulted in an increase in overall cell death on day 1, 7% (±0.3) versus 22% (±2), respectively. Further, double −20°C exposure resulted in the inability of PANC-1 cells to repopulate over the 7-day interval, which was in contrast to the single-exposure samples. Similar observations were made for BxPC-3 repeat freeze samples at −10°C and −15°C (Figure 5B). Repeat exposure of BxPC-3 cells to −10°C resulted in a significant decrease in day 1 postthaw survival compared to single freeze exposure, 25% (±3) versus 71% (±3), respectively. As with PANC-1 samples exposed to −15°C, BxPC-3 samples were able to recover over the 7-day analysis following a repeat exposure to −10°C. Repeat exposure to −15°C in the BxPC-3 samples, however, resulted in complete cell death at 1 day postfreeze with no observed cell recovery (Figure 5B). This was in contrast to a single exposure to −15°C, which resulted in 47% (±2) day 1 survival compared to 5% (±0.3) in double freeze samples.
Figure 5.
Comparison of single versus repeat thermal exposure in PaCa cells. PANC-1 (A) or BxPC-3 (B) cells were subjected to select freezing or heating temperatures either as a single exposure or a double exposure. Viability was assessed over a 7-day recovery period to compare initial death response and recovery in each condition. Data suggest that repeat exposure results in increased cell death at milder temperatures compared to a single exposure. (*,^,#,+P < .05). PaCa indicates pancreatic cancer.
A similar trend in increased cell death following repeat exposure to heating was observed in both PaCa cell lines. Repeat exposure to 50°C resulted in a decline in day 1 survival compared to a single exposure in both PANC-1, 61% (±3) versus 33% (±2), and BxPC-3, 19% (±2) versus 15% (±0.5), samples. As with the single exposures by day 7, complete cell death was achieved (Figure 5). Both single and double exposure to 45°C yielded no detectable cell death posttreatment. Repeat exposure to 48°C resulted in a differential response in both cell lines. At day 1 posttreatment, repeat exposure to 48°C resulted in an increase in cell death compared to single exposures in PANC-1, 69% (±3) versus 77% (±3), and BxPC-3, 29% (±2) versus 90% (±1). In addition to the initial increases in cell death, following repeat exposure to 48°C, a decline in cell viability was noted during the 7-day recovery period in contrast to single 48°C exposure experiments where cell population recovery was observed.
Overall, the data for the repeat thermal exposure experiments suggest that double exposure of PaCa to partially effective temperatures (PANC-1: −20°C and 48°C; BxPC-3: −15°C and 48°C) results in complete cell death, thereby extending the lethal isotherm into milder thermal ranges.
Discussion
Thermal ablation is now providing an effective path for the treatment of numerous cancers.5,8,19,29,33,34,36,37 While effective, thermal therapy has not yet been utilized to a great extent in the treatment of PaCas. Given the potential of thermal therapy as a treatment modality for PaCa, we investigated the response of PaCa cells to mild thermal treatment in an in vitro model. The intent of this study was to evaluate the impact of mild freezing and heating on PaCa using 2 model cell lines in an effort to characterize their thermal response and identify the minimum lethal isotherm necessary to achieve complete PaCa cell destruction in vitro. Mild ablation temperatures were selected as they are representative of temperatures attained in the periphery of an ablation zone distal to a thermal ablation probe in vivo. It is within this peripheral zone (>−40°C or <60°C) that partial cell death has been observed in other cancers and may result in cancer reoccurrence in vivo. While −40°C or 60°C isotherms serve as a reference point, the critical lethal temperature for PaCa has yet to be reported. In addition, given reports of varying sensitivity of different molecular variants of similar cancers by Klossner et al 35 and others, we also investigated 2 distinct molecular phenotypes of PaCa, PANC-1 and BxPC-3 cells.
In examining PaCa response to freeze injury, it was found that the BxPC-3 cells were more sensitive than PANC-1 cells. Despite this, it was found that a single freeze exposure of either cell type to −25°C resulted in complete cell death. The increased sensitivity of BxPC-3 cells to freezing resulting in complete cell destruction at −20°C is hypothesized to be due to phenotypic and genotypic differences between cell lines, as reported by Deer et al.38 This, however, needs to be investigated further. In support of this differential response, it was found that BxPC-3 samples exhibited a larger increase in the level of apoptosis postfreeze than PANC-1 samples within the assessed interval (Figure 4). The lack of apoptosis observed in PANC-1 may be the result of rapid apoptotic induction occurring within minutes postfreeze, as reported by Robilotto et al,37 resulting in this population being perceived as necrotic in the current study. Nonetheless, the increase in apoptotic response in BxPC-3 samples supports a molecular component to the differential sensitivity to mild freezing exposure.
Studies focused on heating revealed near-complete cell death attained in both PANC-1 and BxPC-3 cells following exposure to 50°C. Complete cell death was not seen immediately posttreatment but took several days to fully manifest in vitro. Although the data suggest that complete cell death was attained, the delayed period necessary may provide for a chance for recurrence in vivo where more complex biological factors come into play. While unlikely, it is nonetheless a limitation of this study which cannot be addressed.
The mechanisms responsible for triggering cell death in response to freezing consist of several factors, including nadir exposure temperature, rate of freezing and thawing, microcirculatory reperfusion injury after thawing, waste accumulation, osmotic imbalances, and energy depletion, among others.25 As cells freeze and thaw, ice crystals may cause physical rupturing of the cell membrane, resulting in cellular necrosis of the cells. This necrosis is observed most often in cells nearest the cryoprobe, wherein cells experience the coldest exposure temperatures during freezing.16–21 Furthermore, both intrinsic and extrinsic pathways of apoptotic induction have been shown to be triggered by freezing injury, with the specific pathway influenced by the final freezing temperature within the different areas of the cryogenic lesion.16–21,25 Regarding cell death mechanisms associated with hyperthermal ablation, several reports suggest that protein denaturation results in compromised cell function and onset of coagulative necrosis.22,23 To this end, tissues treated with hyperthermia are observed to result in scarring and complete loss of tissue architecture.25 Given the significant amount of damage that occurs as a result of hyperthermal ablation, including RFA, the observed result of necrosis as the primary mode of cell death with minimal contribution of apoptosis reported in this study appears in line with other reports.22,23 A more detailed analysis of cell death signals remains necessary to determine the exact molecular mechanisms by which heating and freezing induce cellular PaCa destruction.
When comparing the differential response and overall sensitivity of the PaCa cells to that of other cancers, it was found that PaCa cells are highly susceptible to thermal ablation. For example, the −25°C critical isotherm found for PaCa in vitro is much higher than the −40°C critical isotherm reported for prostate cancer.16,26–28 The PaCa response was similar to the −20°C isotherm reported for renal carcinoma.36 For both prostate and renal cancer, decreasing levels of survival have been reported following exposure to −15°C, −20°C, −30°C, and lower; however, it is not until the minimum lethal temperature is attained that complete cell death is achieved.18–21 For instance, Clark et al reported that exposure of renal cancer cells to −10°C or −15°C resulted in 77% and 32% viability, respectively, whereas freezing to temperatures below −20°C resulted in complete cell death.36 Robilotto et al and others reported induction of cell death after prostate cancer exposure to −15°C, resulting in 58% viability at 24 hours post-freeze.37 Despite the approximately 50% cell death expressed following exposure to −15°C, these reports have shown that complete destruction of prostate cancer cells is not achieved until temperatures reach −40°C or below in vitro.26–28 , 35,37 In regard to PaCa cells, in this study, we observed a moderate impact on the viability at −15°C or warmer, whereas temperatures below −20°C resulted in a more severe decrease in cell viability. Gage et al suggest that while temperatures within the range of −20°C are sufficient to cause significant cellular damage, temperatures below −40°C should be sought to ensure complete tissue destruction during freezing.21 Our findings corroborate this assertion as well as provide a more defined minimum lethal temperature to PaCa specifically.
With the establishment of −25°C and 50°C as the critical lethal isotherms for PANC-1 and BxPC-3 cells, our investigations were expanded to examine the impact of a repeat exposure paradigm. A repeated exposure of heating or freezing is common practice clinically for the treatment of numerous cancers.5,19,20 Overall, the trend observed with both the PANC-1 and the BxPC-3 cells following repeat exposure revealed an increase in cell death at milder temperatures which was consistent with literature reports for other cancers. Double freeze exposure of PANC-1 cells resulted in complete cell death at −20°C versus −25°C needed for a single exposure. For BxPC-3 cells, double freeze to −15°C yielded complete cell destruction versus −20°C for a single freeze. When cell survival following a double heat exposure was examined, it was found that the lethal temperature of 50°C was reduced to 48°C in both the PANC-1 and the BxPC-3 cell lines. Interestingly, repeat exposure to 48°C resulted in a shift in response from initial death followed by recovery after a single exposure to increased cell death followed by a decline in cell number throughout the 7-day assessment interval (Figure 5).
The fundamental differences in heating versus freezing cells or tissues offer a mechanistic view into how each function in their own ways. Hyperthermal ablation was found to be effective in the current study when temperatures of 50°C or greater were obtained. While not immediately lethal, complete ablation was observed within 7 days posttreatment in both the PANC-1 and the BxPC-3 cells. Cryoablation was also found to be highly effective, as complete destruction of BxPC-3 and PANC-1 cells was attained at −20 and −25°C, respectively. Although both ablation strategies were effective following attainment of the critical lethal temperature, when correlating the reported side effects and collateral damage associated with both hyperthermal and cryoablation with our findings, one may conclude that cryotherapy may provide for a more attractive therapeutic option.25,27,39 One reason for this conclusion is the extended delayed temporal component (7 days) necessary to achieve complete cell death following hyperthermal ablation. This may provide for an opportunity for cancer recovery in vivo, whereas in cryoablation, once the minimal lethal temperature was attained, no survival or recovery was observed. While appearing beneficial, this remains to be studied clinically. Furthermore, recent studies by our group suggest that the combined application of heat and freezing in tandem may provide for improved ablation beyond that obtained with either as a monotherapy.40
In conclusion, the data in this study suggest that thermal ablation may be an effective means of treating PaCa. Freezing PaCa cells in vitro to temperatures below −25°C results in complete lethality as did heating to 50°C or greater. Double exposure was found to significantly reduce the temperature required for complete ablation. In addition to illustrating that thermal therapy is a viable option for PaCa treatment, our findings also support adjunctive approaches, such as those described by Neoptolemos et al 41 or Santucci et al,42 combining thermal exposure with other agents to offer the potential for enhanced ablation due to the involvement of a molecular-based component of PaCa cell response to thermal injury. Given the benefit of double heat or freeze over single exposures, studies into the combination of both heating and freezing as a dual thermal ablation technique may also be warranted.40 Regardless of the application strategy (single versus repeat exposure), monotherapy or in combination, the results suggest that heat ablation and cryoablation may be an effective means for treating PaCa.
Abbreviations
- AUA
American Urological Association
- LN2
liquid nitrogen
- PaCa
pancreatic cancer
- RFA
radiofrequency
- RFUs
raw fluorescence units
- RT
room temperature
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. K.W. Baumann is a consultant for CPSI Biotech. J.M. Baust, K.K. Snyder, and R.G. Van Buskirk are employees of CPSI Biotech.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by CPSI Biotech and the National Institutes of Health, grant numbers 1R43CA183265-01 and 1R43CA195948-01A1.
References
- 1. National Cancer Institute. SEER Cancer Statistics Factsheets: Pancreatic Cancer, 1975-2012. Bethesda, MD: National Cancer Institute; 2015. Website http://seer.cancer.gov/csr/1975_2012/. Published November 2014, Updated April 2015, Accessed October 21, 2015. [Google Scholar]
- 2. Maitra A, Hruban RH. Pancreatic Cancer. Annu Rev Pathol. 2008;3:157–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Cancer Institute. PDQ® Pancreatic Cancer Treatment. Bethesda, MD: National Cancer Institute; 2015. Website http://www.cancer.gov/types/pancreatic/patient/pancreatic-treatment-pdq#section/_162. Updated July 2, 2015, Accessed October 21, 2015. [Google Scholar]
- 4. Treating Pancreatic Cancer: How is Pancreatic Cancer Treated? Atlanta, GA: American Cancer Society; Website http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-treating-general-info. Published June 11, 2014, Updated January 9, 2015, Accessed October 21, 2015. [Google Scholar]
- 5. Keane MG, Bramis K, Pereira SP, Fusai GK. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol. 2014;20(9):2267–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ricci MS, Zong WX. Chemotherapeutic approaches for targeting cell death pathway. Oncologist. 2006;11(4):342–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909. [DOI] [PubMed] [Google Scholar]
- 8. Chu KF, Dupuy DE. Thermal ablation of tumors: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208. [DOI] [PubMed] [Google Scholar]
- 9. Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, DePinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006:20(10):1218–1249. [DOI] [PubMed] [Google Scholar]
- 10. Hanahan D, Weinburg R. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 11. Buja LM, Eigenbrodt ML, Eigenbrodt EH. Apoptosis and necrosis: basic types and mechanisms of cell death. Arch Pathol Lab Med. 1993;117(12):1208–1214. [PubMed] [Google Scholar]
- 12. Evan G, Finch A, Meier P. Apoptosis in development. Nature. 2000;704(6805):796–802. [DOI] [PubMed] [Google Scholar]
- 13. Majno G, Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol. 1995;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 14. Yan N, Shi Y. Mechanisms of apoptosis through structural biology. Annu Rev Cell Dev Biol. 2005;21:35–56. [DOI] [PubMed] [Google Scholar]
- 15. Lemasters JJ. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol. 1999;276(1 pt 1):g1–g6. [DOI] [PubMed] [Google Scholar]
- 16. Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int. 2005;95(9):1187–1191. [DOI] [PubMed] [Google Scholar]
- 17. Gage AA, Baust JG. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–186. [DOI] [PubMed] [Google Scholar]
- 18. Gage AA, Baust JG. Cryosurgery of tumors—a clinical overview. Technol Cancer Res Treat. 2004;3(2):187–199. [DOI] [PubMed] [Google Scholar]
- 19. Gage AA, Baust JG. Cryosurgery for tumors. J Am Coll Surg. 2007;205(2):342–356. [DOI] [PubMed] [Google Scholar]
- 20. Gage AA, Baust JM, Baust JG. Principles of cryosurgical technique In: Rukstalis DB, Katz A, eds. Handbook of Urologic Cryoablation. Colchester, UK: Informa UK Ltd; 2007: 1–18. [Google Scholar]
- 21. Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59(3):229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curley SA, Izzo F, Ellis LM, Vauthey JN, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232(3):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mirza AN, Fornage BD, Sneige N, et al. Radiofrequency ablation of solid tumors. Cancer J. 2001;7(2):95–102. [PubMed] [Google Scholar]
- 24. Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JD, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford). 2008;10(5):371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baust JG, Gage AA, Robilotto AT, Baust JM. The pathophysiology of thermoablation: optimizing cryoablation. Curr Opin Urol. 2009;19(2):127–132. [DOI] [PubMed] [Google Scholar]
- 26. Baust JG, Bischof JC, Jiang-Hughes S, et al. Re-purposing cryoablation: a combinatorial ‘therapy’ for the destruction of tissue. Prostate Cancer Prostatic Dis. 2015;18(2):87–95. [DOI] [PubMed] [Google Scholar]
- 27. Baust JM, Santucci K, Gage AA, Robilotto A. Optimizing ablative therapy: manipulating the microenvironment In: Polascik TJ, ed. Imaging and Focal Therapy of Early Prostate Cancer. New York, NY: Humana Press; 2013: 355–366. [Google Scholar]
- 28. Babaian RJ, Donnelly B, Bahn D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180(5):1993–2004. [DOI] [PubMed] [Google Scholar]
- 29. Mohammed A, Miller S, Douglas-Moore J, Miller M. Cryotherapy and its applications in the management of urological malignancies: a review of its use in prostate and renal cancers. Urol Oncol. 2014;32(1):39 e19-e27. [DOI] [PubMed] [Google Scholar]
- 30. Korpan N, Hochwarter G, Sellner F. Cryoscience and cryomedicine: new mechanisms of biological tissue injury following low temperature exposure. Experimental study. Klin Khir. 2009;(7-8):80–85. [PubMed] [Google Scholar]
- 31. Korpan NN. Ultrastructural cellular changes in living nature: pancreas cryosurgery in vivo (Chapter 19). In: Modern Cryosurgery for Cancer. Singapore: World Scientific Publishing Co. Pte. Ltd; 2012: 373–394. [Google Scholar]
- 32. Kovach SJ, Hendrickson RJ, Cappadona CR, et al. Cryoablation of unresectable pancreatic cancer. Surgery. 2002;131(4):463–464. [DOI] [PubMed] [Google Scholar]
- 33. Xu K, Niu L, Yang D. Cryosurgery for pancreatic cancer. Gland Surg. 2013;2(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meyer J, Toomay S. Update on treatment of liver metastases: focus on ablation therapies. Curr Oncol Rep. 2015;17(1):420. [DOI] [PubMed] [Google Scholar]
- 35. Klossner DP, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Cryoablative response of prostate cancer cells is influenced by androgen receptor expression. BJU Int. 2008;101(10):1310–1316. [DOI] [PubMed] [Google Scholar]
- 36. Clarke DM, Robilotto AT, Rhee E, et al. Cryoablation of renal cancer: variables involved in freezing-induced cell death. Technol Cancer Res Treat. 2007;6(2):69–79. [DOI] [PubMed] [Google Scholar]
- 37. Robilotto AT, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Temperature-dependent activation of differential apoptotic pathways during cryoablation in a human prostate cancer model. Prostate Cancer Prostatic Dis. 2013;16(1):41–49. [DOI] [PubMed] [Google Scholar]
- 38. Deer EL, Gonzalez-Hernandez J, Coursen JD, et al. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39(4):425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58(1):1–11. [DOI] [PubMed] [Google Scholar]
- 40. Baumann KW, Snyder KK, Baust JM, Baust JG, Van Buskirk RG. C-8: monitoring the effects of dual thermal ablation on pancreatic cancer cell line PANC-1. Cryobiology. 2014;69(3):504–505. [Google Scholar]
- 41. Neoptolemos JP, Cunningham D, Friess H, et al. Adjuvant therapy in pancreatic cancer: historical and current perspectives. Ann Oncol. 2003;14(5):675–692. [DOI] [PubMed] [Google Scholar]
- 42. Santucci K, Snyder K, Baust JM, et al. Use of 1,25α dihydroxyvitamin D3 as a cryosensitizing agent in a murine prostate cancer model. Prostate Cancer Prostatic Dis. 2011;14(2):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]