Abstract
Background:
The mixed lineage kinase domain-like protein has recently been identified as a key downstream component of tumor necrosis factor–induced necroptosis, which is an important pathway of cancer cell death. The goal of the current study is to explore the expression of mixed lineage kinase domain-like protein in colon cancer tissues and evaluate the prognostic value in patients with colon cancer.
Methods:
We collected normal and cancer colon tissues from 135 patients diagnosed with colon cancer after radical operation during July 2007 to April 2009 at The Affiliated Hospital of Qingdao University. Immunohistochemistry analysis was scored using an established scoring system. Kaplan-Meier survival curves were generated for recurrence-free survival and overall survival for all patients and 2 subsets of patients. The relationship between mixed lineage kinase domain-like protein expression and prognosis parameter (recurrence-free survival, overall survival) was analyzed by univariate and multivariate Cox regression analyses.
Results:
The median age of all patients was 67 years and 56.3% were male. Low expression of mixed lineage kinase domain-like protein was associated with decreased overall survival (78.6 vs 81.2 months; P = .011) in all patients. In the subset of 79 patients who received adjuvant chemotherapy, low expression of mixed lineage kinase domain-like protein was associated with decreased recurrence-free survival (60.4 vs 72.8 months; P = .032) and decreased overall survival (66.3 vs 72.9 months; P = .005). Low expression of mixed lineage kinase domain-like protein was associated with decreased overall survival (74.9 vs 79.8 months; P = .006) and recurrence-free survival (69.6 vs 78.8 months; P = .005) among patients with Tumor Node Metastasis (TNM) stage II colon cancer.
Conclusions:
Low expression of mixed lineage kinase domain-like protein was associated with decreased overall survival in all patient-group with resected colon cancer. It is associated with decreased recurrence-free survival and overall survival in the subset of patients who receive adjuvant chemotherapy and patients who were TNM stage II. Mixed lineage kinase domain-like protein may provide important prognostic information in patients with colon cancer.
Keywords: MLKL, necroptosis, prognostic value, colon cancer
Introduction
Colorectal cancer is responsible for 8.5% of all cancer deaths worldwide, induced an estimated 694 000 deaths annually, and it is ranked as the third most common cause of cancer death, and 5-year overall survival (OS) was 64.2% according to stages defined by the American Joint Committee on Cancer fifth edition system.1 The weighted mean local recurrence rate in 5 years is approximately 4.5%.2 It has been estimated that the number of deaths due to colorectal cancer will reach approximately 376 700 by 2020 in Asia.3 Therefore, a better understanding of the molecular events involved in the prognosis of colon cancer is necessary and conducive to cancer detection and treatment.
Necrosis is a common feature observed in tumor center, with different morphological characteristics to apoptosis. Previously, necrosis was thought to be conducted in an uncontrolled way of cell death procedure, but recent studies have shown that it can occur in a controlled and regulated manner, which is called “necroptosis” or programmed cell death. Originally, in the initiation of necroptosis procedure, tumor necrosis factor α (TNF-α) activates TNF receptor type 1 signals to form complex II, which consists of receptor-interacting protein kinase 1 (RIP-1), Fas-associated death domain, and caspase-8,4 and then it phosphorylates and activates RIP-3 to form a complex called “necrosome.” Activated RIP-3 binds and interacts with mixed lineage kinase domain-like protein (MLKL), then migrates to the mitochondria, phosphorylated phosphoglycerate mutase 5, resulting in producing reactive oxygen species, calcium influx, lipid peroxidation, increased mitochondrial membrane permeability, and necroptotic cell death.5 Studies have shown that necroptosis involved in the pathogenesis of a variety of human diseases including cancer.6,7 Later researches demonstrated that MLKL played an indispensable role in necroptosis.8–10,11 Interestingly, recent studies suggested that MLKL expression can serve as a potential prognostic biomarker for patients with pancreatic and ovarian cancer.12,13 However, the role of MLKL proteins in cancer cell death procedure and the prognostic value of MLKL in colon cancer are unknown. In this study, we try to investigate the prognosis value of MLKL expression in patients with colon cancer.
Materials and Methods
Patient Selection
This study was approved by The Affiliated Hospital of Qingdao University. Colon cancer tissue samples were collected from 135 patients diagnosed with primary colon cancer after radical excision in our hospital from July 2007 to April 2009. The other 30 cases of normal colon tissue was dealt with the same conditions as a control. Informed consent was obtained from all patients. Histopathological features, including the tumor node metastasis (TNM) stage, pathologic grade, lymph node invasion (LNI), and lymphovascular invasion (LVI), were performed by 2 experienced pathologists. Recurrence-free survival (RFS) was calculated from the date of surgery to the first radiological evidence of recurrence based on surveillance imaging obtained at regular intervals after surgical resection. Overall survival was calculated from the date of surgery to the date of cancer-caused death or to the date of the last follow-up examination.
Immunohistochemical Analysis
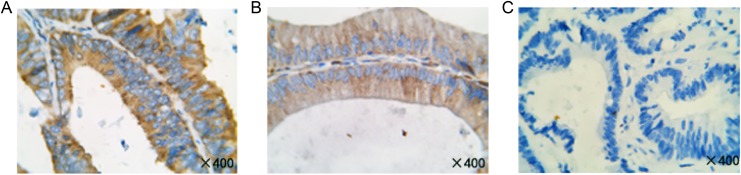
Formalin-fixed paraffin-embedded slides were used to identify representative sections of tumor and normal tissue. The tissues were stained using anti-MLKL (ab118348; Abcam, Cambridge, Massachusetts) monoclonal mouse antibody. The procedure of MLKL expression was performed at a concentration of 1:400. Protein staining was scored independently by 2 pathologists. Specific MLKL-positive staining was observed in the cytoplasm of tumor cells and is visualized as brown-yellow staining. The score was ascertained by consideration of both staining extent and intensity. The extent of the staining was categorized into 5 semiquantitative classes based on the percentages of positive tumor cells—0: <5%; 1+: 5%-25%; 2+: 26%-50%; 3+: 51%-50%; and 4+: >75%. The intensity was also determined semiquantitatively on a scale of 0 to 3 as follows: 0 = weak (no color or light blue), 1 = moderate (light yellow), 2 = strong (yellow-brown), and 3 = very strong (brown). Multiplying the 2 scores gets the overall score. An overall score of ≥4 was defined as high expression, and a score of <4 was defined low expression (Figure 1). According to this definition of high-expression and low-expression groups, 83 (61.5%) patients exhibited high tumoral MLKL expression. Fifty-five (40.7%) patients had >80% of cells staining for MLKL, and the intensity of staining was recorded as high in 25 (18.5%) of the 135 samples. Examples of typical staining patterns for MLKL expression are shown in Figure 1.
Figure 1.
Representative images of mixed lineage kinase domain-like protein (MLKL) immunohistochemical staining in colon cancer tissues (magnification ×400). A, High expression. B, Low expression. C, Negative expression.
Statistical Analysis
The relationship between MLKL expression and patients’ gender, age, pathologic grade, TNM stage, and so on, was analyzed using the χ2 test or Fisher exact test, as appropriate. Kaplan-Meier log-rank survival analysis was performed to determine prognostic factors for RFS and OS. Univariate and multivariate Cox regression analyses were performed for all patients to respectively examine the impact of MLKL expression on both RFS and OS. Factors examined on univariate analysis included TNM stage, pathologic grade, LNI, LVI, CEA levels, and whether received adjuvant chemotherapy and so on, among them, meaningful ones are listed in tables. Clinically relevant covariates significant of P < .2 on univariate analysis for either RFS or OS were included in the multivariate model. Subset analyses were performed for patients who received adjuvant chemotherapy, and for TNM stage II patients, the same methodology of Kaplan-Meier survival analysis and Cox regression analyses was performed. Data were analyzed using the Statistical Package for the Social Sciences software (version 22.0 for Windows; SPSS Inc, Chicago, Illinois).
Results
The demographic and pathologic characteristics of the patients in the current study are summarized in Table 1. Seventy-six (56.3%) of the 135 patients included in the current study were male. Tumor size ranged from 0.5 to 13 cm, with a median of 5 cm. Forty-three (31.8%) patients had positive lymph nodes metastasis. The median follow-up for survivors was 73.6 months (range, 6-95.3 months). At the time of last follow-up, 79 (58.5%) patients received adjuvant therapy, 41 patients (34.1%) had died, 28 patients (20.7%) had tumor recurrence, and 10 (7.4%) patients were lost in follow-up. Patients did not have a history of chemotherapy or radiotherapy before operation.
Table 1.
Patient Demographics, Tumor Characteristics, and Treatment Characteristics of All Patients.a
| MLKL Expression | ||||
|---|---|---|---|---|
| Parameter | n | Negative (%) | Positive (%) | P |
| Gender | ||||
| Male | 76 | 31 | 45 | .538 |
| Female | 59 | 21 | 38 | |
| Age | ||||
| <60 | 52 | 16 | 36 | .143 |
| ≥60 | 83 | 36 | 47 | |
| TNM stage | ||||
| I | 16 | 4 | 12 | |
| II | 75 | 29 | 46 | .441 |
| III | 44 | 19 | 25 | |
| Differentiation | ||||
| Well | 9 | 2 | 7 | .302 |
| Middle | 95 | 35 | 60 | |
| Poor | 31 | 15 | 16 | |
| LNI | ||||
| Negative | 92 | 29 | 63 | .015 |
| Positive | 43 | 23 | 20 | |
| LVI | ||||
| Negative | 112 | 43 | 69 | .047 |
| Positive | 23 | 14 | 9 | |
| Tumor size | ||||
| <5 | 90 | 30 | 60 | .080 |
| ≥5 | 45 | 22 | 23 | |
| CEA | ||||
| Normal | 73 | 27 | 46 | .691 |
| High | 62 | 25 | 37 | |
| Position | ||||
| Left | 50 | 19 | 31 | .449 |
| Transverse | 6 | 6 | 13 | |
| Right | 9 | 9 | 7 | |
| Sigmoid | 18 | 18 | 32 | |
| Median age (range), years | 67 (30-82) | |||
| Median OS (range), months | 69.1 (5-95.3) | |||
| Median RFS (range), months | 78.8 (6-92) | |||
Abbreviations: CEA, carcino embryonie antigen; LNI, positive lymph node invasion; LVI, lymphovascular invasion; MLKL, mixed lineage kinase domain-like protein; OS, overall survival; RFS, recurrence-free survival; TNM, tumor node metastasis.
an = 135.
bBold type denotes statistical significance.
Survival Analyses: All Patients
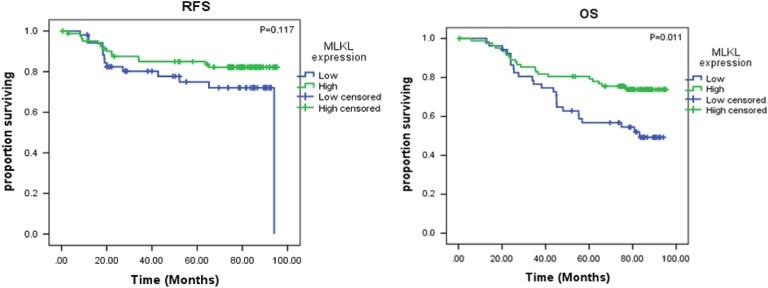
Kaplan-Meier survival analyses for all 135 patients demonstrated significant association between low MLKL expression and decreased OS (78.6 vs 81.2 months; P = .011; Figure 2B), but MLKL expression found no significant association with RFS (75.1 vs 78.1 months; P = .117; Figure 2A). Table 2 shows the association of factors found to be significantly associated with OS and RFS on univariate and multivariate Cox regression analysis. Low MLKL expression was associated with decreased OS on both univariate (hazard ratio [HR]: 0.48; 95% confidence interval [CI]: 0.27-0.86; P = .01) and multivariate analysis (HR: 0.38; 95% CI: 0.16-0.89; P = .03). Although MLKL expression shows association with RFS on univariate analysis (HR: 0.35; 95% CI: 0.13-0.96; P = .04), there was no association with multivariate analysis (Table 2).
Figure 2.
Kaplan-Meier log-rank survival analysis is shown for mixed lineage kinase domain-like protein (MLKL) expression in all patients (n = 135). A, The effect of MLKL expression on recurrence-free survival. B, The effect of MLKL expression on overall survival.
Table 2.
Univariate and Multivariate Cox Regression Analysis of All Patients.a
| Outcome | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| RFS | OS | RFS | OS | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| MLKL | 0.35 (0.13-0.96) | .04 | 0.32 (0.14-0.75) | .01 | 0.41 (0.15-1.12) | .08 | 0.38 (0.16-0.89) | .03 |
| TNM stage | 1.77 (0.76-4.10) | .18 | 2.09 (1.03-4.25) | <.04 | 1.33 (0.44-4.02) | .61 | 2.19 (1.01-4.79) | .05 |
| LVI | 3.81 (1.38-10.50) | .01 | 2.71 (1.12-6.58) | .03 | 2.36 (0.79-7.02) | .12 | 1.05 (1.01-1.09) | .02 |
| LNI | 1.38 (0.71-2.68) | .34 | 1.80 (1.05-3.08) | .03 | 0.64 (0.16-2.64) | .84 | 1.11 (0.35-3.51) | .86 |
| Grade | 1.60 (0.58-4.42) | .36 | 2.38 (1.06-5.32) | .04 | 1.81 (0.45-6.34) | <.01 | 2.76 (1.22-6.25) | .02 |
| CEA | 4.63 (1.49-14.38) | <.01 | 3.01 (1.29-7.05) | .01 | 4.17 (1.33-13.04) | .01 | 1.37 (0.45-4.11) | .58 |
Abbreviations: CEA, carcino embryonie antigen; CI, confidence interval; HR, hazard ratio; MLKL, mixed lineage kinase domain-like protein; LNI, positive lymph node invasion; LVI, lymphovascular invasion; OS, overall survival; RFS, recurrence-free survival; TNM, tumor node metastasis.
an = 135.
bBold type denotes statistical significance.
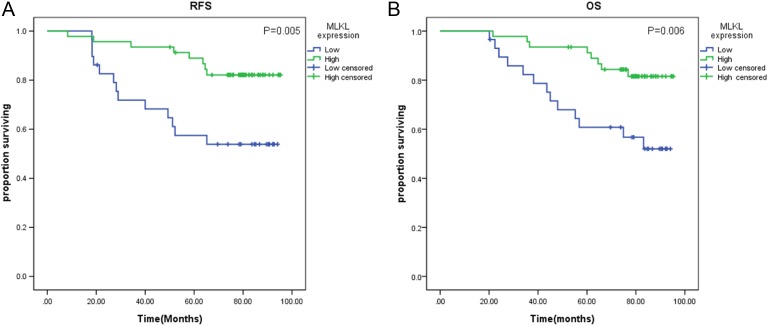
A second subset analysis was performed in TNM stage II patients (n = 75). On Kaplan-Meier analysis, low MLKL expression remained associated with poor RFS (69.6 vs 78.8 months; P = .005; Figure 3A) and poor OS (74.9 vs 79.8 months; P = .006; Figure 3B).
Figure 3.
Kaplan-Meier log-rank survival analysis is shown for mixed lineage kinase domain-like protein (MLKL) expression in patients receiving adjuvant chemotherapy (n = 79). A, The effect of MLKL expression on recurrence-free survival. B, The effect of MLKL expression on overall survival.
Subset Analyses: Patients Receiving Adjuvant Therapy
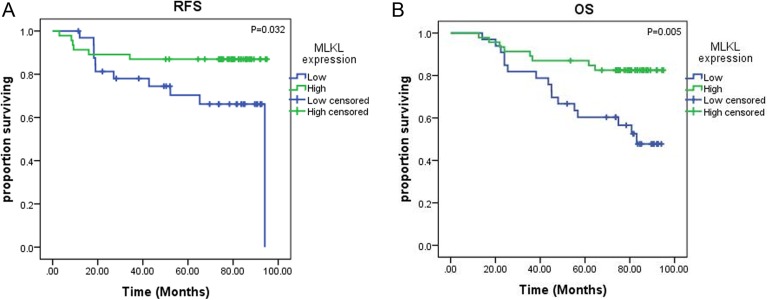
In the subset of patients receiving adjuvant chemotherapy (n = 79), low MLKL expression was associated with decreased RFS (60.4 vs 72.8 months; P = .032; Figure 4A) and decreased OS (66.3 vs 72.9 months; P = .005; Figure 4B) by Kaplan-Meier survival analyses. The relationship between low MLKL expression and decreased 5-year OS persisted on both univariate (HR: 0.48; 95% CI: 0.27-0.86; P = .01) and multivariate Cox regression analyses for OS (HR: 0.54; 95% CI: 0.30-0.97; P = .04). But results showed no significant association of MLKL expression with RFS on univariate and multivariate Cox regression analyses (Table 3).
Figure 4.
Kaplan-Meier log-rank survival analysis is shown for mixed lineage kinase domain-like protein (MLKL) expression in patients receiving adjuvant chemotherapy (n = 79). A, The effect of MLKL expression on recurrence-free survival. B, The effect of MLKL expression on overall survival.
Table 3.
Univariate and Multivariate Cox Regression Analysis of Patients Who Received Adjuvant Chemotherapy.a
| Outcome | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| RFS | OS | RFS | OS | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| MLKL | 0.56 (0.26-1.17) | .12 | 0.48 (0.27-0.86) 0.01 | .01 | 0.61 (0.28-1.30) | .20 | 0.54 (0.30-0.97) | .04 |
| TNM stage | 3.71 (1.86-7.42) | <.01 | 2.96 (1.77-4.95) | <.01 | 3.80 (1.84-7.83) | <.01 | 2.03 (1.03-4.02) | <.04 |
| LVI | 1.05 (1.01-1.09) | <.01 | 1.05 (1.03-1.09) | <.01 | 2.15 (0.80-5.83) | .13 | 1.97 (0.90-4.32) | .09 |
| LNI | 2.44 (1.53-3.88) | <.01 | 2.38 (1.65-3.44) | <.01 | 2.44 (1.53-3.88) | <.01 | 0.99 (0.38-2.54) | .03 |
| Grade | 2.63 (1.23-5.60) | .01 | 2.77 (1.54-4.95) | <.01 | 1.20 (0.56-2.58) | 0.42 | 1.17 (0.53-2.58) | .70 |
| CEA | 0.47 (0.22-1.01) | .05 | 2.08 (1.16-3.76) | .01 | 0.47 (0.22-1.01) | .05 | 1.77 (0.82-3.82) | .14 |
Abbreviations: CEA, carcino embryonie antigen; CI, confidence interval; HR, hazard ratio; MLKL, mixed lineage kinase domain-like protein; LNI, positive lymphatic node invasion; LVI, lymphovascular invasion; OS, overall survival; RFS, recurrence-free survival; TNM, tumor node metastasis.
an = 79.
bBold type denotes statistical significance.
Discussion
Recently, engagement of necroptosis as an alternative mode of programmed cell death provides new opportunities to kill cancer cells10 and becomes hot focus research of many diseases including cancer. The MLKL functions downstream of RIP-3 in necroptosis and plays an indispensable role.11 The expression level of MLKL may suggest the intensity of necroptosis signaling in tumors.
In the present study, we have studied the expression of MLKL protein in colon cancer using immunohistochemistry. We found that MLKL is highly expressed in colorectal cancer tissue, whereas it is little expressed in normal colon tissue, perhaps suggests that necroptosis is not the main death form of colon cancer cell or maybe MLKL participates in other cellular events.
The results of our research show that MLKL expression has nonsignificant association with tumor TNM stage, differentiation, size, location, and so on, but it is significantly associated with tumor lymphatic node and vascular invasion. This prompts that MLKL may be involved in tumor LVI, its prognosis value may just be because of this.
Our study also shows that low MLKL expression was associated with decreased OS and RFS in patients with colon cancer. Univariate Cox regression analysis and multivariate analysis still showed that MLKL expression is significantly associated with OS, demonstrating that MLKL expression may be worth evaluating as a predictive biomarker for prognosis in patients with colon cancer. Thus, MLKL provides a further evidence that a necroptosis protein has prognostic value in patients with cancer, which found prognostic evidence in patients with pancreatic adenocarcinoma and ovarian cancer.12,13 One possible potential mechanism for the association of low MLKL expression with poor prognosis in patients with colon cancer may be a result of decreased necroptosis signaling in these patients, which suggests that necroptosis is an important determinant of cancer cell death and outcome in patients with colon cancer.
It is unclear whether the administration of adjuvant chemotherapy improves the survival in patients with stage II colon cancer. Studying MLKL expression in colon cancer tissues of TNM stage II patients may provide prognosis value in those patients. Our results showed that low expression of MLKL was associated with decreased OS and RFS in TNM stage II patients. What do we need to do next is to study the association of MLKL expression with the prognosis of TNM stage II patients with chemotherapy or not, and therefore come to guide the treatment prescription.
Another result of our study showed that in the subset of patients who received adjuvant chemotherapy, low expression of MLKL was associated with decreased OS and RFS. Low expression of MLKL was associated with decreased RFS and decreased OS on univariate Cox regression analysis and associated with decreased OS on multivariate analysis, and the HR for death associated with low expression of MLKL became stronger in the group of patients treated with adjuvant therapy than in all patients, indicating that MLKL expression may be a helpful biomarker for predicting chemotherapy sensitivity in patients with colon cancer. Lately, studies have shown that necroptosis can be activated in certain cells by chemotherapeutics.14 Therefore, activating necroptosis could be used for anticancer therapy. In future research, we can detect phosphorylated MLKL in cancer cells and analyze the role of MLKL in necroptosis in cells of patients who received chemotherapy or received different chemotherapy regimens. Activating necroptosis of tumor cells may become a new hot spot for tumor treatment.15
In recent years, there were many proteins reported as prognostic factor of colon cancer, such as cyclins, apoptotic protease-activating factor 1, B-cell lymphoma 2, metadherin, and so on.16–20 These proteins are confirmed to participate in the regulation of cell cycle, apoptosis or tumor invasion, and metastasis. Our results show that the proteins in necroptosis play an important role in determining tumor cell death and whether it can benefit from chemotherapy. Evaluating the association of MLKL with other colon cancer prognosis factors, drawing up a prognostic molecular combination of higher sensitivity, and sensitivity is great meaningful for the prognosis of colon cancer.
In conclusion, our research studied the MLKL expression in colon cancer for the first time. We found that low expression of MLKL is closely related to poor prognosis of colon cancer, suggesting that MLKL may be a target for colon cancer treatment. Currently, it is not very clear about the function of MLKL in necroptosis signal path. Further research about necroptosis signaling pathways and its regulation mechanism is likely to improve treatment strategies and predict prognosis of disease.
Abbreviations
- CEA
carcino embryonie antigen
- CI
confidence interval
- HR
hazard ratio
- LNI
lymph node invasion
- LVI
lymphovascular invasion
- MLKL
mixed lineage kinase domain-like protein
- OS
overall survival
- RFS
recurrence-free survival
- RIP
receptor-interacting protein
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
- TNM
tumor node metastasis.
Footnotes
Authors’ Note: Jing Guo contributed equally to this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Natural Science Foundation of China and Shandong Province Higher Educational Science and Technology Program.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Natural Science Foundation of China under award numbers 81472338 to Qiu and a Project of Shandong Province Higher Educational Science and Technology Program award J15LL58 to Lv.
References
- 1. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. [DOI] [PubMed] [Google Scholar]
- 2. Killeen S, Mannion M, Devaney A, Winter DC. Complete mesocolic resection and extended lymphadenectomy for colon cancer: a systematic review. Colorectal Dis. 2014;16(8):577–594. [DOI] [PubMed] [Google Scholar]
- 3. Shin A, Jung KW, Won YJ. Colorectal cancer mortality in Hong Kong of China, Japan, South Korea, and Singapore. World J Gastroenterol. 2013;19(7):979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. [DOI] [PubMed] [Google Scholar]
- 5. Wu W, Liu P, Li J. Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol. 2012;82(3):249–258. [DOI] [PubMed] [Google Scholar]
- 6. Nehs MA, Lin CI, Kozono DE, et al. Necroptosis is a novel mechanism of radiation-induced cell death in anaplastic thyroid and adrenocortical cancers. Surgery. 2011;150(6):1032–1039. [DOI] [PubMed] [Google Scholar]
- 7. Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song BW, Wang L. Necroptosis: a programmed cell necrosis. Sheng Li Ke Xue Jin Zhan. 2013;44(4):281–286. [PubMed] [Google Scholar]
- 9. Wu J, Huang Z, Ren J, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23(8):994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fulda S. The mechanism of necroptosis in normal and cancer cells. Cancer Biol Ther. 2013;14(11):999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moujalled DM, Cook WD, Murphy JM, Vaux DL. Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis. 2014;5:e1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colbert LE, Fisher SB, Hardy CW, et al. Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer. 2013;119(17):3148–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He L, Peng K, Liu Y, Xiong J, Zhu FF. Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. Cancer. 2013;119(17):3148–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galluzzi L, Vanden Berghe T, Vanlangenakker N, et al. Programmed necrosis from molecules to health and disease. Int Rev Cell Mol Biol. 2011;289:1–35. [DOI] [PubMed] [Google Scholar]
- 15. Long JS, Ryan KM. New frontiers in promoting tumour cell death: targeting apoptosis, necroptosis and autophagy. Oncogene. 2012;31(49):5045–5060. [DOI] [PubMed] [Google Scholar]
- 16. Corin I, Larsson L, Bergstrom J, et al. A study of the expression of cyclin e and its isoforms in tumor and adjacent mucosa, correlated to patient outcome in early colon cancer. Acta Oncol. 2010;49(1):63–69. [DOI] [PubMed] [Google Scholar]
- 17. Myklebust MP, Li Z, Tran TH, et al. Expression of cyclin d1a and d1b as predictive factors for treatment response in colorectal cancer. Br J Cancer. 2012;107(10):1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strater J, Herter I, Merkel G, et al. Expression and prognostic significance of apaf-1, caspase-8 and caspase-9 in stageII/IIIcolon carcinoma: caspase-8 and caspase-9 is associated with poor prognosis. Int J Cancer. 2010;127(4):873–880. [DOI] [PubMed] [Google Scholar]
- 19. Ghita C, Vilcea ID, Dumitrescu M, et al. The prognostic value of the immunohistochemical aspects of tumor suppressor genes p53, bcl-2, pten and nuclear proliferative antigen ki-67 in resected colorectal carcinoma. Rom J Morphol Embryol. 2012;53(3):549–556. [PubMed] [Google Scholar]
- 20. Casimiro S, Fernandes A, Oliveira AG, et al. Metadherin expression and lung relapse in patients with colorectal carcinoma. Clin Exp Metastasis. 2014;31(6):689–696. [DOI] [PubMed] [Google Scholar]