Abstract
Autofluorescence bronchoscopy shows good sensitivity and poor specificity in detecting dysplasia and cancer of the bronchus. Through quantitative analysis on the target area of autofluorescence bronchoscopy image, determine the optimal identification index and reference value for identifying different types of diseases and explore the value of autofluorescence bronchoscopy in diagnosis of lung cancer. Patients with 1 or more preinvasive bronchial lesions were enrolled and followed up by white-light bronchoscope and autofluorescence bronchoscopy. Color space quantitative image analysis was conducted on the lesion shown in the autofluorescence image using MATLAB image measurement software. A retrospective analysis was conducted on 218 cases with 1208 biopsies. One hundred seventy-three cases were diagnosed as positive, which included 151 true-positive cases and 22 false-positive cases. White-light bronchoscope associated with autofluorescence bronchoscopy was able to differentiate between benign and malignant lesion with a high sensitivity, specificity, positive predictive value, and negative predictive value (92.1%, 59.3%, 87.3%, and 71.1%, respectively). Taking 1.485 as the cutoff value of receiver operating characteristic of red-to-green value to differentiate benign and malignant diseases, the diagnostic sensitivity reached 82.3% and the specificity reached 80.5%. U values could differentiate invasive carcinoma and other groups well. Quantitative image analysis method of autofluorescence bronchoscopy provided effective scientific basis for the diagnosis of lung cancer and precancerous lesions.
Keywords: lung cancer, diagnosis, autofluorescence bronchoscopy, white-light bronchoscopy, medical image processing
Introduction
Lung cancer is one of the most common malignancies and the main cause of cancer death around the world. The prognosis of lung cancer is closely related to tumor stage. The 5-year survival rate of early-stage lung cancer is significantly higher than that of advanced lung cancer.1–3 Conventional bronchoscopy can only increase the discovery rate of central-type early lung cancer (CELC) slightly.4 As most cases with CELC show only minor changes in the bronchial mucosa, even an experienced bronchoscopist cannot find preinvasive cancer by using white-light bronchoscope (WLB). It is even more difficult to detect moderate to severe dysplasia using WLB by an experienced bronchoscopist.5 Therefore, it is urgent for us to find a more objective and better way to diagnosis of lung cancer.
Autofluorescence bronchoscopy (AFB) is a novel bronchoscope developed with cell autofluorescence and computer image analysis technology. It can significantly improve early diagnosis sensitivity (Se) of lung cancer and precancerous lesions.6 However, AFB also has some limitations, such as low specificity (Sp), because red fluorescence also shows, in cases of bronchial mucosal inflammation, inflammatory granuloma, scar tissue, and mucosal injury, which can easily be confused with precancerous lesions, carcinoma in situ (CIS), and invasive carcinoma.7
Therefore, by means of quantification of fluorescence intensity at different histopathologic stages in the development of lung cancer, the method could play an important role in early diagnosis of lung cancer and assessment of the extent of local cancer.
Materials and Methods
Patients
The work was a retrospective study of patients who underwent WLB associated with AFB at Shanghai Chest Hospital from April 2012 to January 2014. The study has been approved by ethical committees of Shanghai Chest Hospital. All patients signed informed consent before the examination. White-light bronchoscope and AFB were performed according to the following guidelines: (1) newly emerging or changing nature of the original symptoms, such as sputum, cough, hoarseness, hemoptysis, and weight loss, which were clinically suspected to be lung cancer; (2) imaging examination indicating lung mass, atelectasis, or patchy shadows that were suspected to be patients with central lung cancer; (3) abnormalities found in sputum cytology; (4) informed written consent was obtained from all patients and the institutional review board approved this study (KS10-03); and (5) no contraindication to the procedure. There were exclusion criteria for the study: (1) those who had bronchoscopy contraindications (active hemoptysis, unstable angina, anesthetic allergy, etc); (2) those who had bronchoscopy, biopsy, and brush biopsy; (3) those who had taken photosensitizing drugs within 3 months; and (4) those who had conducted cytotoxic chemotherapy within 6 months.
White-Light Bronchoscope and AFB Examination
This study adopted BF-F260 electronic AFB (Olympus Corporation, Japan) that has both WLB and AFB function and can be switched between white-light mode and autofluorescence mode.
White-Light Bronchoscope and AFB Image Characteristics for Lesions
Under conventional white light, the visible lesions were divided into 3 levels8—WLB I: congenital anatomic abnormalities, external pressure lesions, pure widened bronchial ridge, and normal mucosal color, not associated with congestion and edema; WLB II: mucosal congestion, edema, thickening, color change, vascular aggregation, or distortion; WLB III: mucosal granular changes or significant new particle-like creatures. The WLB II and WLB III were classified as abnormal WLB results.
Under the autofluorescent state, the visible lesions were also divided into 3 levels: AFB I: green mucosa; AFB II: mild color changes in mucosa, pale pink or brown; AFB III: mucosa color turning into typical red or purple. The AFB II and III were defined as abnormal results.
White-light bronchoscope and AFB were conducted by 2 experienced physicians, and the images were rated by them unaware of the pathological findings, and if their ratings were not the same, the chief physician would join the discussion and decide the rating.
Evaluation of Pathological Results
The pathological diagnoses were divided into invasive carcinoma, CIS, severe dysplasia, moderate dysplasia, mild dysplasia, hyperplasia, squamous metaplasia, inflammation, and normal cell.8 The pathological diagnosis of severe dysplasia and CIS were defined as high-grade preinvasive (HGD); hyperplasia, squamous metaplasia, and mild and moderate dysplasia were defined as low-grade preinvasive (LGD). And pathological diagnosis of HGD and invasive carcinoma were defined as positive diagnosis; normal mucosa, inflammation, and LGD were defined as negative diagnosis.9
Quantitative Analysis of AFB Images
Autofluorescence bronchoscopy images were processed by MATLAB software (R2012b version, MathWorks Inc, Natick, United states) in the same computer and collected by the same set of system, 16 × 16 pixel target regions in the center and 2 edges of the lesion were measured, and the average values of 3 regions was taken as the final data, which was analyzed to obtain R, G, B, red-to-green (R/G), red-to-blue (R/B), green-to-blue (G/B), Y, U, and V values.
Data Analysis
Statistical analyses were performed using SPSS 11.5 (SPSS Inc, Chicago, Illinois). Measurement data were expressed as mean standard deviation (SD), and count data were expressed as percentage. Significance was considered as P value < .05. Comparison between means of different groups was conducted with least significant difference test method in analysis of variance. The χ2 test or Fisher exact test was performed, when appropriate, for categorical variables.
Results
Pathology Results
After specimens with specific infections such as fungal infections and tuberculosis were removed, a total of 218 cases with 1208 effective pathological specimens were obtained. In the 218 effective cases, there were 72 cases of squamous carcinoma, 31 cases of adenocarcinoma, 31 cases of small cell carcinoma, 16 cases of poorly differentiated carcinoma and unclassified carcinoma, 32 cases of acute and chronic inflammation or inflammatory cell infiltration, 14 cases of severe dysplasia and CIS, 9 cases of LGD, and 13 cases of normal bronchial epithelium.
The Diagnostic Evaluation of WLB and/or AFB
The Se, Sp, positive predictive value, and negative predictive value of WLB were 62.2%, 77.8%, 89.5%, and 40.4%, respectively. The Se, Sp, positive predictive value, and negative predictive value of WLB + AFB were 92.1%, 59.3%, 87.3%, and 71.1%, respectively.
Comparing the sensitivities of the 2 methods using χ2 test, we found a significant difference (P < .01) between WLB associated with AFB (92.1%) and WLB alone (62.2%). And there was also a significant difference (P < .05) in the Sp of finding positive lesions between WLB associated with AFB (59.3%) and WLB alone (77.8%; Table 1). Test results of R/G, R/B, G/B, Y, U, and V values in all pathological groups are shown in Table 2.
Table 1.
The Diagnostic Evaluation of WLB and AFB Associated With WLB.a
| WLB | WLB + AFB | |
|---|---|---|
| Sensitivity | 62.2% (102/164) | 92.1% (151/164)b |
| Specificity | 77.8% (42/54) | 59.3% (32/54)c |
| Positive predictive value | 89.5% (102/114) | 87.3% (151/173) |
| Negative predictive value | 40.4% (42/104) | 71.1 (32/45)b |
Abbreviations: AFB, autofluorescence bronchoscopy; WLB, white-light bronchoscopy.
aThe AFB associated with the WLB group compared with the WLB group.
b P < .01.
c P < .05.
Table 2.
R/G, R/B, G/B, Y, U, and V Values in the Different Groups.
| Groups | N | R/G, Mean (SD) | R/B, Mean (SD) | G/B, Mean (SD) | Y, Mean (SD) | U, Mean (SD) | V, Mean (SD) |
|---|---|---|---|---|---|---|---|
| Invasive carcinoma | 150 | 1.81 ± 0.35 | 1.47 ± 0.15 | 0.84 ± 0.19 | 0.43 ± 0.14 | −0.00 ± 0.03 | 0.15 ± 0.06 |
| HGD | 14 | 1.58 ± 0.30 | 1.47 ± 0.11 | 0.96 ± 0.17 | 0.45 ± 0.16 | −0.02 ± 0.02 | 0.12 ± 0.06 |
| LGD | 9 | 1.27 ± 0.15 | 1.41 ± 0.15 | 1.13 ± 0.21 | 0.54 ± 0.18 | −0.04 ± 0.02 | 0.08 ± 0.03 |
| Inflammation | 32 | 1.34 ± 0.32 | 1.44 ± 0.11 | 1.14 ± 0.33 | 0.53 ± 0.18 | −0.04 ± 0.04 | 0.10 ± 0.08 |
| Normal | 13 | 1.09 ± 0.22 | 1.49 ± 0.11 | 1.44 ± 0.39 | 0.51 ± 0.20 | −0.07 ± 0.03 | 0.05 ± 0.03 |
Abbreviations: G/B, green-to-blue value; HGD, high-grade preinvasive; LGD, low-grade preinvasive; R/B, red-to-blue value; R/G, red-to-green value; SD, standard deviation.
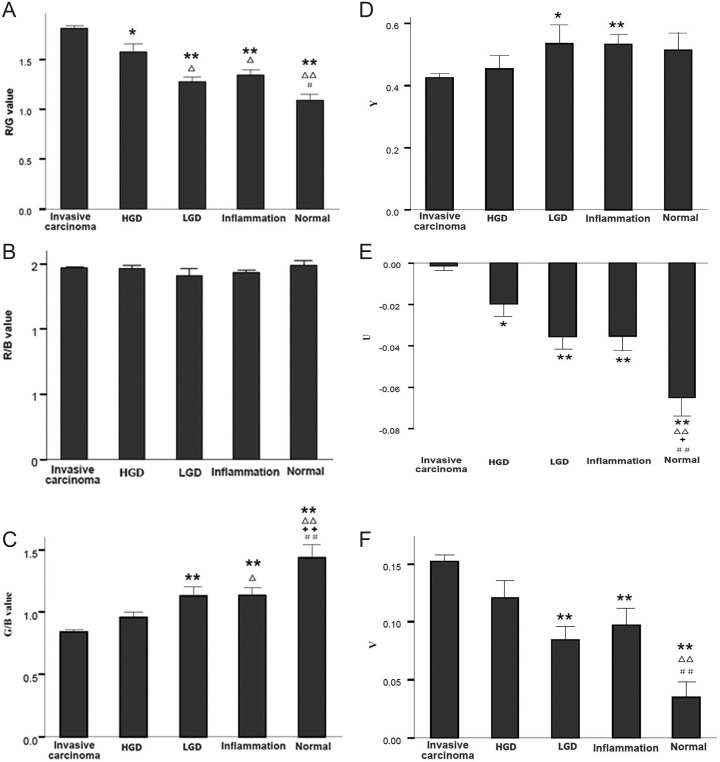
Correlation Between R/G Value and Pathological Diagnosis
There were significant differences between the R/G values in the invasive carcinoma group and in the HGD group as well as the LGD, normal bronchial mucosa, and inflammation groups. There were significant differences of R/G value between the invasive carcinoma group and the LGD group with P value < .001; significant differences also existed between the invasive carcinoma group and the normal bronchial mucosa (P ≤ .001) and the inflammation groups (P < .001).
The results showed that R/G values were different among groups and can be used to discriminate between benign and malignant lesions and identify HGD with benign diseases, as shown in Figure 1A. There were no significant differences in R/B value between each groups, as shown in Figure 1B. There were significant differences in G/B value between the normal bronchial mucosa group and the other groups (P < .001; Figure 1C).
Figure 1.
Red, green, and blue ratio and YUV of AFB in the different groups. 1A, R/G value. 1B, R/B value. 1C, G/B value. 1D, Y value. 1E, U value. 1F, V value. *Compare with invasive carcinoma, P < .05; **compare with invasive carcinoma, P < .01; ▵compare with HGD, P < .05; ▵▵compare with HGD, P < .01; ++compare with LGD, P < .01; #compare with inflammation, P < .05; ##compare with inflammation, P < .01. AFB indicates autofluorescence bronchoscopy; HGD, high-grade preinvasive; LGD, low-grade preinvasive.
The results indicated that G/B value was an ideal index for measurement of normal bronchial mucosa and can also be used for discriminating between invasive carcinoma and benign lesions.
Receiver Operating Characteristic Analysis and the Cutoff Value
The receiver operating characteristic curve of R/G related to the detection of pathological diagnosis for benign and malignant diseases was made based on data obtained in the study, with the area under the curve being 0.857. If R/G value of 1.485 was taken as cutoff value, the diagnostic Se would reach 82.3% and the Sp would reach 80.5%.
Relationship Between Pathological Diagnosis and Quantitative Value of Fluorescence Image (YUV System)
Results showed that U values could better differentiate invasive carcinoma and other groups as well as normal bronchial mucosa and other groups but had little value in differentiating HGD, LGD, and inflammation. Y and V values showed difference in part of groups, and they were meaningful in identification and diagnosis of disease (see in Figure 1D–F).
Discussion and Conclusion
Conventional WLB has provided certain help for the diagnosis of central type lung cancer, whereas AFB can better detect CELC10–13 and precancerous lesions. The Se of AFB associated with WLB in detecting precancerous lesions and cancerous tissues was higher than that of using WLB alone.14–20 However, certain factors, such as the friction damage of airway wall caused by bronchoscopy in the process, airway mucosal inflammation, oral anticoagulation, taking photosensitizing drugs within 3 months, cytotoxic chemotherapy carried within 6 months, may lead to false-positive results in AFB.10 Furthermore, many nonneoplastic diseases showed positive in AFB. So its Sp was low, which reduces its clinical application in airway lesions to some extent. Therefore, the correlation between different quantitative indicators and diseases was made to obtain more accurate diagnosis and identification of diseases.
Red, green, and blue (RGB) space hue analysis was used in the quantitative analysis. It is an international standard about hue defined by International Electrotechnical Commission that obtains various colors through changes of the 3 color channels of RGB and their mutual superposition. Red, green, and blue space hue analysis is currently one of the most widely used color systems. In 2000, Kusunoki et al first used the ratio of RGB hue space to identify benign and malignant diseases, and the research discovered that red/green = 0.53 was the boundary for the identification of benign and malignant diseases through analysis of 216 biopsy specimens and AFB images of 65 patients using LIFE System (Xillix Technology, Vancouver, British Columbia, Canada).21 Consistent with related studies, our results showed that R/G value as the red/green ratio in the RGB color space system can play an important role in the identification of benign and malignant diseases. Nakanishi et al 22 made similar conclusions by using PDS-2000 system (Hamamatsu Photonics K.K., Hamamatsu, Japan). Lee et al 8 studied AFB biopsy specimens of 738 patients using Onco-LIFE System (Xillix Technology, Vancouver, British Columbia, Canada) through multicenter collaboration and found R/G could significantly distinguish benign diseases from malignant diseases and had a guiding role for biopsy. If the R/G value was determined as 0.54, the Se would reach 85% and the Sp would reach 80%.
As for the AFB system (Olympus), especially for populations in China and other oriental countries, there is no clear research that has obtained similar conclusions of determining benign and malignant diseases with R/G value at present, whereas our study has made a preliminary exploration in this area.
Our study found that R/G value could not only be used as the guidance of differentiating benign and malignant diseases for AFB system but also identify precancerous lesions.
The study also found that G/B value of normal bronchial mucosa was significantly higher and would decrease with the malignancy progress of bronchial mucosa. The reason may be that microvascular filling existed in the inflammation, HGD, LGD, and invasive carcinoma, the epithelial thickening and tissue congestion reduced incident light, and reducing substances in tumor tissue reduced the amount of the fluorescent carriers; meanwhile, blood porphyrin structure of hemoglobin in the congestion organization increased absorption of green fluorescent, which further weakened the fluorescence phenomenon. Thus, G/B value is an ideal indicator for distinguishing normal bronchial mucosa from benign and malignant diseases.
YUV system is a common method for image analysis. In which, Y means the visibility of color, that is, brightness; actually, Y is gray scale value of images and U and V are called chroma, showing the tone and saturation of images, respectively. Tone mainly reflects the classification of color and determines the basic characteristics of color, whereas saturation reflects the purity of a certain color, that is, light and dark degree of a color. Since images processing RGB hue space also utilize more computing space, the conversion speed is slow. Many broadcasting, television systems, and imaging criteria use brightness and color difference video signal, that is, YUV hue space. So YUV hue space also takes up an important position in computer image processing field.23 One of the main advantages of YUV hue space is that brightness signal (Y) and chroma signal (U, V) are independent from each other. Therefore, this study innovatively utilized the different manifestations of AFB in YVU system to conduct statistical analysis. Results showed that U values could better differentiate invasive carcinoma and other groups as well as normal bronchial mucosa and other groups, and it could be considered as one of the quantitative indicators of early diagnosis of lung cancer.
The AFB image quantitative method of this study can further increase the diagnosis rate of lung cancer, early lung cancer in particular, and improve the Sp of diagnosis. With the development of scientific technologies and the promotion of quantitative method of AFB, the fluorescence intensity at different pathological stages in the development of lung cancer will be quantified. In addition, with in-depth study of excitation light source and image processing of AFB and further improvement in the imaging system, AFB will play a more important role and be of greater clinical significance in terms of detection and diagnosis of lung diseases.
Abbreviations
- AFB
autofluorescence bronchoscopy
- CELC
central-type early lung cancer
- CIS
carcinoma in situ
- G/B
green-to-blue value
- HGD
high-grade preinvasive
- IEC
International Electrotechnical Commission
- LGD
low-grade preinvasive
- R/B
red-to-blue value
- R/G
red-to-green value
- RGB
red, green, and blue
- Se
sensitivity
- Sp
specificity
- U
chroma
- V
saturation
- WLB
white-light bronchoscope
- Y
grey-scale value.
Footnotes
Authors’ Note: Jiayuan Sun and Baohui Han designed and performed the study. Xiaoxuan Zheng collected data, participated in the study, and wrote the article. Hongkai Xiong participated in the study. Yong Li analyzed the data.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Research Fund for the Scientific and Technical Project of Shanghai Chest Hospital (No. YZ13-35), Shanghai Jiao Tong University “Biomedical Engineering Cross Research Fund” Project (No. YG2011MS48), The Research Special Fund for Public Welfare Industry of Health (No. 201402024), and The Shanghai Municipal Health Bureau (No. 20124270).
References
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 2. Bechtel JJ, Petty TL, Saccomanno G. Five year survival and later outcome of patients with X-ray occult lung cancer detected by sputum cytology. Lung Cancer. 2000;30(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3. Lam S, MacAulay C, LeRiche JC, Palcic B. Detection and localization of early lung cancer by fluorescence bronchoscopy. Cancer. 2000;89(11 suppl):2468–2473. [DOI] [PubMed] [Google Scholar]
- 4. Aihara H, Sumiyama K, Saito S, Tajiri H, Ikegami M. Numerical analysis of the autofluorescence intensity of neoplastic and non-neoplastic colorectal lesions by using a novel videoendoscopy system. Gastrointest Endosc. 2009;69(3 pt 2):726–733. [DOI] [PubMed] [Google Scholar]
- 5. Moghissi K, Dixon K, Stringer MR. Current indications and future perspective of fluorescence bronchoscopy: a review study. Photodiagnosis Photodyn Ther. 2008;5(4):238–246. [DOI] [PubMed] [Google Scholar]
- 6. Froudarakis ME. New challenges in medical thoracoscopy. Respiration. 2011;82(2):197–200. [DOI] [PubMed] [Google Scholar]
- 7. Ueno K, Kusunoki Y, Imamura F, et al. Clinical experience with autofluorescence imaging system in patients with lung cancers and precancerous lesions. Respiration. 2007;74(3):304–308. [DOI] [PubMed] [Google Scholar]
- 8. Lee P, van den Berg RM, Lam S, et al. Color fluorescence ratio for detection of bronchial dysplasia and carcinoma in situ. Clin Cancer Res. 2009;15(14):4700–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gibbs AR, Thunnissen FB. Histological typing of lung and pleural tumors: third edition. J Clin Pathol. 2001;54(7):498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thakur A, Gao L, Ren H, Yang T, Chen T, Chen M. Descriptive data on cancerous lung lesions detected by auto-fluorescence bronchoscope: a five-year study. Ann Thorac Med. 2012;7(1):21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bard MP, Amelink A, Skurichina M, et al. Improving the specificity of fluorescence bronchoscopy for the analysis of neoplastic lesions of the bronchial tree by combination with optical spectroscopy: preliminary communication. Lung Cancer. 2005;47(1):41–47. [DOI] [PubMed] [Google Scholar]
- 12. Kennedy TC, Lam S, Hirsch FR. Review of recent advances in fluorescence bronchoscopy in early localization of central airway lung cancer. Oncologist. 2001;6(3):257–262. [DOI] [PubMed] [Google Scholar]
- 13. Divisi D, Di Tommaso S, De Vico A, Crisci R. Early diagnosis of lung cancer using a SAFE-3000 autofluorescence bronchoscopy. Interac Cardiovasc Thorac Surg. 2010;11(6):740–744. [DOI] [PubMed] [Google Scholar]
- 14. Sun J, Garfield DH, Lam B, et al. The value of autofluorescence bronchoscopy combined with white light bronchoscopy compared with white light alone in the diagnosis of intraepithelial neoplasia and invasive lung cancer. J Thorac Oncol. 2011;6(8):1336–1344. [DOI] [PubMed] [Google Scholar]
- 15. Jang TW, Oak CH, Chun BK, Jung MH. Detection of pre-invasive endobronchial tumors with D-light/autofluorescence system. J Korean Med Sci. 2006;21(2):242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch FR, Prindiville SA, Miller YE, et al. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst. 2001;93(18):1385–1391. [DOI] [PubMed] [Google Scholar]
- 17. Hanibuchi M, Yano S, Nishioka Y, et al. Autofluorescence bronchoscopy, a novel modality for the early detection of bronchial premalignant and malignant lesions. J Med Inves. 2007;54(3-4):261–266. [DOI] [PubMed] [Google Scholar]
- 18. Häussinger K, Becker H, Stanzel F, et al. Autofluorescence bronchoscopy with white light bronchoscopy compared with white light bronchoscopy alone for the detection of precancerous lesions: a European randomised controlled multicentre trial. Thorax. 2005;60(6):496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edell E, Lam S, Pass H, et al. Detection and localization of intraepithelial neoplasia and invasive carcinoma using fluorescence-reflectance bronchoscopy: an international, multicenter clinical trial. J Thorac Oncol. 2009;4(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ernst A, Simoff MJ, Mathur PN. D-light autofluorescence in the detection of premalignant airway changes: a multicenter trial. Bronchol. 2005;12(3):133–138. [Google Scholar]
- 21. Kusunoki Y, Imamura F, Uda H, Mano M, Horai T. Early detection of lung cancer with laser-induced fluorescence endoscopy and spectrofluorometry. Chest. 2000;118(6):1776–1782. [DOI] [PubMed] [Google Scholar]
- 22. Nakanishi K, Ohsaki Y, Kurihara M, et al. Color auto-fluorescence from cancer lesions: improved detection of central type lung cancer. Lung Cancer. 2007;58(2):214–219. [DOI] [PubMed] [Google Scholar]
- 23. Khattab D, Ebied HM, Hussein AS, Tolba MF. Color image segmentation based on different color space models using automatic GrabCut. ScientificWorldJournal. 2014;2014:126025. [DOI] [PMC free article] [PubMed] [Google Scholar]