Abstract
Cd (Cd) is a nephrotoxic environmental pollutant that causes generalized proximal tubule dysfunction. Even though the specific mechanisms by which Cd damages the kidney have yet to be fully elucidated, there is evidence to suggest that some of these nephrotoxic effects may result from the ability of Cd to alter the levels and function of metals such as Cu, Se, Zn and Fe within the kidney. In order to further explore this issue, we examined the effects of subchronic Cd exposure on tissue levels of a panel of metals (Ca, Cu, Fe, K, Mg, Na, Se and Zn) in the rat renal cortex. Adult male Sprague-Dawley rats were treated with CdCl2 (0.6 mg Cd/kg body weight in isotonic saline by subcutaneous injection, 5 days per week for 6, 9 or 12 weeks). At each time point, 24 h urine samples were collected and assayed for levels of protein, creatinine, β2 microglobulin and cystatin C. Samples of renal cortex were removed and assayed for levels of the metals of interest by inductively-coupled mass spectrometry at Michigan State University. Results showed that at 9 and 12 weeks, Cd caused significant increases in urine volume and urinary protein with no change in creatinine excretion. Increases in the excretion of the urinary biomarkers β2 microglobulin and cystatin C were evident after 6 weeks of Cd exposure. Results of the metal analyses showed that Cd caused significant increases in tissue levels of Cu and Se at all of the time points examined. Tissue levels of Zn were transiently elevated at 6 weeks but declined to control levels at 9 and 12 weeks. Cd caused a significant decrease in levels of Fe at 9 and 12 weeks. Cd had no effects on any of the other metals. Tissue levels of Cd were 530 ± 52, 863 ± 23, 837 ± 23 ppm dry weight at 6, 9 and 12 weeks, respectively. These results indicate that the early stages of Cd nephrotoxicity are associated with alterations in renal tissue levels of Cu, Se, Zn and Fe. The fact that the changes in levels of the metals occurred during the early stages of Cd toxicity raises the possibility that the alterations in renal cortical metal content may play some role in the pathophysiology or Cd-induced injury.
Keywords: Kidney, Cadmium, Copper, Selenium, Zinc, Iron
1. Introduction
Cadmium (Cd) is an important nephrotoxic metal that currently ranks 7th on the United States Environmental Protection Agencies Priority List of Hazardous Substances [2]. With the chronic, low-level patterns of Cd exposure that are common in humans, the kidney is the primary target of toxicity. Cd accumulates in the epithelial cells of the proximal tubule, where it causes a generalized reabsorptive dysfunction characterized by polyuria and proteinuria (for reviews see [18], [19], [36]. These nephrotoxic effects occur when circulating Cd that is bound to low molecular proteins or thiol compounds is filtered at the glomerulus and/or presented to the basolateral cell surface and then taken up by the epithelial cells of the proximal tubule (for reviews see [36], [63], [64]. Over time, Cd accumulates in the cells to reach levels of 150–200 μg/g wet weight (equivalent to 450–600 μg/g dry weight) that cause toxic injury [18], [23], [43], [44].
While these general effects of Cd on the kidney have been well-characterized, the specific molecular mechanisms through which Cd produces these effects have yet to be fully elucidated. Studies to address this issue have shown that Cd can produce a myriad of biochemical effects in proximal tubule cells including, the induction of oxidative stress, reorganization of the cytoskeleton, disruption of cadherin-mediated homotypic cell adhesion, and activation or inhibition of various cellular signaling cascades (for reviews see [13], [14], [21], [27], [45], [51]. However, in most cases the specific relationships between these various biochemical effects and the overt cytotoxic effects of Cd remain unclear.
One of the more widely accepted general mechanisms by which Cd can affect cell function involves disruption of the actions or alterations of the physiologic disposition of essential metals. These essential metals include agents such as Cu, Zn, Se, Fe and Mg, which serve many important physiologic functions. As a stable divalent cation, Cd is able to interact with many of the same molecular targets on which these essential metals act. In addition, Cd can interact with transport proteins and ion channels that regulate the physiologic disposition of these metals. In a number of important reviews over the past 2 decades, various authors have highlighted the fact that alterations in the homeostasis or transport of essential metals may play a major role in the pathophysiology of Cd-induced kidney injury [4], [15], [31], [52], [57], [58], [61]. However, the relationships between alterations in the homeostasis of individual metals and specific nephrotoxic actions of Cd remain unclear.
Over the past 10–15 years, we have been engaged in studies to elucidate mechanisms underlying Cd-induced kidney injury and to identify more sensitive biomarkers of Cd nephrotoxicity [40], [39], [41], [36], [42]. Most of our in vivo studies have involved the use of a sub-chronic model of Cd exposure in the rat. This dosing regimen, which involves the daily, subcutaneous administration of CdCl2, at a daily dose of 0.6 mg/kg, 5 days per week for up to 12 weeks, has been extensively used by other investigators and is an accepted model in the Cd research field [1], [8], [22], [40], [41], [46], [48], [50]. In addition, previous studies from our laboratory have shown that this treatment protocol can be used to reproducibly induce the early stages of Cd nephrotixicty [41], [38], [42]. In the present study, we have analyzed the levels of a panel of essential metals in archived, frozen tissue samples from our recent Cd biomarker studies [42]. In addition, we consider the results of the metal analyses in relationship to the severity of Cd-induced kidney injury, as assessed by histopathology and alterations in specific urinary biomarkers. The results indicate that the early stages of Cd nephrotoxicity are associated with changes in the tissue levels of several essential metals, particularly Cu, Se, Zn and Fe.
2. Materials and methods
The samples of kidney tissue that were used in the present studies were harvested from rats that had been treated with Cd in a series of urinary biomarker studies that were conducted between 2012 and the end of 2015. Results of the biomarker studies have been published recently [42]. These animal studies were conducted in compliance with the United States NIH Guide for the Care and Use of Laboratory Animals [32], and they were approved by the Institutional Animal Care and Use Committee of Midwestern University. Adult male Sprague-Dawley rats weighing 250–300 g (Harlan, Indianapolis, IN) were housed socially (two rats per plastic cage) on a 12 h light cycle/12 h dark cycle. Animals in the Cd treatment groups (n = 6 for each time point) received daily (Monday-Friday) subcutaneous injections of CdCl2 at a dose of 0.6 mg Cd (5.36 μmol) in 0.24–0.35 ml isotonic saline for up to 12 weeks. Control group animals (n = 6 for each time point) received daily injections of the saline vehicle alone. After 6, 9 and 12 weeks of treatment, animals were placed in individual metabolic cages and 24 h urine samples were collected. The animals were allowed free access to water at all times. Food was also available ad libitum, except during the period in which the urine samples were being collected. The 12 week Cd treatment protocol was repeated at least twice and data from the multiple treatment protocols were pooled whenever possible. Before the start of each experiment, the Cd concentrations of the stock solutions used for the various treatment protocols were verified by Chemical Solutions, Ltd. (Harrisburg, PA) using the technique of inductively coupled plasma mass spectroscopy.
2.1. Urine and blood analysis
After collection, the 24 h urine samples were aliquoted into 0.5–1.0 ml portions. The aliquots were frozen at −80 °C and later assayed for protein, creatinine, cystatin C and β2 microglobulin as described previously [42]. Blood samples were obtained at the time the animals were euthanized, and serum levels of cystatin C were determined by an enzyme-linked immunoabsorbent assay as described by Prozialeck et al. [42].
2.2. Metal analyses
As noted previously, the tissue samples that were analyzed in the present study were generated from animal treatment protocols that were carried out in our laboratories between 2012 and 2015. At the time the animals were euthanized, samples of renal cortex (∼50–100 mg each) were harvested and frozen at −80 °C until they were analyzed for metal content, which was within 6 months after the end of the treatment period. For the metal analyses, the samples were shipped on dry-ice to the Diagnostic Center for Population and Animal Health, at Michigan State University (East Lansing, MI). The tissues were dried overnight at 75 °C and then digested in approximately 10× the dry tissue mass of nitric acid. The digested samples were diluted with water to 100× the dried tissue mass. Elemental analyses were conducted according to the method of Wahlen et al. [59] using an Agilent 7500ce Inductively Coupled Plasma – Mass Spectrometer (ICP/MS) (Aligent Technologies, Inc.) Briefly, the tissue digests and standards were diluted 20-fold with a solution containing 0.5% EDTA and Triton X-100, 1% ammonium hydroxide, 2% propanol and 20 ppb of scandium, rhodium, indium and bismuth as internal standards. The ICP/MS was tuned to yield a minimum of 7500 cps sensitivity for 1 ppb yttrium (mass 89), less than 1.0% oxide level as determined by 156/140 mass ratio and less than 2.0% double charged ions as determined by the 70/140 mass ratio. Elemental concentrations were calibrated using a 4-point linear curve of the analyte-internal standard response ratio. Standards were from GFS Chemicals (Powell, OH). Results were expressed as μg metal/g dry tissue weight. This was done to avoid any variability that might be caused by the presence of frost or water condensation on the tissue samples. The concentration of the metals based on the wet weight of the tissue samples can be estimated by multiplying the concentration/per unit of dry weight by a factor of 0.33.
2.3. Histopathologic analyses
Transverse slices of renal cortical tissue (∼5 mm thick) were placed in 4% formalin and sent for analysis to Colorado Histo-Prep Services (Ft. Collins, CO), where the samples were embedded in paraffin, sectioned at a thickness of 4 μ and stained with hematoxylin and eosin. The samples were viewed and histologic changes were semi-quantitatively evaluated by a trained veterinary histopathologist. The slides were evaluated for inflammation, necrosis, and apoptosis of the proximal tubules, as well as any other lesions that might have been present. Histopathologic changes were rated on a 6 point scale as follows: 0 = No appreciable changes, 1 = Minimal (individually scattered changes), 2 = Mild (changes in less than 10% of tubules), 3 = Moderate (changes in 10–25% of tubules), 4 = Moderately Severe (changes in 25–50% of tubules), 5 = Severe (changes in more than 50% of tubules).
Photographic images of representative tissue sections were obtained in our laboratory using a Nikon Eclipse E-400 microscope equipped with a 20× objective lens. Images were captured with a digital camera (Media Cybernetics), using automated exposure times and gain settings, and then processed using Adobe Photoshop (Version CS6) to enhance brightness and contrast. Identical adjustments were made in all images from the control and the Cd-treated samples.
2.4. Statistical analysis
Statistical analyses were done using the GraphPad Prism Computer Program. Data for the various urinary parameters, which showed heterogeneity of variances, were evaluated by the non-parametric Kruskal-Wallis test and Dunn’s post hoc test, as described by Prozialeck et al. [41]. Data for the tissue metal levels, which showed homogeneity of variances, were analyzed by ANOVA and Tukey’s post-hoc test.
3. Results
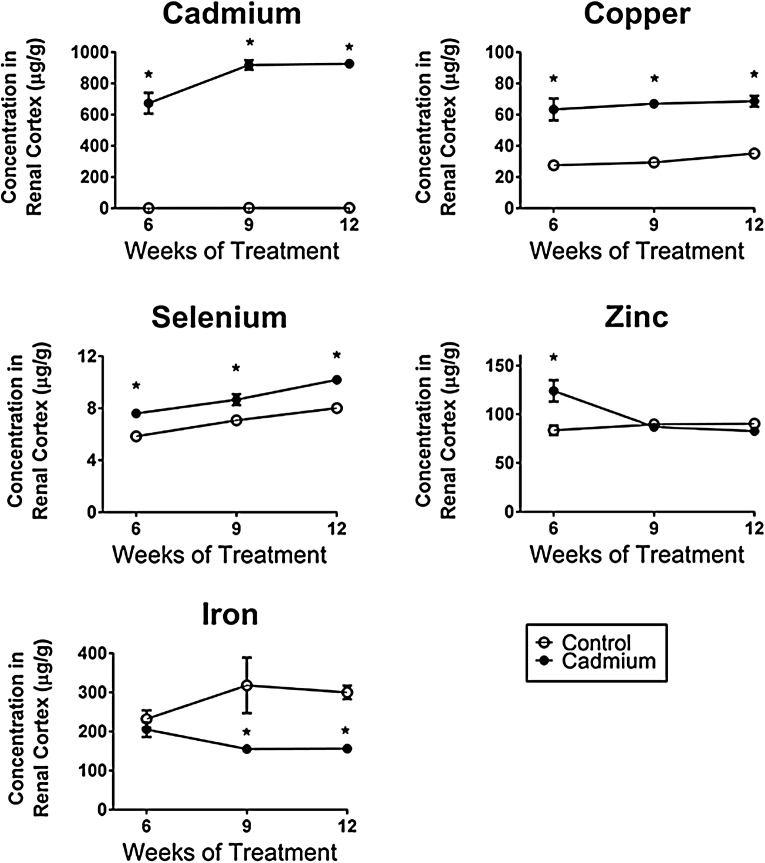
The graphs in Fig. 1 show the changes in levels of the metals that were significantly altered by Cd exposure. Several key findings are immediately apparent. First, Cd differentially affected the tissue levels of the various metals. In some cases, these effects were relatively straightforward, but in other cases, they were more complex and variable. For example, Cd caused a significant (2–3 fold) increase in levels of Cu that were evident as early as 6 weeks and then persisted at this same level throughout the 12 week treatment period. Cd treatment also increased tissue levels of Se. It is noteworthy that the levels of Se in the control animals tended to increase throughout the treatment period. At the same time, the level of Se in the samples from the Cd-treated animals were consistently (about 25–30%) higher than in the time-matched controls. By contrast, Cd decreased renal cortical levels of Fe to about 50% of control values, an effect that was statistically significant at 9 and 12 weeks. Cd appeared to have biphasic, time-dependent effects on renal cortical levels of Zn. After 6 weeks of Cd treatment, tissue levels of Zn were significantly elevated to about 140% control values. However, by 9 weeks, Zn levels fell back to control levels and by 12 weeks, they appeared to be slightly below control levels, although the difference was not statistically significant. Cd had no overall effect on tissue levels of several other key metals including Ca, Mg, K and Na (Table 1).
Fig. 1.
Effects of Cd on renal cortical levels of Cd, Cu, Se, Zn and Fe.
Table 1.
Lack of effect of Cd on levels of Mg, Ca, Na and K in renal cortex.
| Treatment | Metal |
|||
|---|---|---|---|---|
| Mg | Ca | Na | K | |
| Control 6 Weeks | 705 ± 4.04 | 227 ± 8.9 | 4138 ± 588 | 11,876 ± 441 |
| Cd 6 weeks | 655 ± 8.2 | 241 ± 13.3 | 4450 ± 524 | 11,216 ± 166 |
| Control 9 Weeks | 759 ± 3.9 | 272 ± 1.9 | 4227 ± 533 | 12,165 ± 414 |
| Cd 9 weeks | 724 ± 27 | 253 ± 18.5 | 3786 ± 231 | 12,764 ± 546 |
| Control 12 Weeks | 751 ± 9.9 | 304 ± 15.8 | 3883 ± 137 | 12,694 ± 234 |
| Cd 12 weeks | 754 ± 15.5 | 320 ± 9.6 | 4575 ± 310 | 14,271 ± 426 |
In evaluating the results of the metal analyses, a key factor that needs to be considered is the level of kidney injury during the time frame in which the early Cd-induced changes in renal cortical metal content are occurring. This Cd dose and treatment protocol have been shown to trigger the early stages of Cd-induced kidney injury [38], [39], [37]. Results of the biomarker studies from which the present tissue samples were obtained showed that after 9 weeks of exposure, Cd increased urine volume and total urinary protein excretion but had no significant effect on the urinary excretion of creatinine even after 12 weeks. The development of polyuria and proteinuria, with no change in creatinine is characteristic of Cd-induced proximal tubule dysfunction [41], [36]. With regard to the present studies, it is noteworthy that urinary levels of the biomarkers cystatin C and β2 microglobulin in the samples from the Cd-treated animals were significantly elevated after only 6 weeks of Cd exposure, and then continued to increase throughout the remainder of the 12 week Cd treatment period. These results are similar to those in previous reports [41], [39], [36], [35], [42] and they indicate that between 9 and 12 weeks, this dose of Cd induces a mild to moderate level of proximal tubule dysfunction (polyuria and proteinuria). However, more subtle changes in proximal tubule dysfunction can be detected about 3 weeks earlier as evidenced by increases in the urinary excretion of biomarkers such as cystatin C and β2 microglobulin. Additional studies in which we utilized this same treatment protocol showed that Cd had no effect on serum levels of either creatinine [41], [39] or cystatin C [42], indicating that Cd had no effect on glomerular function and that the proximal tubule is the primary target of nephrotoxic injury.
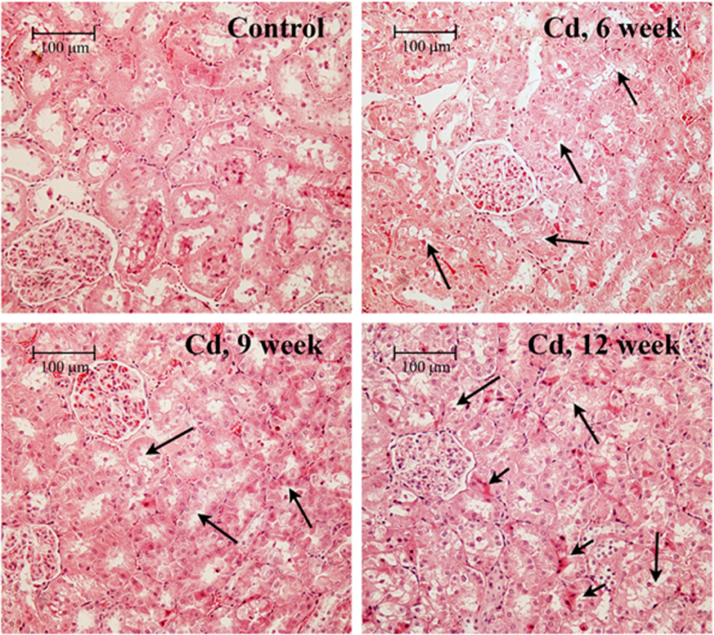
To further evaluate the severity of Cd-induced kidney injury, a series of histopathologic analyses of hematoxylin and eosin stained tissue sections were conducted. The first analysis involved the qualitative evaluation of the tissue sections in our laboratory. Fig. 2 shows typical images of tissue sections of outer renal cortex from control and Cd-treated animals. Note that proximal tubule epithelial cells in control samples (top left) exhibited cuboidal shapes, well-defined nuclei, and a uniform cytoplasm, with no spaces or gaps between the cells. By contrast, the epithelial cells in samples from Cd-treated animals (top right and bottom row) showed an irregular appearance, with gaps between cells (long arrows). However, the cells remained attached to the basement membrane and showed little overt evidence of necrosis. In addition, the samples from the 12 week Cd treatment group showed some areas of enhanced eosinophilic staining in the proximal tubules (short arrows in bottom right image). Analyses of the glomeruli and distal segments of the nephron revealed no evidence of pathology.
Fig. 2.
Effects of Cd on the general morphology of the renal cortex.
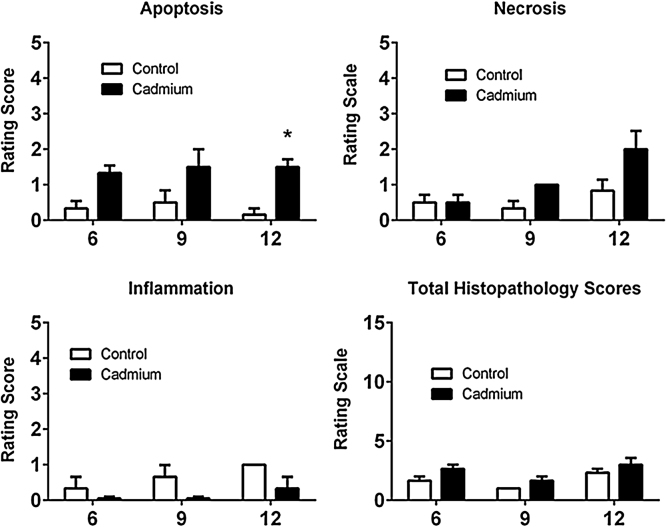
For the second phase of the histopathologic analyses, hematoxylin and eosin-stained tissue sections were evaluated semi-quantitatively by a trained pathologist at Colorado Histo-Prep Services. Results of these histopathologic analyses are summarized in Fig. 3. As may be seen from the graphs, Cd had only modest effects on over-all proximal tubule morphology. The most consistent change was a slight increase in the number of apoptotic cells in the proximal tubule that only reached statistical significance at 12 weeks. Cd had no significant effect on the evaluation scores for necrosis or inflammation. In addition, the total histopathologic scores (calculated by adding scores for apoptosis, necrosis and inflammation) were not altered by Cd. This finding is consistent with previous studies showing that this Cd dosing regimen causes apoptosis in a low, but consistent percentage (2.0–5%) of proximal tubule cells [38], while producing little evidence of general inflammation or necrosis. Together, the biomarker studies summarized in Prozialeck et al. [42] and the morphologic analyses in Fig. 2, Fig. 3 indicate that even after 12 weeks, using this treatment protocol, the level of Cd-induced kidney injury is relatively mild.
Fig. 3.
Summary of effects of Cd on Histopathological changes in the proximal tubule.
4. Discussion
The results of the present studies show that after subchronic administration of a modest dose (0.6 mg/kg SC, 5 days per week for 6–12 weeks), Cd causes significant changes in the renal cortical levels of several key metals, particularly Cu, Se, Fe and Zn. It should be noted that even though many previous studies have shown that Cd can alter renal levels of these metals [11], [26], [55], [53], there are several aspects of the present studies that are unique and merit special attention.
First, the present studies involved the use of specific Cd dosing and treatment protocols that have been widely used by numerous investigators to characterize the toxicokinetics of Cd in the body, to examine possible mechanisms by which Cd damages the kidney, and to validate the use of various biomarkers of Cd-induced kidney injury [1], [8], [22], [40], [41], [39], [38], [46], [48], [50]. However, there is almost no information in the literature regarding the effects of this specific Cd treatment regimen on levels of other metals in the kidney. Most of the previous studies to address this issue involved vastly different treatment regimens in which relatively high doses of Cd were given either orally or intra-peritoneally for relatively short periods of time, typically less than 5 weeks [9], [10], [53], [55], [61]. Since this model has been so widely used in the Cd field, information regarding the effects of this particular Cd treatment on the levels of metals in the kidney may be especially relevant to previous mechanistic and biomarker studies.
A second unique aspect of the present work is that the present metal analyses focused on renal cortical tissue. This is significant because the primary target of Cd toxicity is the renal cortex, with an approximate 4-fold greater concentration of Cd in the cortex as compared to the medulla [56]. In addition, most of the metals examined in the present study tend to concentrate in the proximal tubules [45]. However, most of the previous studies on the effects of Cd on metals in the kidney involved the analyses of whole kidneys or kidney homogenates [53], [55], [61] an approach that could obscure possible Cd-induced changes in the metal content of the renal cortex. The results of the present studies specifically highlight the Cd-induced changes in the metal content of the renal cortex.
Probably, the most unique and important aspect of the present work is that it includes quantitative histopathologic analyses, along with a discussion of changes in various biomarkers of Cd-nephrotoxicity. By considering the histopathological and biomarker findings, we clearly show that at the time the initial changes in renal metal content are occurring, the level of Cd-induced proximal tubule injury is mild. This suggests that alterations in renal metal content may represent early events in the pathophysiology of Cd nephrotoxicity. Few, if any of the previous studies on the effects of Cd on levels of metals in the kidney included such detailed histopathological and biomarker analyses.
As a consequence of the differences in the way various investigators have reported renal levels of these metals (mass vs. molar units; dry weight vs wet weight; whole kidney vs renal cortex, etc.), direct comparisons of results from different laboratories can be difficult. Nevertheless, after making adjustments for these variables, the renal cortical levels of the metals that we observed in the present studies are in ranges reported by other investigators who examined various types of renal pathology in the rat [24], [25], [55], [61]. Of particular note, the levels of Cd that we found to be associated with the onset of renal injury are in ranges reported by other investigators [17], [18], [20], [26], [43], [23].
In addition, the Cd-induced changes in renal metal content that we observed are qualitatively similar to various results reported in the literature. For example, Lee et al. [26] showed that Cd given via subcutaneous injection to female rats for 5 days caused significant increases in renal levels of Cu and Zn. In another study in which Cd was given to pregnant rats via the drinking water, Turgut et al. [55] found that Cd increased renal levels of Zn and reduced Fe levels. In addition, Toman et al. [53] reported that Cd increased renal levels of Se. On the other hand, Erdem et al. [11] reported that Cd administered via drinking water to male rats actually decreased kidney levels of Cu and Zn. It should be noted that the levels of metals reported in the latter study were several orders of magnitude lower than in the other cited studies. While we cannot explain these apparent discrepancies, they may be related to differences in the routes of Cd administration, strain and gender differences, tissue collection procedures, analytical methods and/or methods for the expression of results that were used in the various studies.
An important question, of course, is what are the mechanisms by which Cd alters renal cortical metal content? From a conceptual perspective, it would seem that there are only a few general mechanisms through which Cd could alter the renal cortical concentrations of the various metals. For those metals that showed increased levels, (Cu and Se), Cd could be either enhancing the cellular uptake of the metals and/or inducing the synthesis of proteins that bind/store the metals within the cells. For a metal such as Fe, which decreases in concentration with Cd exposure, Cd could be either inhibiting the cellular uptake and/or inducing the binding/storage of Fe within the cells of the proximal tubule. For a metal such as Zn, which shows biphasic effects, Cd could be exerting multiple, time-dependent effects on uptake and/or storage. There is evidence in the literature to support many of these possibilities. For example, it has long been recognized that Cd can influence tissue levels of Fe and vice versa (for review see [52]. In general, Cd interferes with the absorption and storage of Fe and low levels of Fe can lead to increased Cd accumulation in tissues such as the kidney [52]. Likewise, the association between Cd exposure and increases in renal levels of Zn have long been recognized. Nordberg and coworkers examined the effects of Cd on levels of Zn in horse kidney [23], [33]. As renal levels of Cd increased, there was a steady increase in levels of Zn. The authors attributed this effect to the induction of metallothionein in the proximal tubule, which acts to sequester both Cd and Zn. It is noteworthy that with longer term exposure, the ratio of Zn/Cd bound to metallothionein actually decreased. Such effects could account for the biphasic effect (increase followed by decrease) that we observed in our rat model. For additional information regarding the possible mechanisms through which Cd can affect the homeostasis of various metals, the reader is referred to an outstanding review by Moulis [31].
One final point to consider is that the present findings could also have important implications regarding exposures to mixtures of metals. Simultaneous exposure to multiple metals is common, both in the workplace and through the environment (for reviews see [5], [6], [7], [29], [34], [47], [54]. Despite overwhelming evidence that humans can be exposed to multiple metals, there are relatively few experimental studies examining the potential synergistic or antagonistic effects of exposure to multiple types of metals. Most of these studies show increased kidney dysfunction and necrosis of tubular epithelial cells with exposure to Cd in combination with other metals [6], [12], [28], [62].
Our results show that Cd increases renal cortical levels of Cu and Fe under controlled conditions in which the animals were only exposed to non-toxic levels of Cu and Fe, mainly through their diet. An intriguing question is how might Cd influence renal cortical levels of these metals in subjects exposed to Cd along with toxic levels of Cu and/or Fe? Our findings indicate that Cd may increase the renal accumulation of Cu, which may have either adverse or protective effects in the kidney depending on the level of exposure [10]. On the other hand, it is well known that metals such as Zn and Se can greatly reduce the nephrotoxic effects of Cd [3], [9], [16], [30], [49], [60]. Co-exposure to Cd and these metals may actually attenuate the toxic effects of Cd in the kidney. It remains to be determined how these Cd-metal interactions may relate to mixed metal exposure in humans.
Acknowledgements
The authors sincerely thank Laura Phelps and Vicki Sears of Midwestern University, for their help in preparing the manuscript and the figures. Portions of this work were supported by restricted funds from the Department of Pharmacology, the Biomedical Sciences Program and the Chicago College of Osteopathic Medicine of Midwestern University.
References
- 1.Aoyagi T., Hayakawa K., Miyaji K., Ishikawa H., Hata M. Cadmium nephrotoxicity and evacuation from the body in a rat modeled subchronic intoxication. Int. J. Urol. 2003;10:332–338. doi: 10.1046/j.1442-2042.2003.00627.x. [DOI] [PubMed] [Google Scholar]
- 2.ATSDR . 2008. Toxicological Profile for Cadmium.http://www.atsdr.cdc.gov/cercla/toxprofiles/tp5.html [PubMed] [Google Scholar]
- 3.Babaknejad N., Moshtaghie A.A., Nayeri H., Hani M., Bahrami S. Protective role of zinc and magnesium against cadmium nephrotoxicity in male wistar rats. Biol. Trace Elem. Res. 2016;8:1160–1167. doi: 10.1007/s12011-016-0671-x. [DOI] [PubMed] [Google Scholar]
- 4.Bridges C.C., Zalups R.K. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J.W., Chen H.Y., Li W.F., Liou S.H., Chen C.J., Wu J.H., Wang S.L. The association between total urinary arsenic concentration and renal dysfunction in a community-based population from central Taiwan. Chemosphere. 2011;84:17–24. doi: 10.1016/j.chemosphere.2011.02.091. [DOI] [PubMed] [Google Scholar]
- 6.Cobbina S.J., Chen Y., Zhou Z., Wu X., Zhao T., Zhang Z., Feng W., Wang W., Li Q., Wu X., Yang L. Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J. Hazard. Mater. 2015;294:109–120. doi: 10.1016/j.jhazmat.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 7.de Burbure C., Buchet J.P., Leroyer A., Nisse C., Haguenoer J.M., Mutti A., Smerhovsky Z., Cikrt M., Trzcinka-Ochocka M., Razniewska G., Jakubowski M., Bernard A. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ. Health Perspect. 2006;114:584–590. doi: 10.1289/ehp.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley R.E., Gammal L.M., Klaassen C.D. Cadmium-induced hepatic and renal injury in chronically exposed rats: likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. Appl. Pharmacol. 1985;77:414–426. doi: 10.1016/0041-008x(85)90181-4. [DOI] [PubMed] [Google Scholar]
- 9.El-Sharaky A.S., Newairy A.A., Badreldeen M.M., Eweda S.M., Sheweita S.A. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology. 2007;235:185–193. doi: 10.1016/j.tox.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Enli Y., Turgut S., Oztekin O., Demir S., Enli H., Turgut G. Cadmium intoxication of pregnant rats and fetuses: interactions of copper supplementation. Arch. Med. Res. 2010;41:7–13. doi: 10.1016/j.arcmed.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Erdem O., Yazihan N., Kocak M.K., Sayal A., Akcil E. Influence of chronic cadmium exposure on the tissue distribution of copper and zinc and oxidative stress parameters in rats. Toxicol. Ind. Health. 2015 doi: 10.1177/0748233714566875. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Fowler B.A., Mahaffey K.R. Interactions among lead, cadmium, and arsenic in relation to porphyrin excretion patterns. Environ. Health Perspect. 1978;25:87–90. doi: 10.1289/ehp.782587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiwara Y., Lee J.Y., Tokumoto M., Satoh M. Cadmium renal toxicity via apoptotic pathways. Biol. Pharm. Bull. 2012;35:1892–1897. doi: 10.1248/bpb.b212014. [DOI] [PubMed] [Google Scholar]
- 14.Garrett S.H., Clarke K., Sens D.A., Deng Y., Somji S., Zhang K.K. Short and long term gene expression variation and networking in human proximal tubule cells when exposed to cadmium. BMC Med. Genomics. 2013;6(Suppl. 1):S2. doi: 10.1186/1755-8794-6-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He L., Wang B., Hay E.B., Nebert D.W. Discovery of ZIP transporters that participate in cadmium damage to testis and kidney. Toxicol. Appl. Pharmacol. 2009;238:250–257. doi: 10.1016/j.taap.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamakala O., Rani U.A. Amelioration effect of zinc and iron supplementation on selected oxidative stress enzymes in liver and kidney of cadmium-treated male Albino rat. Toxicol. Int. 2015;22:1–9. doi: 10.4103/0971-6580.172289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamall I.S., Smith J.C. Effects of cadmium treatment on selenium-dependent and selenium-independent glutathione peroxidase activities and lipid peroxidation in the kidney and liver of rats maintained on various levels of dietary selenium. Arch. Toxicol. 1985;58:102–105. doi: 10.1007/BF00348317. [DOI] [PubMed] [Google Scholar]
- 18.Jarup L. Cadmium overload and toxicity. Nephrol. Dial. Transplant. 2002;17(Suppl. 2):35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- 19.Jarup L., Akesson A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Jihen e.H., Imed M., Fatima H., Abdelhamid K. Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: histology and Cd accumulation. Food Chem. Toxicol. 2008;46:3522–3527. doi: 10.1016/j.fct.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 21.Joseph P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238:272–279. doi: 10.1016/j.taap.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Kaur J., Sharma N., Attri S., Gogia L., Prasad R. Kinetic characterization of zinc transport process and its inhibition by cadmium in isolated rat renal basolateral membrane vesicles: in vitro and in vivo studies. Mol. Cell. Biochem. 2006;283:169–179. doi: 10.1007/s11010-006-2676-9. [DOI] [PubMed] [Google Scholar]
- 23.T. Kjellstrom, 1986. Renal Effects. In Cadmium and Health: a Toxicological and Epidemiological Appraisal (L. Friberg, C.-G. Elinder, T. Kjellstrom, G. Nordberg, Eds.), pp. 21–109.
- 24.Krosniak M., Kowalska J., Francik R., Grybos R., Blusz M., Kwiatek W.M. Influence of vanadium-organic ligands treatment on selected metal levels in kidneys of STZ rats. Biol. Trace Elem. Res. 2013;153:319–328. doi: 10.1007/s12011-013-9688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucukatay V., Turgut S., Kocamaz E., Emmungil G., Bor-Kucukatay M., Turgut G., Akca H., Bagci H. Effect of sulfite exposure on zinc, iron, and copper levels in rat liver and kidney tissues. Biol. Trace Elem. Res. 2006;114:185–195. doi: 10.1385/BTER:114:1:185. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y.H., Shaikh Z.A., Tohyama C. Urinary metallothionein and tissue metal levels of rats injected with cadmium, mercury, lead, copper or zinc. Toxicology. 1983;27:337–345. doi: 10.1016/0300-483x(83)90029-x. [DOI] [PubMed] [Google Scholar]
- 27.Liu J., Qu W., Kadiiska M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madden E.F., Akkerman M., Fowler B.A. A comparison of 60, 70, and 90 kDa stress protein expression in normal rat NRK-52 and human HK-2 kidney cell lines following in vitro exposure to arsenite and cadmium alone or in combination. J. Biochem. Mol. Toxicol. 2002;16:24–32. doi: 10.1002/jbt.10015. [DOI] [PubMed] [Google Scholar]
- 29.Mari M., Nadal M., Schuhmacher M., Barberia E., Garcia F., Domingo J.L. Human exposure to metals: levels in autopsy tissues of individuals living near a hazardous waste incinerator. Biol. Trace Elem. Res. 2014;159:15–21. doi: 10.1007/s12011-014-9957-z. [DOI] [PubMed] [Google Scholar]
- 30.Messaoudi I., El H.J., Hammouda F., Said K., Kerkeni A. Protective effects of selenium, zinc, or their combination on cadmium-induced oxidative stress in rat kidney. Biol. Trace Elem. Res. 2009;130:152–161. doi: 10.1007/s12011-009-8324-y. [DOI] [PubMed] [Google Scholar]
- 31.Moulis J.M. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals. 2010;23:877–896. doi: 10.1007/s10534-010-9336-y. [DOI] [PubMed] [Google Scholar]
- 32.National Reaseach Council of the National Academies . National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 33.Nordberg M., Elinder C.-G., Rahnster B. Cadmium, zinc and copper in horse kidney metallothionein. Environ. Res. 1979;20:341–350. doi: 10.1016/0013-9351(79)90010-0. [DOI] [PubMed] [Google Scholar]
- 34.Poreba R., Gac P., Poreba M., Antonowicz-Juchniewicz J., Andrzejak R. Relation between occupational exposure to lead, cadmium, arsenic and concentration of cystatin C. Toxicology. 2011;283:88–95. doi: 10.1016/j.tox.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Prozialeck W.C. Biomarkers for cadmium. In: Uversky V., Kretsinger R.H., Permyakov E., editors. Encyclopedia of Metalloproteins. Springer; New York: 2013. pp. 272–277. [Google Scholar]
- 36.Prozialeck W.C., Edwards J.R. Early biomarkers of cadmium exposure and nephrotoxicity. Biometals. 2010;23:793–809. doi: 10.1007/s10534-010-9288-2. [DOI] [PubMed] [Google Scholar]
- 37.Prozialeck W.C., Edwards J.R. Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012;343:2–12. doi: 10.1124/jpet.110.166769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prozialeck W.C., Edwards J.R., Lamar P.C., Liu J., Vaidya V.S., Bonventre J.V. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol. Appl. Pharmacol. 2009;238:306–314. doi: 10.1016/j.taap.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prozialeck W.C., Edwards J.R., Vaidya V.S., Bonventre J.V. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol. Appl. Pharmacol. 2009;238:301–305. doi: 10.1016/j.taap.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prozialeck W.C., Lamar P.C., Lynch S.M. Cadmium alters the localization of N-cadherin, E-cadherin, and beta-catenin in the proximal tubule epithelium. Toxicol. Appl. Pharmacol. 2003;189:180–195. doi: 10.1016/s0041-008x(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 41.Prozialeck W.C., Vaidya V.S., Liu J., Waalkes M.P., Edwards J.R., Lamar P.C., Bernard A.M., Dumont X., Bonventre J.V. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prozialeck W.C., VanDreel A., Ackerman C.D., Stock I., Papaeliou A., Yasmine C., Wilson K., Lamar P.C., Sears V.L., Gasiorowski J.Z., DiNovo K.M., Vaidya V.S., Edwards J.R. Evaluation of cystatin C as an early biomarker of cadmium nephrotoxicity in the rat. Biometals. 2016;29:131–146. doi: 10.1007/s10534-015-9903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roels H., Bernard A., Buchet J.P., Goret A., Lauwerys R., Chettle D.R., Harvey T.C., Haddad I.A. Critical concentration of cadmium in renal cortex and urine. Lancet. 1979;1:221. doi: 10.1016/s0140-6736(79)90630-5. [DOI] [PubMed] [Google Scholar]
- 44.Roels H., Lauwerys R., Dardenne A.N. The critical level of cadmium in human renal cortex: a reevaluation. Toxicol. Lett. 1983;15:357–360. doi: 10.1016/0378-4274(83)90156-x. [DOI] [PubMed] [Google Scholar]
- 45.Sabolic I. Common mechanisms in nephropathy induced by toxic metals. Nephron Physiol. 2006;104:107–114. doi: 10.1159/000095539. [DOI] [PubMed] [Google Scholar]
- 46.Shaikh Z.A., Vu T.T., Zaman K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999;154:256–263. doi: 10.1006/taap.1998.8586. [DOI] [PubMed] [Google Scholar]
- 47.Shelley R., Kim N.S., Parsons P., Lee B.K., Jaar B., Fadrowski J., Agnew J., Matanoski G.M., Schwartz B.S., Steuerwald A., Todd A., Simon D., Weaver V.M. Associations of multiple metals with kidney outcomes in lead workers. Occup. Environ. Med. 2012;69:727–735. doi: 10.1136/oemed-2012-100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki Y. Cadmium metabolism and toxicity in rats after long-term subcutaneous administration. J. Toxicol. Environ. Health. 1980;6:469–482. doi: 10.1080/15287398009529866. [DOI] [PubMed] [Google Scholar]
- 49.Tang W., Sadovic S., Shaikh Z.A. Nephrotoxicity of cadmium-metallothionein: protection by zinc and role of glutathione. Toxicol. Appl. Pharmacol. 1998;151:276–282. doi: 10.1006/taap.1998.8465. [DOI] [PubMed] [Google Scholar]
- 50.Tanimoto A., Hamada T., Koide O. Cell death and regeneration of renal proximal tubular cells in rats with subchronic cadmium intoxication. Toxicol. Pathol. 1993;21:341–352. doi: 10.1177/019262339302100401. [DOI] [PubMed] [Google Scholar]
- 51.Thevenod F. Cadmium and cellular signaling cascades: to be or not to be? Toxicol. Appl. Pharmacol. 2009;238:221–239. doi: 10.1016/j.taap.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Thevenod F., Wolff N.A. Iron transport in the kidney: implications for physiology and cadmium nephrotoxicity. Metallomics. 2015;8:17–42. doi: 10.1039/c5mt00215j. [DOI] [PubMed] [Google Scholar]
- 53.Toman R., Golian J., Siska B., Massanyi P., Lukac N., Adamkovicova M. Cadmium and selenium in animal tissue and their interactions after an experimental administration to rats. Slovak J. Anim. Sci. 2009;42:115–118. [Google Scholar]
- 54.Trzeciakowski J.P., Gardiner L., Parrish A.R. Effects of environmental levels of cadmium, lead and mercury on human renal function evaluated by structural equation modeling. Toxicol. Lett. 2014;228:34–41. doi: 10.1016/j.toxlet.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turgut S., Enli Y., Emmungil G., Turgut G., Demir S., Kaptanoglu B., Genc O. Influence of cadmium and copper on tissue element levels of pregnant rats. Cent. Eur. J. Med. 2007;2:447–457. [Google Scholar]
- 56.Uetani M., Kobayashi E., Suwazono Y., Honda R., Nishijo M., Nakagawa H., Kido T., Nogawa K. Tissue cadmium (Cd) concentrations of people living in a Cd polluted area, Japan. Biometals. 2006;19:521–525. doi: 10.1007/s10534-005-5619-0. [DOI] [PubMed] [Google Scholar]
- 57.Van Kerkhove E., Pennemans V., Swennen Q. Cadmium and transport of ions and substances across cell membranes and epithelia. Biometals. 2010;23:823–855. doi: 10.1007/s10534-010-9357-6. [DOI] [PubMed] [Google Scholar]
- 58.Vesey D.A. Transport pathways for cadmium in the intestine and kidney proximal tubule: focus on the interaction with essential metals. Toxicol. Lett. 2010;198:13–19. doi: 10.1016/j.toxlet.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Wahlen R., Evans L., Hearn R. The use of collision/reaction cell ICP-MS for the determination of elements in blood and serum samples. Spectometry. 2005;20:84–89. [Google Scholar]
- 60.Webb M. Protection by zinc against cadmium toxicity. Biochem. Pharmacol. 1972;21:2767–2771. doi: 10.1016/0006-2952(72)90024-x. [DOI] [PubMed] [Google Scholar]
- 61.Yang X.F., Wang S.Y., Zhao R.C., Ao S.Q., Xu L.C., Wang X.R. Changes in tissue metals after cadmium intoxication and intervention with chlorpromazine in male rats. Biomed. Environ. Sci. 2000;13:19–25. [PubMed] [Google Scholar]
- 62.Yuan G., Dai S., Yin Z., Lu H., Jia R., Xu J., Song X., Li L., Shu Y., Zhao X. Toxicological assessment of combined lead and cadmium: acute and sub-chronic toxicity study in rats. Food Chem. Toxicol. 2014;65:260–268. doi: 10.1016/j.fct.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 63.Zalups R.K. Evidence for basolateral uptake of cadmium in the kidneys of rats. Toxicol. Appl. Pharmacol. 2000;164:15–23. doi: 10.1006/taap.1999.8854. [DOI] [PubMed] [Google Scholar]
- 64.Zalups R.K., Ahmad S. Molecular handling of cadmium in transporting epithelia. Toxicol. Appl. Pharmacol. 2003;186:163–188. doi: 10.1016/s0041-008x(02)00021-2. [DOI] [PubMed] [Google Scholar]