Highlights
-
•
Atmospheric geogenic dust comprised of a mineral-metal mixture is a source of exposure in Clark County, Nevada.
-
•
Lung exposures over a period of one month to NDRA atmospheric geogenic dust suppressed immune function in a mouse model.
-
•
Similar geological desert surfaces emit dust in southern Nevada and elsewhere in the world.
-
•
This study is representative of a desert environment; dust composition may vary by source.
Keywords: Geogenic dust, Heavy metals, Minerals, Lung exposure, Immunotoxicity, Neurotoxicity
Abstract
Desert areas are usually characterized by a continuous deposition of fine airborne particles. Over time, this process results in the accumulation of silt and clay on desert surfaces. We evaluated health effects associated with regional atmospheric dust, or geogenic dust, deposited on surfaces in the Nellis Dunes Recreation Area (NDRA) in Clark County, Nevada, a popular off-road vehicle (ORV) recreational site frequented daily by riders, families, and day campers. Because of atmospheric mixing and the mostly regional origin of the accumulated particles, the re-suspended airborne dust is composed of a complex mixture of minerals and metals including aluminum, vanadium, chromium, manganese, iron, cobalt, copper, zinc, arsenic, strontium, cesium, lead, uranium, and others. Geogenic dust with a median diameter of 4.1 μm was administered via oropharyngeal aspiration to female B6C3F1 mice at doses of 0.01 to 100 mg dust/kg body weight, four times, a week apart, for 28-days. Immuno- and neurotoxicological outcomes 24 h following the last exposure were evaluated. Antigen-specific IgM responses were dose-responsively suppressed at 0.1, 1.0, 10 and 100 mg/kg/day. Splenic and thymic lymphocytic subpopulations and natural killer cell activity also were significantly reduced. Antibodies against MBP, NF-68, and GFAP were not affected, while brain CD3+ T cells were decreased in number. A lowest observed adverse effect level (LOAEL) of 0.1 mg/kg/day and a no observed adverse effect level (NOAEL) of 0.01 mg/kg/day were derived based on the antigen-specific IgM responses.
1. Introduction
The Nellis Dunes Recreation Area (NDRA) provides the only no-permit-required publicly accessible area in southern Nevada for legal off-road vehicle (ORV) driving in a natural desert setting. Annual visitors have been estimated at over 300,000 [13] and ORV driving emits significant amounts of dust that vary greatly with the type of sediment and the characteristics of surface over which the vehicle is driving [14]. The primary pathway of exposure to these dusts is from inhalation, yet there is insufficient evidence to identify differences in the health effects of dust particles with different chemical compositions [31]. As breathing natural, or geogenic, dust from natural settings is an emerging public health concern [25], we undertook this study to understand some of the potential toxicological effects of geogenic dusts via lung exposure.
Airborne dust derived from natural desert environments is often a complex mixture of mineral and organic components with highly variable chemical compositions and sizes ranging from nanometers to several tens of micrometers. Human dust exposures will vary with location and activity, and can range from locally-derived dust originating from a specific soil or geologic deposit [7], to regionally-derived dust that is a complex mixture of dust particles that originated from multiple distant and local sources. Due to their small size, these particles can travel long distances before they settle, and in multimodal wind regimes, dust accumulated in the topsoil may have been supplied by several, sometimes very distant sources. This regional dust is always present in desert areas and plays a large role in shaping landforms and affecting soil characteristics. The continuous deposition of fine airborne particles results in the accumulation of silt and clay in the near-surface and forms vesicular soil horizons that are often associated with desert pavements and biological soil crusts (e.g. [22], [33], [37]). Although natural geomorphic processes affect these dust deposits through time, human disturbance is one of the strongest mechanisms for airborne resuspension of this dust [13] and therefore human exposure.
This study is the third of seven reports characterizing unique surface types, associated dust, and toxicological health effects from a site located only minutes from a major metropolitan population. The surface type studied in this article and designated here as “CBN 3” is a combination of 5 subtypes that were originally delineated based on their dust emission capacity (see Ref. [23] for more details). They include various stages of desert pavement formation that range from young, bar and swale surfaces with poorly developed desert pavement, to older, planar, well-developed desert pavements with tightly interlocking surface clasts, to even older degraded pavements where the clasts are widely distributed across the surface [36]. The well-developed desert pavements have a silty eolian deposit (vesicular horizon) directly underneath the surface rocks that forms as a result of eons of dust deposition (e.g. [22], [33]). The less developed pavements have a similar dust accumulation, but the rock cover is discontinuous and much of the accumulated dust lies directly at the surface. Lastly, CBN 3 also includes areas where bedrock or petrocalcic horizons crop out and have eolian silt filling in cracks, or accumulating near shrubs [23]. The surfaces of CBN 3 cover 77.2% of the area of NDRA.
The surface sediment mineralogy for CBN 3 is typical for arid climates. Quartz, calcite, gypsum, and feldspar minerals are ubiquitous [30]. Clay minerals include palygorskite, smectite, kaolinite, illite, and some chlorite [30]. Fibrous amphiboles are present and recent work suggests they are eolian in origin [4]. These minerals have within their crystal structure or adsorbed on their surfaces, many different heavy metals and metalloids including: aluminum (Al), iron (Fe), strontium (Sr), manganese (Mn), zinc (Zn), vanadium (V), copper (Cu), arsenic (As), uranium (U), cobalt (Co), cadmium (Cd), antimony (Sb), cesium (Cs), thallium (Tl), lead (Pb) and chromium (Cr).
Understanding health impacts of heavy-metal dust exposure is a global concern [1], [6], [35]. This study presents a profile of toxicological effects with specific focus on select immune and nervous system endpoints. Adult female B6C3F1 mice were exposed via oropharyngeal aspiration to CBN 3 geogenic dust with a median grain size of 4.1 μm at concentrations of 0.01–100 mg of dust per kg of body weight for four exposures spaced a week apart over a 28-day period to model a month of weekend exposures. Hematology, clinical chemistry, and descriptive and functional assays to assess toxicity to the immune and nervous systems were performed 24 h after the final exposure. Characterization included the establishment of no observed adverse effect levels (NOAEL) and/or lowest observed adverse effect levels (LOAEL).
2. Materials and methods
2.1. Collection of geogenic dust
Composite samples were collected from the surface sediment (upper 0–4 cm) of each of the five subunits of CBN 3 using a plastic scoop and placed into a clean plastic bag, which was hermetically closed after collection. GPS position of the center of the collection areas was carefully recorded. After drying, the samples were treated in a Soil Fine Particle Extractor (see [12] to extract a sample with a median diameter of approximately 4 μm. The extracts were then combined to create a CBN 3 sample. The exact particle size distribution of the CBN 3 sample was determined with laser diffraction using a Malvern Mastersizer S laser particle size analyzer (Malvern Instruments Ltd., Malvern, UK).
2.2. ICP-MS analyses of geogenic dust
The CBN 3 sample was digested in accordance with the USGS Four-Acids Method (Briggs and Meier [39]) and subsequently analyzed using an Agilent 7700 inductively coupled plasma/mass spectrometry (ICP-MS) device (Agilent Technologies, Santa Clara, USA). To ensure quality control for the ICP-MS analyses, all quality control procedures set forth by US EPA Method 6020A [34] were followed. In addition, NIST SRM 8704 (Buffalo River Sediment) and NIST SRM 2711A (Montana II Soil) were used as standard reference materials (SRMs).
2.3. Arsenic speciation
Arsenite, arsenate, monomethylarsonic acid, and dimethylarsinic acid quantification speciation was performed using IC-ICP-CRC-MS at Brooks Rand Labs, LLC (formerly Applied Speciation and Consulting, LLC) according to an in-house developed method based on [18].
2.4. Quantitation of silicon in geogenic dust
Analysis for the element silicon utilized a Thermo Scientific Niton XL3t GOLDD+ portable XRF (X-ray fluorescence) instrument. For calibration, the NIST Standard Reference Material 2711A was run and the XRF results were in agreement with the certified values for silicon and within the margins of uncertainty for the soil standard. Twelve samples were analyzed from CBN 3, and each sample was run twice for a total of 120 s for each analysis.
2.5. Preparation, stability and verification of geogenic dust for animal exposures
CBN 3 dust was added to ETF-PBS to ascertain stable time frames in which the solution could be used for mouse exposures. Solutions were prepared, and samples of the solutions were collected immediately after preparation, and then at 1, 2, 4, and 6 h. Samples were immediately centrifuged, supernatants removed, and examined using an ICP-MS to quantitate total soluble element concentrations. The analysis indicated that leaving the dust samples in an ETF-PBS solution for up to six hours did not substantially change the distribution of elements in solution. At six hours, soluble element concentrations in supernatant began to increase, indicating that insoluble:soluble portions remained constant for six hours in solution. We did not test for changes in speciation, but only total values of elemental metals. This additional quality control measure verified our dosing solution concentrations, potential for flux, and accounted for potential contamination from PBS or other steps in our preparation process. To control for contamination in this preparation process, no metal spatulas or any other metal items were used for weighing, storage, manipulation, or transport of dust samples. Based on these preperation studies, CBN 3 extracts were carefully labeled, stored in sealed and dry containers, protected from light, and secured in a lock box in the laboratory. Dust samples were prepared in sterile, endotoxin-free, phosphate buffered saline (ETF-PBS) at concentrations adjusted to a delivery volume of 10 uL that would represent 0.01, 0.1, 1, 10, or 100 mg of dust/kg of body weight. Mice were exposed via the lungs within 1–2 h of dust solution preparation. Addition of dust samples to ETF-PBS for delivery into the mouse may have changed the distribution of insoluble elements versus concentration of those elements in solution. To verify that adding the dust samples in ETF-PBS did not substantially alter the solubility of elements, a stability study was performed with the lowest concentration (0.01 mg/kg) and a higher concentration (10 mg/kg).
2.6. Animals
Mice were obtained from Charles River Laboratories (headquartered in Wilmington, MA) and were acclimated for 7 days to the conditions of the treatment room (12-h light/dark cycle, 22 ± 2 °C, 60–65% relative humidity) at the University of Nevada Las Vegas (UNLV) animal facilities, which are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The UNLV Institutional Animal Care and Use Committee approved all experiments. Mice were housed in ventilated polycarbonate shoebox cages with corncob bedding and were given unlimited access to food and water.
2.7. Animal exposures
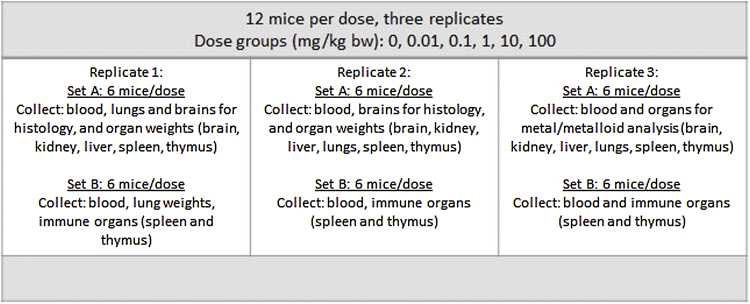
To simulate the potential health impacts of a month of weekend exposures to CBN 3 dust from the NDRA, adult female B6C3F1 mice were exposed to CBN 3 extracts with a median diameter of 4.1 μm (Table 1) at 0, 0.01, 0.1, 1.0, 10, or 100 mg/kg of body weight once weekly for four weeks. B6C3F1 mice have been recommended in immunotoxicity studies by the National Toxicology Program and female mice were selected as they tend to be less aggressive than male mice [5]. Aggressiveness is linked to changes in corticosterone levels in mice, which is a well-known factor affecting immune responses. Each dose administered was adjusted to body weight. Therefore, based on a 20 g mouse, 20 μg was administered to the lung via oropharyngeal aspiration. Mice in the 0 mg/kg group received PBS only and served as a vehicle control group. To ensure availability of tissue for toxicology assays, each dose group was comprised of 12 mice, housed six per cage. In addition, three separate groups of mice were used for a total of three replicates for each exposure. Samples for toxicity studies were collected from each group and replicate as depicted in Fig. 1.
Table 1.
Total elemental concentration (μg/g) in dry geogenic dust from CBN 3.
| Mediana | Al | V | Cr | Mn | Fe | Co | Cu | Zn | As | Sr | Cd | Sb | Cs | Tl | Pb | U | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.1 | 59,993 | 62 | 44 | 552 | 28,564 | 11 | 32 | 94 | 17 | 328 | <0.47 | <3.0 | 9.3 | <8.3 | 23 | 2.2 | 202,660 |
< indicates value is below method quantitation limit (MQL) and that value presented is MQL.
Data are reported with a maximum of three significant figures.
Median diameter (μm).
Fig. 1.
Toxicology sample collection arrangement.
Mice were exposed by oropharyngeal aspiration using isofluorane as an anesthetic agent. The dose was based on current body weight of the mouse. An average volume of 10 μl was administered to each mouse; however, based on individual body weight changes, this volume was adjusted to between 9–13 μl per mouse. One day after the final dose was delivered, samples for toxicity studies were collected from animals euthanized by carbon dioxide asphyxiation. The following methods describe the assays used to determine a profile of toxicological effects specific to NDRA geogenic dust from CBN 3.
2.8. Body weight, organ weights, and immune organ cellularity
Body weight was monitored weekly during the study and terminal body weights were collected for all animals the day of euthanasia. Brain, kidney, liver, lung, spleen, and thymus were removed and weighed. Weights were adjusted for terminal body weights to determine absolute and relative organ weights. Spleens and thymuses were suspended in complete medium (RPMI, 10% fetal calf serum, 50 IU penicillin and 50 μg streptomycin) and were aseptically processed into single-cell suspensions by gentle grinding between two sterile, frosted microscope slides. An aliquot of each spleen or thymus suspension was manually counted on a hemocytometer to determine the number of live cells (viability of cells for each organ was generally greater than 95%). The total number of cells per spleen and thymus (cellularity), adjusted by the weight of each organ, was determined for each animal from Set B (see Fig. 1).
2.9. Endpoints collected for toxicological assays
Briefly, as described in [17], sera, spleens, thymuses, and brain were prepared and evaluated in the following assays.
2.10. Blood and sera
For hematology and clinical chemistry endpoints, blood from anesthetized animals was collected into a microtainer tube containing EDTA, which kept the blood from coagulating, or in a microtainer with no anticoagulant for serum collection. Once collected, samples were sent overnight to the Montana Veterinary Diagnostic Laboratory (MVDL) in Bozeman, MT, for hematology (whole EDTA blood) and clinical chemistry analysis (serum). Hematologies were run on all samples; however clinical chemistries performed were dependent on the volume of sample provided and not all samples were of sufficient volume. For this reason, hematology and clinical chemistry analyses were not performed in duplicate or triplicate as were other assays. For determination of blood metal and metalloid concentrations, blood from anesthetized mice was collected into a microtainer tube containing heparin. Using an analytical balance, each blood collection tube was weighed before and after collection of blood to determine the weight of each blood sample. Once collected, samples were frozen at −80 °C and then shipped to the Laboratory Services Bureau of the Montana Department of Public Health and Human Services for analysis of total levels of metal and metalloid concentrations. Blood metal/metalloid concentrations were not performed in duplicate or triplicate as were other assays. Due to limited volumes, whole blood values were determined only.
2.11. Immunophenotyping of B lymphocytes and CD4/CD8 lymphocytes
The number of splenic B cells (B220) and splenic and thymic T cells (CD4+, CD8+, CD4+/CD8+, and CD4-/CD8-) were counted in single-cell suspensions diluted to a concentration of 1 × 107 cells/mL. Optimal concentrations of flow antibodies and reagents were determined in previous experiments [17]. All experimental replicates included isotype controls (to estimate non-specific binding), unstained cells as negative controls, and single color controls as positive controls to determine color compensation. Monoclonal antibodies coupled to fluorochromes specific for the following markers were used: anti-mouse B220-PE, anti-mouse CD4-FITC, and anti-mouse CD8-PE. Flow cytometric analysis was performed using a BD FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA) and 10,000 events were collected from each sample. The total number of each cell type was determined from the spleen or thymus cellularity.
2.12. Immunophenotyping of regulatory T lymphocytes (Tregs)
Splenic lymphocytes were adjusted to a concentration of 1 × 106 cells per well and depleted of red blood cells via a 5-min incubation in NH4Cl lysis buffer at 37 °C. Monoclonal antibodies coupled to fluorochromes specific for the following markers were used at a concentration of 1 μg/106 cells: anti-mouse CD25-FITC, rat IgG1-PE isotype control, rat IgG2b-AF647 isotype control, and rat IgG2b-FITC isotype control (BD Pharmingen, San Diego, CA, USA). FoxP3, CD4, and IL-17 cells were stained using a commercial kit (BD Pharmingen, San Diego, CA, USA or eBiosciences, San Diego, CA, USA) according to manufacturer’s instructions. Appropriate positive, negative, and isotype controls were added to wells containing cells only. Treg subsets were quantified using a BD FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA). 10,000 events were acquired for each sample. CD4+ lymphocytes in the lymphocyte fraction were gated, and the percentages of CD25 + foxP3+ cells, CD25 + foxP3- cells, IL-17+, and IL-17− cells were calculated.
2.13. Plaque forming cell (PFC) assay
The primary IgM response to sheep red blood cells (SRBC; Rockland, Gilbertsville, PA) was determined using a hemolytic plaque assay. Five days before euthanasia, mice were given an intraperitoneal injection of 100 μl of 25% SRBC in PBS. Single-cell suspensions (as previously described) were prepared from spleens of mice injected with SRBC and diluted to a concentration of 1.0 × 106 cells/mL. A 10-μl aliquot of the single-cell suspension was added to a tube containing 100 μl of 25% SRBC in PBS, 40 μl of RPMI medium (without additives), and 50 μl of guinea pig complement. Aliquots of the solution were placed into Cunningham chamber slides. The slides were sealed with paraffin and were incubated at 37 °C and 5% CO2 for 1–2 h. PFCs were counted microscopically and were reported as PFCs/million splenocytes.
2.14. Natural killer cell activity
Natural killer (NK) cell activity was assessed via an in vitro cytotoxicity assay using 51Cr-labeled Yac-1 cells as described previously [8], [15]. To minimize radioactive waste, the procedure was adapted to 96-well plates that were read on a Packard Top Count scintillation counter. Spleen single-cell suspensions were adjusted to 2 × 107 cells/mL in complete medium and then the spleen cells and Yac-1 cells were added, in triplicate wells, in ratios of 100:1, 50:1, 25:1, and 12.5:1 spleen cells:labeled Yac-1 cells, and in a final volume of 0.2 ml per well. Maximum release was determined by lysing 51Cr-labeled Yac-1 cells with 0.1% Triton X-100 in complete medium. Spontaneous release was determined by incubating Yac-1 cells only in complete medium. After a four-hour incubation at 37 °C and 5% CO2, the plates were centrifuged (1200 rpm, 3 min), and 25 μl of supernatant was then transferred to a 96-well plate containing solid scintillant (LumaPlate). Plates were air dried overnight and within 24 h, were counted for 5 min, after a 10-min dark delay, using a Packard Top Count-NXT. The results were expressed in lytic units per 107 splenocytes using 10% lysis as the reference point as described by Ref. [3], equation 10. Essentially, this measure considers the target tumor cell activity in the context of both maximum and spontaneous release. This method has been validated in interlaboratory studies by the National Toxicology Program [21].
2.15. Neuronal autoantibody formation
Blood was collected, held at room temperature for at least 30 min, and then centrifuged at 4 °C to separate serum, which was frozen at −80 °C until analysis of IgM and IgG antibody concentrations. IgM and IgG antibodies against glial fibrillary acidic protein (GFAP; American Research Products, Waltham, MA), myelin basic protein (MBP; Sigma-Aldrich, St. Louis, MO), or neurofilament 68 (NF-68; American Research Products, Waltham, MA) were determined with an ELISA assay developed by Ref. [9]. Plates were read at 405 nm on a BioTek Synergy HT plate reader. Optical density values were converted to ng/mL concentrations using values obtained from a standard curve. All sera were assayed twice to verify results. Values that fell below the limits of detection were assigned a value of zero. Values that fell above the limits of detection were diluted upon the second evaluation. Values that still remained above the limits of detection after dilution were eliminated from the overall calculations due to insufficient amounts of sample.
2.16. Brain histology
Two sections of cerebellum from each mouse brain, each 10 μm thick, were stained with either anti-CD3+ antibody (abcam, Cambridge, MA) or anti-myelin basic protein (MBP) antibody (abcam, Cambridge, MA). In sections stained with anti-CD3+, the number of T cells present throughout both sections was counted at 20× magnification. In sections stained with anti-MBP, the relative intensity of the stain was gauged relative to the intensity of the staining of the sections from the control brains. Control brains were scored as weak (1), mild (2), moderate (3), or strong (4). The intensity of the stain in brains from exposed animals was assigned a numerical value according to the following scale: 0 = no change in staining intensity relative to controls; 1 = very weak staining intensity relative to controls; 2 = mild intensity in staining relative to controls; 3 = moderate intensity in staining relative to controls; 4 = strong intensity in staining relative to controls; 5 = very strong intensity in staining relative to controls.
2.17. Metal-free particle
As described in Ref. [17], six separate groups of mice (N = 6/group) were exposed to titanium dioxide (TiO2), a particle with none of associated metals found on geogenic dusts from the NDRA, to help us discern particle-only effects from the effects of metals and particles together. Mice were given matching concentrations of TiO2 (0.01 to 100 mg/kg of body weight), and were exposed via the same paradigm as the geogenic dust. Toxicity testing was evaluated in the TiO2-exposed mice in parallel with CBN 3-exposed mice. Data, although relevant to this study too, have been reported previously in our first Nellis Dunes publication [17].
2.18. Statistical analysis
Data were tested for normality and homogeneity and, if needed, appropriate transformations were made. A one-way analysis of variance (ANOVA) was used to determine differences among doses for each endpoint using JMP 9 (SAS Institute Inc., Cary, NC) in which the standard error used a pooled estimate of error variance. When significant differences were detected by the F-test (p < 0.05), Dunnett’s t-test was used to compare treatment groups to the 0 mg/kg group. A Dunnett’s t-test also was used to compare results of the 0 mg/kg group to the TiO2 group.
2.19. Quality assurance
This study was conducted as under the conceptual guidance of Good Laboratory Practices (GLP). Within this guidance, periodic audits of all aspects of the project were conducted as well as extensive independent review of all documentation and data. In addition, each of the participating university sites conducting experiments (UNLV, MSU and ECU) were audited by an internal but independent Quality Assurance team. All final notebooks were reviewed and initialed by the Quality Assurance Team.
3. Results
3.1. CBN 3 geogenic dust characterization
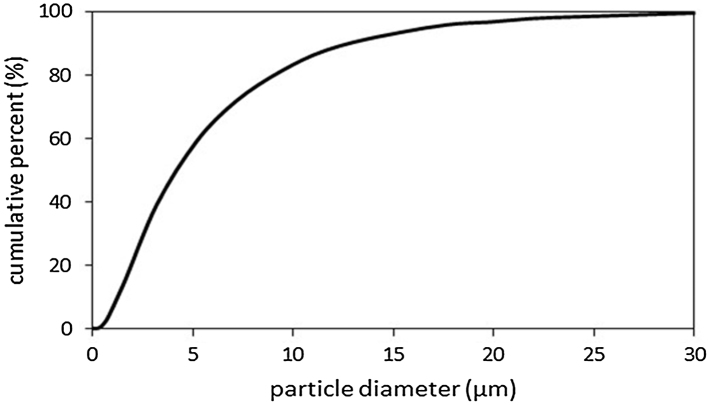
Dust from CBN 3 used in this study had a median diameter of 4.1 μm (Table 1 and Fig. 2). The chemical composition of the dust after total digestion is shown in Table 1. Arsenic speciation for naturally-occurring concentrations illustreated that of the measured arsenic species, As(V) was the predominant species with a concentration of 4.04 μg/g, monomethylarsonic acid and dimethylarsinic acid in the dust samples were not detected at the applied dilutions, and other speices of arsenic were not evaluated. Of the metals in this sample, aluminum and iron concentrations were found to have the highest concentrations: 59,993 μg/g and 28,564 μg/g respectively (Table 1).
Fig. 2.
Particle size distribution of the CBN 3 geogenic dust used in this study. The median diameter for this dust was 4.1 μm.
3.2. Body weight, organ weights, and immune organ cellularity
Following exposure to CBN 3 geogenic dust, mice did not demonstrate signs of overt toxicity in terms of body condition or a significant change in body weight as compared to the 0 mg/kg group. No significant changes in immune organ weights, kidney, or brain weights were observed. The lung somatic index (SI: organ weight corrected for body weight), liver SI, and spleen and thymus cellularity adjusted for organ weight were altered in some of the dosed groups relative to the 0 mg/kg group during one or more replicates; however this difference was within 20% of the control group values and was not dose-responsive.
3.3. Hematology, clinical chemistry, and blood metals
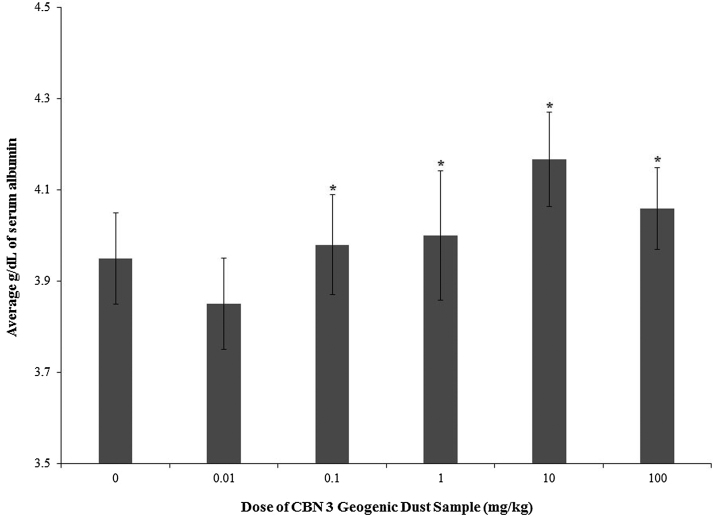
No statistically significant changes in hematological endpoints were observed at any dose. Of the clinical chemistry endpoints measured in mice dosed with CBN 3 dust samples, only serum albumin levels collected from animals exposed to 10 or 100 mg/kg were statistically and significantly increased (by about 10%) relative to the 0 mg/kg group (Fig. 3). Mice exposed to dust from CBN 3 had detectable whole blood concentrations of the metals/metalloids (Table 2). Statistical analyses could not be run on these concentrations as blood of animals from each dose group was pooled for analyses.
Fig. 3.
Serum albumin levels in adult female B6C3F1 mice following oropharyngeal aspiration exposure to CBN 3 geogenic dust samples from NDRA each week for 28 days. Data are presented as mean percentage ± standard deviation. Sample size for each group was 5–6 animals. Data presented represent one replicate. The (*) indicates a response statistically different from the 0 mg/kg group (p < 0.05).
Table 2.
Total elemental concentration (μg/g in wet sample) in whole blood of animals dosed with CBN 3 geogenic dust each week for 28 days.
| 0 mg/kg | 0.01 mg/kg | 0.1 mg/kg | 1 mg/kg | 10 mg/kg | 100 mg/kg | |
|---|---|---|---|---|---|---|
| As | 0.0023 | 0.0028 | 0.0032 | 0.0032 | 0.0030 | 0.0034 |
| Cd | 0.0051b | a | a | a | a | a |
| Cr | a | a | a | a | a | a |
| Mg | 34.7 | 35.8 | 35.4 | 34.8 | 35.6 | 34.8 |
| Mn | 0.0206 | 0.0218 | 0.0214 | 0.0424 | 0.0206 | 0.0208 |
| Mo | 0.0254 | 0.0172 | 0.0226 | 0.0162 | 0.0247 | 0.0226 |
| Ni | a | a | a | a | a | a |
| Pb | a | 0.0036 | 0.0025 | 0.0013 | 0.0023 | a |
| Sr | 0.0075 | 0.0061 | 0.0061 | 0.0065 | 0.0086 | 0.0078 |
| V | 0.0097 | 0.0097 | 0.0092 | 0.0099 | 0.0098 | 0.0098 |
| Zn | 3.96 | 4.18c | 4.20c | 4.21c | 4.30c | 4.14 |
values not on the standard curve were not used.
This was based on one animal with a concentration above the LOQ. All other animals were below the LOQ.
Indicates a concentration statistically different from the 0 mg/kg group (p < 0.05).
3.4. Immunophenotype
Total numbers of splenic B cells and regulatory T cells (Tregs) were not statistically altered by exposure to CBN 3 dust samples (Table 3), whereas the number of thymic and splenic T cells were statistically altered by exposure to dust samples from CBN 3. In the spleen, CD4+, CD8+, and CD4+/CD8+ T cells were reduced by about 30% following exposure to 10 and 100 mg/kg relative to the 0 mg/kg group. In the thymus, all four subtypes of T cells (CD4+, CD8+, CD4+/CD8+, and CD4-/CD8-) were reduced by 37.4%, on average, following exposure to 100 mg/kg. CD8+ and CD4+/CD8+ T cells also were reduced by about 30% following exposure to 0.01 and 10 mg/kg relative to the 0 mg/kg group. Additionally, CD4+/CD8+ T cells were reduced by 21.6% after exposure to 0.1 mg/kg relative to the control group. No significant changes were observed in mice exposed to particle control only, TiO2, reported in Ref. [17].
Table 3.
Spleen and thymus B and T cell lymphocytes in adult female B6C3F1 mice following oropharyngeal aspiration exposure to CBN 3 geogenic dust each week for 28 days.
| Spleen |
|||||
|---|---|---|---|---|---|
| Geogenic dust (mg/kg) | CD4+ (cells × 107) |
CD8+ (cells × 107) |
CD4+/CD8+ (cells × 106) |
CD4-/CD8- (cells × 108) |
B220 (cells × 107) |
| 0 | 4.09 ± 0.715 | 2.02 ± 0.422 | 1.36 ± 0.412 | 1.11 ± 0.213 | 5.78 ± 1.27 |
| 0.01 | 3.93 ± 0.440 | 1.93 ± 0.287 | 1.25 ± 0.301 | 1.08 ± 0.192 | 6.11 ± 1.78 |
| 0.1 | 3.70 ± 0.899 | 1.81 ± 0.358 | 1.24 ± 0.312 | 1.00 ± 0.181 | 5.35 ± 0.515 |
| 1 | 3.44 ± 1.22 | 1.76 ± 0.574 | 1.24 ± 0.474 | 1.05 ± 0.347 | 5.70 ± 2.91 |
| 10 | 2.91 ± 0.591* | 1.43 ± 0.300* | 0.850 ± 0.216* | 0.869 ± 0.196 | 4.83 ± 1.36 |
| 100 | 2.90 ± 0.633* | 1.50 ± 0.326* | 0.797 ± 0.238* | 0.888 ± 0.181 | 5.71 ± 0.877 |
| Thymus |
||||
|---|---|---|---|---|
| Geogenic dust (mg/kg) | CD4+ (cells × 107) |
CD8+ (cells × 106) |
CD4+/CD8+ (cells × 107) |
CD4-/CD8- (cells × 106) |
| 0 | 1.60 ± 0.274 | 8.63 ± 1.44 | 11.54 ± 1.77 | 8.53 ± 1.64 |
| 0.01 | 1.19 ± 0.349 | 6.29 ± 1.65* | 8.58 ± 1.85* | 6.00 ± 1.41* |
| 0.1 | 1.29 ± 0.166 | 6.42 ± 0.827 | 9.05 ± 0.738* | 6.20 ± 1.29 |
| 1 | 1.50 ± 0.416 | 6.60 ± 1.96 | 9.71 ± 2.04 | 6.60 ± 1.32 |
| 10 | 1.20 ± 0.135 | 5.84 ± 0.971* | 8.17 ± 1.08* | 6.39 ± 1.25 |
| 100 | 1.08 ± 0.389* | 4.89 ± 1.62* | 6.96 ± 0.800* | 5.59 ± 1.57* |
| Spleen |
|||||
|---|---|---|---|---|---|
| Geogenic dust (mg/kg) | 4+/25+/fp3+ (cells × 107) |
4+/25-fp3+ (cells × 107) |
4+/25+/fp3- (cells × 105) |
4+/IL17A+ (cells × 106) |
4-/IL17A+ (cells × 106) |
| 0 | 1.74 ± 0.438 | 1.48 ± 0.505 | 6.38 ± 2.32 | 0.755 ± 0.274 | 0.960 ± 0.482 |
| 0.01 | 1.85 ± 0.364 | 1.72 ± 0.347 | 6.60 ± 2.42 | 1.31 ± 0.213 | 2.02 ± 0.458 |
| 0.1 | 1.69 ± 0.278 | 1.78 ± 0.426 | 5.96 ± 1.31 | 1.10 ± 0.172 | 1.23 ± 0.269 |
| 1 | 1.68 ± 0.484 | 1.73 ± 0.602 | 6.45 ± 3.12 | 0.755 ± 0.620 | 1.42 ± 0.418 |
| 10 | 1.59 ± 0.384 | 1.60 ± 0.390 | 6.37 ± 1.66 | 0.836 ± 0.219 | 1.34 ± 0.431 |
| 100 | 1.44 ± 0.297 | 1.28 ± 0.306 | 6.65 ± 2.04 | 0.718 ± 0.181 | 1.56 ± 0.144 |
Data are presented as mean cell number ± standard deviation. Sample size for each group was 5–6 animals and are representative of three trial days.
indicates aresponse statistically different from the 0 mg/kg group (p < 0.05) as determined from log transformed data.
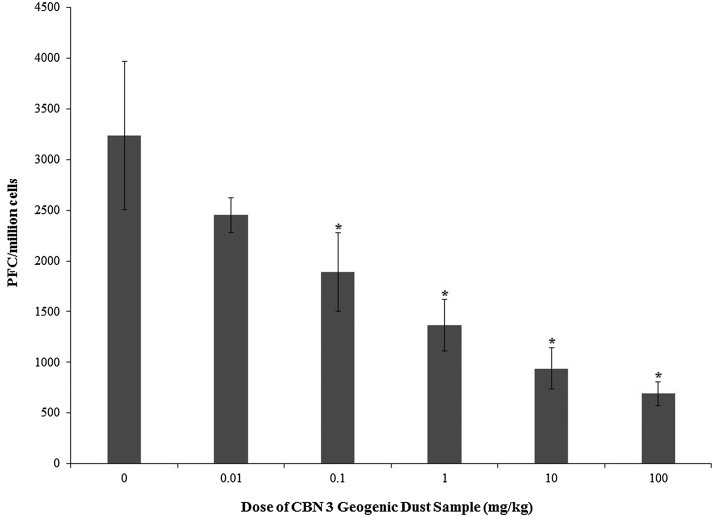
3.5. Plaque forming cell (PFC) assay
Exposure to geogenic dust samples from CBN 3 dose-responsively reduced the number of plaque forming cells secreting IgM antibody following SRBC challenge at all administered doses (Fig. 4). PFC/million of spleen cells was reduced by 51.7–92.8% in these treated groups relative to the 0 mg/kg group. A LOAEL of 0.1 mg/kg/day and a NOAEL of 0.01 mg/kg/day were determined based on this immunological parameter. No significant changes were observed in mice exposed to TiO2 as reported in [17].
Fig. 4.
Sheep red blood cell-specific-IgM antibody production in adult female B6C3F1 mice following oropharyngeal aspiration exposure to CBN 3 geogenic dust samples from NDRA each week for 28 days. Data are presented as mean PFC/million cells ± standard deviation. Sample size for each group was 5–6 animals. Data presented represent one replicate. The (*) indicates a response statistically different from the 0 mg/kg group (p < 0.05) and was determined from log transformed data.
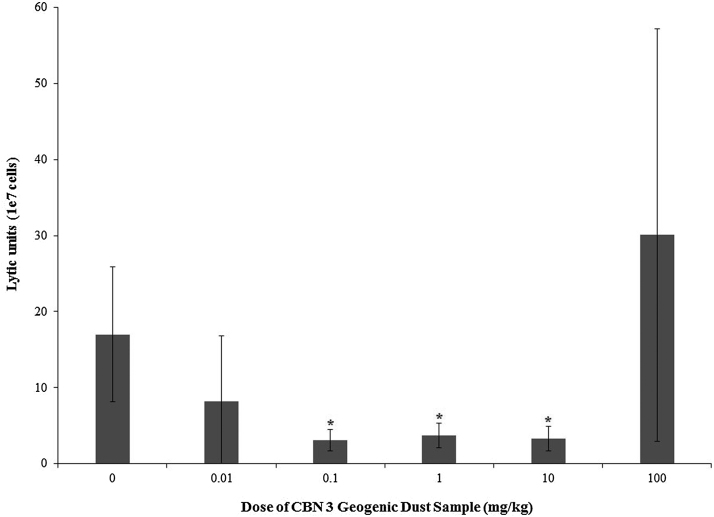
3.6. Natural killer cell activity
Exposure to dust samples from CBN 3 reduced natural killer cell activity at concentrations of 0.01–10 mg/kg (Fig. 5). Activity was reduced by 51.8–81.8% in dosed groups relative to the 0 mg/kg group. No significant changes were observed in mice exposed to TiO2 as reported in [17].
Fig. 5.
Splenic natural killer cell activity was assessed in adult female B6C3F1 mice following oropharyngeal aspiration exposure to CBN 3 geogenic dust samples from NDRA each week for 28 days. Data are presented as mean lytic unit ± standard deviation. Sample size for each group was 5–6 animals. Data presented represent one replicate. The (*) indicates data significantly different from the 0 mg/kg group (p < 0.05).
3.7. Neuronal autoantibody formation
Exposure to dust samples from CBN 3 did not alter IgM or IgG antibody production against MBP, NF-68, or GFAP, relative to antibody production in the 0 mg/kg group.
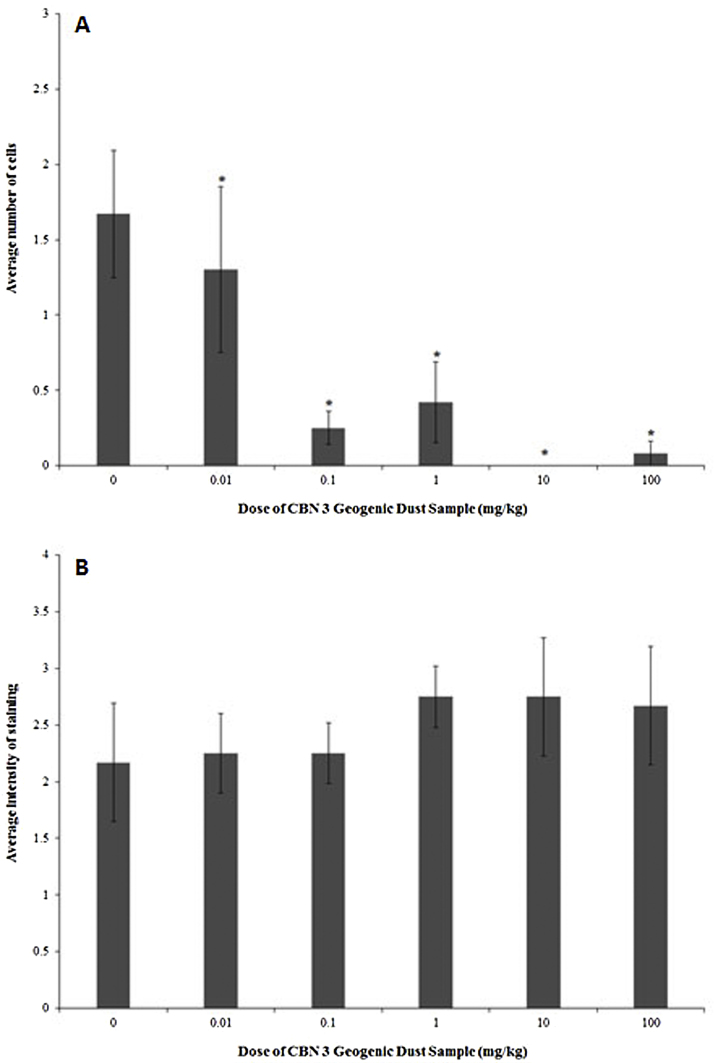
3.8. Brain histology
The number of CD3+ T cells was 22.2–100% higher in the brains of animals exposed to 0 mg/kg relative to all dosed groups (Fig. 6A). No difference between the 0 mg/kg group and any dosed group was observed in the intensity of MBP staining in the brains (Fig. 6B).
Fig. 6.
Histological markers in the brains of adult female B6C3F1 mice following oropharyngeal aspiration exposure to CBN 3 geogenic dust samples. Data are presented as mean ± standard error of mean. Sample size for each group was 5–6 animals. The (*) indicates a response statistically different from the 0 mg/kg group (p< 0.05). (A) CD3+ T cells; (B) myelin basic protein (MBP).
4. Discussion
Due to the high visitor rate and risk of exposure at the NDRA, concerns about health risks arose. Consequently, this study addressed toxicological endpoints relevant to human health that were associated with geogenic dust from desert pavements, bedrock, and other surfaces at NDRA where the silt and clay particles do not come from underlying geologic deposits but are a direct result of atmospheric deposition. Similar surfaces occur abundantly in southern Nevada and elsewhere in the world, which makes this study representative for many deserts although the chemical and mineral composition of the dust may vary according to the specific source(s).
Previously, we published the results of experiments with TiO2, a particle used with none of the associated heavy metals found on geogenic dusts from the NDRA [17]. Clearly, we needed a particle control for this study. Yet, with a complex dust sample that contains various minerals and metals, there was not a “one size fits all” for this study. The authors’ realize that some published data suggest that titanium dioxide is immunomodulatory. In our case, TiO2 did not affect adaptive or innate immunity following oropharyngeal aspiration via the same protocol applied to CBN 3. Based on our observations, TiO2 served as an effective particle control for evaluating systemic measures of immunotoxicity [17]. We had hypothesized that the metals on our dust samples were systemically available and could contribute to other effects besides those specific to the lung. Our data at this time, appear to support this. Hence, the bioavailability of absorbed metals from the geogenic CBN 3 dust mixture were likely to account for the observed toxicological effects.
A strength as well as a limitation of this study is that we report cumulative responses to a characterized, metal dust mixture; yet we cannot tease out the metal(s) that might be the worst offenders or potentiate or cancel each other’s effects. Zn, for example, was higher in whole blood of dosed animals relative to control animals and it was higher in concentration in the dust samples relative to some of the other metals. Several published studies of the effects of zinc (via dietary supplementation) on adaptive immunity suggest that Zn enhances humoral immune responses [29], [19], [2] rather than suppressing them, as observed in this study. In μg/g, the total metal load was similar across doses as was the concentration of all metals except Mn. Mn spiked in the 1 mg/kg group relative to the other groups, but as responses in this group did not deviate from a dose-response, it is unlikely that this concentration of Mn alone induced measurable effects. Therefore, we postulate that the toxicology profile presented in this study reflects cumulative effects of this metals mixture.
The NOAEL identified in this study was 0.01 m/kg and the LOAEL was 0.1 mg/kg. The NOAEL and LOAEL were based on dose-responsive suppression of the primary T cell-dependent antibody response (TDAR). This immunomodulation occurred at dust concentrations where no overt toxicity was indicated by a change in body weight from control animals, which is often an indicator of overt or systemic toxicity. The LOAEL of 0.1 mg/kg was associated with a 66.7% reduction in IgM antibody production relative to the responses measured in the 0 mg/kg group, with a dose-responsive suppression observed in the remaining groups. Subsets of T cells from both the spleen and thymus were reduced relative to the 0 mg/kg group across all dust concentrations but the 1 mg/kg group, suggesting that effects of the dust on the TDAR are directed against T cells rather than against B cell numbers. Processes involved in antigen production and clonal expansion at the level of the B cell also are possible targets, but the 20–30% reduction in several T cell subpopulations implicate T cells as likely targets of this geogenic dust.
The NOAEL and LOAEL for other sites at the NDRA were also established using the outcomes from TDAR. That is, CBN 1 dust sample represented the sand dunes, the most popular site at the NDRA. The LOAEL was established as 0.01 mg/kg based on both TDAR and a kidney effect [17]. CBN 2 sample consisted of geogenic dust generated from the Muddy Creek Formation, a northern site within the NDRA, dust derived from the white claystone and yellow sandstone facies found throughout the northern portion of the NDRA. Exposure to CBN 2 resulted in a NOAEL of 0.01 mg/kg and a LOAEL of 0.1 mg/kg established by TDAR and natural killer cell activity [7]. In each of these three exposure scenarios, TDAR was key to establishing the NOAEL and LOAEL.
Additionally, the ability of NK cells to effectively lyse target cells was impaired in the 0.01–10 mg/kg dose groups. However, a NOAEL or a LOAEL could not be established for this endpoint as NK cells in the 100 mg/kg group exhibited high variability. We realize that the standard deviation is very high for the 100 mg/kg and would call these data into question. We acknowledge that only one replicate is presented as we did not have the opportunity to repeat this experiment. However, we are assured by the fact that (1) the negative control is within historical ranges, (2) The negative control and the 0.01- 10 mg/kg treatment groups have acceptable standard deviation ranges. Taken together, we chose to present these data despite the variability observed in the 100 mg/kg group.
Serum albumin was elevated relative to the 0 mg/kg group after exposure to 10 and 100 mg/kg. Albumin is produced in the liver and can serve as a general marker of liver health. Typically, reductions in serum albumin can be indicative of liver disease; however, elevations are more challenging to interpret as it is not generally considered a marker specific to disease. Based on our observations and body weight data, animals in these dose groups showed no signs of overt toxicity. However, this change may have reflected mild dehydration, a common cause of increases in serum albumin. The increased whole blood levels of Zn in animals exposed to 0.01–10 mg/kg could reflect mild dehydration [32]. This is consistent with the observed increase in serum albumin. No liver enzymes were affected (data not shown) nor was there any cause to consider a measurement error as all of the other clinical chemistry or hematology results were in normal ranges.
Inhalation of fine particulate matter containing heavy metals has been linked to diminished intellectual capacity in children, microglial activation, neurotoxicity, and triggering of the production of free radicals associated with oxidative stress [16], [24], [28], [38]. Oxidative stress associated with many heavy metals can damage cells and liberate proteins inducing an autoimmune response measurable as serum autoantibodies [11]. For example, El-Fawal and O’Callaghan reported increases in IgM and IgG antibodies against neural and glial proteins in rats exposed to only one dose of trimethyltin that were detectable up to three weeks post-exposure [10]. Premised on these studies and several other reports linking metals to neurotoxicity, we included some of these endpoints in this study.
Even though we did not observe changes in IgM or IgG antibody production against MBP, NF-68, or GFAP, relative to antibody production in the 0 mg/kg group we observed unexpected changes in T cell brain infiltration. Not well understood were the reduced numbers of CD3+ T cells infiltrating the brain in the dosed groups relative to the 0 mg/kg group. Generally, neuronal cell injury or death can lead to an influx of T cells into the central nervous system (CNS), although T cells can be present in low levels in the CNS proper in an uninjured brain [27]. The lack of a notable influx of T cells into the CNS is suggestive of either a) lack of neuronal injury associated with exposure to dust from CBN 3, or b) a very localized and/or small area of injury that we were unable to detect as we did not evaluate every region of the brain.
5. Conclusions
Exposure to natural dust representing CBN 3 from the NDRA, administered via the lung in mice in concentrations of 0.1–100 mg/kg, reduced primary antigen-specific antibody production. This suppression of antibody production was accompanied by a reduction in the number of T cells in the spleen and the thymus and in the ability of natural killer (NK) cells to lyse target cells. The LOAEL and NOAEL established in this study were based on measures of adaptive immunity that are supported by a measure of innate immunity. These endpoints are measures of immune function that when observed in experimental animal models, are suggestive of immunotoxicological risk in similarly exposed persons. Therefore, exposure to geogenic dust in CBN 3 may present a human health risk when considering an exposure scenario similar to that presented in our study.
Transparency document
Acknowledgements
This project was funded by the U.S. Bureau of Land Management (URL: http://www.blm.gov/nv/st/en/fo/lvfo.html), Grant number: L11AC20058. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are grateful for the assistance of Margie Peden-Adams, Ph.D., Rebecca (Mehana) Chow, Winnie David, Mallory Leetham, MS, MLS(ASCP), Lacey Murphy MS, MLS(ASCP), Sharon Young, Ph.D., Mari Eggers, Ph.D., and our grant legal consultant, Curtis B. Coulter.
Contributor Information
Deborah E. Keil, Email: deborah.keil@montana.edu, deborah.e.keil@gmail.com.
Brenda Buck, Email: buckb@unlv.nevada.edu.
Dirk Goossens, Email: dirk.goossens@kuleuven.be.
Yuanxin Teng, Email: tengy@unlv.nevada.edu.
James Pollard, Email: pollardj@unlv.nevada.edu.
Brett McLaurin, Email: bmclauri@bloomu.edu.
Russell Gerads, Email: Russ@brooksapplied.com.
Jamie DeWitt, Email: dewittj@ecu.edu.
References
- 1.Aldabe J., Elustondo D., Santamaria C., Lasheras E., Pandolfi M., Alastuey A. Chemical characterisation and source apportionment of PM2.5 and PM10 at rural, urban and traffic sites in Navarra (North of Spain) Atmos. Res. 2011;102:191–205. [Google Scholar]
- 2.Bartlett J.R., Smith M.O. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult. Sci. 2003;82:1580–1588. doi: 10.1093/ps/82.10.1580. [DOI] [PubMed] [Google Scholar]
- 3.Bryant J., Day R., Whiteside T.L., Herberman R.B. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J. Immunol. Methods. 1992;146:91–103. doi: 10.1016/0022-1759(92)90052-u. [DOI] [PubMed] [Google Scholar]
- 4.Buck B.J., Metcalf R.V., Berry D., Kent D., McLaurin B., Goossens D., Merkler D., Januch J. october. International Society of Exposure Science; Henderson NV: 2015. Naturally Occurring Asbestos in the Southern Nevada Region: Characterization and Potential for Human Exposure.http://www.ises2015.org/Images/ISES2015_AbstractBook%20FINAL.pdf Final Abstract Book, p. 146. [Google Scholar]
- 5.Cameron T.P., Hickman R.L., Kornreich M.R., Tarone R.E. History, survival, and growth patterns of B6C3F1 mice and F344 rats in the National Cancer Institute Carcinogenesis Testing Program. Fundam. Appl. Toxicol. 1985;5(3):526–538. doi: 10.1016/0272-0590(85)90100-9. [DOI] [PubMed] [Google Scholar]
- 6.de Miranda R.M., de Fatima Andrade M., Fornaro A., Astolfo R., de Andre P.A., Saldiva P. Urban air pollution: a representative survey of PM2.5 mass concentrations in six Brazilian cities. Air Qual. Atmos. Health. 2012;5:63–77. doi: 10.1007/s11869-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeWitt J., Buck B., Goossens D., Hu Q., Chow R., David W., Young S., Teng Y., Leetham-Spencer M., Murphy L., Pollard J., McLaurin B., Gerards R., Keil D.E. Health effects following subacute exposure to geogenic dusts from arsenic-rich sediment at the Nellis Dunes Recreation Area, Las Vegas, NV. Toxicol. Appl. Pharm. 2016;304:79–89. doi: 10.1016/j.taap.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Duke S.S., Schook L.B., Holsapple M.P. Effects of N-nitrosodimethylamine on tumor susceptibility. J. Leukoc. Biol. 1985;37:383–394. doi: 10.1002/jlb.37.4.383. [DOI] [PubMed] [Google Scholar]
- 9.El-Fawal H.A.N., Gong Z., Little A.R., Evans H.L. Exposure to methyl mercury results in serum autoantibodies to neurotypic and gliotypic protiens. Neurotoxicology. 1996;17:267–276. [PubMed] [Google Scholar]
- 10.El-Fawal H.A., O'Callaghan J.P. Autoantibodies to neurotypic and gliotypic proteins as biomarkers of neurotoxicity: assessment of trimethyltin (TMT) Neurotoxicology. 2008;29:109–115. doi: 10.1016/j.neuro.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 11.El-Fawal H.A., Waterman S.J., De Feo A., Shamy M.Y. Neuroimmunotoxicology: humoral assessment of neurotoxicity and autoimmune mechanisms. Environ. Health Perspect. 1999;107(Suppl (5)):767–775. doi: 10.1289/ehp.99107s5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goossens D. A method for dry extracting large volumes of fine particulate matter from bulk soil samples. Air Qual. Atmos. Health. 2012;5:425–431. [Google Scholar]
- 13.Goossens D., Buck B. Dust emission by off-road driving: experiments on 17 arid soil types, Nevada, USA. Geomorphology. 2009;107:118–138. [Google Scholar]
- 14.Goossens D., Buck B.J., McLaurin B. Contributions to total dust production of natural and anthropogenic emissions in a recreational area designated for off-road vehicular activity (Nellis Dunes, Nevada, USA) J. Arid Environ. 2012;78:80–99. [Google Scholar]
- 15.Holsapple M.P., White K.L., Jr., McCay J.A., Bradley S.G., Munson A.E. An immunotoxicological evaluation of 4,4'-thiobis-(6-t-butyl-m-cresol) in female B6C3F1 mice 2. Humoral and cell-mediated immunity, macrophage function, and host resistance. Fundam. Appl. Toxicol. 1988;10:701–716. doi: 10.1016/0272-0590(88)90197-2. [DOI] [PubMed] [Google Scholar]
- 16.Jomova K., Vondrakova D., Lawson M., Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010;345(1–2):91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 17.Keil D.E., Buck B., Goossens D., Teng Y., Spencer M., Murphy L., Pollard J., Eggers M., McLaurin B., Gerads R., DeWitt J. Immunotoxicological and neurotoxicological profile of health effects following subacute exposure to geogenic dust from sand dunes at the Nellis Dunes Recreation Area Las Vegas, NV. Toxicol. Appl. Pharmacol. 2016;291:1–12. doi: 10.1016/j.taap.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Kubachka K.M., Shockey N.V., Hanley T.A., Conklin S.D., Heitkemper D.T. 2012. FDA Elemental Analysis Manual: Section 4.11: Arsenic Speciation in Rice and Rice Products Using High Performance Liquid Chromatography-Inductively Coupled Plasma-Mass Spectrometric Determination Version 1.1.http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm328363.htm [Google Scholar]
- 19.Lastra M.D., Pastelin R., Herrera M.A., Orihuela V.D., Aguilar A.E. Increment of immune responses inmice perinatal stages after zinc supplementation. Arch. Med. Res. 1997;28:67–72. [PubMed] [Google Scholar]
- 21.Luster M.I., Portier C., Pait D.G., Rosenthal G.J., Germolec D.R., Corsini E., Blaylock B.L., Pollock P., Kouchi Y., Craig W., White K.L., Munson A.E., Comment C.E. Risk assessment in immunotoxicology: II. Relationships between immune and host resistance tests. Fundam. Appl. Toxicol. 1993;21:71–82. doi: 10.1006/faat.1993.1074. [DOI] [PubMed] [Google Scholar]
- 22.McFadden L.D., Wells S.G., Jercinovich M.J. Influences of eolian and pedogenic processes on the origin and evolution of desert pavements. Geology. 1987;15:504–508. [Google Scholar]
- 23.McLaurin B.T., Goossens D., Buck B.J. Combining surface mapping and process data to assess, predict, and manage dust emissions from natural and disturbed land surfaces. Geosphere. 2011;7:260–275. [Google Scholar]
- 24.MohanKumar S.M., Campbell A., Block M., Veronesi B. Particulate matter, oxidative stress and neurotoxicity. Neurotoxicology. 2008;29(3):479–488. doi: 10.1016/j.neuro.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Mormon S.A., Plumlee G.S. Chapter 15. Dust and human health. In: Knippertz P., Stuut J.-B.W., editors. Mineral Dust: A Key Player in the Earth System. Springer Science + Business Media; Dordrecht: 2014. p. 385. [Google Scholar]
- 27.Raivich G., Jones L.L., Kloss C.U., Werner A., Neumann H., Kreutzber G.W. Immune surveillance in the injured nervous system: t-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuornal degeneration. J. Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Risom Lotte, Møller Peter, Loft Steffen. Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res.: Fundam. Mol. Mech. Mutagen. 2005;592(1):119–137. doi: 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Schiffer R.B., Sunderman F., Jr., Baggs R.B., Moynihan J.A. The effects of exposure to dietary nickel and zinc upon humoral and cellular immunity in SJL mice. J. Neuroimmunol. 1991;34:229–239. doi: 10.1016/0165-5728(91)90134-s. [DOI] [PubMed] [Google Scholar]
- 30.Soukup D., Buck B.J., Goossens D., Teng Y., Baron D. Mineralogical composition of soil samples in the nellis dunes recreation area. In: Goossens D., Buck B.J., editors. Assessment of Dust Emissions, Chemistry, and Mineralogy for Management of Natural and Disturbed Surfaces at Nellis Dunes Recreation Area, Nevada, Final Report to Bureau of Land Management for Task Agreement Number FAA010017. 2011. pp. 171–187. [Google Scholar]
- 31.Stanek L.W., Brown J.S., Stanek J., Gift J., Costa D.L. Air pollution toxicology—a brief review of the role of the science in shaping the current understanding of air pollution health risks. Toxicol. Sci. 2011;120(Suppl (1)):S8–27. doi: 10.1093/toxsci/kfq367. [DOI] [PubMed] [Google Scholar]
- 32.Strand T.A., Adhikari R.K., Chandyo R.K., Sharma P.R., Sommerfelt H. Predictors of plasma zinc concentrations in children with acute diarrhea. Am. J. Clin. Nutr. 2004;79:451–456. doi: 10.1093/ajcn/79.3.451. [DOI] [PubMed] [Google Scholar]
- 33.Turk J.K., Graham R.C. Distribution and properties of vesicular horizons in the western United States. Soil Sci. Soc. Am. J. 2011;75:1449–1461. [Google Scholar]
- 34.US EPA (U.S. Environmental Protection Agency) 2007. Method 6020A. Inductively Coupled Plasma-Mass Spectrometry. 6000 Series Methods.www.epa.gov/osw/hazard/testmethods/sw846/pdfs/6020a.pdf [Google Scholar]
- 35.Wang J., Hu Z., Chen Y., Chen Z., Xu S. Contamination characteristics and possible sources of PM10 and PM2.5 in different functional areas of Shanghai, China. Atmos. Environ. 2013;68:221–229. [Google Scholar]
- 36.Wells S.G., McFadden L.D., Dohrenwend J.C. Influence of Late Quaternary climatic changes on geomorphic and pedogenic processes on a desert piedmont, eastern Mojave Desert, California. Quat. Res. 1987;27:130–146. [Google Scholar]
- 37.Williams A.J., Buck B.J., Beyene M.A. Biological soil crusts in the Mojave Desert, USA: micromorphology and pedogenesis. Soil Sci. Soc. Am. J. 2012;76:1685–1695. [Google Scholar]
- 38.Xia Tian, Kovochich Michael, Nel Andre. The role of reactive oxygen species and oxidative stress in mediating particulate matter injury. Clin. Occup. Environ. Med. 2005;5(4):817–836. doi: 10.1016/j.coem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Briggs P.H., Meier A.L., 2002. The determination of forty-two elements in geological materials by inductively coupled plasma—mass spectrometry. In: Taggart J.E., Editor. Analytical Methods for Chemical Analysis of Geologic and Other Materials, Chapter 1. U.S. Geological Survey Open-File Report 02-223-I. Available: http://pubs.usgs.gov/of/2002/ofr-02-0223/I20NAWQAPlus_M.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.