Abstract
Butyl paraben (BPB) is an antimicrobial used in a variety of consumer products. Due to widespread human exposure and reported estrogenic activity, the National Toxicology Program quantified internal exposure during critical periods of development. Time-mated female Hsd:Sprague Dawley SD rats were administered 0, 1500, 5000 or 15,000 ppm BPB via NIH-07 feed, ad libitum, from gestation day (GD) 6 to postnatal day (PND) 28. Dam plasma, amniotic fluid and fetuses were collected on GD18 and pup and dam plasma were collected on PNDs 4, 10, 14, 21 and 28 and analyzed for free (unconjugated) and total (unconjugated and conjugated) BPB using liquid chromatography-tandem mass spectrometry. Free BPB was below the limit of quantitation in fetuses (LOQ 1.91 ng BPB/g fetus) and amniotic fluid (LOQ 0.17 ng BPB/mL amniotic fluid) at 1500 ppm. Analyte levels in amniotic fluid were less than 1% of maternal plasma, suggesting limited placental transfer. Total BPB in PND4 pup plasma was less than 5% of dam plasma in all exposure groups, suggesting low lactational transfer. However, at nearly all time points and exposure groups, there were higher levels of free BPB in pup versus dam plasma, suggesting limited conjugation in pups. Pup conjugation of BPB was age-dependent, not reaching the percent-conjugation in dams (>99%) until PNDs 21 to 28. These data illustrate low placental and lactational transfer of dietary BPB and that poor conjugation in pups during early lactation results in higher exposure to free BPB in pups compared to dams.
Keywords: Butyl paraben, Maternal transfer, Placental transfer, Lactational transfer, Conjugation, Metabolism ontogeny
1. Introduction
Butyl paraben (n-butyl-p-hydroxybenzoate; BPB) is a member of the paraben family, consisting of esters of p-hydroxybenzoic acid. Parabens are antimicrobials commonly used in food and personal care products, e.g. shampoo, lotion, sunscreen and cosmetics. Paraben levels measured in food products sampled in Albany, New York showed an increase in parabens, as a class, from 2008 to 2012, with the concentration of butyl paraben tripling from 2008 to 2012 [10].
Several studies have evaluated human paraben exposure. National Health and Nutrition Examination Survey (NHANES) data of four parabens (butyl, n-propyl, ethyl and methyl) measured in urine samples between 2005 and 2012 [2], showed that women had higher levels of all four parabens compared to men, likely due to the prevalence of parabens in cosmetics. Another study has reported that BPB was detectable in the urine of over 70% of the female population [23]. The level of BPB detected in urine is frequently lower than other parabens, potentially due to a higher use of shorter-chain parabens in food products [10]. Based on the NHANES data, BPB exposure has decreased from 2005 to 2012, particularly in females: the 95th percentile of BPB in female urine was reported as 34.7 and 17.6 μg total BPB/g creatinine in 2005–2006 and 2011–2012, respectively [2]. The observed decrease in urine levels is in contrast to the increase reported of BPB in foodstuffs [10]. A small study (n = 100) of parabens in urine, collected from adults between 2003 and 2005, reported 29.5 μg/L as the 95th percentile of BPB in urine [29]. Few studies report the level of BPB in serum; one study of Danish men (n = 60) reported a maximal concentration of 0.87 BPB ng/mL serum, with 58/60 samples falling below the limit of detection (LOD 0.33 ng/mL) [5].
BPB exposure during critical windows of development has been reported in rats and humans. A study in rats reported higher levels (ng/mL) of total BPB in amniotic fluid compared to dam plasma following subcutaneous injection of the dam from GD 7–21 at all doses (100, 200 or 400 mg/kg) [6]. In humans, potential BPB exposure to the developing fetus has been reported in a small survey investigating free BPB levels in paired maternal plasma and cord blood samples: 4/50 maternal samples and 8/50 cord samples had levels of free BPB above the limit of detection, 7 ng/L [24].
Human post-natal exposure to parabens has also been investigated by measuring levels in human breast milk. In two small studies (n = 4 and n = 20–54), total BPB was measured in human breast milk, and was below the limits of detection in all samples, however low levels of other parabens were detected [21], [28]. A paired study (n = 46) in humans comparing BPB in urine from pregnant women (collected within 1 day of delivery) and their infant (collected within 2 days after birth) reports that 13/46 pregnant women and 19/46 infants had detectable levels (LOD 0.5 μg/L) of total BPB in urine [9]. This infant exposure may have originated from various sources, including placental transfer, breastfeeding or use of BPB-containing personal care products after birth.
While the estrogenic activity of BPB has been reported to be 10,000 to 100,000 times less potent than estradiol, exposure to these compounds during pre- and early post-natal development is of major concern [7]. Studies have suggested that the estrogenic activity of parabens is positively correlated to the alkyl chain length and branching complexity [25]. BPB has been reported to have effects on the male reproductive system in both mice (Crj:CD-1) and rats (Wistar) following dietary exposure for 10 or 8 weeks, respectively [17], [18]. Lower serum testosterone was observed in both rats (>100 ppm) and mice (1000 ppm). In mice, there were lower levels of round and elongated sperm in stage VII–VIII seminiferous tubules at 100 and 1000 ppm [18]. In rats, lower epididymal weights (1000 ppm) and lower total caudal sperm (>10 ppm) and caudal sperm concentrations (1000 ppm) were observed [17]. Similar effects, lower sperm counts and motility, were reported by another group in the F1 generation of Sprague-Dawley rats exposed to BPB from gestation day (GD) 6 to post-natal day (PND) 20 via subcutaneous injection to 100 or 200 mg/kg/day [8]. A human study of Danish men (n = 60) measured BPB in biological fluids and reported higher prevalence and higher maximal levels of BPB in seminal fluid (detected in 52% of samples, maximum 1.73 ng/mL) compared to serum (detected in 3% of samples, maximum 0.87 ng/mL) [5].
Parabens were nominated to the National Toxicology Program (NTP) for toxicity testing due to widespread human exposure and concern for reproductive toxicity because of reported endocrine disrupting activity. BPB was selected as the test article for the NTP’s testing program based on estrogenic potency in vitro, reported male reproductive toxicity, and widespread usage.
The objective of this investigation was to elucidate the extent to which BPB can be transferred to offspring during gestation and lactation as well as understand the development of the F1 metabolic capabilities as they relate to dietary BPB exposure. In order to evaluate F1 exposure to dietary BPB during gestation and lactation, the NTP conducted a feed exposure study in time-mated Hsd:Sprague Dawley SD rats administered 0, 1500, 5000 or 15,000 ppm BPB via NIH-07 feed, ad libitum, from GD6 to PND28. Exposure concentrations were selected for comparison to the NTP reproductive and developmental toxicity study. Dams were removed for sampling on GD18 and dams and litters were removed on PNDs 4, 10, 14, 21 or 28 to measure free and total BPB and a metabolite, butyl 3,4-dihydroxybenzoate (3-OH BPB) in plasma, amniotic fluid or fetuses.
2. Materials and methods
2.1. Materials
Butyl paraben (CAS# 94-26-8, Lot No. 20081101) was procured from Ivy Fine Chemicals Corporation (Cherry Hill, NJ). The identity of the chemical was confirmed by infrared spectroscopy, nuclear magnetic resonance spectroscopy and mass spectrometry. The purity determined by high performance liquid chromatography with ultraviolet detection (HPLC/UV) and gas chromatography with flame ionization detection was ≥99.5%. Glass jars used for feed containers were obtained from Lab Products Inc., Seaford DE. Solid-bottom polycarbonate cages were obtained from Lab Products, Inc. Seaford, DE. Cage filters were obtained from Granville Milling Co, Creedmoor NC. Certified irradiated San-Chips® hardwood cage bedding was obtained from PJ Murphy Forest Products, Montville NJ). (13C6)BPB (ring labeled, 99%, 1 mg/mL in methanol) was obtained from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA). 3-Hydroxybutylparaben (3-OH BPB) was prepared by the esterification of 3,4 dihydroxybenzoic acid [13]. Ascorbic acid, sodium fluoride, and β-glucuronidase and sulfatase from Helix pomatia were obtained from Sigma-Aldrich, St. Louis, MO. Ammonium acetate, acetic acid, and formic acid were from Fluka (Sigma-Aldrich). UV-grade acetonitrile and methanol and HPLC-grade DI water were obtained from Honeywell Burdick & Jackson (Muskegon, MI). Maternal and pup (PND 4 and 28) plasma, amniotic fluid and fetuses used for method validation, quality control (QC) sample preparation and matrix calibration curves were collected on GD18 and PND4 from Hsd:Sprague Dawley SD donor rats.
2.2. Animals
Time-mated female Hsd:Sprague Dawley SD rats were delivered from Harlan (Dublin, VA) to RTI International (Research Triangle Park, NC), aged 11–12 weeks and weighing 185–260 g upon arrival. Animals were randomly assigned exposure groups based on GD3 body weight (n = 40 controls, n = 35 for treated groups) and quarantined for 14 days upon arrival. NIH-07 feed and water were provided ad libitum. Dams were housed individually throughout gestation and with pups during lactation in a climate controlled (45–73% humidity, 70–75 °F) room on a 12-h light/dark cycle (light from 0600 to 1800). Dams were weighed once daily during gestation and dams and pups were weighed individually on PNDs 1, 4, 7, 10, 13, 14, 16, 19, 21, 25 and 28 during lactation. Animals were observed for mortality at least twice daily throughout gestation and lactation. Litters were standardized to 5 males and 5 females, when possible, on PND4.
The study protocol was in accordance with the AAAALAC and NRC guidelines and was approved by the RTI International IACUC. Studies were conducted in accordance with the US Public Health Service animal care policy and the Guide for the Care and Use of Laboratory animals. In addition, these studies were conducted in compliance with FDA Good Laboratory Practice Regulations established by the Food and Drug Administration.
2.3. Exposure
Dosed-feed was prepared at 1500, 5000 and 15,000 ppm BPB in irradiated and certified NIH-07 diet (Zeigler Brothers, Inc., Gardeners, PA) and analyzed by HPLC/UV using a validated analytical method. Three batches of dosed feed were formulated for administration during the study. Each batch was used for up to 42 days based on previously demonstrated stability of BPB in feed. Dosed feed was analyzed prior to administration and post-administration and all measurements were within +/−10% of the target concentration. The homogeneity of samples taken from different locations of the feed blender was within +/−5% for all concentrations. BPB was below the limit of detection (6 ppm) in control feed (0 ppm) in all batches analyzed.
Dosed (or control) NIH-07 feed was provided ad libitum beginning on GD6, throughout the quarantine period, gestation and lactation until PND28. Cage food consumption was measured at intervals of 3 days during gestation and lactation (e.g. GDs 6–9, 9–12, 12–15 etc.). Food consumption and chemical consumption per body weight were calculated for the same time intervals based on dam weight.
2.4. Sample collection
Animals were removed for sample collection on GD18 (dams, n = 5 per group) and PNDs 4, 10, 14, 21 and 28 (dams and pups) (n = 4–5 dams and litters per group per time point). Disposition of the dams and litters for sampling are shown in Table 1.
Table 1.
Disposition of Dams during study.
| 0 | 1500 | 5000 | 15,000 | |
|---|---|---|---|---|
| Time-Mated Females | 40 | 35 | 35 | 35 |
| Non-pregnant | 7 | 8 | 5 | 6 |
| Pregnant, no delivery | 1 | 2 | 2 | 1 |
| GD 18 Sampling | 5 | 5 | 5 | 5 |
| No. Littered | 27 | 20 | 23 | 23 |
| PND 4 Sampling | 4 | 4 | 4 | 4 |
| PND 10 Sampling | 5 | 4 | 5 | 4 |
| PND 14 Sampling | 4 | 4 | 4 | 5 |
| PND 21 Sampling | 5 | 4 | 5 | 5 |
| PND 28 Sampling | 5 | 4 | 5 | 5 |
| Terminal Euthanasia | 4 | 0 | 0 | 0 |
Dam plasma, amniotic fluid, and fetuses were collected on GD18 to assess placental transfer of BPB following exposure. Dams were euthanized by CO2 asphyxiation on GD18; fetuses were then euthanized via decapitation upon removal by caesarian section. Blood was collected into K2EDTA tubes from dams via cardiac puncture. Blood samples were maintained on wet ice at collection and centrifuged within one hour of collection for plasma isolation. Amniotic fluid and fetuses were pooled per litter. Fetuses were flash frozen in liquid nitrogen. All samples were stored at −70 °C after collection.
Plasma, from pups and dams, was collected on PND 4, 10, 14, 21 and 28 to assess exposure during lactation. Dams and pups, PND14 and older, were euthanized by CO2 asphyxiation. Younger pups (PND 4 and 10) were euthanized by decapitation. Blood was collected from dams and pups, PND14 and older, by cardiac puncture immediately following euthanasia. Trunk blood was collected from younger pups (PND4 and PND10) following decapitation. All blood samples were collected into K2EDTA tubes. Blood samples were maintained on wet ice at collection and centrifuged within one hour of collection for plasma isolation. Plasma was collected via centrifugation and stored at −70 °C.
All samples were collected between 0900 and 1200. On a given day, the time between first and last collection did not exceed two hours.
2.5. Sample analysis
Analytical methods were developed and validated to quantitate free (unconjugated) and total (combined unconjugated and conjugated) BPB and 3-OH BPB in dam and pup plasma, amniotic fluid, and fetal homogenate (1 g fetus homogenized in 3 vol of water using a Polytron PT3100 tissue homogenizer). While the 3-OH BPB metabolite is not the major metabolite of BPB, it was selected for analysis because it is a unique metabolite where the butyl group is retained; in addition, it is a catechol metabolite which may be easily oxidized to a reactive quinone. Methods were first validated in plasma and pup homogenate. The plasma and pup homogenate methods were subsequently cross-validated to amniotic fluid and fetus homogenate, respectively.
For analysis of total BPB and 3-OH BPB, to 25 μL of plasma, amniotic fluid or fetal homogenate, 5 μL of 24 mM sodium fluoride/240 mM ascorbic acid and 5 μL of the internal standard solution ((800 ng/mL (13C6)BPB in methanol)) were added. To each sample, 25 μL of 2 M ammonium acetate (pH 5) containing ß-glucuronidase (2 units/μL) and sulfatase (0.4 units/μL) were added and samples were incubated at 37 °C for 2 h. Samples for the determination for free analytes were prepared parallel to total analytes without ß-glucuronidase and sulfatase, and were kept on ice during the incubation of the companion samples for total determinations. After the 2 h incubation or hold, 100 μL of acetonitrile were added to each sample, and the samples were vortexed and then centrifuged for 6 min at 4 °C. Approximately 80 μL of the supernatant was removed for analysis by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC–MS/MS) as given below.
Matrix calibration curves were prepared by adding BPB and 3-OH BPB to respective blank matrix to achieve final concentrations as given in Table 2. Linearity, accuracy, precision (inter- and intraday), selectivity, and recovery were estimated for all matrices. Stability of BPB and 3-OH BPB in extracted samples was evaluated in samples stored at room and refrigeration temperatures. Dilution verification was conducted to demonstrate that concentrations outside the validated range could be accurately quantitated after dilution. Stability of the analytes in plasma was evaluated following three freeze-thaw cycles and in plasma stored at −70 °C for up to 206 days.
Table 2.
Summary of Analytical Method Validation and Stability Data of BPB and 3-OH BPB.
| Plasma/ Amniotic Fluidh |
Pup/Fetal Homogenatei |
|||
|---|---|---|---|---|
| BPB | 3-OH-BPB | BPB | 3-OH-BPB | |
| Free BPB | ||||
| LOD | ||||
| ng/mLa | 0.17 | 0.0697 | 0.477 | 0.121 |
| ng/gb | N/A | N/A | 1.91 | 0.484 |
| ELOQs | ||||
| ng/mLa | 1.01 | 0.5 | 2.02 | 1.01 |
| ng/gb | N/A | N/A | 8.08 | 4.04 |
| Concentration Range | ||||
| ng/mLa | 1 to 1000 | 0.5 to 500 | 2 to 1000 | 1 to 500 |
| ng/ga | N/A | N/A | 8 to 4000 | 4 to 2000 |
| Total BPB | ||||
| LOD | ||||
| ng/mLa | 0.292 | 0.133 | 0.573 | 0.219 |
| ng/gb | N/A | N/A | 2.29 | 0.876 |
| ELOQ | ||||
| ng/mLa | 1.01 | 0.5 | 2.01 | 0.988 |
| ng/gb | N/A | N/A | 8.04 | 3.95 |
| Concentration Range | ||||
| ng/mLa | 1 to 1000 | 0.5 to 500 | 2 to 1000 | 1 to 500 |
| ng/gb | N/A | N/A | 8 to 4000 | 4 to 2000 |
| Mean Accuracy Rangec, d, e | -8.2 to 15% | -8.9 to 11.3% | -6.8 to 6.0% | -7.6 to 11% |
| Intra-Day Precisiond, e | 1.6 to 9.3% | 1.5 to 11% | 2.2 to 6.2% | 3.4 to 6.7% |
| Inter-Day Precisiond, e | 1.6 to 25%j | 1.1 to 14% | 1.4 to 3% | 1.6 to 10% |
| Mean Extraction Recoverye | ||||
| Free | 88.2 to 120% | 91 to 115% | 85.3 to 120% | 77.4 to 95.4% |
| Total | 82.9 to 132% | 82.3 to 124% | 90.6 to 135% | 64.9 to 86.9% |
| Stability | ||||
| Extract Stability (4 days)e, f | 80 to 99.8% | 89.4 to 115% | 91.5 to 108% | 99.2 to 122% |
| Matrix Freeze/Thaw Stability at −70°C(4 or 9 days)g | 87.7 to 97.4% | 88 to 99.3% | 93.7 to 118% | 99.6 to 119% |
| Matrix Stability at −70 °C | ||||
| (60 days) | 104 to 106% | 108 to 110% | 100 to 105% | 101 to 110% |
| Matrix Stability at −70°C | ||||
| (206 days) | 90.6 to 116% | 86.8 to 108% | N/A | N/A |
The limit of detection (LOD) and experimental limit of quantitation (ELOQ) values are shown for free (unconjugated) BPB, total (unconjugated+ conjugated) BPB, free 3-OH BPB and total 3-OH BPB. ELOQ is the lowest standard and LOD was estimated as three times the standard deviation of the ELOQ.
Values indicate either maternal plasma or pup homogenate (1 g pup in 3 mL water, total volume 4 mL).
Values indicate mass concentration per gram of pup.
Calculated as the percent relative error.
Accuracy and Precision were calculated using calibration and QC samples.
Values indicate the range across 8 concentrations.
Values indicate mean percent recovered after refrigerator, autosampler or ambient air storage for 4 days.
Spiked matrix stored at −70 °C and subjected to 3 freeze-thaw cycles over 4 days (plasma) or 9 days (pup homogenate).
Amniotic fluid and pup plasma were cross-validated using maternal plasma calibration curves.
Fetal homogenate was cross-validated using pup homogenate calibration curves.
The 1 ng/mL sample had an inter-day precision of 25%, all other concentrations were within 11%.
All samples were analyzed by UPLC–MS/MS using a 4000 QTRAP (Sciex, Framingham, MA) coupled to a Waters Acquity UPLC (Milford, MA). Waters Acquity UPLC HSS T3 column (1.8 μm, 2.1 mm × 50 mm) was used for analyte separation. Mobile phases used were 0.1% aqueous formic acid (A) and acetonitrile (B) with a linear gradient of 40%B to 95%B in 2.5 min at a flow rate of 0.6 mL/min. The electrospray ion source was operated in the negative ion mode with an ion source temperature of 450 °C and an ion spray voltage of −4000 V. Transitions monitored were m/z 193 → 92 for BPB, 209 → 108 for 3-OH BPB, and 199 → 98 for (13C6)BPB. The retention times were 0.83 and 1.11 min for 3-OH BPB and BPB, respectively.
A summary of validation parameters and data is shown in Table 2 demonstrating the suitability of the analytical methods for quantitation of BPB and 3-OH BPB in samples. Standards, as high as 10,000 ng/mL of BPB and 5000 ng/mL 3-OH BPB could successfully be diluted into the validated concentration range. The experimental limit of quantitation (ELOQ) was set as the lowest calibration standard concentration. The limit of detection (LOD) was defined as three times the standard deviation of the ELOQ. Analytes in extracted samples were stable for 4 days when stored at ambient temperature, refrigerator (∼5 °C) or in auto-sampler (∼10 °C). Analytes were stable when undergoing 3 freeze-thaw cycles in 4 or 9 days in plasma or pup homogenate, respectively. Analytes were stable when stored in a freezer (∼−70 °C) for up to 60 or 206 days for plasma and pup homogenate, respectively.
Samples from the study were prepared and analyzed as described above. For samples with insufficient volume to provide a 25 μL aliquot, a recorded amount of deionized water was added to reach a total volume of 25 μL. Samples that exceeded the matrix calibration range were diluted 1:10 (20 μL extract plus 180 μL extracted control plasma containing internal standard) into the range of the calibration curve and reanalyzed.
Matrix calibration curves with up to eight matrix standards were run at the beginning and end of each sample run. All 16 points were used to generate the calibration curve. The performance of the calibration curve was evaluated prior to the analysis of each sample set. A successful calibration was indicated by the followings: correlation coefficient (r) ≥ 0.99 and relative error (RE) ≤ ± 15% for the standards (except at ELOQ RE ≤ ± 20%). Data from study samples were considered valid if they were bracketed by valid QC sets. In general, each sample set, method blanks and controls were bracketed by two quality control (QC) sets, which consisted of two concentrations of calibration standards prepared at the low and high ends of the calibration curve, with the number of QC samples prepared at each concentration corresponding to ≥20% of total samples analyzed per each analysis day. A QC set passed when the measured concentration for at least 75% of the QC standards were within 15% of the nominal values. If the QC samples failed, it was necessary to reanalyze the bracketed samples. To determine reproducibility of analysis, ≥10% of samples were repeated per each analysis day.
The concentration of each analyte was calculated using its individual response, the regression equation, sample volume and dilution when applicable. The concentration of BPB and 3-OH BPB in plasma and amniotic fluid was expressed as ng/mL and that of fetus was expressed as ng/g fetus.
2.6. Statistical analysis
Data presented in Table 5, Table 6, Table 7 and Fig. 2, Fig. 3 include some individual sample values that were below the ELOQ (ELOQs are presented in Table 2). These values were used to have a better understanding and analysis of the variance across the group. In some cases, within a single litter or group, there were plasma samples that were above the LOD while others were below. In these cases, if at least 20% of the values within a litter (pups) or a group (dams) were above the LOD, the samples that were below the LOD in those groups were replaced with ½ of LOD value for calculations of the average and standard error. If greater than 80% of the values within a litter or dose group were below the LOD, a zero was used for calculations. This was done to better characterize low exposures and allow for statistical comparison.
Table 5.
Maternal and Fetal Levels of Butyl Paraben and Metabolites at GD18.
| Feed Concentration (ppm) | Analyte | Dam Plasma (ng/mL) | Amniotic Fluid (ng/mL) | Fetuses (ng/g) |
|---|---|---|---|---|
| 0 | ||||
| Free BPB | ND | ND | ND | |
| Total BPB | ND | ND | ND | |
| Free 3-OH BPB | ND | ND | ND | |
| Total 3-OH BPB | ND | ND | ND | |
| 1500 | ||||
| Free BPB | 1.7 ± 0.2 | ND | ND | |
| Total BPB | 191.4 ± 19.1 | 1.1 ± 0.3 | 3.8 ± 0.4 | |
| Free 3-OH BPB | ND | ND | ND | |
| Total 3-OH BPB | 51.8 ± 11.2 | ND | ND | |
| 5000 | ||||
| Free BPB | 6.0 ± 0.6 | ND | 4.3 ± 0.5 | |
| Total BPB | 583.6 ± 59.9 | 3.3 ± 0.7 | 13.9 ± 1.4 | |
| Free 3-OH BPB | ND | ND | ND | |
| Total 3-OH BPB | 152.0 ± 27.9 | 0.3 ± 0.2 | 1.6 ± 0.2 | |
| 15,000 | ||||
| Free BPB | 7.1 ± 0.8 | 0.4 ± 0.1 | 9.5 ± 0.6 | |
| Total BPB | 889.8 ± 75.9 | 7.7 ± 0.7 | 27.2 ± 2.9 | |
| Free 3-OH BPB | ND | ND | ND | |
| Total 3-OH BPB | 261.0 ± 38.0 | 0.6 ± 0.1 | 4.3 ± 0.5 | |
Values represent the average ± SEM, with 5 dams/litters per group. BPB and 3-OH BPB (free and total) are measured in ng/mL dam plasma (individual) and amniotic fluid (pooled per litter). In fetuses (pooled per litter), analaytes are measured in ng/g. For analyte data, not detected (ND) indicates that at least 80% of the values in the group were below the limit of detection (LOD), shown in Table 3. Values below the limit of detection (LOD) were assigned half LOD to calculate group averages if at least 20% of the samples in a group were above the LOD.
Table 6.
Dam Plasma Concentration to Butyl Paraben and Metabolites During Lactation.
| Feed Concentration (ppm) | Analyte (ng/mL) | PND4 | PND10 | PND14 | PND21 | PND28 |
|---|---|---|---|---|---|---|
| 0 | ||||||
| Free BPB | ND | ND | 0.1 ± 0.1 | ND | ND | |
| Total BPB | ND | 0.3 ± 0.3 | ND | ND | ND | |
| Free 3-OH BPB | ND | ND | ND | ND | ND | |
| Total 3-OH BPB | ND | ND | ND | ND | ND | |
| 1500 | ||||||
| Free BPB | 2.8 ± 0.6 | 3.0 ± 0.7 | 5.2 ± 1.1 | 3.8 ± 0.7 | 1.4 ± 0.5 | |
| Total BPB | 289.8 ± 44.3 | 252.8 ± 46.8 | 591.3 ± 134.0 | 466.8 ± 75.4 | 214.1 ± 41.4 | |
| Free 3-OH BPB | ND | ND | ND | ND | ND | |
| Total 3-OH BPB | 62.4 ± 7.9 | 55.7 ± 10.0 | 135.8 ± 24.7 | 126.2 ± 38.3 | 38.9 ± 4.2 | |
| 5000 | ||||||
| Free BPB | 6.3 ± 1.3 | 9.4 ± 2.1 | 9.6 ± 1.3 | 10.8 ± 2.1 | 2.8 ± 0.4 | |
| Total BPB | 790.5 ± 147.6 | 949.0 ± 186.1 | 1447.5 ± 167.7 | 1145.0 ± 217.7 | 340.6 ± 74.7 | |
| Free 3-OH BPB | ND | ND | ND | ND | 0.6 ± 0.2 | |
| Total 3-OH BPB | 175.3 ± 37.8 | 235.6 ± 27.0 | 286.0 ± 16.4 | 253.3 ± 44.2 | 104.3 ± 9.6 | |
| 15,000 | ||||||
| Free BPB | 21.0 ± 5.6 | 31.5 ± 3.2 | 28.6 ± 10.1 | 22.6 ± 3.9 | 12.6 ± 2.0 | |
| Total BPB | 2792.5 ± 582.4 | 3672.5 ± 428.7 | 3434.0 ± 452.2 | 3776.0 ± 463.6 | 1482.2 ± 225.6 | |
| Free 3-OH BPB | ND | ND | ND | ND | 1.8 ± 0.5 | |
| Total 3-OH BPB | 668.0 ± 136.0 | 730.3 ± 93.1 | 669.8 ± 61.7 | 958.4 ± 79.9 | 310.8 ± 43.8 | |
Values represent the average ± SEM, with 4 or 5 dams per group. Total BPB, BPB, total 3-OH BPB and 3-OH BPB were measured in ng/ml dam plasma. ND or not detected, implies that at least 80% of the values in the group were below the limit of detection (LOD), shown in Table 3. Values below the limit of detection (LOD) were assigned half LOD to calculate group averages if at least 20% of the samples in a group were above the LOD.
Table 7.
Pup Plasma Concentration to Butyl Paraben and Metabolites During Lactation.
| Feed Concentration (ppm) | Analyte (ng/mL) | PND4 | PND10 | PND14 | PND21 | PND28 |
|---|---|---|---|---|---|---|
| 0 | ||||||
| Free BPB | 0.1 ± 0.1 | 2.1 ± 1.8 | ND | 56.2 ± 53.7 | 13.1 ± 10.7 | |
| Total BPB | 0.1 ± 0.1 | 2.5 ± 2.2 | 0.2 ± 0.2 | 67.0 ± 60.0 | 12.7 ± 9.8 | |
| Free 3-OH BPB | ND | ND | ND | 0.3 ± 0.3 | ND | |
| Total 3-OH BPB | ND | 1.3 ± 1.2 | ND | 0.7 ± 0.2 | 0.1 ± 0.1 | |
| 1500 | ||||||
| Free BPB | 3.0 ± 1.0 | 13.6 ± 8.3 | 0.7 ± 0.2 | 6.7 ± 3.4 | 2.8 ± 0.2 | |
| Total BPB | 5.9 ± 1.5 | 19.4 ± 10.4 | 3.9 ± 0.6 | 608.1 ± 304.0 | 362.3 ± 49.3 | |
| Free 3-OH BPB | ND | 0.1 ± 0.1 | ND | 0.3 ± 0.1 | 0.2 ± 0.1 | |
| Total 3-OH BPB | 1.1 ± 0.3 | 1.3 ± 0.3 | 1.1 ± 0.2 | 91.1 ± 45.6 | 45.6 ± 6.4 | |
| 5000 | ||||||
| Free BPB | 9.0 ± 2.9 | 21.1 ± 3.1 | 3.5 ± 1.2 | 61.6 ± 27.5 | 9.6 ± 0.7 | |
| Total BPB | 19.0 ± 5.1 | 28.7 ± 3.2 | 18.3 ± 10.0 | 2575.5 ± 1151.8 | 1163.2 ± 92.2 | |
| Free 3-OH BPB | ND | 0.1 ± 0.0 | ND | 0.9 ± 0.4 | 0.8 ± 0.1 | |
| Total 3-OH BPB | 2.7 ± 0.3 | 2.8 ± 0.4 | 4.6 ± 1.4 | 392.3 ± 175.4 | 158.8 ± 12.7 | |
| 15,000 | ||||||
| Free BPB | 62.3 ± 19.5 | 118.3 ± 36.3 | 8.6 ± 1.2 | 81.1 ± 37.4 | 30.2 ± 3.2 | |
| Total BPB | 98.8 ± 26.8 | 158.9 ± 44.6 | 51.3 ± 18.0 | 1146.4 ± 156.4 | 4361.7 ± 274.0 | |
| Free 3-OH BPB | 0.3 ± 0.0 | 0.7 ± 0.2 | 0.1 ± 0.0 | 0.9 ± 0.2 | 1.8 ± 0.3 | |
| Total 3-OH BPB | 9.3 ± 1.8 | 12.2 ± 1.7 | 15.8 ± 5.2 | 245.5 ± 30.2 | 564.9 ± 72.8 | |
Values represent the average of litter averages ± SEM, the number of pups per litter ranges from 2 to 6 and the number of litters per group ranges from 3 to 5. Total BPB, BPB, total 3-OH BPB and 3-OH BPB were measured in ng/ml pup plasma. ND or not detected, implies that at least 80% of the values in the group were below the limit of detection (LOD), shown in Table 3. Values below the limit of detection (LOD) were assigned half LOD to calculate group averages if at least 20% of the samples in a group were above the LOD.
Fig. 2.
Levels of Percent-Free Butyl Paraben During Lactation. Ontogeny of BPB Conjugation in Pups. Data represent the average of the litter averages for percent free BPB + SEM (n = 2 to 6 pups per litter, 4–5 litters per group/time point) detected in pup plasma. Percent free BPB is calculated as [(free BPB (ng/ml)/Total BPB (ng/ml))*100]. Thin black horizontal line at 1% indicates the average percent free BPB in dams as a reference. White bars represent animals in the 1500 ppm exposure group, grey bars for the 5000 ppm group and black bars for the 15,000 ppm group. Statistical significance indicated by asterisks (*p < 0.05, ** p < 0.01, *** p < 0.001) are for the percent free BPB in pup plasma versus dam plasma in the same exposure group.
Fig. 3.
Comparison of Dam and Pup Plasma Levels During Lactation. Dam and pup exposure to dietary butyl paraben (5000 ppm) during lactation. Data represent average (dam, n = 4 to 5) and average of the litter averages (pups, n = 2 to 7 pups per litter, 3 to 5 litters per group) + SEM. (A) represents the total butyl paraben and (B) represents the unconjugated BPB (ng/mL plasma). White bars represent dam plasma and black bars represent pup plasma. These data are for the 5000 ppm exposure group only, however they are representative of the 1500 ppm and 15,000 ppm groups. Data for all groups are available in Supplementary material.
For the dams, regression analysis was used to determine if there were dose-dependent effects on BPB conjugation (shown as percent-free BPB) and 3-OH BPB metabolite formation. Since multiple pups were assessed in each litter, mixed effects analysis of variance was used to test for dose-dependent effects in pups on BPB conjugation and metabolite formation. For both dams and pups, Dunnett’s test was used, where control group levels were detectable, to compare each dose group to the control group. Within each dose group, changes in BPB conjugation and metabolite formation over postnatal times were evaluated using analysis of variance (dams) or mixed effects analysis of variance (pups) with Tukey’s multiple comparisons method to test for differences among pairs of time points. In addition, dam and pup body weights were compared among dose groups with analyses of variance and Dunnett’s tests. P-values of less than 0.05 were considered statistically significant.
3. Results
3.1. Chemical consumption and body weights
Average chemical consumption, based on food consumption per cage, during gestation and lactation is shown in Table 3. BPB exposure was not considered to affect food consumption (g food consumed/dam kg/day) throughout gestation and lactation since values were generally within 10% of control values. One exception was from GDs 6–9, when consumption measurements in the 15,000 ppm group were 80% higher than controls. This was attributed to dams adjusting to the diet with possible food spillage; the later intervals were 0–6% higher than control values. Average daily BPB exposure during gestation was 106, 360 and 1218 mg BPB/dam kg/day in the 1500, 5000, 15,000 ppm groups, respectively (Table 3). Average dam BPB exposure from PNDs 1 to 16 (assuming little to no active pup feeding) was 240, 729 and 2478 mg BPB/dam kg/day, for the 1500, 5000 and 15,000 ppm groups, respectively (Table 3). Chemical consumption, per cage, from PND 16 to 28 likely accounts for both dam and pup consumption, but is presented in Table 3 as mg BPB/dam kg/day.
Table 3.
Chemical Consumption (mg BPB/kg BW/day) During Gestation and Lactation.
| 1500 ppm | 5000 ppm | 15,000 ppm | |
|---|---|---|---|
| GD 6–21 | 106.0 ± 1.3 | 360.3 ± 4.9 | 1217.8 ± 27.9 |
| PND 1–16 | 240.1 ± 10.3 | 728.6 ± 53.7 | 2477.8 ± 40.2 |
| PND 1–28 | 339.2 ± 20.6 | 1224.5 ± 29.7 | 3493.8 ± 55.8 |
Average chemical intake ± SEM during gestation (GD 6–21) and lactation (PND 1–16, 1–28) based on cage food consumption. Note: dam weight was used to calculate food or chemical consumption per weight and values after PND 16 may be an overestimate. The number of dams/litters per group ranges from 22 to 33 depending on time point. BPB in the 0 ppm diet was below the limit of detection (6 ppm).
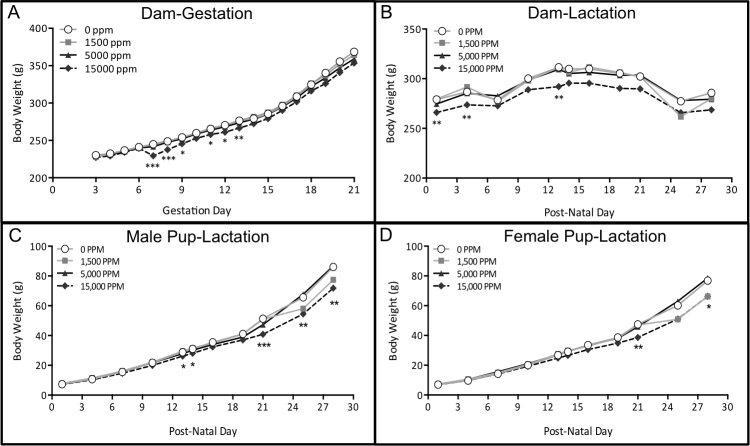
Throughout gestation and lactation, maternal body weights and body weight gain in the 1500 and 5000 ppm groups were comparable to the controls. Minimal, yet significant, differences (≤−6% from control values) in body weight in the 15,000 ppm group were observed during early gestation and may have been related to the adjustment to the diet (Fig. 1A). During lactation, minimal differences (≤−6% from control values) in dam body weights in the 15,000 ppm group were observed on PNDs 1, 4 and 13, with no difference from controls after PND14 (Fig. 1B). Pup body weights in the 15,000 ppm group were significantly lower than controls starting at PND13 (−8.7% compared to controls, total pups), with persisting effects in male (−16.5%) and female (−13.7%) pups observed on PND28 (Fig. 1C, D). There was no effect of BPB exposure on littering rate (Table 1), litter size, live litter size or sex ratio (Table 4).
Fig. 1.
Gestational and Lactational Body Weights. (A) dam body weight during gestation, (B) dam body weights during lactation, (C) male pup body weights during lactation and (D) female pup body weights during lactation. Data shown are averages (dams) and average of litter averages (pups) + SEM. Group sizes (n) vary from 4 to 27 due to biological sampling. Asterisks indicate statistical significance (ANOVA and post-hoc using Dunnett 2- sided test) between control group and 15,000 ppm group. * p-value < 0.05, ** p-value < 0.01 and *** p-value < 0.001.
Table 4.
Litter Size After Gestational Butyl Paraben Exposure.
| 0 | 1500 ppm | 5000 ppm | 15,000 ppm | ||
|---|---|---|---|---|---|
| Total Pups PND 0 |
Mean Litters |
12.2 ± 0.7 27 |
11.9 ± 0.8 20 |
11.6 ± 0.7 23 |
12.8 ± 0.4 23 |
| Live Pups PND 0 |
Mean Litters |
11.6 ± 0.7 27 |
10.9 ± 0.8 20 |
10.7 ± 0.7 23 |
12.3 ± 0.5 23 |
| Live Pups PND 4 |
Mean Litters |
10.6 ± 0.9 26 |
11.4 ± 0.5 19 |
10.0 ± 0.9 23 |
12.0 ± 0.5 23 |
| % Males PND 0 |
Mean Litters |
49.4 ± 2.4 26a |
46.7 ± 3.5 19a |
49.1 ± 4.2 23 |
44.1 ± 3.3 23 |
Values represent litter means ± SEM. There were no statistically significant differences between any of the BPB-exposed groups and the control group.
One litter excluded because there were no surviving pups on PND0.
3.2. Placental transfer
Placental transfer of dietary BPB was assessed on GD18 by measuring free and total BPB in dam plasma, amniotic fluid and fetus; amniotic fluid and fetuses were pooled by litter. A metabolite of BPB, 3-OH BPB, was also measured.
In dam plasma, free BPB was detected at all exposure concentrations, at levels approximately 1% of total BPB indicating extensive conjugation of BPB in dams following dietary BPB exposure (Table 5). Total 3-OH BPB was detected in all exposure groups (25–30% of total BPB), while free 3-OH BPB was below the LOD in all groups. Analyte levels (free and total BPB and total 3-OH BPB) increased in proportion (∼3-fold) to the feed concentration for the 1500 and 5000 ppm groups, while levels at 15,000 ppm were less than proportional (1–2 fold) to increased feed concentration from 5000 to 15,000 ppm (Table 5).
In the fetus, free and total BPB was detected in all exposure groups, except at 1500 ppm where free BPB levels were below the LOD of the analytical method (Table 5). The percent-free BPB (free BPB/total BPB*100) detected in fetus ranged from 30 to 35% of total BPB, compared to 1% in dam plasma (Table 5). Total 3-OH BPB was only detected in fetus in the 5000 and 15,000 ppm groups and free 3-OH BPB was below the LOD in fetus at all exposure concentrations (Table 5). Total 3-OH BPB levels were 10–15% of total BPB levels in fetus while in dam plasma the value was closer to 25% (Table 5). Consistent with the pattern observed in the dam, the analyte levels in fetus increased in proportion to feed concentration (∼3-fold) from the 1500 to 5000 ppm group, but increased slightly less than proportional from the 5000 to 15,000 ppm group.
In amniotic fluid, total BPB (1.1–7.7 ng/mL) and total 3-OH BPB (0.4–0.6 ng/mL) were detected at very low levels. Values in amniotic fluid were 0.57–0.87% and 0.23–0.26% of maternal plasma, for total BPB and total 3-OH BPB, respectively (Table 5). Free BPB was below the LOD in the 1500 and 5000 ppm groups and free 3-OH BPB was below the LOD in all groups (Table 5). There was no BPB or 3-OH BPB, free or total, detected in tissues from control animals at GD18 (Table 5).
3.3. Exposure during lactation
Compared to gestation, dams consumed more feed during lactation for milk production, resulting in higher plasma analyte levels (Table 3, Table 6). Free and total BPB were quantified at several time points in dam plasma during lactation. Levels of free BPB in dam plasma were generally 1% of total BPB across all time points and exposure groups. During early lactation (PNDs 4–14) dam plasma levels of free and total BPB were proportionate to feed concentration in all groups (Table 6). At PNDs 21 and 28, dam plasma levels were more variable in relation to feed concentrations and did not increase in proportion to exposure. Because cage food consumption at PNDs 21 and 28 accounts for dams and pups, it is unknown if this observation is due to a change in the amount of feed consumed by dams.
Total 3-OH BPB was detected in dams in all BPB-exposed groups and at all time points, ranging from 18 to 30% of total BPB (Table 6). Free 3-OH was below the LOD for most time points and exposure groups, except on PND28 in the 5000 ppm (0.6 ± 0.1 ng/mL) and 15,000 ppm (1.8 ± 0.5 ng/mL) groups (Table 6). When detected, free 3-OH BPB was less than 1% of the total 3-OH BPB. Total 3-OH BPB levels were exposure-proportional on lactation days PNDs 4–14 and less so on PNDs 21 and 28, similar to the pattern observed for total BPB.
For pups, exposure transitioned from lactational to direct dietary exposure as pups began to consume the diet, generally around PNDs 16–19, although small amounts of consumption may begin earlier. Based on data from PNDs 4, 10 and 14, lactational transfer of BPB was limited. The total BPB levels in pup plasma averaged 2–3% of maternal levels during this time, in all BPB-exposed groups. (Fig. 2, Tables 6,7). On PND14, free and total BPB in pup plasma appeared lower in all BPB-exposed groups compared to PND10 (Table 7). Lower analyte levels on PND14, versus PND10, cannot be explained and may be due to changes in pup habits during lactation. Due to increased feed consumption by pups between PNDs 14 and 21, the total BPB in pup plasma increased by two orders of magnitude in the 1500 and 5000 ppm groups, reaching peak levels during lactation: in the 5000 ppm group, the total BPB increased from 18.3 ± 10.0 ng/mL on PND14 to 2575.5 ± 1151.8 ng/mL on PND21 (Fig. 3, Table 7).
Free and total BPB levels in pup plasma during lactation were more variable compared to maternal levels and did not always increase in proportion to feed concentration (Table 7). For example, pup plasma levels of all analytes in the 15,000 ppm group on PND21 were similar or less than the 5000 ppm group (Table 7). The reason for this observation is unknown. Although consumption measurements are reported on a per cage basis (maternal versus offspring consumption is not defined), there was no decrease in measured food consumption at PND21 in the 15,000 ppm group. Additionally, maternal levels of these analytes did not display a similar pattern. Sampling time of blood was also investigated, in case later sampling times biased the measurements, but this was not the case. Unlike the other groups, pup exposure in the 15,000 ppm group, based on total BPB, did not reach peak lactational exposure until PND28.
Prior to direct consumption of dietary BPB by pups (PNDs 4, 10, 14), percent-free BPB in pup plasma was significantly higher than in dams for all BPB-exposed groups (Fig. 2); percent-free (free BPB/total BPB) in pups ranged from 47–74% at PNDs 4 and 10 and decreased to 20% or less by PND14. While total BPB was low in pup plasma at PND4 and 10, higher levels of free BPB, compared to dams, were observed in all exposure concentrations, suggesting limited metabolizing capabilities in pups at PNDs 4–10 (Tables 6, 7); Fig. 3 compares the levels of total BPB and free BPB in pup versus dam plasma for the 5000 ppm group. At PND21, BPB conjugation was still higher in dams (<1% free BPB) compared to pups (<7% free BPB), but not significantly different; by PND28 the percent-free BPB in pups was equivalent to dams (Fig. 2).
Formation of 3-OH BPB on PNDs 21 and 28, as a ratio of total 3-OH BPB to total BPB, ranged from 18 to 31% and 13–22% for dams and pups, respectively (Tables 6, 7). At PNDs 21 and 28, dams had consistently higher metabolite formation (total 3-OH/total BPB) compared to pups, reaching significance at PND21 in 1500 ppm (p < 0.05) and at PND28 in 5000 ppm (p < 0.01) and 15,000 ppm (p < 0.05).
BPB was detected in plasma samples from control pups during lactation; most striking were the levels of free and total BPB at PND21 (Table 7). Free BPB was detected in 11/21 pups and total BPB was detected in 20/21 pups in the 0 ppm group at PND21. Based on the percent-free BPB in 0 ppm pups at this time point (60%), it is unlikely that control animals were directly exposed to BPB since the percent-free would have been much lower (<10%) and may be due to contamination during sample collection, processing or analysis. Some potential contamination was also evident in control dams at PNDs 10 and 14, at a much lower level compared to that seen in pup samples (Table 6).
BPB conjugation (free BPB/total BPB) and the formation of 3-OH BPB (total 3-OH/total BPB) in pups or dams were not dependent on feed concentration at any time point (did not increase or decrease with feed concentration) and there were no apparent sex differences in free or total BPB and 3-OH BPB levels in pups.
4. Discussion
To quantify internal exposure to dietary BPB during critical periods of development, the NTP has evaluated BPB-derived analytes in BPB-exposed Hsd:Sprague Dawley SD rats and offspring during gestation and lactation. Parabens are of human health concern due to widespread exposure and reported effects on reproductive endpoints Centers for Disease Control and Prevention [2], [17], [18]. A previous study by the NTP demonstrated that BPB is well absorbed in adult male and female Hsd:Sprague Dawley SD rats following oral gavage exposure (>80%) [13]. Due to the concern of endocrine activity and susceptibility of the developing fetus, the present study investigated gestational and lactational exposure to dietary BPB. Here we show that total BPB exposure to offspring via placental and lactational transfer was low compared to maternal levels. However, the percent-free BPB was higher in fetuses and pups compared to dams, with the level of free BPB in pup plasma exceeding that of dams at most time points during lactation. There are no known studies that quantitate the estrogenic activity of BPB compared to BPB metabolites, however in vitro activity of BPB in metabolically incompetent assays suggests that the parent molecule has some estrogenic activity [19], [27] (Wróbel and Gregoraszczuk, 2014). It is unknown if the metabolites are more or less active, however it is presumed that conjugation of the parent or metabolites would lower estrogenic activity. The observed lack of conjugation in pups could have a significant impact on toxicity.
During gestation, plasma levels of free and total BPB in the dams increased less than proportionally with feed concentration and estimated chemical consumption in the 15,000 ppm group. The lack of feed concentration proportionality may be the result of one or more factors, including saturation of gastrointestinal absorption pathways or induction of metabolism/clearance mechanisms, other than conjugation pathways, that have been shown to be major pathways of BPB metabolism. Independent of feed concentration, the conjugation of BPB by dams was consistently high throughout gestation and lactation, with less than 1% of total BPB existing as the free analyte. The percent of free BPB observed in fetuses was much higher, with approximately 30–35% of total BPB present in the unconjugated form. Similar findings were reported in another study following oral exposure to bisphenol A (BPA) during late gestation (GD16) in CD Sprague-Dawley rats, resulting in 3.6% free BPA in dam plasma and 58% free BPA in fetus [3]. A small human survey reported in Towers et al. [24] also suggests higher free BPB in fetus compared to maternal plasma; twice as many cord blood samples (8/50) had measureable levels of BPB compared to paired maternal plasma (4/50) when measuring free BPB only.
A higher percentage of free versus conjugated BPB in the fetus may result from preferential transport by the placenta of free BPB. Alternatively, both conjugated and free BPB may be transported by the placenta and the conjugated form is de-conjugated by the fetus. De-conjugation in the placenta/fetus would lead to a higher percentage of free BPB in the fetus compared to dam plasma, particularly if there was limited expression of conjugation enzymes in the fetus. De-conjugation in the placenta/fetus has been observed in pregnant Sprague-Dawley rats exposed (intravenously) to glucuronide-conjugated bisphenol A (BPA-GA); BPA-GA was able to cross the placenta and was subsequently de-conjugated [16]. Nishikawa et al. also characterized the RNA expression of uridine 5′-diphospho-glucuronosyltransferase (UGT) enzymes in the rat fetal liver, showing that low expression of UGTs most likely resulted in a limited ability of the fetus to conjugate the free compound.
During lactation, prior to direct pup feeding, total BPB exposure in pups was very low. However, despite low overall exposure to pups during lactation, what appears to be poor conjugation of BPB in pups, resulted in higher exposure to free BPB compared to dams. The average percent-free BPB in dam plasma at all time points and exposure concentrations was less than 1%, with more than 99% conjugated.
The present study did not characterize the specific BPB conjugates that were formed. However, in a previous NTP study investigating the metabolism and disposition of BPB, both glucuronide and sulfate conjugates of BPB and BPB metabolites were excreted in the urine of adult male HSD rats following oral exposure [13], [15]. Ontogeny of UGT enzymes may explain the delay in conjugation observed in pups in the present study. Fetal and pup glucuronidation activity has been characterized in rats using phenolic substitutes and liver homogenate or microsomal fractions [4], [14], [22]. Scragg et al. report that fetal glucuronidation activity is nearly equivalent to adult levels, however activity decreases dramatically at birth and does not reach adult levels again until PND 30–40. Low post-birth glucuronidation activity is consistent with observations in the present study. In humans, fetal liver UGT expression is 114 times lower than adult expression and does not reach adult levels until 3 to 6 months postpartum [20], [26]. During the in utero and early lactation period when UGTs are low, the role of other enzymes, such as sulfotransferases (SULTs) would be important for fetal and young pup conjugation. Unlike UGTs, SULT enzyme expression in humans is reported to be only 3.5 times lower in fetal liver, compared to adult liver [20]. Characterization of SULT ontogeny in rats has shown that different isoforms have different ontogeny patterns; some isoforms are highest just after birth and decrease in adulthood while others are very low at birth and do not plateau until ∼PND60 [11], [12]. These studies illustrate some sex differences in sulfotransferase activity dependent on the substrate, however major sex differences were only observed after PND30.
The level of total 3-OH BPB (as a ratio to total BPB) in the fetus at GD18 was lower in comparison to dams, however it is difficult to determine if this is due to limited transport of the 3-OH metabolite compared to the parent compound, or lower oxidative metabolism in the fetus. Similarly, the ability of the pup to form oxidative metabolites cannot be accurately assessed prior to direct pup feeding; the percent 3-OH BPB in dams and pups was similar and it is impossible to distinguish between lactational transfer and pup formation of the metabolite. During late lactation (PNDs 21 and 28) the dam had slightly higher, yet consistent, formation of 3-OH BPB, compared to pups.
In summary, BPB exposure to the developing fetus appears to be minimal following exposure of dams to BPB via feed. Pup exposure to BPB prior to weaning was not substantial until pups began to consume BPB-feed suggesting low lactational transfer, however, poor BPB conjugation in pups led to considerable exposure to the free BPB. Based on available data, the ontogeny of UGTs in humans is similar to that of rodents, meaning that maternal levels of free BPB are likely not reflective of infant exposure and could be of human health concern.
Acknowledgments
This work was performed by RTI International (RTP, NC) for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S Department of Health and Human Services, under contract No. N01-ES-75564, No. N01-ES-75563 and No. HHSN273201400003C. Prior to submission this work was internally reviewed by Brad Collins and Dr. Mike DeVito.
References
- 2.Centers for Disease Control and Prevention (CDC) U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2015. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data.http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf [Google Scholar]
- 3.Domoradzki J.Y., Pottenger L.H., Thornton C.M., Hansen S.C., Card T.L., Markham D.A., Dryzga M.D., Shiotsuka R.N., Waechter J.M. Metabolism and pharmacokinetics of bisphenol A (BPA) and the embryo-fetal distribution of BPA and BPA-monoglucuronide in CD Sprague-Dawley rats at three gestational stages. Toxicol. Sci. 2003;76:21–34. doi: 10.1093/toxsci/kfg206. [DOI] [PubMed] [Google Scholar]
- 4.Dutton G.J. Glucuronide synthesis in foetal liver and other tisses. Biochem. J. 1959;71(1):141–148. doi: 10.1042/bj0710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frederiksen H., Jørgensen N., Andersson A.M. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC–MS/MS) J. Expo. Sci. Environ. Epidemiol. 2011;21:262–271. doi: 10.1038/jes.2010.6. [DOI] [PubMed] [Google Scholar]
- 6.Frederiksen H., Taxvig C., Hass U., Vinggaard A.M., Nellemann C. Higher levels of ethyl paraben and butyl paraben in rat amniotic fluid than in maternal plasma after subcutaneous administration. Toxicol. Sci. 2008;106(2):376–383. doi: 10.1093/toxsci/kfn171. [DOI] [PubMed] [Google Scholar]
- 7.Golden R., Gandy J., Vollmer G. A review of the endocrine activity of parabens and implications for potential risks to human health. Crit. Rev. Toxicol. 2005;35:435–458. doi: 10.1080/10408440490920104. [DOI] [PubMed] [Google Scholar]
- 8.Kang K.S., Che J.H., Ryu D.Y., Kim T.W., Li G.X., Lee Y.S. Decreased sperm number and motile activity on the F1 offspring maternally exposed to butyl p-hydroxybenzoic acid (butyl paraben) J. Vet. Med. Sci. 2002;64(3):227–235. doi: 10.1292/jvms.64.227. [DOI] [PubMed] [Google Scholar]
- 9.Kang S., Kim S., Park J., Kim H.J., Lee J., Choi G., Choi S., Kim S., Kim S.Y., Moon H.B., Kim S., Kho Y.L., Choi K. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci. Total Environ. 2013;461–462:214–221. doi: 10.1016/j.scitotenv.2013.04.097. [DOI] [PubMed] [Google Scholar]
- 10.Liao C., Liu F., Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ. Sci. Technol. 2013;47:3918–3925. doi: 10.1021/es400724s. [DOI] [PubMed] [Google Scholar]
- 11.Liu, Klaassen Ontogeny and hormonal basis of female-dominant rat hepatic sulfotransferases. J. Pharmacol. Exp. Ther. 1996;279:386–391. [PubMed] [Google Scholar]
- 12.Liu, Klaassen Ontogeny and hormonal basis of male-dominant rat hepatic sulfotransferases. Mol. Pharm. 1996;50:565–572. [PubMed] [Google Scholar]
- 13.Mathews J.M., Brown S.S., Patel P.R., Black S.R., Banks T.T., Etheridge A.S., Fennell T.R., Snyder R.W., Blystone C.R., Waidyanatha S. Metabolism and disposition of [14C]n-butyl-p-hydroxybenzoate in male and female Harlan Sprague Dawley rats following oral administration and dermal application. Xenobiotica. 2013;43(2):169–181. doi: 10.3109/00498254.2012.702935. [DOI] [PubMed] [Google Scholar]
- 14.Matsui M., Watanabe H.K. Developmental alteration of hepatic UDP-glucuronosyltransferase and sulphotransferase towards androsterone and 4-nitrophenol in Wistar rats. Biochem. J. 1982;204:441–447. doi: 10.1042/bj2040441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Toxicology Program (NTP). 2012. ADME Study Report M88007. Disposition and Metabolism of n-Butyl-p-Hydroxybenzoate (CAS# 94-26-8) in Rats Following Dermal, Oral and Intravenous Exposure. Research Triangle Park, NC.
- 16.Nishikawa M., Iwano H., Yanagisawa R., Koike N., Inoue H., Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ. Health Perspect. 2010;118(9):1196–1203. doi: 10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oishi S. Effects of butylparaben on the male reproductive system in rats. Toxicol. Ind. Health. 2001;17(1):31–39. doi: 10.1191/0748233701th093oa. [DOI] [PubMed] [Google Scholar]
- 18.Oishi S. Effects of butyl paraben on the male reproductive system in mice. Arch. Toxicol. 2002;76(7):423–429. doi: 10.1007/s00204-002-0360-8. [DOI] [PubMed] [Google Scholar]
- 19.Okubo T., Yokoyama Y., Kano K., Kano I. ER-dependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ERα and PR. Food Chem. Toxicol. 2001;39:1225–1232. doi: 10.1016/s0278-6915(01)00073-4. [DOI] [PubMed] [Google Scholar]
- 20.Pacifici G.M., Franchi M., Giulani L., Rane A. Development of the glucuronyltransferase and sulphotransferase towards 2-naphthol in human fetus. Dev. Pharmacol. Ther. 1989;14(2):108–114. [PubMed] [Google Scholar]
- 21.Schlumpf M., Kypke K., Wittassek M., Angerer J., Mascher H., Mascher D., Vokt C., Birchler M., Lichtensteiger W. Exposure patterns of UV filters fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere. 2010;81:1171–1183. doi: 10.1016/j.chemosphere.2010.09.079. [DOI] [PubMed] [Google Scholar]
- 22.Scragg I., Michael P., Burchell B., Dutton G.J. The temporary postnatal decline in glucuronidation of certain phenols by rat liver. Biochem. J. 1983;214:533–537. doi: 10.1042/bj2140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith K.W., Braun J.M., Williams P.L., Ehrlich S., Correia K.F., Calafat A.M., Ye X., Ford J., Keller M., Meeker J., Hauser R. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ. Health Perspect. 2012;120:1538–1543. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Towers C.V., Terry P.D., Lewis D., Howard B., Chambers W., Armistead C., Weitz B., Porter S., Borman C.J., Kennedy R.C.M., Chen J. Transplacental passage of antimicrobial paraben preservatives. J. Expo. Sci. Environ. Epidemiol. 2015:1–4. doi: 10.1038/jes.2015.27. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe Y., Kojima H., Takeuchi S., Uramaru N., Ohta S., Kitamura S. Comparative study on transcriptional activity of 17 parabens mediated by estrogen receptor α and β and androgen receptor. Food Chem. Toxicol. 2013;57:227–234. doi: 10.1016/j.fct.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Wildt S.N., Kearns G.L., Leeder J.S., van den Anker J.N. Glucuronidation in humans; pharmacogenic and developmental aspects. Clin. Pharmacokinet. 1999;36(6):439–452. doi: 10.2165/00003088-199936060-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wróbel A.M., Gregoraszczuk E.Ł. Actions of methyl-, propyl- and butylparaben on estrogen receptor-α and -β and the progesterone receptor in MCF-7 cancer cells and non-cancerous MCF-10A cells. Toxicol. Lett. 2014;230(3):375–381. doi: 10.1016/j.toxlet.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Ye X., Bishop A.M., Needham L.L., Calafat A.M. Automated on-line column-switchin HPLC–MS/MS method with peak focusing for measuring parabens: tricolsan and other environmental phenols in human milk. Anal. Chim. Acta. 2008;622:150–156. doi: 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 29.Ye X., Bishop A.M., Reidy J.A., Needham L.L., Calafat A.M. Parabens as urinary biomarkers of exposure in humans. Environ. Health Perspect. 2006;114(12):1843–1846. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]