Abstract
Environmental xenoestrogen contaminant bisphenol A (BPA), widely used as a monomer in the manufacture of epoxy, polycarbonate plastics and polystyrene resins. However, exposure to BPA has raised concerns, and the negative impacts of its exposure on reproduction have been controversial. The purpose of this work was directed to assess the potential adverse effects of BPA on dam rats and their first generation females in a comparative toxicological study. Fifteen pregnant female rats were classified into three equal groups; first group was orally administered corn oil and served as control (group1), second and third groups were orally administered BPA at dose levels of 50 and 200 mg/kg b.wt respectively (groups 2 & 3). The administration was carried out daily from zero day through the gestation period (21 days) until the last day of the lactation period (21days) and was extended after weaning for three months, in which female off springs of first generation (F1) of the three groups of dams were classified into; F1control group (group 4), F1 group treated with low dose of BPA (group 5) and F1 group treated with high dose of BPA (group 6) which continued in daily oral administration of BPA at the same previously mentioned doses for three months. The results elucidated a clear marked DNA fragmentation in the ovary of both dam and F1 female groups especially at higher examined concentration. Also, the data demonstrated a significant increase in the serum levels of GGT, ALP, glucose, total cholesterol, triglycerides, LDH and also in the serum level of estrogen hormone. Meanwhile, our study recorded a significant decrease in total protein, catalase, GST, HDL and FSH hormone in both treated dam and F1 female groups which was more significantly decreased in F1 female rats. Moreover, our experiment illustrated up-regulation in the immunoexpression of ERβ in ovary, uterus and liver of dam and F1 female groups. The histopathological investigation showed degeneration in the epithelial lining of ovarian follicles, submucosal leukocytic infiltration and increase in vaculation of hepatic cells with proliferation of kupffer cells. The lesions were more sever in groups 3 & 6 of both dam and their F1 females. Our data speculated that long- term exposure to BPA at 50 and 200 mg/kg.b.wt. depicted total genomic damage, significant alterations in liver enzymes, lipid profile, antioxidant enzymes and reproductive hormones with up-regulation in the expression of ERβ which were more significantly perturbed in group 3 and group 6 of both dam and F1 female rats.
Keywords: DNA damage, Estrogen receptors, Reproductive organs, Female rats
1. Introduction
Many environmental chemicals recently, are considered as xenoestrogens and endocrine disruptors [31], [7]. Nowadays, there is growing concern regarding the impact of environmental chemicals on animal and human reproduction [19]. Such endocrine disruptors may represent a major toxicological and public health issue [35]. The xenoestrogen bisphenol A (BPA) has received much attention due to its high production volume and wide spread exposure [24].
Bisphenol A {BPA; 2,2-bis-(4-hydroxyphenyl) propane} is a plasticizer that is widely used to produce polycarbonate plastic, epoxy resin and unsaturated polystyrene. BPA can leach from linings of food cans, polycarbonate baby bottles and other beverage containers, dental sealants and composites, polyvinyl chloride plastics and recycled thermal paper [60]. This compound released to the environment both accidentally and through permitted discharges [53] and its wide spread distribution has been a major cause of concern to regulatory agencies and others [45]. Its lifetime in the environment is sufficient for it to be virtually permanently detectable and it has been described as ubiquitous in surface waters [29].
Christiansen et al. [11] examined the influence of BPA (5 and 50 mg/kg b.wt.) on early sexual development in male and female rats, they recorded significant decrease in anogenital distance of both sexes besides; the incidence of nipple retention in male appeared to increase.
Studies on BPA genotoxicity have conflicting results; both genotoxic [37] and non- genotoxic [21] effects of BPA have been reported. Where, studies of BPA genotoxicity have yielded conflicting results; BPA is considered non-genotoxic because it was negative to a set of basic genotoxicity tests [49], did not induce gene mutations [57] or chromosomal aberrations [23]. In contrast, BPA induced numerical chromosomal aberrations and morphological changes in cultured Syrian Hamster Embryo cells [57]. In addition, BPA metabolites were shown to bind to DNA in a cellular system [13].
It was reported that BPA induce oxidative stress [28], in which reactive oxygen species (ROS) are cytotoxic agents that lead to significant oxidative damage by attacking biomolecules such as membrane lipids and DNA in cells [27].
A few studies have focused on whether BPA exerts its action through nuclear receptors such as estrogen [50], androgen [56] and thyroid [44] receptors. Further, Peretz et al. [40] have shown that BPA does not exert its toxic effects via the genomic estrogenic pathway in mouse ovarian follicles.
Meanwhile, in vitro studies demonstrated that BPA binds to the estrogen receptors induces estrogen − dependant gene expression responses [30] and Caserta et al. [9] mentioned that BPA has estrogenic activity and binds to α and to a lesser extent to β-estrogen receptors. Further BPA can acts as antiestrogen, blocking these estrogen response by competing with endogenous estrogen [42].
In this spirit and because there is paucity of information concerning studying the comparative toxic impacts from long-term exposure to BPA on dam rats and their female generations. We show in this study for the first time the effects of BPA in a comparative manner on dam rats and their first generation females concerning DNA damage, expression of estrogen receptor β, with some biochemical parameters and histopathological examination of liver and reproductive organs.
2. Materials and methods
2.1. Chemical compound
Bisphenol A (purity 97% of CAS number 80-50-7) was obtained from sigma- Aldrich Company and dissolved in corn oil [32].
2.2. Animals and dosing
Eighty mature albino rats (sixty of females weighing 150–200 gm b.wt. and twenty of males weighing 220–260 gm b.wt., used for mating) were obtained from experimental Animal Unit of Faculty of Veterinary Medicine, Zagazig University, Egypt. Animals were kept in metal cages under hygienic conditions, fed on well balanced ration and provided with water ad-libitum throughout the experiment.
Experiments were conducted in accordance with the guidelines set by Animals Health Research Ethics Training Initiative, Egypt, and experimental protocols were approved by the institutional animal ethics committee.
Female dam rats were daily examined to ensure estrous phase using vaginal smear technique, females in estrous were paired with mature males, the presence of sperms in vaginal smear indicating zero day of gestation [4]. Fifteen pregnant female rats were classified into three equal groups; first dam group orally administered corn oil and served as control (group1), second dam group orally administered BPA at dose level of 50 mg/kg b.wt. [61] (group2) and third dam group orally administered BPA at dose level of 200 mg/kg b.wt. [48] (group3). The administration was carried out daily from zero day throughout the gestation period (21days) until the last day of lactation period (21days) and extended after weaning for three months.
After weaning, female off springs of first generation (F1) of the three groups of dams; each group contain seven females of F1 were classified into; F1control group (group 4), F1 group treated with low dose of BPA (group 5) and F1 group treated with high dose of BPA (group 6) which continued in oral daily administration of BPA at the same previously mentioned doses for three months.
At the end of the experiment, blood samples were collected from the retro-orbital sinus without anticoagulant in sterile test tubes for separation of serum which kept at −20 °C till biochemical analysis and then dam rats of the three groups and their F1 females that examined for pro-estrus phase were anesthetized and euthanized by decapitation. Tissue samples from ovaries of all groups were taken and kept at −20 °C for applying DNA fragmentation assay. For immunohistochemical and histopathological studies, specimens from ovary, uterus and liver were collected and fixed in 10% buffered neutral formalin solution.
2.3. DNA fragmentation assay
DNA damage determined by DNA fragmentation assay according to Bortner et al. [8] that could be summarized as following:
Small pieces of ovarian tissues were put in 1.5 ml microfuge tube. Extraction buffer was added to 0.3 ml mark. Tissues were crushed and then extraction buffer was added till 0.5 ml mark. 50.0 μl of proteinase-K solution (10.0 mg/ml) was added then the tubes were closed and inverted to mix. Tubes were incubated at 50 °C for 12 h: 3 days with occasional vigorous mixing. DNA was extracted with a mixture of phenol, chloroform and isoamyl alcohol (25: 24: 1) and vortex samples 5.0 s. Samples were centrifuged at 12000 rpm for 5.0 min. Then 500 μl of aqueous layer for each sample was removed carefully into a new tube and 50.0 μl of 3.0 M sodium acetate (pH = 5.3) was added to each tube. Pure ethanol (100%) was added till mark 1.5 ml. The tubes were inverted for mixing and DNA precipitation then let to be set at −20 °C overnight and centrifuged at 12000 rpm for 20 min. The supernatant was removed and 50.0 μl of Tris EDTA buffer was added overnight till complete dissolving. Samples were run on electrophoresis using 1.2% agarose gel at 50.0 voltages, gel was stained using ethidium bromide. Samples were analyzed using image analyses software.
2.4. Immunohistochemical examination for determination of estrogen receptor–β (ERβ)
The paraffin embedded ovaries, uteri and livers were fixed in 10% formalin, sectioned into 5 μm sections, and mounted on positively charged slides for immunostaining of ERβ. Sections were deparaffinised, rehydrated and autoclaved at 120 °C for 10 min in 10 Mm citrate buffer (pH 6) for ERβ. After washing with PBS endogenous peroxidase was blocked using 0.3% hydrogen peroxide in methanol (15 min). Thereafter, slides were washed in PBS again and blocking was performed by adding blocking buffer and incubated for 30 min at room temperature. Primary antibody for ERβ (Cat. No. RB- 10658-R7, Thermo Scientific Co., UK). It was diluted by PBS (1:100) then added to the slides and incubated for 30 min. The slides subjected to washing with PBS three times for 3 min each. Biotinylated polyvalent secondary antibody (Cat. No. 32230, Thermo Scientific Co., UK) was applied to tissue sections and incubated for 30 min. The slides were washed three times for 3 min each with wash buffer and then incubated with avidin-biotincomplex (ABC peroxidase kit, Santa Cruz, USA). Slides were incubated for ten minutes with DAB peroxidase enzyme substrate. They were washed with buffer two times for 3 min each. Sections were counterstained with hematoxylin stain then slides were dehydrated, cleared and mounted [3]. For negative controls, sections were treated with similar steps with the exception of the primary antibodies. Positive immunoreactivity was recognized as brown staining. Images were captured from ovaries, uteri and livers using a Canon power Shot digital camera (Canon, Inc., Tokyo, Japan) for light microscopic examination.
2.5. Serum biochemical analysis
The sera were analyzed for estimation of gamma glutamyl transferase (GGT), alkaline phosphatase (ALP) and total protein using commercially available kits (Biosystem S.A., Costa Brava., 30, Barcelona, Spain) according to instructions of manufacture. Glucose was calculated according to Glick et al. [17], catalase activity (CAT) according to the method described by Sinha [52], glutathione S transferase activity (GST) according to Habig et al. [20], total cholesterol according to Allain et al. [1], triglycerides according to Fossatic and Prenicined [14], high density lipoprotein C (HDL-c) according to Lopes- Vurella et al. [33], low density lipoprotein −c (LDL-c) was calculated according to Friedwald et al. [15], follicular stimulating hormone (FSH) was estimated using the assay described by Beastall et al. [5] and estrogen hormone was measured using commercial immunoassay kit (vat estradiol ELISA kit, cubio, USA).
2.6. Histopathological examination
Ovary, uterus and liver specimens were routinely processed by dehydration in gradual ethanol (70–100%), cleared in xylene and the paraffin embedded tissues were cut 5 μm thick and then routinely stained with haematoxylin and eosin (H&E) dyes according to Bancroft and Gamble [2].
2.7. Statistical analysis
Data are expressed as mean values ± SE. Statistical analysis was performed using one way analysis of variance (ANOVA) using SPSS statistical version 21 software package (SPSS, Inc, USA). Duncan’s test was used for making a multiple comparisons among the groups for testing the inter-grouping homogeneity. The significance was declared at (P < 0.05).
3. Results
3.1. Effect of BPA on ovarian total genomic damage of DNA
Fig. 1 showed electrophoretic pattern of ovarian total genomic DNA fragmentation of control and treated groups with BPA at both concentrations (50 mg & 200 mg/kg B.wt.) of dam and F1 female rats. Bisphenol A revealed strong DNA damage at the examined doses which was more obvious at higher concentration comparing with the low toxic dose in both dam and F1 female treated groups (groups 3&6). DNA damage represented by fragments migrated from the wells. On the contrary, control groups of dam and F1 female did not revealed fragmentation of DNA.
Fig. 1.
Effect of BPA administration at dose levels of (50 & 200 mg/kg b.wt) on ovarian DNA fragmentation of dam rats and their F1 females. Lane 1: no DNA fragmentation of control group of dam (group 1). Lane 2: mild DNA fragmentation of dam treated with low dose of BPA (group 2). Lane 3: strong DNA fragmentation of dam treated with high dose of BPA (group 3). Lane 4: no DNA fragmentation of control group of F1 female (group 4). Lane 5: moderate DNA fragmentation of F1 female treated with low dose of BPA (group 5). Lane 6: strong DNA fragmentation of F1 female treated with high dose of BPA (group 6).
3.2. Effect of BPA on serum biochemical parameters of dam rats and their F1 females
Table 1 indicated that dam rats and their F1 females orally administered BPA at dose levels of (50 & 200 mg/kg b.wt.) showed significant increase (P < 0.05) in serum levels of GGT, ALP and glucose comparing with their corresponding control ones and when comparing between the treated groups meanwhile, glucose level was not significantly increased when comparing group 3 of dam with group 5 of F1 female. The levels of GGT, ALP and glucose were more significantly increased in F1 female groups when comparing with their corresponding dam groups.
Table 1.
Effects of PBA orally administered at dose levels of (50 mg & 200 mg/kg.b.wt.) to dam rats and their first generation females on serum liver enzymes, total protein, glucose, catalase and glutathione S − transeferase. (Mean ± SE) (n = 5).
| Groups | Parameters |
|||||
|---|---|---|---|---|---|---|
| GGT (u/L) | ALP (IU/L) | Total protein (gm/dl) | Glucose (mg/dl) | Catalase (u mol H2O2 Decomposed/ml) | GST (U/L) | |
| Group1 (Control dam) | 24.99±0.14e | 69.14±0.3e | 8.1 8±0.30a | 84.08±0.73d | 41.10±0.28a | 13.32±0.15a |
| Group 2 (Dam treated with Low dose of BPA) | 32.66±0.16d | 78.83±0.24d | 8.01±0.06b | 96.34±0.22c | 39.86±0.19b | 11.43±0.41b |
| Group 3 (Dam treated with High dose of BPA) | 38.64±0.32b | 86.84±0.21c | 7.14±0.20cd | 104.61±0.32b | 35.59±0.16c | 9.59±0.21c |
| Group 4 (Control F1 female) | 24.51±0.13e | 68.49±0.24e | 8.30±0.41a | 85.23±0.36d | 40.61±0.83a | 13.61±0.18a |
| Group 5 (F1 female treated with Low dose of BPA) | 36.90± 0.23c | 91.90±0.64b | 7.12±0.52de | 102.41±0.67b | 34.03±0.17d | 9.75±0.21c |
| Group 6 (F1 female treated with High dose of BPA | 41.92±0.19a | 112.70±0.80a | 6.96±0.81e | 127.52±0.58a | 30.43±0.26e | 7.52±0.91d |
Means within the same column having the different superscripts are significantly different at (P < 0.05).
On the contrary, Table 1 illustrated significant decrease (P < 0.05) in the serum levels of total protein, catalase and GST in all treated groups comparing with the control rats of both dam and F1 female groups but the levels of total protein and GST were not significantly decreased when comparing group 3 of dam with group 5 of F1 female and also, when comparing both group 5 and group 6 of F1 females concerning the level of total protein.
Regarding the picture of lipid profile, Table 2 recorded significant increase (P < 0.05) in the levels of total cholesterol, triglycerides and HDL and significant decrease (P < 0.05) in the level of LDL in the treated groups of both dam rats and their F1 female groups comparing with control and when comparing with each other, otherwise the level of HDL was not significantly increased when comparing group 3 of dam with group 5 of F1 females and when comparing both treated groups of F1 females with each other.
Table 2.
Effects of PBA orally administered at dose levels of (50 mg & 200 mg/kg.b.wt.) to dam rats and their first generation females on serum levels of lipid profile, FSH and estrogen hormones. (Mean ± SE) (n = 5).
| Groups | Parameters |
|||||
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dl) | Triglycerides (mg/dl) | HDL (mg/dl) | LDL (mg/dl) | FSH (IU/L) | Estrogen (pg/ml) | |
| Group1 (Control dam) | 90.81±0.58e | 80.57±0.41e | 50.20±0.59a | 30.48±1.02e | 3.71±0.80ab | 26.71±0.25d |
| Group 2 (Dam treated with Low dose of BPA) | 98.21±0.84d | 85.59±0.27d | 46.61±0.41 | 34.41± 0.51d | 3.43±0.32bc | 29.46±0.31c |
| Group 3 (Dam treated with High dose of BPA) | 105.20±0.37c | 88.58±0.23c | 42.46±0.29cd | 45.32± 0.92b | 2.95±0.30d | 31.49±0.55b |
| Group 4 (Control F1 female) | 92.40±0.42e | 82.30±0.58e | 49.35±0.31a | 31.47± 0.36e | 3.87±0.61a | 25.93±0.43d |
| Group 5 (F1 female treated with Low dose of BPA) | 107.80±0.34b | 106.41±0.28b | 43.41± 052c | 41.34± 0.62c | 3.29±0.45c | 30.75±0.53bc |
| Group 6 (F1 female treated with High dose of BPA) | 112.10±0.53a | 108.18±0.72a | 41.23±0.65d | 49.16±0.93a | 2.57±0.41e | 36.66±0.75a |
Means within the same column having the different superscripts are significantly different at (P < 0.05).
Also, our data depicted in Table 2 showed significant decrease (P < 0.05) in the serum level of FSH and significant increase in the estrogen level in the treated rats of both dam and F1 female groups comparing with the control rats. Meanwhile, the level of FSH was not significantly decreased when comparing group 2 of dam with the corresponding control one and also, FSH level was not significantly decreased when comparing both group 2 and group 5 of dam rats and their F1 females. Besides, the level of estrogen was significantly increased when comparing group 3 of dam with group 5 of F1 females. Also, the level of estrogen in group 6 was significantly higher than that of group 5.
3.3. Immunhistochemical detection of estrogen receptor–β (ERβ)
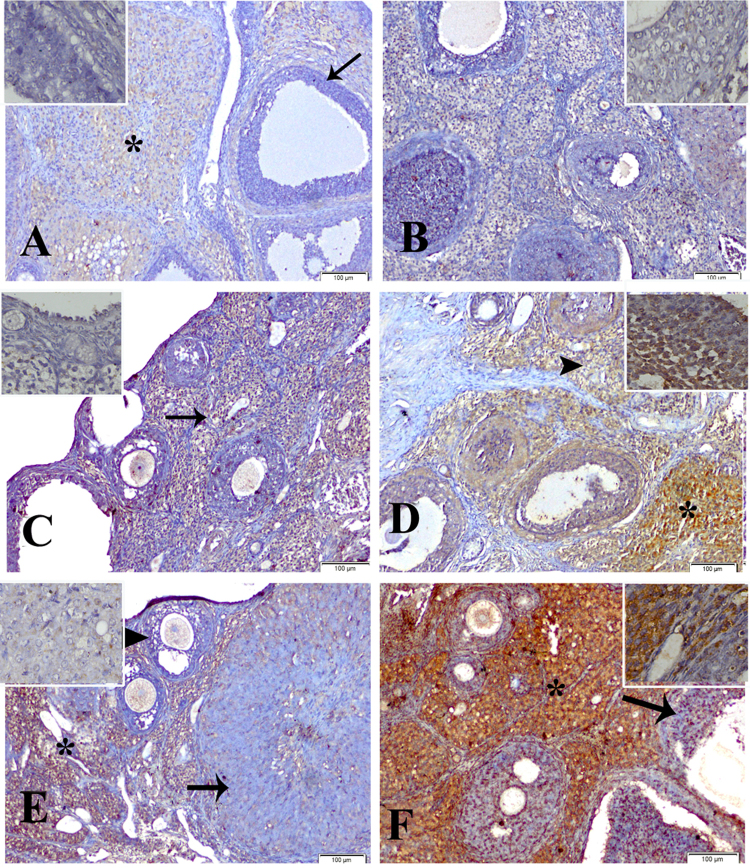
Our study showed faint immunoreactions of ERβ expression in ovary, uterus and liver of control groups (groups 1 & 4) of dam and F1 female (Fig. 2A, B & Fig. 3A, B & Fig. 4A, B). The ovary of dam treated with BPA (50 mg/kg b.wt) (group2) revealed moderate reaction (Fig. 2C) and of F1 female (group 5) showed intense immunoreaction (Fig. 2D). Groups treated with BPA (200 mg/kg b.wt) of dam and F1 female (groups 3 & 6) showed moderate and strong reaction respectively (Fig. 2E, F).
Fig. 2.
Immunolocalization of estrogen receptor-β in proestrus phase in ovary of: control group of dam (group1) (A) and F1 female (group 4) (B) showing faint immunoreactivity in the cellular component of corpus luteum (*), the ovarian follicles were devoid of staining affinity (arrow). Group 2 of dam (C) showed moderate immunoreactivity (arrow) and F1 female (group 5) (D) showing strong immunoreactivity in the corpus luteum (*), ovarian follicles and stromal cells (arrowhead). Group 3 of dam (E) showing moderate reaction in the stromal cells (*), ovarian follicles (arrowhead) and corpus luteum (arrow), and F1 female (group 6) (F) showing an intense staining affinity of the stromal cells (*) and moderate in the ovarian follicles (arrow) (immunostaining scale bar 100 μm and inset scale bar 20 μm).
Fig. 3.
Immunolocalization of estrogen receptor-β in proestrus phase in uterus of: control group of dam(group 1) (A) showing faint immunoreactivity throughout the stromal cells, the lining epithelium of the endometrium and uterine gland were devoid of immunoreactivity (*) and F1 female (group 4) (B) showing faint reaction in the deep stromal cells (*), the lining epithelium of the endometrium was more intense than that of uterine gland. Group 2 of dam (C) showing moderate reaction in the stromal cells and endometrial epithelium and F1 female (group 5) (D) showing more intense reaction in the superficial stromal cells (arrow). Group 3 of dam (E) showing moderate reaction in the epithelium of endometrium and uterine glands and showing mild to moderate reaction in the stromal cells (arrow) and F1 female (group 6) (F) showing the most intense immunoreactivity in the stromal cells (arrow) (immunostaining scale bar 50 μm and inset scale bar 20 μm).
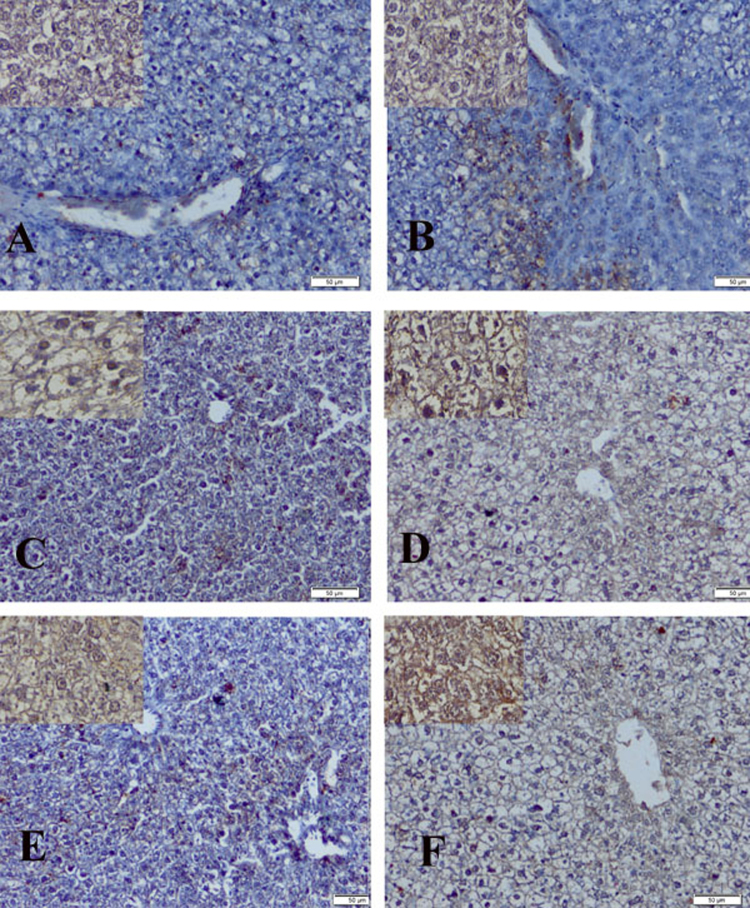
Fig. 4.
Immunolocalization of estrogen receptor-β in proestrus phase in liver of control group of: dam (group 1) (A) and F1 female (group 4) (B) sowing faint immunoractivity. Group 2 of dam (C) sowing mild to moderate reaction and F1 female (group 5) (D) showing moderate to strong reaction. Group 3 of dam (E) showing moderate reaction and F1 female (group 6) (F) showing strong reaction (immunostaining scale bar 50 μm and inset scale bar 20 μm).
The uterus of group 2 of dam revealed moderate immunoreaction (Fig. 3C) and of F1 female (group 5) showed more intense reaction (Fig. 3D). In addition, group 3 of dam indicated mild to moderate reaction (Fig. 3E) and of F1 female (group 6) revealed most intense immunoreaction (Fig. 3F).
The liver showed mild to moderate reaction of group 2 of dam (Fig. 4C) and revealed moderate to strong reaction of F1 female group (group 5) (Fig. 4D). Group 3 and group 6 of dam and F1 female showed moderate and strong immunoreaction respectively (Fig. 4E, F).
3.4. Histopathological findings
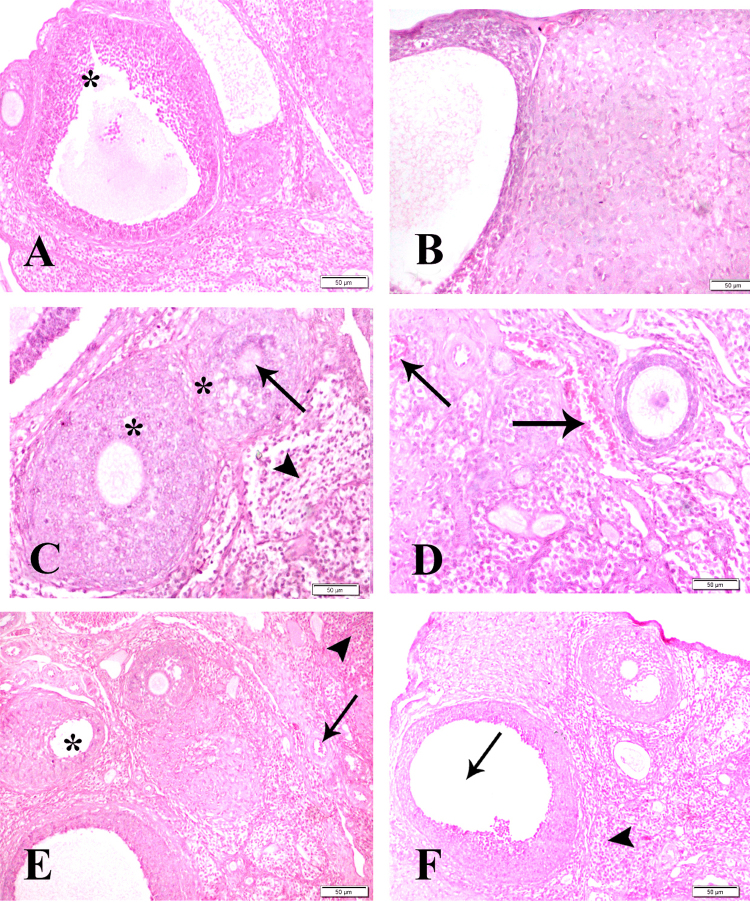
The ovary showed normal histological architecture of control groups of dam and their F1 females (Fig. 5A, B). Groups treated with BPA (50 mg/kg b.wt.) of dam (group 2) showed degeneration in the epithelial lining of ovarian follicles (arrow) and leukocytic infiltration in interstitial tissue (arrowhead) (Fig. 5C) and of F1 females (group 5) showed secondary follicle and mild congested blood vessel (arrow) (Fig. 5D). Groups treated with BPA (200 mg/kg b.wt.) of dam (group 3) showed congestion (arrow), hemorrhage (arrowhead) and degenerated ovum (*) (Fig. 5E) and of F1 females (group 6) showed degenerated ovum (arrow) and interstitial leukocytic infiltration (arrowhead) (Fig. 5F).
Fig. 5.
Ovarian sections of control group of dam (group 1) (A) and F1 female (group 4) (B) showing normal histological structure of ovarian follicles (*). Group 2 of dam treated with low dose of BPA (C) showing degeneration in the epithelial lining of ovarian follicles (arrow) and leukocytic infiltration in the interstitial tissue (arrowhead) and F1 female (group 5) (D) showing secondary follicle and mild congested blood vessel (arrow). Group 3 of dam treated with high dose of BPA (E) showing congestion (arrow), haemorrhage (arrowhead) and degenerated ovum (*) and of F1 female (group 6) (F) showing degenerated ovum (arrow) and interstitial leukocytic infiltration (arrowhead) HE x 400.
The uterus of control groups (groups 1 & 4) of dam and F1 females showed normal histological structures of epithelial lining (*), and glandular units (arrow) and stromal tissue (arrowhead) (Fig. 6A,B). Group 2 of dam revealed cystic dilatation of endometrial gland (arrow), submucosal leukocytic infiltration and hyperplasia of endometrial epithelium (arrowhead) (Fig. 6C) and of F1 females (group 5) showed endometrial hyperplasia with invaginations (arrow) (Fig. 6D). Group 3 of dam showing diffuse leukocytic infiltration (arrow) with vacuolar degeneration in endometrial epithelium (arrowhead) and glandular epithelium (*) (Fig. 6E) and of F1 female (group 6) showing multiple cyst formation in endometrial glands (arrow) with diffuse interstitial leukocytic infiltration (Fig. 6F).
Fig. 6.
Uterine sections of control groups of dam (group 1) (A) & F1 female (group 4) (B) showing normal histological structure of epithelial lining (*), and glandular units (arrow) and stromal tissue (arrowhead). Group 2 of dam treated with low dose of BPA (C) showing cystic dilatation of endometrial gland (arrow), submucosal leukocytic infiltration and hyperplasia of endometrial epithelium (arrowhead) and of F1 female (group 5) (D) showing endometrial hyperplasia with invaginations (arrow). Group 3 of dam treated with high dose of BPA (E) showing diffuse leukocytic infiltration (arrow) with vacuolar degeneration in endometrial epithelium (arrowhead) and glandular epithelium (*) and of F1 female (group 6) (F) showing multiple cyst formation in endometrial glands (arrow) with diffuse interstitial leukocytic infiltration. HE x 400.
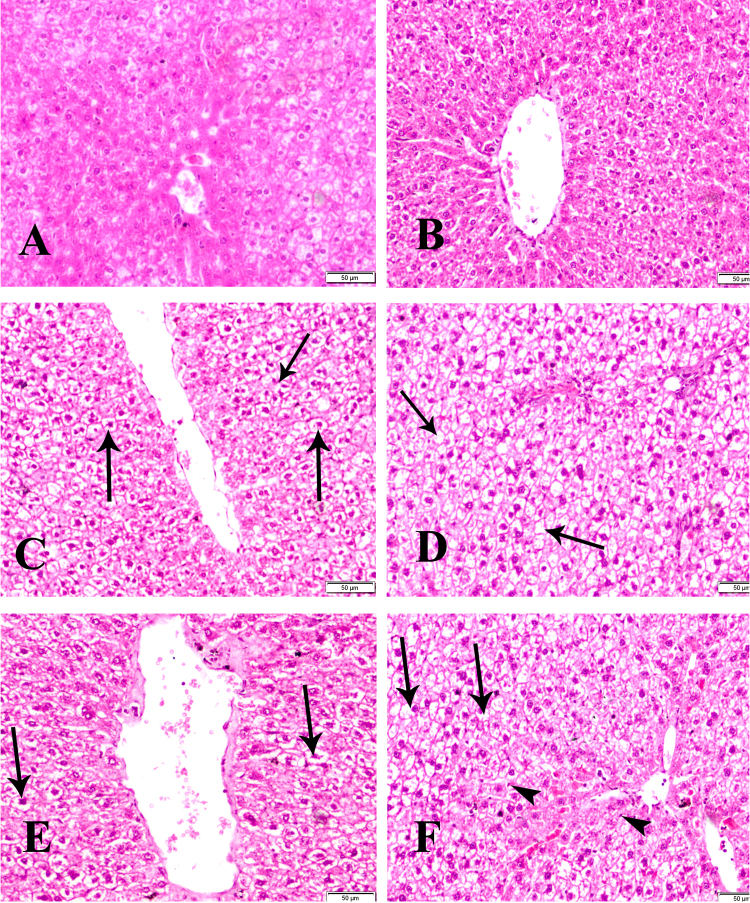
The liver revealed normal histological structure of control groups (groups 1 & 4) (Fig. 7A, B). Group 2 of dam showed hydropic degeneration and pyknosis of the nuclei (arrow) (Fig. 7C) and revealed sever hydropic degeneration and coagulative necrosis (arrow) in F1 female group (group 5) (Fig. 7D). In addition group 3 of dam showing increase in vacoulation of hepatic cells (arrows) (Fig. 7E) and revealed sever hydropic degeneration (arrows), coagulative necrosis, sinusoidal dilatation, pyknosis and proliferation of Kupffer cells (arrowheads) in F1 female group (group 6) (Fig. 7F).
Fig. 7.
Sections of liver of control group (group 1) (A) and F1 female (group 4) (B) showing normal histological structure. Group 2 of dam treated with low dose of BPA (C) showing hydropic degeneration and pyknosis of the nuclei (arrow) and of F1 female (group 5) (D) showing sever hydropic degeneration and coagulative necrosis (arrow). Group 3 of dam treated with high dose of BPA (E) showing increase in vacuolation of hepatic cells (arrows) and of F1 female (group 6) (F) showing sever hydropic degeneration (arrows), coagulative necrosis, sinusoidal dilatation, pyknosis and proliferation of kupffer cells (arrowheads) HE x 400.
4. Discussion
The current study is considered the first work for evaluating in a comparative manner the toxic impacts of BPA exposed dam rats and their F1 females concerning ovarian genotoxicity, effect on the expression of ERβ accompanying with biochemical and histopathological investigations.
Using the genotoxicity tests in vivo models plays a crucial role in the risk assessment process [58]. Few short − term studies have been carried out on the genotoxic potential of BPA using in vivo test models [26], In addition few papers have described the effects of BPA on somatic cells, and most of those are studies in males [16].
The present study indicated that BPA at both examined concentrations revealed marked DNA damage using DNA fragmentation assay in the ovary of all treated rats of dam and their F1 female groups in which the fragmentation was more clear in groups 3 & 6 comparing with groups 2 & 5 (Fig. 1). In this manner Tayama et al. [55] detected an increase in DNA migration after treatment of CHO −K1 cells with PBA. On the other hand De flora et al. [12] recorded that there was no increase of single strand DNA breaks in cells of BPA − treated rats, including peripheral blood lymphocytes and bone marrow erythrocytes. In which Ulutas et al. [58] reported that the genotoxic effects of BPA is controversial.
One pathway of BPA metabolism is the hydroxylation of one of its symmetric phenyl rings to form its catechole, O-OH BPA, which can oxidize to O-quinone BPA [47] which in turn, react with DNA.
O-Quinone BPA forms predominantly depurinating adducts O-OH-BPA −6-N3 Ade and O-OH-BPA −6-N7Gua [28]. Moreover, Sakuma et al. [46] detected that O-quinone BPA could increase ROS formation and oxidize the guanine moiety of deoxyguanosine in the DNA of primary rat hepatocyte culture.
In the same context our data of the present study recorded significant decrease in serum levels of antioxidant enzymes; catalase and GST in groups 2 & 3 of dam, which were more significantly decreased in groups 5& 6 of F1 females (Table 1).
Our results came in harmony with those reported by Kabuto et al. [27] who found that intraperitoneal administration of 50 mg/kg b.wt. of BPA reduced the activity of detoxifying enzymes, including superoxide dismutase, glutathione peroxidase and catalase in mouse tissue. Also, Popa et al. [41] recorded increased lipid peroxidation and decreased activity of some antioxidant enzymes such as catalase, glutathione peroxidase, glutathione reductase and glutathione S-transferase in female rats treated with BPA. In which, catalase may reflect inability of liver mitochondria and microsomes to eliminate hydrogen peroxide produced after exposure to BPA [6] and GST protects cells or tissue against oxidative stress and damage by detoxifying various toxic substrates derived from cellular oxidative processes [51].
Previous study strongly suggested that DNA damage induced by estrogen is dependent on estrogen receptors (ER) [22].
Regarding to the effect of both examined doses of BPA on the expression of estrogen receptor (ERβ), our study revealed that there were mild to strong immunoreactions of ERβ in ovary, uterus and liver of both dam (groups 2 & 3) and F1 female groups (groups 5 & 6) in which the expression was more clear in F1 groups comparing with dam rats (Figs. 2–4). Similarly, Rodiguez et al. [43] found that PBA caused an increase in the expression of ERβ in rat's ovary. Conversely, Vandenberg et al. [59] reported that BPA is a weak estrogen agonist and so its effects observed in animal studies are difficult to reconcile with the actions as estrogen agonists. Our study was supported by the results of the hormonal levels which showed a significant increase in serum level of estrongen hormone accompanied with a significant decrease of FSH level of both examined concentrations of BPA treated dam and F1 female groups which were more significantly altered in F1 female groups. Additionally, our data recorded significant increase in the level of lipid profile; cholesterol, triglycerides, LDL with a significant decrease of the level of HDL (Table 2).
Our results coincide with those reported by Grasselli et al. [18] who found that PBA treatment altered steroid hormone production in rat ovary. The precise mechanism remains unclear [10] but they speculated that steroidogenic acute regulatory protein (StAR) and aromatase cytochrome P450 appeared to be targeted by BPA. Moreover, Peretz et al. [39] concluded that BPA may interfere with the steroidogenesis by inhibiting cholesterol uptake, which consistent with our study concerning the results of lipid profile picture. Also, our data of lipid profile came in harmony with those recorded by Jiang et al. [25] who found that Wister rats treated with BPA up–regulated hepatic lipid metabolism and up-regulated genes involved in lipogenesis pathway.
Concerning the histopathological findings of the examined organs of our study which showed various lesions including degeneration in the epithelial lining of ovarian follicles, cystic dilatation of endometrial gland, submucosal leukocytic infiltration and increase in vaculation of hepatic cells with proliferation of kupffer cells, which were more sever in groups 3& 6 of both dam and their F1 female (Figs. 5–7) that could be explained by the results of DNA fragmentation previously mentioned. Sakuma et al. [46] supported our attribution where they mentioned that oxidative DNA damage has been implicated in a wide variety of pathological conditions. Additionally, our findings regarding the effect on the expression of ERβ, could attribute our histopathological lesions. In the same context Popa et al. [41] recorded that histopathologicial changes can be explained by estrogen activity of BPA. In this respect, our histopathologicial findings are in agreement with those findings detected by Newbold et al. [38] who mentioned that BPA has been reported to cause significant histological changes in the reproductive tract.
The histopathological perturbations in the hepatic tissues which investigated in the present study could be reflect the functional impairment of the liver which may be attribute the alteration in the serum levels of GGT, ALP, total protein and glucose of the treated rats of both dam and their F1 females. Previous studies of Tarantino et al. [54] mentioned that BPA increased insulin resistance and Makaji et al. [34] who found that BPA disturb glucose homeostasis in animals. Similarly, Yildiz and Barlas [62] recorded that BPA treated rats revealed alteration in total protein, glucose and alkaline phosphatase. Also, Melzer et al. [36] mentioned that BPA significantly correlated with elevations in the liver enzymes.
To sum up, our findings contribute to show that long- term exposure to BPA at 50 and 200 mg/kg.b.wt. reflected total genomic damage of DNA, significant alterations in liver enzymes, lipid profile, antioxidant enzymes and reproductive hormones with up-regulation in the expression of ERβ which were more significantly perturbed in group 3 and group 6 of both dam and F1 female rats.
Conflict of interests
The authors declare that they have no conflict of interests.
Transparency document
References
- 1.Allain C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 2.Bancroft J.D., Gamble M. 6th ed. Churchill Livingstone Elsivier; Philadelphia, PA: 2008. Theory and Practice of Histological Technique; p. 319. [Google Scholar]
- 3.Bancroft J.D., Cook H.D. 2nd edition. W.B. Saunders Company; 1994. Manual of Histological Techniques and Their Diagnostic Applications; pp. 263–325. [Google Scholar]
- 4.Barcelona R.S., Fanelli O., Campana A. Teratological study in rat and rabbit. Toxicology. 1977;2:87–94. doi: 10.1016/0300-483x(77)90026-9. [DOI] [PubMed] [Google Scholar]
- 5.Beastall G.H., Freguson K.M., D.S.J, Reilly O., Seth J., Sheridan B. Assays for follicle stimulating hormone and luteinizing hormone: guidelines for the provision of a clinical biochemistry service. Ann. Clin. Biochem. 1987;24:246–262. doi: 10.1177/000456328702400303. [DOI] [PubMed] [Google Scholar]
- 6.Bindhumol V., Chitra K.C., Mathur P.P. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188:117–124. doi: 10.1016/s0300-483x(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 7.Blake C.A., Boockfor F.R., Nair-Menon J.U., Millette F.C., Raychoundhury S.S., McCoy G.L. Effects of 4-tert-octylphenol given in drinking water for 4 months on the male reproductive system of Fischer 344 rats. Reprod. Toxicol. 2004;18:43–51. doi: 10.1016/j.reprotox.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Bortner C.D., Oldenburg N.B., Cidlowski J.A. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995;5:21–26. doi: 10.1016/s0962-8924(00)88932-1. [DOI] [PubMed] [Google Scholar]
- 9.Caserta D., Mantovani A., Marci R., Fazi A., Ciardo F., La Rocca C., Maranghi F., Moscarini M. Environment and women’s reproductive health. Hum. Reprod. Update. 2011;17:418–433. doi: 10.1093/humupd/dmq061. [DOI] [PubMed] [Google Scholar]
- 10.Caserta D., Di Segni N., Mallozzi M., Giovanale V., Mantovani A., Marci R., Moscarini M. Bisphenol a and the female reproductive tract: an overview of recent laboratory evidence and epidemiological studies. Reprod. Biol. Endocrinol. 2014;12:37–46. doi: 10.1186/1477-7827-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christiansen S., Axelstad M., Boberg J., Vinggaard A.M., Pedersen G.A., Hass U. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction. 2014;147:477–487. doi: 10.1530/REP-13-0377. [DOI] [PubMed] [Google Scholar]
- 12.De Flora S., Micale R.T., La Maestra S., Izzotti A., D’Agostini F., Camoirano A., Davoli S.A., Troglio M.G., Rizzi F., Davalli P., Bettuzzi S. Upregulation of clusterin inprostate and DNA damage in spermatozoa from bisphenol A-treated rats and formation of DNA adducts in cultured human prostatic cells. Toxicol. Sci. 2011;122:45–51. doi: 10.1093/toxsci/kfr096. [DOI] [PubMed] [Google Scholar]
- 13.Edmonds J.S., Nomachi M., Terasaki M., Morita M., Skelton B.W., White A.H. The reaction of bisphenol a 3,4-quinone with DNA. Biochem. Biophys. Res. Commun. 2004;319:556–561. doi: 10.1016/j.bbrc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Fossati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- 15.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Gajowik A., Radzikowska J., Dobrzýnska M.M. Genotoxic effects of bisphenol A on somatic cells of female mice, alone and in combination with X-rays. Mutat. Res. 2013;757:120–124. doi: 10.1016/j.mrgentox.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Glick M.R., Ryder K.W., Jackson S.A. Graphical comparisons of interferences in clinical chemistry instrumentation. Clin. Chem. 1986;32:470–475. [PubMed] [Google Scholar]
- 18.Grasselli F., Baratta L., Baioni L., Bussolati S., Ramoni R., Grolli S. Bisphenol A disrupts granulosa cell function. Domest. Anim. Endocrinol. 2010;39:34–39. doi: 10.1016/j.domaniend.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Gregoraszczuk E.L., Ptak A. Endocrine-disrupting chemicals: some actions of POPs on female reproduction. Int. J. Endocrinol. 2013;828532:1–9. doi: 10.1155/2013/828532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habig W.H., Pabst M.J., Jacoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 21.Honma M., Hayashi M., Shimada H., Tanaka N., Wakuri S., Awogi T., Yamamoto K.I., Kodani M.U., Nishi Y., Nakadate M., Sofuni T. Evaluation of the mouse lymphoma tk assay (microwell method) as an alternative to the in vitro chromosomal aberration test. Mutagenesis. 1999;14:5–22. doi: 10.1093/mutage/14.1.5. [DOI] [PubMed] [Google Scholar]
- 22.Iso T., Watanabe T., Iwamoto T., Shimamoto A., Furuichi Y. DNA damage caused by bisphenol a and estradiol through estrogenic activity. Biol. Pharm. Bull. 2006;29:206–210. doi: 10.1248/bpb.29.206. [DOI] [PubMed] [Google Scholar]
- 23.Ivett J.L., Brown B.M., Rodgers C., Anderson B.E., Resnick M.A., Zeiger E. Chromosomal aberrations and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. IV. Results with 15 chemicals. Environ. Mol. Mutagen. 1989;14:165–187. doi: 10.1002/em.2850140306. [DOI] [PubMed] [Google Scholar]
- 24.Izzotti A., Kanitz S., D’Agostini F., Camoirano A., De Flora S. Formation of adducts by bisphenol A an endocrine disruptor, in DNA in vitro and in liver and mam-mary tissue of mice. Mutat. Res. 2009;679:28–32. doi: 10.1016/j.mrgentox.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y., Xia W., Zhu Y., Li X., Wang D., Liu J., Chang H., Li G., Xu B., Chen X., Xu Y.S. Mitochondrial dysfunction in early life resulted from perinatal bisphenol A exposure contributes to hepatic steatosis in rat offspring. Toxicol. Lett. 2014;228:85–92. doi: 10.1016/j.toxlet.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Johnson G.E., Parry E.M. Mechanistic investigations of low dose exposures to the genotoxic compounds Bisphenol-A and rotenone. Mutat. Res. 2008;651:56–63. doi: 10.1016/j.mrgentox.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Kabuto H., Hasuike S., Minagawa N., Shishibori T. Effects of bisphenol A on the active oxygen species in mouse tissues. Environ. Res. 2003;93:31–35. doi: 10.1016/s0013-9351(03)00062-8. [DOI] [PubMed] [Google Scholar]
- 28.Kolšek K., Mavri J., Sollner Dolenc M. Reactivity of bisphenol A-3, 4-quinone with DNA. A quantum chemical study. Toxicol. In Vitro. 2012;26:102–106. doi: 10.1016/j.tiv.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Kuch H.M., Ballschmiter K. Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC-(NCI)-MS in the pictogram per liter range. Environ. Sci. Technol. 2001;35:3201–3206. doi: 10.1021/es010034m. [DOI] [PubMed] [Google Scholar]
- 30.Kuiper G.G., Carlsson B., Grandien K., Enmark E., Haggblad J., Nilsson S., Gustafsson J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 31.Laws S.C., Carey S.A., Ferrel J.M., Bodman G.J., Copper R.L. Estrogenic activity of octylphenol, nonylphenol, bisphenol A, and methoxychlor in rats. Toxicol. Sci. 2000;54:154–167. doi: 10.1093/toxsci/54.1.154. [DOI] [PubMed] [Google Scholar]
- 32.Li Y.J., Song T.B., Cai Y.Y., Zhou J.S., Song X., Zhao X., Wu X.L. Bisphenol an exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol. Sci. 2009;108:427–436. doi: 10.1093/toxsci/kfp024. [DOI] [PubMed] [Google Scholar]
- 33.Lopes-Virella M.F., Stone P., Ellis S., Colwell J.A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem. 1977;23:882–884. [PubMed] [Google Scholar]
- 34.Makaji E., Raha S., Wade M.G. Effect of environmental contaminants on Beta cell function. Int. J. Toxicol. 2011;30:410–418. doi: 10.1177/1091581811405544. [DOI] [PubMed] [Google Scholar]
- 35.Markey C.M., Rubin B.S., Soto A.M., Sonnenschein C. Endocrine disruptors from wingspread to environmental developmental biology. J. Steroid Biochem. Mol. Biol. 2002;83:235–244. doi: 10.1016/s0960-0760(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 36.Melzer D., Rice N.E., Lewis C., Henley W.E., Galloway T.S. Association of uri-nary bisphenol a concentration with heart disease: evidence from NHANES2003/06. PLoS One. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naik P., Vijayalaxmi K.K. Cytogenetic evaluation for genotoxicity of Bisphenol-A in bone marrow cells of Swiss albino mice. Mutat. Res. 2009;676:106–112. doi: 10.1016/j.mrgentox.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Newbold R.R., Jefferson W.N., Padilla-Banks E. Prenatal exposure to bisphenol A at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ. Health Perspect. 2009;117:879–885. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peretz J., Gupta R.K., Singh J., Hernandez-Ochoa I., Flaws J.A. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol. Sci. 2011;119:209–217. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peretz J., Craig Z.R., Flaws J.A. Bisphenol A inhibits follicle growth and induces atre-sia in cultured mouse antral follicles independently of the genomic estrogenicpathway. Biol. Reprod. 2012;87:1–11. doi: 10.1095/biolreprod.112.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popa D.S., Bolfa P., Kiss B., Vlase L., Păltinean R., Pop A., Cătoi C., Crişan G., Loghin F. Influence of genista tinctoria l or methylparaben on subchronic toxicity of bisphenol a in rats. Biomed. Environ. Sci. 2014;27:85–96. doi: 10.3967/bes2014.021. [DOI] [PubMed] [Google Scholar]
- 42.Richter C.A., Birnbaum L.S., Farabollini F., Newbold R.R., Rubin B.S., Talsness C.E. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez H.A., Santambrosio N., Santamria C.G., Munoz-de-Toro M., Luque E.H. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod. Toxicol. 2010;30:550–557. doi: 10.1016/j.reprotox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Rubin B.S. Bisphenol A: an endocrine disruptor with widespread exposureand multiple effects. J. Steroid Biochem. Mol. Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Safe S. Bisphenol A and related endocrine disruptors. Toxicol. Sci. 2000;56:251–252. doi: 10.1093/toxsci/56.2.251. [DOI] [PubMed] [Google Scholar]
- 46.Sakuma S., Nakanishi M., Morinaga K., Fujitake M., Wada S., Fujimoto Y. Bisphenol A 3, 4-quinone induces the conversion of xanthine dehydrogenase into oxidase in vitro. Food Chem. Toxicol. 2010;48:2217–2222. doi: 10.1016/j.fct.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt J., Kotnik P., Trontelj J., Knez Z., Mašič L.P. Bioactivation of bisphenol A and its analogs (BPF, BPAF BPZ and DMBPA) in human liver .microsomes. Toxicol. In Vitro. 2013;27:1267–1276. doi: 10.1016/j.tiv.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt S., Degen G.H., Seibel J., Hertrampf T., Vollmer G., Diel P. Hormonal activity of combinations of genistein, bisphenol A and 17beta-estradiol in the female Wister rat. Arch. Toxicol. 2006;80:839–845. doi: 10.1007/s00204-006-0102-4. [DOI] [PubMed] [Google Scholar]
- 49.Schweikl H., Schmalz G., Rackebrandt K. The mutagenic activity of unpolymerized resin monomers in Salmonella typhimurium and V79 cells. Mutat. Res. 1998;415:119–130. doi: 10.1016/s1383-5718(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 50.Sengupta S., Obiorah I., Maximov P., Curpan R., Jordan V. Molecular mechanismof action of bisphenol and bisphenol A mediated by oestrogen receptor alphain growth and apoptosis of breast cancer cells. Br. J. Pharmacol. 2013;169:167–178. doi: 10.1111/bph.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma R., Yang Y., Sharma A., Awasthi S., Awasthi Y.C. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid. Redox Signaling. 2004;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- 52.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 53.Staples C.A., Dorn P.B., Klecka G.M., Block S.T., Harris L.R. A review of the environmental fate, effects, and exposure of bisphenol A. Chemosphere. 1998;36:2149–2173. doi: 10.1016/s0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- 54.Tarantino G., Valentino R., Di Somma C., D'Esposito V., Passaretti F., Pizza G., Brancato V., Orio F., Formisano P., Colao A., Savastano S. Bisphenol A in polycystic ovary syndrome and its association with liver-spleen axis. Clin. Endocrinol. 2013;78:447–453. doi: 10.1111/j.1365-2265.2012.04500.x. [DOI] [PubMed] [Google Scholar]
- 55.Tayama S., Nakagawa Y., Tayama K. Genotoxic effects of environmen-tal estrogen-like compounds in CHO-K1 cells. Mutat. Res. 2008;649:114–125. doi: 10.1016/j.mrgentox.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Teng C., Goodwin B., Shockley K., Xia M., Huang R., Norris J. Bisphenol-A affects androgen receptor function via multiple mechanisms. Chem. Biol. Interact. 2013;203:556–564. doi: 10.1016/j.cbi.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsutsui T., Tamura Y., Yagi E., Hasegawa K., Takahashi M., Maizumi N., Yamaguchi F., Barrett J.C. Bisphenol-A induces cellular transformation, aneuploidy and DNA adduct formation in cultured Syrian hamster embryo cells. Int. J. Cancer. 1998;75:290–294. doi: 10.1002/(sici)1097-0215(19980119)75:2<290::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 58.Ulutas O.K., Yildiz N., Durmaz E., Ahbab M.A., Ismet Çok N.B. An in vivo assessment of the genotoxic potential of bisphenol A and 4-tert-octylphenol in rats. Arch. Toxicol. 2011;85:995–1001. doi: 10.1007/s00204-010-0620-y. [DOI] [PubMed] [Google Scholar]
- 59.Vandenberg L.N., Maffini M.V., Sonnenschein C., Rubin B.S., Soto A.M. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vandenberg L.N., Hauser R., Marcus M., Olea N., Welshons W.V. Human exposure to bisphenol a (BPA) Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Vom Saal F.S., Hughes C. An extensive new literature concerning low-dose effects of bisphenol a shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yıldız N., Barlas N. Hepatic and renal functions in growing male rats after bisphenol A and octylphenol exposure. Hum. Exp. Toxicol. 2013;32:675–686. doi: 10.1177/0960327112464796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.