Abstract
Perfluoralkylated substances (PFAS) are classified as persistent, bioaccumulative and toxic substances and are widespread environmental contaminants. Humans are exposed through food, drinking water and air. We have previously reported that bisphenol A accelerates spontaneous diabetes development in non-obese diabetic (NOD) mice and observed in the present study that perfluoroundecanoic acid, PFUnDA, increased insulitis development, a prerequisite for diabetes development in NOD mice. We exposed NOD mice to PFUnDA in drinking water (3, 30 and 300 μg/l) at mating, during gestation and lactation and until 30 weeks of age. After 300 μg/l PFUnDA exposure, we report (i) increased pancreatic insulitis, (ii) increased number of apoptotic cells in pancreatic islets prior to insulitis and (iii) decreased phagocytosis in peritoneal macrophages. There was also a trend of decreased number of tissue resident macrophages in pancreatic islets prior to insulitis after exposure to 300 μg/l, and altered cytokine secretion in activated splenocytes after exposure to 3 μg/l PFUnDA. Although insulitis is a prerequisite for autoimmune diabetes, the accelerated insulitis was not associated with accelerated diabetes development. Instead, the incidence of diabetes tended to be reduced in the animals exposed to 3 and 30 μg/l PFUnDA, suggesting a non-monotonic dose response. The effects of PFUnDA exposure on increased apoptosis in pancreas and reduced macrophage function as well as accelerated insulitis development in NOD mice, may also be relevant for human insulitis. Further observational autoimmune diabetes clinical cohort studies and animal experiments for PFUnDA as well as other PFASs are therefore encouraged.
Keywords: Perfluoralkylated substances, PFUnDA, T1DM, Diabetes, NOD mice, Insulitis
1. Introduction
Development of type 1 Diabetes Mellitus (T1DM) involves autoimmune destruction of pancreatic beta-cells, leading to insulin deficiency, with a disease development at early age in genetically predisposed individuals. There is evidence for genetic predisposition, but the increased incidence of T1DM worldwide suggests a role of environmental factors in triggering disease development (reviewed in [7], [9]. Lack of specific nutrients during pregnancy (e.g. Vitamin D and long chain n-3 fatty acids) and viral infection during early life as well as exposure to N-nitroso compounds, air pollutants and persistent organic pollutants have been suggested to influence T1DM development in epidemiological studies [39], [61], [32]. There are however limited numbers of epidemiological studies investigating association between T1DM and environmental factors and causal experimental data is sparse [9].
Environmental toxins, as bafilomycin from Streptomyces infected vegetables, as well as the toxicant rodenticide Vacor, are reported to specifically decrease beta-cell function and to accelerate diabetes type 1 development in non-obese diabetic (NOD) mice, a model for T1DM [31], [44], illustrating that environmental chemicals can promote T1DM development in this animal model [9].
Further, we have in earlier studies shown that exposure to bisphenol A (BPA) accelerates spontaneous diabetes development in NOD mice [6], [7], [8]. One mechanism of this accelerated diabetes development due to BPA exposure was suggested to be reduced phagocytosis by macrophages [6], leading to increased number of apoptotic cells in the pancreas and thereby accelerated insulitis development. Impaired macrophage phagocytosis was also seen after in vitro BPA exposure in peritoneal macrophages from both non-exposed C57/Bl6 mice and Wistar rats (Friis Berntsen et al. manuscript submitted for publication). In that study, we also observed a reduced phagocytosis in isolated mouse and rat peritoneal macrophages after in vitro exposure to the perfluorinated compound perfluoroundecanoic acid (PFUnDA), to a similar degree as observed after BPA exposure. Other perfluoroalkyl and polyfluoroalkyl substances (PFASs) like PFOS, PFNA and PFDA did not impair macrophage function to the same extent Reduced macrophage phagocytosis is one of several mechanisms in T1DM development in NOD mice [48]. Thus, we hypothesized that exposure to PFUnDA, via impaired macrophage function, will accelerate insulitis and T1DM in the NOD mouse model.
PFASs are substances with lipid- and water-repelling properties and are used in a wide range of consumer and industrial products including non-stick, stain-repellent, water-repellent, and fire-retardant coatings [41]. PFASs are classified as persistent, bio accumulative and toxic substances. Humans are exposed through intake of marine food and game, but also via air and dust from the indoor environment [30]. PFASs have been detected in human serum, breast milk and adipose tissue in more than 98% of the analysed samples. The previously most used PFASs, like perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) have due to their known toxicity now widely been replaced in many consumer products with PFASs with longer carbon-chains, like PFUnDA. A longer fluorinated carbon chain has however been associated with increased toxicity [24], [37], [49]. The increased use of PFUnDA is accompanied by an increased PFUnDA serum level, with an increase (8%) in human sera in Northern Norway between 2001 and 2007 [46]. Typical mean human serum levels reported for PFUnDA are 0.1–0.4 ng/ml and maximal levels 0.70–1.4 ng/ml, compared to 11–23 ng/ml and 3–9 ng/ml for maximal PFOS and PFOA serum levels respectively [11], [25], [36], [46], [52], [58], [62].
Epidemiologic studies report immunosuppressive effects of PFAS, measured as associations between PFAS serum levels and reduced vaccine response and increased risk of infections in early childhood [26], [27]. Although the evidence from epidemiologic studies regarding the association between PFAS exposure and asthma and allergy is inconsistent, the available data suggest that PFAS exposure is associated with immunotoxic effects [2], [3], [17], [27], [33], [50], [59], [64]. Studies of immunotoxic effects of PFASs related to autoimmunity are, however, sparse, but recently, higher PFOS serum levels have been reported in T1DM patients compared to controls [54]. The aim of the present study was to investigate the effect of one selected PFAS, PFUnDA, on early stages of T1DM development in the NOD mouse model. PFUnDA was chosen since it is one of the PFASs with rising exposure levels [46], and since it was shown to impair macrophage function in our in vitro systems.
2. Material and methods
2.1. Mice and exposure conditions
Female (94) and male (46) NOD/ShiLtJ mice from Jackson Laboratory (Maine, USA) were used for breeding at 8 and 10 weeks of age, respectively. The female mice were randomized into 4 groups that were exposed through drinking water from the time of mating and throughout the life time of the offspring. The mating period was set to one week with vaginal plug-check twice a day and exchange of males if no vaginal plug was detected during the first two days. The males were euthanized after the mating period of one week. The 4 exposure groups included: (1) negative control (autoclaved water only), (2) PFUnDA3 μg/ml (CAS: 2058-94-8, >96% purity, Santa Cruz Biotechnology, Dallas, US), (3) PFUnDA 30 μg/ml and (4) PFUnDA 300 μg/ml. The exposure doses correspond to about 0.417, 4.17 and 41.7 μg/kg bw/day (calculated based on mean mouse weight of 23 g and mean measured volume of drinking water consumption of 3.2 ml/day at 10 weeks of age). The lowest exposure level of PFUnDA was chosen at a dose corresponding to 3 times the TDI (tolerable daily intake) for PFOS (0.15 μg/kg bw/day as set by EFSA [18]), since there is no TDI or estimated intake available for PFUnDA. Estimated total intake of PFOS and PFOA is reported to be between 0.71–2 and 13–83 ng/kg bw/day respectively in adults, while calculated levels in infants ranges between 9.4–26 and 13–83 ng/kg bw/day respectively for PFOS and PFOA [23], [30]. The lowest exposure dose chosen for PFUnDA to NOD mice in this study is in the range of human environmental exposure, about five times higher than the maximal calculated intake of PFOA in human infants.
Only female offspring were selected at the time of weaning, since insulitis and diabetes development is most prevalent in female mice [35]. The perfluorinated substance PFUnDA was dissolved in deionized autoclaved water heated to 60 °C. Controls received similar water without PFUnDA. The water bottle was changed once every second week and filled up once a week. The mice had ad libitum access to feed (Harlan Teklad 2919 irradiated) and water and were exposed to a 12 h light/12 h dark cycle and 35–75% humidity. To keep the dams as the statistical unit, female siblings from each dam were separated and divided into two sub-groups, 4–5 mice per cage; (i) short term exposure for histological examination, splenocyte and peritoneal macrophage function and (ii) long time exposure for diabetes incidence surveillance. The short term exposure group was divided into groups with exposure until 7 and 11 weeks of age, used for histological examination of pancreas and collection of splenocytes and peritoneal macrophages. All experiments were performed in conformity with the laws and regulations for experiments with live animals in Norway and were approved by the local representative of the Norwegian Animal Research Authority (FOTS number 6687).
2.2. Serum glucose measurements
Serum glucose was monitored weekly (from 6 to 30 weeks of age) in female offspring in the long term exposure group in blood sample from vena saphena using an Accu-Check Aviva blood glucose meter (Roche Diagnostics GmbH, Mannheim, Germany). Animals with two subsequent (with 24 h interval) glucose measurements at or above 13.9 mmol/l were considered diabetic, and were euthanized.
2.3. Histological evaluation
For histological evaluation, pancreas were collected from 8 mice (at 7 and 11 weeks of age respectively), fixed in formalin, embedded in paraffin and processed as previously described before hematoxylin and eosin staining [8]. For each mouse 6 sections at different depth of the pancreas were examined and all islets present in the sections (10–15 islets/section) were graded for insulitis according to the area of an islet infiltrated by lymphocytes. 0% infiltration = grade 0, periinsulitis and up to 10% infiltration = grade 1, 10–49% infiltration = grade 2, 50–74% infiltration = grade 3 and 75–100% infiltration = grade 4, as illustrated previously in [8]. For each section, an overall grade was assigned which correlated to the highest grade detected in at least 3 islets, since all pancreas samples studied had islets with all insulitis grades represented. Then, the final grade for each pancreas/mouse was set to the highest grade determined for the 6 analysed sections. The mean insulitis grade for each exposure group corresponds to the mean of the final grade for each pancreas/mouse.
Sections of the formalin fixed pancreas were also stained over-night with antibodies towards F4/80 (tissue resident macrophages, AbD Serotec, Oxford, UK, 1:50) and active caspase-3 (apoptotic cells, Cell Signalling Technology, Beverly, MA, USA, 1:400), as previously described [8]. For each antibody staining and insulitis grade, the number of positive cells per islet was counted in two pancreatic sections per mouse. The counts per islet were compared between the exposure groups according to the insulitis grade of each islet. Since most female NOD mice spontaneously develop insulitis with age, the pre-insulitis islets are expected to give most information on early accelerating effects, thus results from islets with grade 0 are presented.
2.4. Phagocytic function of peritoneal macrophages
Peritoneal macrophages were collected from mice at 7 and 11 weeks (n = 8 for both time points) by peritoneal lavage with in total 6 ml PBS as previously described [6]. The cell suspension was centrifuged at 250 × g for 10 min and erythrocytes were removed by dissolving the cell pellet in 0.2% NaCl on ice for 4 min. The cell number was determined by a cell counter (Coulter Z1, Beckman Coulter Corporation, Miami, Florida) and cells were seeded at 2 × 106 cells/ml in 48 wells plates with RPMI cell culture medium (Biowest, Nuaillé, France). After 1 h in culture, 85–95% of the attached cells are assumed to be macrophages [55]. Then the medium with unattached cells was discarded, replaced with fresh medium, and the cells were incubated over night to assess viable cells. To analyse the phagocytic function, FITC-labelled Zymosan particles were added at a ratio of 20 particles per cell (Invitrogen Life Technologies, Carlsbad, California, United States) and incubated for 30 min at 37 °C. The cells were carefully washed twice in PBS and incubated with Accutase (In vitrogen Life Technologies) for at least 15 min to remove particles from the cell surfaces and to detach cells. Thereafter, the cells were washed twice with PBS, fixed in 0.2% paraformaldehyde in RPMI 1640 phenol red-free cell culture medium (Biowest) and analysed for phagocytic activity (FITC intensity per cell) by flow cytometry (LSR II, BD Bioscience, Franklin Lakes, NJ, USA).
2.5. Cytokine secretion from ex vivo stimulated splenocytes
Splenocytes were isolated from 7 and 11 weeks old mice (n = 8) as previously described [7]. Then, cytokine secretion upon ex vivo stimulation with LPS or Concanavalin A (ConA) was determined for all exposure groups, to examine possible effects on systemic immune functions, as described previously [8], [29]. Stimulation with LPS for 48 h activates B-cells and macrophages (10 μg/ml, Sigma Aldrich, GmbH Steinheim, Germany), while ConA stimulation for 48 h activates T-cells (50 μg/ml, Sigma Aldrich). The cytokine levels (INFγ, TNFα, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-13 and IL-17) in the supernatants from stimulated splenocytes were determined with cytometric bead array (FlexSet, BD Bioscience) analysed on a LSRII flow cytometer (BD Bioscience).
2.6. Statistical analysis
Data are presented as group means ± standard errors of the mean (SEM). Differences in diabetes incidence between exposure groups were investigated by Cox regression analysis, while all other data sets were analysed by one-way analysis of variance (ANOVA), using SigmaPlot13.0 software. The Holm–Sidak post hoc test was performed to evaluate significant differences between the exposure groups. For all analyses, p-values <0.05 were considered statistically significant.
3. Results
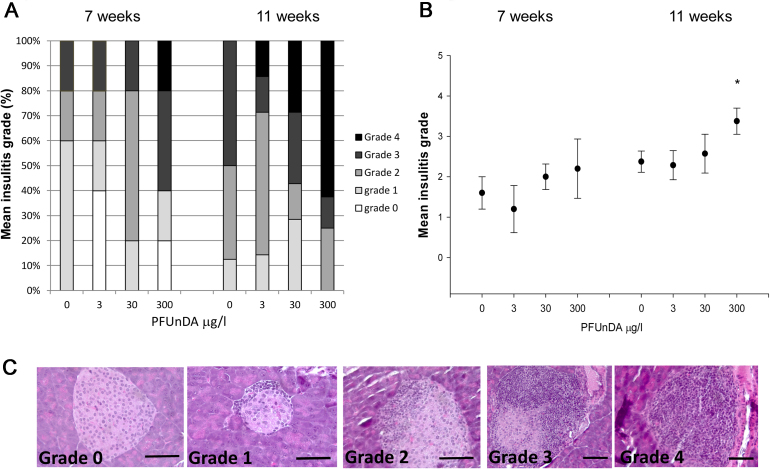
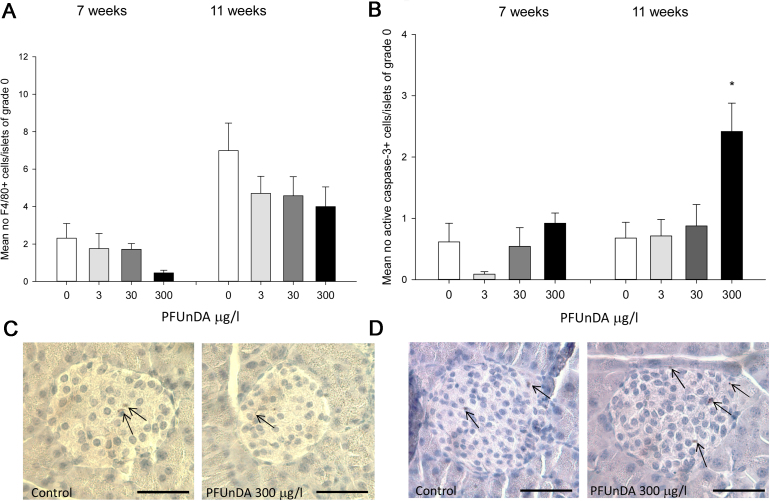
The litter size and sex ratios were not different between the exposure groups (data not shown). Analysis of early signs of diabetes development in the NOD mice, expressed as alteration in pancreatic histology at 7 and 11 weeks of age, revealed an increased insulitis grade after high PFUnDA exposure (Fig. 1). This was significant at 11 weeks of age, and a trend was also observed at 7 weeks of age. As expected due to the spontaneous T1DM development in NOD mice, the degree of insulitis was increased from 7 to 11 weeks age, in all treatment groups. Further, immunohistological analysis of the number of tissue resident macrophages in pancreatic islets prior to insulitis (in islets with insulitis grade 0) indicated a decreased number of F4/80+ macrophages with increasing PFUnDA exposure at both 7 and 11 weeks of age, although it did not reach statistical significance (Fig. 2a). The number of apoptotic, active caspase-3 positive cells, in pancreatic islets was significantly increased prior to insulitis in pancreas at 11 weeks of age due to high PFUnDA exposure (Fig. 2b).
Fig 1.
Insulitis grade in pancreas from female NOD mice after PFUnDA exposure (0, 3, 30 and 300 μg/ml in drinking water) starting from before gestation and continuing until termination at 7 and 11 weeks of age. (A) Formalin fixed pancreas were sectioned and stained with hematoxylin and eosin and evaluated for severity of infiltrating lymphocytes using light microscopy (n = 8). Grade 0 = no infiltration, score 1 = periinsulitis to 10% infiltration, grade 2 = 10–50% infiltration, grade 3 = 50–75% infiltration and grade 4 = 75–100% infiltration. The figure shows the proportion of the animals with the respective insulitis grades within the four exposure groups. (B) Average insulitis grade in NOD mouse pancreas at 7 and 11 weeks of age (n = 8), presented as group mean ± SEM. (C) Representative histological sections of hematoxylin and eosin-stained pancreatic islets showing insulitis grade 0–4. Scale bar indicates 40 μm. *Indicate significant difference from the control group, p < 0.05.
Fig. 2.
Histological changes in pancreatic islets after exposure to 0, 3, 30 and 300 μg/ml PFUnDA in drinking water, starting from before gestation and continuing until termination (A) Mean number of tissue resident macrophages (F4/80 positive cells) in pancreatic islets prior to insulitis (grade 0) from 7 and 11 weeks old female NOD mice (group mean ± SEM, n = 8). (B) Mean number of active caspase-3 positive cells in pancreatic islets with insulitis grade 0 from 7 and 11 weeks old female NOD mice after PFUnDA exposure (group mean ± SEM, n = 8). *Indicates significant difference from the control group p < 0.05. C) Representative pancreatic islets immunostained for tissue resident F4/80 positive macrophages from control and PFUnDA 300 μg/l exposed mice and apoptotic active caspase-3 positive cells respectively (D). Scale bar indicates 40 μm.
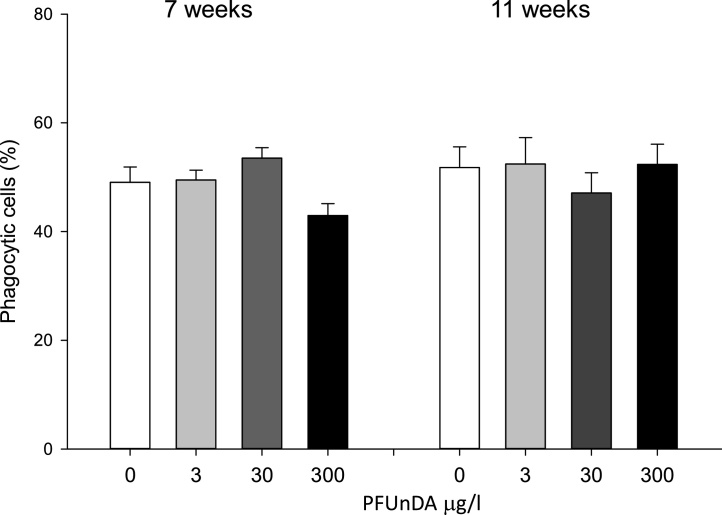
Phagocytic activity in isolated peritoneal macrophages from animals after in vivo PFUnDA exposure was reduced, although not significantly, in the highest exposure group at 7 weeks of age, compared to the control, with 42.9 ± 4.4% and 49.1 ± 5.6% phagocytic cells respectively (Fig. 3).
Fig. 3.
Peritoneal macrophage phagocytosis of FITC-conjugated Zymosan beads at 7 and 11 weeks of age in female NOD mice after PFUnDA exposure (0, 3, 30 and 300 μg/ml in drinking water), % phagocytic macrophages determined by flow cytometry (group mean ± SEM, n = 8).
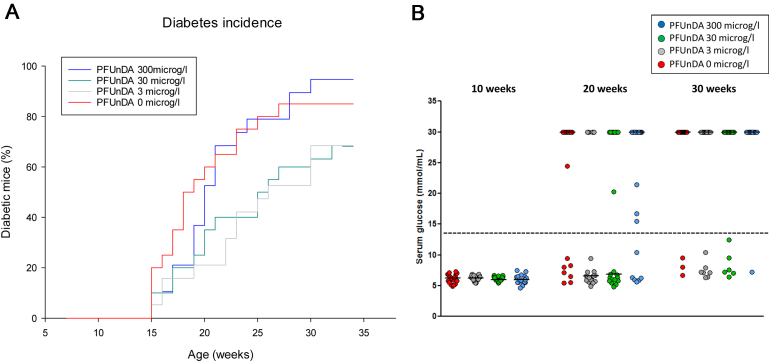
Diabetes incidence cumulatively increased from 15 weeks of age. It was not significantly different between the exposure groups, although the low and intermediate exposures seemed to delay the spontaneous diabetes development (Fig. 4). The blood glucose levels for the NOD animals were low (typically between 5 and 7 mmol/l) until week 15 when diabetes onset started to appear. Most often, the individual levels changed from normal values to above 13.9 (diabetes diagnosis) within a week or two. This fast rise in glucose level explains the relatively few animals with an intermediate glucose level (at week 20 and 30; Fig. 4B) and the small change in the group median values unless the majority of the animals were displaying diabetes. Early signs of diabetes are decreased body weight, polyuria and increased water intake [68]. The body weight and water intake/mouse/day analysed at 10 and 20 weeks of age did not differ between the exposure groups (data not shown).
Fig. 4.
Spontaneous cumulative diabetes incidence, shown as percentage of diabetic female NOD mice exposed to PFUnDA in the drinking water from before gestation and throughout life (n = 25), panel A. Diabetes was diagnosed when the blood glucose levels was above 13.9 mmol/l. Glucose levels at 10, 20 and 30 weeks of age for all exposure groups are presented in panel B. The values at 30 mmol/l represents mice already terminated due to diabetes diagnosis. The dotted line represents diabetes diagnosis. The red line/dots represents animals receiving 0 μg/l PFUnDA exposure, the grey line/dots 3 μg/l PFUnDA exposed mice, the green line/dots 30 μg/l PFUnDA and blue line/dots 300 μg/l PFUnDA exposure group.
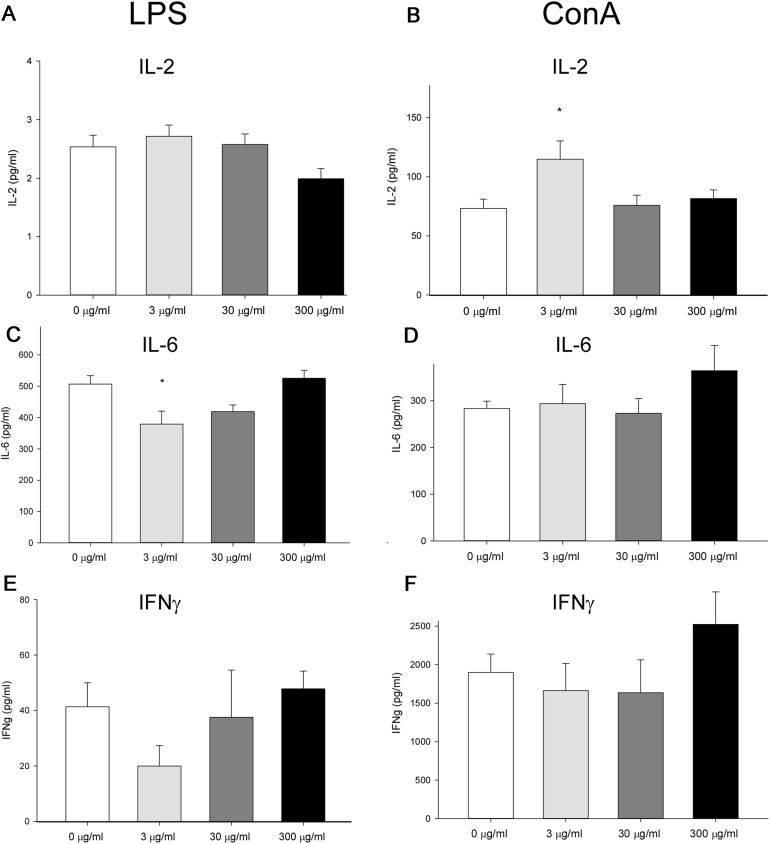
Ex vivo in vitro LPS and ConA-induced cytokine secretion in isolated splenocytes revealed significant differences between the exposure groups (Fig. 5), seen as increased ConA-induced IL2- secretion and reduced LPS-induced IL-6 secretion upon the lowest in vivo PFUnDA exposure. There was also a trend of increased ConA-induced IL-6 and IFNγ secretion from splenocytes from animals with the highest in vivo PFUnDA exposure. There were no significant effects of PFUnDA exposure on the induced secretion of TNFα, IL-1β, IL-4, IL-10, IL-13 and IL-17 (data not shown).
Fig. 5.
In vitro cytokine release from splenocytes isolated from female 11 weeks old NOD mice, exposed to PFUnDA (0, 3, 30 and 300 μg/ml in drinking water) from before gestation and until termination at 11 weeks of age (group mean ± SEM, n = 8). LPS-induced cytokine release is shown in A, C and D and ConA-induced cytokine release is shown in B, D and F (all cultures stimulated for 48 h). *Indicates significant difference from the control group, p < 0.05.
4. Discussion
In this study, peroral PFUnDA exposure during gestation, lactation and throughout life increased pancreatic insulitis, impaired peritoneal macrophage phagocytosis and altered cytokine secretion in splenocytes, mechanisms involved in type 1 diabetes development in NOD mice, but did not increase the incidence of diabetes.
4.1. Insulitis development
The accelerated insulitis development due to high PFUnDA exposure in NOD mice was shown to be associated with a reduced number and/or function of tissue resident macrophages in pancreatic islets prior to insulitis, which may lead to an increased number of apoptotic cells and autoantigens present for autoimmune T-cell activation. These effects in the pancreas showed an apparent dose-response relationship. Insulitis has been reported in the NOD mouse model, as early as at 4 weeks of age [5]. It has been shown that severe insulitis leads to T1DM development via infiltrating dendritic cells that activate autoreactive T-cells subsequently causing reduced beta-cell number for sufficient insulin production [56].
Impaired macrophage phagocytosis and reduced clearance of apoptotic cells is one mechanism contributing to insulitis development [47]. We have previously identified the apoptotic cells in pancreatic islets prior to insulitis as beta-cells, alpha-cells and tissue resident macrophages [8]. The presently observed increase in apoptotic cells in pancreatic islets prior to insulitis could be a result of a lower number or reduced phagocytic function of tissue resident macrophages, or direct cytotoxicity of PFUnDA exposure. In fact, we did observe an apparent reduction in the number of tissue resident macrophages in pancreatic islets prior to insulitis, and impaired phagocytic function in peritoneal macrophages at 7 weeks of age after PFuNDA exposure. The same trend was not seen for macrophage function at 11 weeks of age, consistent with previous data on BPA exposure showing that an accelerated diabetes incidence was associated with reduced phagocytosis function in peritoneal macrophages from mice at 7, but not 11 weeks of age [6]. It has been reported that the majority of the murine peritoneal macrophages have high expression of F4/80 [12], a similarity in phenotype to tissue resident macrophages in pancreatic islets. Decreased phagocytic function in peritoneal macrophages may therefore be a good surrogate marker for a reduced phagocytic function also in macrophages in the pancreas, a mechanism associated with T1DM development that may be similar between NOD mice and humans.
4.2. Diabetes development
Increased insulitis grade at 11 weeks of age in female NOD mice has previously been associated with an accelerated diabetes development [6], [7], [8] via infiltrating dendritic cells that activate autoreactive T-cells subsequently causing reduced beta-cell number for sufficient insulin production [56]. Surprisingly, the accelerated insulitis in the present study due to the highest PFUnDA exposure was not accompanied with an accelerated diabetes development. The low and intermediate PFUnDA exposure doses even seemed to have a delaying effect on the diabetes development, although not statistically significant. This may be explained by different modes of action of PFASs (as discussed below) including mechanisms contributing to lowering the glucose levels at lower PFUnDA doses. The PFuNDA exposure levels for humans are on a rise [46] and the doses used in this study is regarded to be within a relevant range, starting from 3 times higher than the tolerable daily intake (TDI) available for PFOS and with maximal reported PFUnDA serum levels of about 4–11% of the PFOS levels [25], [36], [52].
It has been shown that severe insulitis leads to T1DM development via infiltrating dendritic cells that activate autoreactive T-cells subsequently causing reduced beta-cell number for sufficient insulin production. The molecular mechanisms involved in previously reported the immunotoxic effects of PFASs has been hard to establish [34]. However, our results from the T1DM mouse model is important in light of the recently reported epidemiological observation that PFOS serum levels were elevated in T1DM patients compared to controls [54] and the similarities in histology and immunity of disease development between human and NOD mice [63].
4.3. Cytokine secretion
In addition, PFUnDA treatment also showed different effects on IL-2, IL-6 and IFNγ cytokine secretion from splenocytes at low compared to high PFUnDA exposure doses. PFUnDA induced an increase in ConA-induced IL-2 secretion and decreased LPS-induced IL-6 secretion at the low dose and an apparent increase in ConA-induced IL-6 and IFNγ secretion at the high dose (also discussed below). Higher IFNγ secretion in pancreatic islet infiltrates in female compared to male NOD mice is reported by others and this enhancement of Th1 immune response was suggested to be responsible for the higher T1DM incidence always seen in female compared to male NOD mice in spite of similar degree of insulitis [5], [57]. The present observed differences in cytokine secretion at different exposure doses could indicate a non-monotonic dose response, with different receptor activation or different receptors being activated at different exposure levels. A similar response has been suggested after BPA exposure inducing insulin release in beta-cells via estrogen receptors at high exposure levels and estrogen like-receptor at lower doses [1], [8], [45]. Activation of estrogen receptors in the NOD mouse has been reported to reduce the spontaneous diabetes incidence [4], [14], [21]. PFASs can activate peroxisome proliferator-activated receptor alpha, PPARα, constitutive androstane receptor (CAR) and the pregnane receptor (PXR). Binding of these receptors have been shown to result in different cellular responses, such as cellular stress, toxicity, induction of hepatic CYP2B enzymes, epigenetic changes and cytokine or insulin secretion, inhibition of tight junctional intercellular communication or alterations in circulatory miRNA [16], [19], [66]. It has also been reported that PFOS exposure modulates calcium homeostasis in the spleen, leading to induction of cytotoxic T-cells [42], cells that are involved in driving autoimmune reactions.
PFAS exposure of human leukocytes in vitro has been reported to increase IL-6 and TNFα cytokine secretion [10], [15], whereas after an in vivo challenge IL-6 production by B cells was decreased in B6C3F1mice [20]. Our results are in line with these in vivo results, showing decreased LPS-induced IL-6 secretion from isolated splenocytes at the low dose in vivo PFUnDA exposure. PFAS in vivo exposure has also been reported to reduce secretion of Th1 cytokines (IL-2 and INFγ) in favour of Th2 cytokines (IL-4, IL-10) in C57BL/6 mice [67]. However, our finding of increased ConA-induced IL-2 cytokine secreted from isolated splenocytes after low in vivo PFUnDA exposure is contrary to that report. These differences may be related to varying receptor activation between different mouse strains [13]. However, IL-2 is important for the homeostasis of regulatory T-cells (Tregs) [43], and the observed increase in IL-2 signal could contribute to expand the Treg pool. Likewise, lowered IL-6 signalling would favour Treg differentiation, since IL-6 influence the balance between Treg/Th17cells favouring the differentiation of Th17 cells [22], [38]. Thus, we can speculate that the observed reduction in IL-6 and increase in IL-2, via Treg upregulation could have contributed to a protective effect on diabetes development in the NOD mouse, as seen after low dose PFUnDA exposure.
Only a few studies have been performed with regard to effects and mechanisms of PFUnDA. Higher maternal serum level of PFUnDA has been associated with decreased maternal and cord blood thyroid hormones [65] and alterations in thyroid hormone homeostasis during fetal development may affect fetal growth, neuronal development and glycaemic control [28]. In hypothyroidism a reduced rate of liver glucose production is observed and accounts for a decrease in insulin requirement [51]. If PFUnDA exposure results in altered thyroid hormone homeostasis and potentially decreased insulin requirement, this may be one mechanism explaining how PFUnDA exposure did reduce diabetes development in the low and medium dosed NOD mice, although with possible counteracting immunotoxic effects, like increased insulitis, at higher exposure doses. In humans, imbalance in thyroid homeostasis is often seen in T1DM patients (hyper-as well as hypothyroidism) [60]. Further, prenatal PFUnDA-exposure was reported to be associated with lower birth weight [40] and an upregulation of immunomodulatory genes were associated with a range of prenatal PFASs exposure [53].
In conclusion, we have shown that PFUnDA exposure during gestation, lactation and early life accelerates pancreatic insulitis development, decreases peritoneal macrophage phagocytosis and alters splenocyte cytokine secretion in a mouse model of T1DM. This may suggest that PFUnDA also have similar effects on insulitis development in humans and possible consequences for human T1DM development. Therefore, further epidemiological investigations of associations between PFUnDA and other PFAS exposures and T1DM development are encouraged.
Acknowledgements
This project was funded by the Norwegian Research Council, project number 213060 and the Norwegian Institute of Public Health, Norway. We would like to acknowledge the animal care takers Trude Olsen and Kari Gulbrandsen Løken and the technicians Bodil Hasseltvedt, Asti Grestad and Berit Stensby at the Norwegian Institute of Public Health, Norway.
References
- 1.Alonso-Magdalena P., Ropero A.B., Soriano S. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol. Cell. Endocrinol. 2012;355(2):201–207. doi: 10.1016/j.mce.2011.12.012. S0303-7207(11)00738-6 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Anderson-Mahoney P., Kotlerman J., Takhar H., Gray D., Dahlgren J. Self-reported health effects among community residents exposed to perfluorooctanoate. New solutions: J. Environ. Occup. Health Policy: NS. 2008;18(2):129–143. doi: 10.2190/NS.18.2.d. [DOI] [PubMed] [Google Scholar]
- 3.Ashley-Martin J., Dodds L., Levy A.R., Platt R.W., Marshall J.S., Arbuckle T.E. Prenatal exposure to phthalates, bisphenol A and perfluoroalkyl substances and cord blood levels of IgE, TSLP and IL-33. Environ. Res. 2015;140:360–368. doi: 10.1016/j.envres.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Atwater I., Gondos B., DiBartolomeo R., Bazaes R., Jovanovic L. Pregnancy hormones prevent diabetes and reduce lymphocytic infiltration of islets in the NOD mouse. Ann. Clin. Lab. Sci. 2002;32(1):87–92. [PubMed] [Google Scholar]
- 5.Bao M., Yang Y., Jun H.S., Yoon J.W. Molecular mechanisms for gender differences in susceptibility to T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol. 2002;168(10):5369–5375. doi: 10.4049/jimmunol.168.10.5369. [DOI] [PubMed] [Google Scholar]
- 6.Bodin J.B.A., Wendt A., Eliasson L., Becher R., Kuper F., Løvik M., Nygaard U.C. Exposure to bisphenol A, but not phthalates, increases spontaneous diabetes type 1 development in NOD mice. Toxicol. Rep. 2015;2 doi: 10.1016/j.toxrep.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodin J., Bolling A.K., Becher R., Kuper F., Lovik M., Nygaard U.C. Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice. Toxicol. Sci. 2014;137(2):311–323. doi: 10.1093/toxsci/kft242. kft242 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Bodin J., Bolling A.K., Samuelsen M., Becher R., Lovik M., Nygaard U.C. Long-term bisphenol A exposure accelerates insulitis development in diabetes-prone NOD mice. Immunopharmacol. Immunotoxicol. 2013 doi: 10.3109/08923973.2013.772195. [DOI] [PubMed] [Google Scholar]
- 9.Bodin J., Stene L.C., Nygaard U.C. Can exposure to environmental chemicals increase the risk of diabetes type development? BioMed Res. Int. 2014:1–19. doi: 10.1155/2015/208947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brieger A., Bienefeld N., Hasan R., Goerlich R., Haase H. Impact of perfluorooctanesulfonate and perfluorooctanoic acid on human peripheral leukocytes. Toxicol. In Vitro. 2011;25(4):960–968. doi: 10.1016/j.tiv.2011.03.005. S0887-2333(11)00062-2 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Calafat A.M., Wong L.Y., Kuklenyik Z., Reidy J.A., Needham L.L. Polyfluoroalkyl chemicals in the U.S. population: data from the national health and nutrition examination survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007;115(11):1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassado Ados A., D'Imperio Lima M.R., Bortoluci K.R. Revisiting mouse peritoneal macrophages: heterogeneity, development, and function. Front. Immunol. 2015;6:225. doi: 10.3389/fimmu.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang E.T., Adami H.O., Boffetta P., Wedner H.J., Mandel J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit. Rev. Toxicol. 2016:1–53. doi: 10.3109/10408444.2015.1122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi M.S., Jung U.J., Yeo J., Kim M.J., Lee M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008;24(1):74–81. doi: 10.1002/dmrr.780. [DOI] [PubMed] [Google Scholar]
- 15.Corsini E., Avogadro A., Galbiati V. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs) Toxicol. Appl. Pharmacol. 2011;250(2):108–116. doi: 10.1016/j.taap.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Corton J.C., Cunningham M.L., Hummer B.T. Mode of action framework analysis for receptor-mediated toxicity: the peroxisome proliferator-activated receptor alpha (PPARalpha) as a case study. Crit. Rev. Toxicol. 2014;44(1):1–49. doi: 10.3109/10408444.2013.835784. [DOI] [PubMed] [Google Scholar]
- 17.Dong G.H., Tung K.Y., Tsai C.H. Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of Taiwanese children. Environ. Health Perspect. 2013;121(4):507–513. doi: 10.1289/ehp.1205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EFSA Perfluoroalkylated substances in food: occurrence and dietary exposure. EFSA J. 2012;10:55. [Google Scholar]
- 19.Elcombe C.R., Peffer R.C., Wolf D.C. Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: a case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit. Rev. Toxicol. 2014;44(1):64–82. doi: 10.3109/10408444.2013.835786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fair P.A., Driscoll E., Mollenhauer M.A. Effects of environmentally-relevant levels of perfluorooctane sulfonate on clinical parameters and immunological functions in B6C3F1 mice. J. Immunotoxicol. 2011;8(1):17–29. doi: 10.3109/1547691X.2010.527868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu Z., Zhang W., Zhen W. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151(7):3026–3037. doi: 10.1210/en.2009-1294. en. 2009–1294 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao W., Thompson L., Zhou Q. Treg versus Th17 lymphocyte lineages are cross-regulated by LIF versus IL-6. Cell Cycle (Georgetown, Tex) 2009;8(9):1444–1450. doi: 10.4161/cc.8.9.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebbink W.A., Glynn A., Darnerud P.O., Berger U. Perfluoroalkyl acids and their precursors in Swedish food: the relative importance of direct and indirect dietary exposure. Environ. Pollut. (Barking, Essex: 1987) 2015;198:108–115. doi: 10.1016/j.envpol.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Goecke-Flora C.M., Reo N.V. Influence of carbon chain length on the hepatic effects of perfluorinated fatty acids. A 19F- and 31P-NMR investigation. Chem. Res. Toxicol. 1996;9(4):689–695. doi: 10.1021/tx950217k. [DOI] [PubMed] [Google Scholar]
- 25.Goralczyk K., Pachocki K.A., Hernik A. Perfluorinated chemicals in blood serum of inhabitants in central Poland in relation to gender and age. Sci. Total Environ. 2015;532:548–555. doi: 10.1016/j.scitotenv.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 26.Grandjean P., Andersen E.W., Budtz-Jorgensen E. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307(4):391–397. doi: 10.1001/jama.2011.2034. 307/4/391 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granum B., Haug L.S., Namork E. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunotoxicol. 2013;10(4):373–379. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- 28.Hage M., Zantout M.S., Azar S.T. Thyroid disorders and diabetes mellitus. J. Thyroid Res. 2011;2011:439463. doi: 10.4061/2011/439463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen J.S., Alberg T., Rasmussen H., Lovik M., Nygaard U.C. Determinants of experimental allergic responses: interactions between allergen dose, sex and age. Scand. J. Immunol. 2011;73(6):554–567. doi: 10.1111/j.1365-3083.2011.02529.x. [DOI] [PubMed] [Google Scholar]
- 30.Haug L.S., Huber S., Becher G., Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds-comparing exposure estimates with biomarkers of exposure. Environ. Int. 2011;37(4):687–693. doi: 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Hettiarachchi K.D., Zimmet P.Z., Myers M.A. Transplacental exposure to bafilomycin disrupts pancreatic islet organogenesis and accelerates diabetes onset in NOD mice. J. Autoimmun. 2004;22(4):287–296. doi: 10.1016/j.jaut.2004.02.004. S0896841104000204 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Howard S.G., Lee D.H. What is the role of human contamination by environmental chemicals in the development of type 1 diabetes? J. Epidemiol. Community Health. 2012;66(6):479–481. doi: 10.1136/jech.2011.133694. [DOI] [PubMed] [Google Scholar]
- 33.Humblet O., Diaz-Ramirez L.G., Balmes J.R., Pinney S.M., Hiatt R.A. Perfluoroalkyl chemicals and asthma among children 12–19 years of age: NHANES (1999–2008) Environ. Health Perspect. 2014:12–19. doi: 10.1289/ehp.1306606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keil D.E. Immunotoxicity of perfluoroalkylated compounds. In: DeWitt J., editor. Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. Springer Molecular and Integrative Toxicology; 2015. pp. 239–248. [Google Scholar]
- 35.Kelemen K. The role of T cells in beta cell damage in NOD mice and humans. Adv. Exp. Med. Biol. 2004;552:117–128. [PubMed] [Google Scholar]
- 36.Kim D.H., Kim U.J., Kim H.Y., Choi S.D., Oh J.E. Perfluoroalkyl substances in serum from South Korean infants with congenital hypothyroidism and healthy infants-its relationship with thyroid hormones. Environ. Res. 2016;147:399–404. doi: 10.1016/j.envres.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 37.Kim M., Son J., Park M.S. In vivo evaluation and comparison of developmental toxicity and teratogenicity of perfluoroalkyl compounds using Xenopus embryos. Chemosphere. 2013;93(6):1153–1160. doi: 10.1016/j.chemosphere.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 38.Kimura A., Kishimoto T. Th17 cells in inflammation. Int. Immunopharmacol. 2011;11(3):319–322. doi: 10.1016/j.intimp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Knip M., Simell O. Environmental triggers of type 1 diabetes. Cold Spring Harbor Perspect. Med. 2012;2(7):a007690. doi: 10.1101/cshperspect.a007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon E.J., Shin J.S., Kim B.M. Prenatal exposure to perfluorinated compounds affects birth weight through GSTM1 polymorphism. J. Occup. Environ. Med. Am. Coll. Occup. Environ. Med. 2016;58(6):e198–e205. doi: 10.1097/JOM.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 41.Lehmler H.J. Synthesis of environmentally relevant fluorinated surfactants—a review. Chemosphere. 2005;58(11):1471–1496. doi: 10.1016/j.chemosphere.2004.11.078. [DOI] [PubMed] [Google Scholar]
- 42.Lv Q.Y., Wan B., Guo L.H., Yang Y., Ren X.M., Zhang H. In vivo immunotoxicity of perfluorooctane sulfonate in BALB/c mice: identification of T-cell receptor and calcium-mediated signaling pathway disruption through gene expression profiling of the spleen. Chem. Biol. Interact. 2015;240:84–93. doi: 10.1016/j.cbi.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Malek T.R., Bayer A.L. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 2004;4(9):665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 44.Myers M.A., Hettiarachchi K.D., Ludeman J.P., Wilson A.J., Wilson C.R., Zimmet P.Z. Dietary microbial toxins and type 1 diabetes. Ann. N. Y. Acad. Sci. 2003;1005:418–422. doi: 10.1196/annals.1288.071. [DOI] [PubMed] [Google Scholar]
- 45.Nadal A., Alonso-Magdalena P., Soriano S., Quesada I., Ropero A.B. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol. Cell. Endocrinol. 2009;304(1–2):63–68. doi: 10.1016/j.mce.2009.02.016. S0303-7207(09)00150-6 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Nøst T.H., Vestergren R., Berg V., Nieboer E., Odland J.O., Sandanger T.M. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environ. Int. 2014;67:43–53. doi: 10.1016/j.envint.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien B.A., Geng X., Orteu C.H. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J. Autoimmun. 2006;26(2):104–115. doi: 10.1016/j.jaut.2005.11.006. S0896-8411(05)00183-6 [pii] [DOI] [PubMed] [Google Scholar]
- 48.O'Brien B.A., Huang Y., Geng X., Dutz J.P., Finegood D.T. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes. 2002;51(8):2481–2488. doi: 10.2337/diabetes.51.8.2481. [DOI] [PubMed] [Google Scholar]
- 49.O'Brien J.M., Williams A., Yauk C.L., Crump D., Kennedy S.W. In vitro microarray analysis identifies genes in acute-phase response pathways that are down-regulated in the liver of chicken embryos exposed in ovo to PFUdA. Toxicol. In Vitro. 2013;27(6):1649–1658. doi: 10.1016/j.tiv.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Okada E., Sasaki S., Saijo Y. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ. Res. 2012;112:118–125. doi: 10.1016/j.envres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Okajima F., Ui M. Metabolism of glucose in hyper- and hypo-thyroid rats in vivo. Minor role of endogenous insulin in thyroid-dependent changes in glucose turnover. Biochem. J. 1979;182(2):577–584. doi: 10.1042/bj1820577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papadopoulou E., Sabaredzovic A., Namork E., Nygaard U.C., Granum B., Haug L.S. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): the association with breastfeeding and maternal PFAS concentrations. Environ. Int. 2016;94:687–694. doi: 10.1016/j.envint.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Pennings J.L., Jennen D.G., Nygaard U.C. Cord blood gene expression supports that prenatal exposure to perfluoroalkyl substances causes depressed immune functionality in early childhood. J. Immunotoxicol. 2016;13(2):173–180. doi: 10.3109/1547691X.2015.1029147. [DOI] [PubMed] [Google Scholar]
- 54.Predieri B., Iughetti L., Guerranti C., Bruzzi P., Perra G., Focardi S.E. High levels of perfluorooctane sulfonate in children at the onset of diabetes. Int. J. Endocrinol. 2015;2015:234358. doi: 10.1155/2015/234358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reichner J.S., Fitzpatrick P.A., Wakshull E., Albina J.E. Receptor-mediated phagocytosis of rat macrophages is regulated differentially for opsonized particles and non-opsonized particles containing beta-glucan. Immunology. 2001;104(2):198–206. doi: 10.1046/j.0019-2805.2001.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shinomiya M., Nadano S., Shinomiya H., Onji M. In situ characterization of dendritic cells occurring in the islets of nonobese diabetic mice during the development of insulitis. Pancreas. 2000;20(3):290–296. doi: 10.1097/00006676-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Signore A., Procaccini E., Toscano A.M. Histological study of pancreatic beta-cell loss in relation to the insulitis process in the non-obese diabetic mouse. Histochemistry. 1994;101(4):263–269. doi: 10.1007/BF00315913. [DOI] [PubMed] [Google Scholar]
- 58.Sjogren P., Montse R., Lampa E. Circulating levels of perfluoroalkyl substances are associated with dietary patterns—a cross sectional study in elderly Swedish men and women. Environ. Res. 2016;150:59–65. doi: 10.1016/j.envres.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 59.Smit L.A., Lenters V., Hoyer B.B. Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy. 2015;70(6):653–660. doi: 10.1111/all.12605. [DOI] [PubMed] [Google Scholar]
- 60.Solá E., Morillas C., Garzon S., Gomez-Balaguer M., Hernandez-Mijares A. Association between diabetic ketoacidosis and thyrotoxicosis. Acta Diabetol. 2002;39(4):235–237. doi: 10.1007/s005920200040. [DOI] [PubMed] [Google Scholar]
- 61.Stene L.C., Gale E.A. The prenatal environment and type 1 diabetes. Diabetologia. 2013 doi: 10.1007/s00125-013-2929-6. [DOI] [PubMed] [Google Scholar]
- 62.Taniyasu S., Kannan K., Horii Y., Hanari N., Yamashita N. A survey of perfluorooctane sulfonate and related perfluorinated organic compounds in water, fish, birds, and humans from Japan. Environ. Sci. Technol. 2003;37(12):2634–2639. doi: 10.1021/es0303440. [DOI] [PubMed] [Google Scholar]
- 63.Thayer T.C., Wilson S.B., Mathews C.E. Use of nonobese diabetic mice to understand human type 1 diabetes. Endocrinol. Metab. Clin. North Am. 2010;39(3):541–561. doi: 10.1016/j.ecl.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang I.J., Hsieh W.S., Chen C.Y. The effect of prenatal perfluorinated chemicals exposures on pediatric atopy. Environ. Res. 2011;111(6):785–791. doi: 10.1016/j.envres.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Rogan W.J., Chen P.C. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ. Health Perspect. 2014;122(5):529–534. doi: 10.1289/ehp.1306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan S., Wang J., Zhang W., Dai J. Circulating MicroRNA profiles altered in mice after 28 days exposure to perfluorooctanoic acid. Toxicol. Lett. 2013 doi: 10.1016/j.toxlet.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 67.Zheng L., Dong G.H., Zhang Y.H., Liang Z.F., Jin Y.H., He Q.C. Type 1 and Type 2 cytokines imbalance in adult male C57BL/6 mice following a 7-day oral exposure to perfluorooctanesulfonate (PFOS) J. Immunotoxicol. 2011;8(1):30–38. doi: 10.3109/1547691X.2010.537287. [DOI] [PubMed] [Google Scholar]
- 68.Leiter E.H. Obesity genes and diabetes induction in the mouse. Crit. Rev. Food Sci. Nutr. 1993;33(4-5):333–338. doi: 10.1080/10408399309527629. [DOI] [PubMed] [Google Scholar]