Abstract
Purpose:
To present our clinical workflow of incorporating AlignRT for left breast deep inspiration breath-hold treatments and the dosimetric considerations with the deep inspiration breath-hold protocol.
Material and Methods:
Patients with stage I to III left-sided breast cancer who underwent lumpectomy or mastectomy were considered candidates for deep inspiration breath-hold technique for their external beam radiation therapy. Treatment plans were created on both free-breathing and deep inspiration breath-hold computed tomography for each patient to determine whether deep inspiration breath-hold was beneficial based on dosimetric comparison. The AlignRT system was used for patient setup and monitoring. Dosimetric measurements and their correlation with chest wall excursion and increase in left lung volume were studied for free-breathing and deep inspiration breath-hold plans.
Results:
Deep inspiration breath-hold plans had significantly increased chest wall excursion when compared with free breathing. This change in geometry resulted in reduced mean and maximum heart dose but did not impact lung V20 or mean dose. The correlation between chest wall excursion and absolute reduction in heart or lung dose was found to be nonsignificant, but correlation between left lung volume and heart dose showed a linear association. It was also identified that higher levels of chest wall excursion may paradoxically increase heart or lung dose.
Conclusion:
Reduction in heart dose can be achieved for many left-sided breast and chest wall patients using deep inspiration breath-hold. Chest wall excursion as well as left lung volume did not correlate with reduction in heart dose, and it remains to be determined what metric will provide the most optimal and reliable dosimetric advantage.
Keywords: deep inspiration breath-hold, surface imaging, breast cancer therapy, Real-Time Position Management, chest wall excursion
Introduction
The current paradigm of breast cancer therapy commonly includes adjuvant radiation therapy (RT) following lumpectomy or mastectomy. Several randomized trials and a large meta-analysis consisting of 42 000 women with breast cancer and 78 treatment comparisons have proven that the addition of radiation to appropriately selected patients results in significant improvement in local control as well as cancer-specific survival and overall survival (OS).1–4 Further studies continue to define which patients have the highest risk for disease recurrence, and therefore, the greatest benefit from adjuvant RT; however, due to these clear advantages, the use of RT adjuvant to breast conservation surgery (BCS) and mastectomy remains the standard of care for many patients.
Several studies have demonstrated that radiation exposure to the heart and lungs can increase the risk for late cardiac and lung toxicity including ischemic heart disease and radiation pneumonitis or lung cancer, respectively.5–9 The risk of pneumonitis is thought to be less significant as these rates remain very low even when treating internal mammary (IM) and supraclavicular lymph nodes.6 Furthermore, studies have shown a 2.04 relative increased risk of ipsilateral lung cancer ≥10 years following RT for breast cancer in patients who are smokers.7,10 Potential risks from heart exposure could include angina, pericarditis, valvular heart disease, or vascular disease, which could result in increased cardiac-associated mortality, and a 1.5- to 2-fold increased risk has been demonstrated for left-sided breast cancer when compared to right-sided breast cancer.5,7–9,11 Currently, there are no heart doses that are declared safe, and therefore, strategies are used to reduce heart dose as much as possible.
One strategy to minimize heart dose is by using a deep inspiration breath-hold (DIBH) technique, which increases the distance from the target volume to the heart, resulting in reduced heart volume receiving a significant radiation dose.11–15 This can be accomplished by using one of two different methods. Voluntary DIBH is a process where the patient’s breathing is verbally coached and is simultaneously tracked by an external surrogate (ie, Real-Time Position Management [RPM] system from Varian Medical Systems, Palo Alto, California).15–17 The RPM system uses the vertical displacement of the 6-dot infrared tracker placed on the patient (ie, sternum or abdomen) and provides a relative position value with respect to the patient’s breathing cycle.18 The alternate method, termed moderate DIBH, includes limiting the patient’s inspiration to a predefined lung volume determined by the user (usually 75% of the maximum inspiration). This is accomplished by using an active breathing control (ABC) device (Elekta Oncology Systems, Stockholm, Sweden) that moderates the breathing cycle by controlling the lung volume.13,19 Neither strategy is able to monitor or gate the dose delivery based on the position of the target volume (breast or chest wall [CW]), which may introduce some ambiguity in actual dose delivered to the target. Using a surrogate that more clearly defines the target volume, such as an active surface monitoring system, coupled with respiratory gating may resolve this issue.
The AlignRT system (Vision RT Ltd, London, United Kingdom) is an optical tracking system that reconstructs and presents 3-dimensional (3D) surface anatomy through 2 or 3 pairs of ceiling-mounted cameras. Data from each pair of cameras are analyzed and triangulated by a computer vision algorithm to derive 3D surface information from the corresponding perspective. Different perspectives from the cameras construct a real-time reconstructed surface image that can be compared to body contour outlined from the computed tomography (CT) image, which serves as a reference surface model (RSM). Therefore, it can be used for patient positioning prior to treatment, real-time target monitoring, and respiratory tracking. Studies have been published regarding the feasibility and accuracy of using the AlignRT system on the brain, thorax, and breast.20–23 Furthermore, 3D surface imaging has been shown to improve position errors when compared to skin marks for clinical setup and to reduce interfraction setup.24 We recently published our data suggesting that AlignRT can result in more accurate treatment delivery than RPM alone during DIBH.25 In the current study, we hypothesized that the CW excursion from DIBH would be quantitatively associated with reductions in cardiac and lung doses, and the use of AlignRT would help ensure treatment accuracy. The detailed clinical protocol of incorporating AlignRT for left breast DIBH treatments and the dosimetric considerations with the DIBH protocol are presented in this study.
Materials and Methods
Patient Selection and Treatment Planning
We retrospectively reviewed 15 patients with left-sided breast cancer who underwent either lumpectomy or mastectomy followed by irradiation to the whole breast (n = 7) or CW (n = 8) with or without regional nodes (supraclavicular, undissected axillary, and IM). Patient and tumor characteristics were collected for all patients and evaluated. Target volumes and normal structures were contoured in reference to the Radiation Therapy Oncology Group (RTOG) breast-contouring atlas guidelines. Dose prescriptions for target volumes also followed the RTOG guidelines (Table 1). These volumes were separately delineated on the free-breathing (FB) CT and the DIBH CT, and optimized plans were created using Eclipse treatment planning software (Varian Medical Systems, Palo Alto, CA). All treatment plans used conformal planning with tangential fields to the breast or CW. Field-in-field or electronic compensator techniques were applied to improve dose homogeneity, and conformal field arrangements were used for regional nodal targets. To achieve a fair comparison, a single dosimetrist was assigned to generate both FB and DIBH plans for each patient. Optimal coverage of the breast or CW target was attempted on FB and DIBH CT. The decision to use DIBH for treatment was determined by the physician based on the lowest mean heart dose.
Table 1.
Dose Prescription Guidelines for All Targets.
| Volume | Whole Breast Irradiation With No Boost | Whole Breast Irradiation With Boost | Chest Wall or Breast and Regional Nodes |
|---|---|---|---|
| Target breast volume (TBV) | |||
| Ideal | 95% TBV receives ≥47.5 Gy (95% of 50 Gy) | 95% TBV receives ≥47.5 Gy (95% of 50 Gy) | 95% TBV receives ≥47.5 Gy (95% of 50 Gy) |
| Acceptable | 90% TBV receives ≥45 Gy (90% of 50 Gy) | 90% TBV receives ≥45 Gy (90% of 50 Gy) | 90% TBV receives ≥45 Gy (90% of 50 Gy) |
| Lumpectomy cavity PTV | |||
| Ideal | 100% of 50 Gy covers lumpectomy PTV | Final boost dose covers 100% lumpectomy PTV | Final boost dose covers 100% lumpectomy PTV |
| Acceptable | Final boost dose covers 95% of lumpectomy PTV | Final boost dose covers 95% of lumpectomy PTV | |
| Breast (ipsilateral) | Hotspot (<3 cm3) ≤ 108% | Hotspot (<3 cm3) ≤ 108% of final boost dose, V54 < 50%, V(breast at final boost dose) ≤ 30% | |
| Target chest wall volume (TCV) | NA | NA | Same constraints as with TBV only using the chest wall volume |
| Mastectomy scar (PTV) | NA | NA | |
| Supraclavicular nodes (SCN) | NA | NA | 95% receives ≥47.5 Gy (95% of 50 Gy) |
| Axillary nodal volume (Ax) | NA | NA | 95% receives ≥45 Gy |
| Internal mammary nodes (IMN) | |||
| Ideal | NA | NA | 95% receives ≥45 Gy |
| Acceptable | NA | NA | 90% receives ≥40 Gy |
| Lung | |||
| Ideal | V20 ipsilateral lung ≤ 10% | V20 ipsilateral lung ≤ 10% | V20 ipsilateral lung ≤ 25% |
| Heart | |||
| Ideal | V25 < 5%, mean heart dose <3 Gy | V25 < 5%, mean heart dose <3 Gy | V25 < 5%, mean heart dose <3 Gy |
| Acceptable | V25 < 9%, mean heart dose <5 Gy | ||
Abbreviations: NA, not available; PTV, planning target volume.
Deep Inspiration Breath-Hold Treatment Workflow
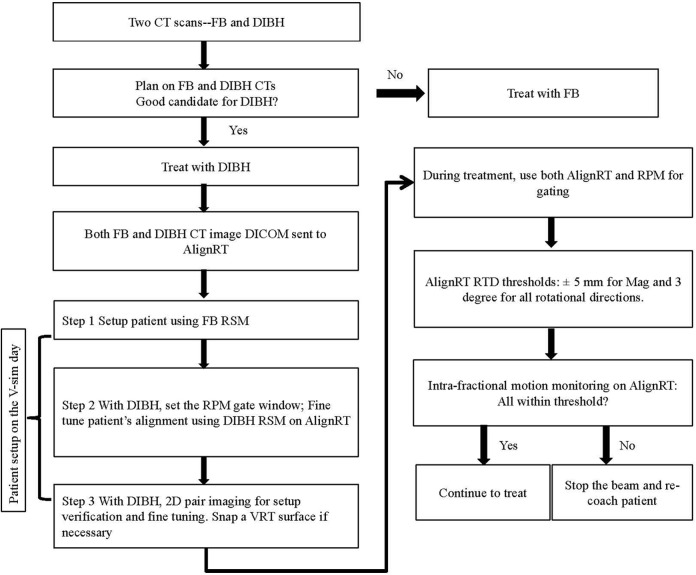
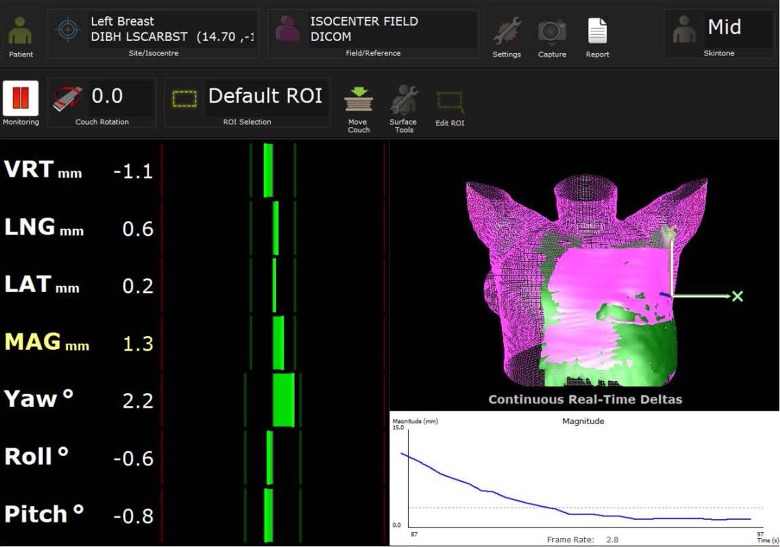
Figure 1 shows the clinical workflow for DIBH treatment. Prior to treatment, the reference respiratory curve from the RPM taken at CT simulation is imported into the TrueBEAM (Varian Medical Systems, Palo Alto, CA) console to establish a 5-mm treatment gate window (RPM reference ±2.5 mm). The RSMs from both FB and DIBH CTs are imported into AlignRT, and the breast/CW regions of interest are defined. On the day of verification simulation, patients are aligned using skin tattoos placed at simulation with FB, which is followed by positioning adjustment based on the registration of the FB RSM using AlignRT. After that, patients are audibly coached on deep inspiration, while alignment is monitored by the RPM system and fine-tuned using the DIBH RSM. The setup and positioning are verified with 2-dimensional (2D) kV orthogonal imaging, which are subsequently taken on a weekly basis. For patients with significant shifts based on 2D imaging, a new surface image (marked as “VRT” in the system) is taken by AlignRT and set as a new RSM during breath-hold, and new skin marks are placed. During daily treatment, AlignRT is used to report the real-time deltas (RTDs) for 3 translational and 3 rotational directions. The vector of all 3 translational RTDs is also reported and named “MAG.” The RTDs are obtained by comparing the surface position at the time of treatment with the reference surface from the planning CT (DIBH CT). A threshold of ±5 mm for MAG and ±3° for rotations was established based on our previous study.25 The RTDs should reach threshold as soon as the patient’s surface at breath-hold matches the RSM and the target is in the treatment position, as shown in Figure 2. At the same time, the RPM trace should reside in the gating window, which triggers the radiation beam. The beam gating is then controlled using both the RPM trace and the AlignRT DIBH RSM.
Figure 1.
Treatment workflow for left breast patients treated with deep inspiration breath-hold (DIBH) technique.
Figure 2.
Screen capture of AlignRT for real-time monitoring of patient’s breath-hold accuracy.
Dosimetric Comparison
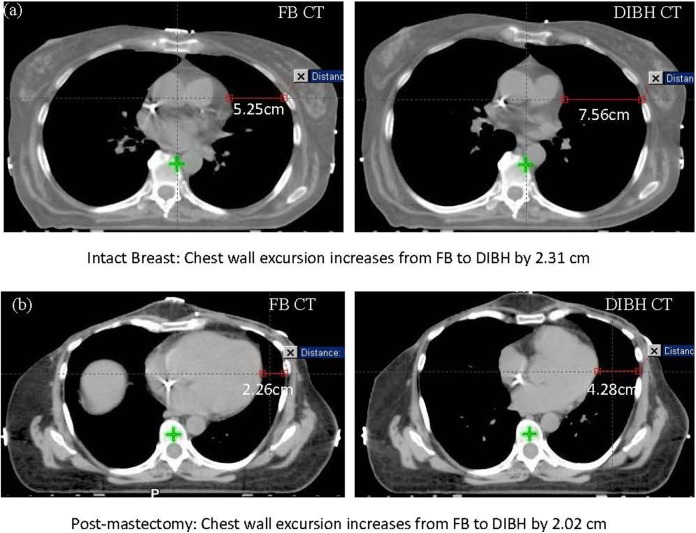
In addition to the DIBH and FB plans generated for each patient, a verification plan was created by applying the DIBH plan to the FB CT, keeping the same plan isocenter. Target coverage and normal tissue dosimetry were analyzed on all 3 plans for each patient. Dosimetric analysis for the normal tissues included maximum heart dose, mean heart dose, left lung V20, total lung V20, and mean lung dose. The CW excursion was measured on the central axial slices (Z = 0) for each study and was defined as the distance from the left heart border to the inner CW, as shown in Figure 3. Our definition of CW excursion was selected because it represents a surrogate for lung inflation and also is a measurement of the displacement of the target from the heart on a representative CT slice that is comparable across plans. The CW excursion was measured and recorded for FB and DIBH CTs, and this difference was computed for each patient, and its correlation to heart and lung dose reduction was evaluated. Figure 3 shows examples of obtaining CW excursion measurements from both FB and DIBH CTs for a breast (Figure 3A) and a CW patient (Figure 3B). As noted in the figure, by taking a deep breath, the CW excursion increased by 2.31 cm for the breast patient and 2.02 cm for the CW patient. Lung volume measurements were completed using the volume measurement tools included in Eclipse. The mean and standard deviation were calculated for the FB and DIBH patients in both the breast and CW groups. These mean values were then compared using the Student t test, with a P value of <.05 representing statistically significant differences. Pearson correlation coefficient was generated to evaluate association between CW excursion or lung inflation and changes in lung or heart doses. All statistical analyses were completed using SPSS version 20 (SPSS Inc, Chicago, Illinois).
Figure 3.
Chest wall excursion comparisons between free breathing (FB) and deep inspiration breath-hold (DIBH) computed tomography (CT) for a intact breast (A) and a postmastectomy patient (B).
Results
Patient Characteristics
The median age for the BCS cohort was 60 years and 14% was premenopausal, whereas the median age for the mastectomy group was 44 years with 88% being premenopausal. All patients in the BCS cohort received a dose of 50 Gy in 25 fractions, and 86% received a boost to the lumpectomy site. For the mastectomy cohort, 63% (n = 5) of patients received 50 Gy in 25 fractions and 37% (n = 3) of patients received 50.4 Gy in 28 fractions, with 75% (n = 6) of patients receiving an electron boost consisting of 10 Gy in 5 fractions to the mastectomy scar. Regional nodal irradiation was delivered in 88% (n = 7) of mastectomy patients and in 0% of BCS patients. The boost dose was not included in the dosimetric evaluation for either group.
Dosimetric Comparison
A total of 15 patients were reviewed for dosimetric comparison between FB and DIBH. A detailed dosimetric comparison is tabulated in Table 2. Average CW excursion was 1.36- and 1.42-fold higher in the breast group and the CW group, respectively. In both the breast and CW groups, DIBH provided a significant volume increase in the left lung (P < .001) and total lungs (P < .001), averaging 874 cm3 for the left lung and 1779 cm3 for total lungs. Even with this difference in volume, the left lung and total lung mean dose and V20 were comparable (P > .05). The significant differences in CW excursion resulted in a 0.4 Gy absolute reduction (P = .067) in the mean heart dose and a 22.2 Gy absolute reduction (P = .0002) in the maximum heart dose for the breast group. Furthermore, the CW group experienced a 3.3 Gy absolute reduction (P = .014) in the mean heart dose and 8.1 Gy absolute reduction (P = .069) in the maximum heart dose.
Table 2.
Dosimetric Comparisons of Regions of Interests Between FB and DIBH Plans for Breast and CW Patients.
| Breast | CW with nodes | |||||
|---|---|---|---|---|---|---|
| DIBH | FB | P | DIBH | FB | P | |
| CW excursion, cm | 6.26 ± 0.93 | 4.60 ± 1.09 | .0097a | 5.10 ± 0.80 | 3.60 ± 1.10 | .0043a |
| Heart mean, Gy | 0.90 ± 0.18 | 1.30 ± 0.49 | .0672 | 1.90 ± 0.90 | 5.20 ± 2.00 | .0143a |
| Heart maximum, Gy | 9.96 ± 3.88 | 32.16 ± 10.45 | .0002a | 39.50 ± 10.50 | 47.60 ± 4.80 | .0686 |
| Left lung V20, % | 11.20 ± 4.21 | 11.11 ± 4.87 | .9725 | 28.10 ± 3.10 | 28.30 ± 6.70 | .9404 |
| Left lung volume, cm3 | 2055.39 ± 384.70 | 1181.34 ± 279.21 | .0004a | 1803.1 ± 355.50 | 1117.1 ± 300.80 | .0010a |
| Left lung mean dose, Gy | 6.61 ± 1.75 | 6.58 ± 2.19 | .975 | 13.94 ± 1.88 | 15.31 ± 1.85 | .164 |
| Total lung V20, % | 5.10 ± 1.90 | 4.89 ± 2.27 | .8513 | 13.20 ± 1.20 | 12.70 ± 2.90 | .6703 |
| Total lung volume, cm3 | 4490.14 ± 723.07 | 2711.06 ± 541.93 | .0001a | 3833.8 ± 695.00 | 2471.8 ± 603.60 | .0009a |
| Total lung mean dose, Gy | 3.05 ± 0.80 | 2.90 ± 1.04 | .771 | 6.67 ± 0.82 | 7.03 ± 0.78 | .376 |
Abbreviations: CW, chest wall; DIBH, deep inspiration breath-hold; FB, free breathing.
a P < .05. Two samples being significantly different (Student t test, 2 tailed, 2-sample equal variance).
Importance of DIBH Positioning Accuracy
As shown in Table 3, both the FB and DIBH plans for the breast group were normalized to 100% of the dose delivered to 95% of the clinical target volume (CTV). These generated plans also had homogenous dose delivery as demonstrated by the very similar dose to 2% and 98% of the CTV as well as consistency between treatment plans as evidenced by the small standard deviations. The verification plan, generated by overlaying the DIBH plan on the FB CT, highlights a possible situation whereby the plan was created using DIBH but was not delivered correctly due to insufficient breath holding. It is evident that the verification plan generated has less homogeneity and appears to be missing the target as denoted by the significantly reduced values of the dose to 98% and 95% of the CTV for breast and 98% of the CTV for CW. Furthermore, the individual plans have reduced homogeneity and target coverage by very wide and unpredictable values as shown by the large standard deviation, indicating that there may be large dose misses if breath-holds are not performed correctly. The result of large variations in dose coverage from the verification plans indicates the importance of target positioning at the time of breath-hold. Clearly, when patients do not take an adequate breath, there can be misses in dose delivery and large and unpredictable variations in how much dose is missed.
Table 3.
Dosimetric Comparisons of Target Coverage for FB Versus DIBH Plans and DIBH Versus Verification Plans for Breast and CW Patients.
| DIBH | FB | P (DIBH Versus FB) | Verification | P (DIBH Versus Verification) | |
|---|---|---|---|---|---|
| Breast group | |||||
| CTV D98%, Gy | 48.1 | 48.2 | .916 | 27.9 | .005 |
| Std | 1.9 | 1.0 | – | 15.5 | – |
| CTV D2%, Gy | 54.3 | 54.5 | .586 | 54.6 | .856 |
| Std | 1.0 | 1.0 | – | 2.7 | – |
| CTV D95%, Gy | 50.00 | 50.00 | 0.99 | 37.8 | .026 |
| Std | 0.00 | 0.00 | – | 12.8 | – |
| CW group | |||||
| CTV D98%, Gy | 40.5 | 42.9 | .303 | 32.2 | .035 |
| Std | 4.6 | 4.6 | – | 9.0 | – |
| CTV D2%, Gy | 54.0 | 53.8 | .837 | 71.6 | .732 |
| Std | 4.6 | 4.4 | – | 11.5 | – |
| CTV D95%, Gy | 44.8 | 45.2 | .751 | 42.4 | .324 |
| Std | 2.4 | 3.0 | – | 6.0 | – |
| Nodes D98%, Gy | 41.6 | 39.5 | .743 | 35.3 | .290 |
| Std | 6.7 | 15.4 | – | 16.2 | – |
| Nodes D2%, Gy | 51.7 | 54.4 | .973 | 51.7 | .313 |
| Std | 2.2 | 2.4 | – | 6.5 | – |
| Nodes D95%, Gy | 45.0 | 46.0 | .516 | 44.5 | .846 |
| Std | 3.5 | 2.5 | – | 5.5 | – |
Abbreviations: CTV, clinical target volume; CW, chest wall; DIBH, deep inspiration breath-hold; FB, free breathing; Std, standard deviation.
Optimal CW Excursion Change
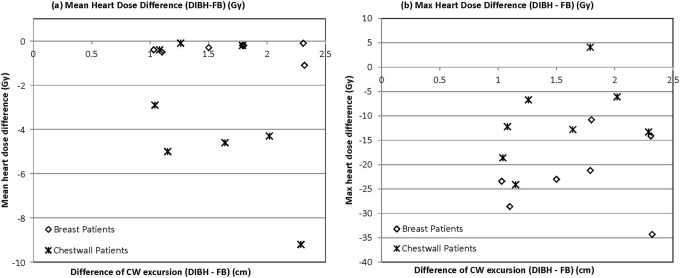
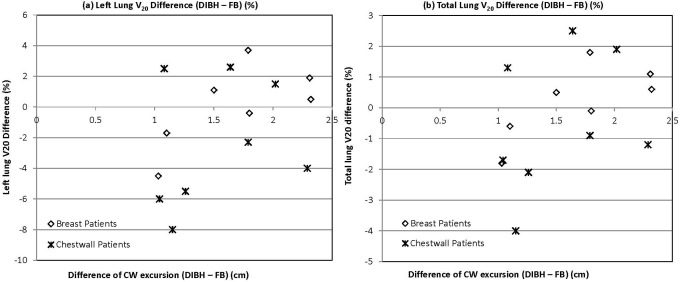
To determine the optimal CW excursion that provides maximum normal tissue sparing while still delivering acceptable target dose, the difference in CW excursion between DIBH and FB was plotted against heart (Figure 4) and lung (Figure 5) doses. Neither mean nor maximum heart dose correlated with CW excursion difference for breast or CW patients (Figure 4A and B). All breast patients exhibited a difference of approximately 1 Gy or less in the mean heart dose. Interestingly, half of the CW patients had a mean heart reduction between 3 and 5 Gy, whereas the other half had very little reduction in the mean heart dose. When evaluating maximum heart dose reduction (Figure 4B), all breast patients with excursion difference from 1 to 2.5 cm exhibited a reduction between 10 and 30 Gy, and the patient achieving the greatest CW excursion had a maximum reduction of about 35 Gy. Similarly, for CW patients, there was a reduction in maximum heart dose for a CW excursion between 1 and 1.5 cm, but as the chest excursion continued from 1.5 to 2.5 cm, there appeared to be less dose reduction, and actually in 1 patient, there was an increase in maximum heart dose (Figure 4B). Although no significant correlation was appreciated, it appeared that both the breast and CW patients followed a similar trend when comparing left lung V20 or total lung V20 to CW excursion (Figure 5). Specifically, with CW excursion between 1 and 1.5 cm, 5 (83%) of 6 patients had reduction in V20, whereas between 1.5 and 2.5 cm, only 3 (33%) of 9 had V20 improvement.
Figure 4.
The correlation between chest wall excursion and (A) mean heart dose reduction and (B) maximum heart dose reduction from free breathing (FB) to deep inspiration breath-hold (DIBH).
Figure 5.
The correlation between chest wall excursion and (A) left lung V20 dose reduction and (B) total lung V20 dose reduction from free breathing (FB) to deep inspiration breath-hold (DIBH).
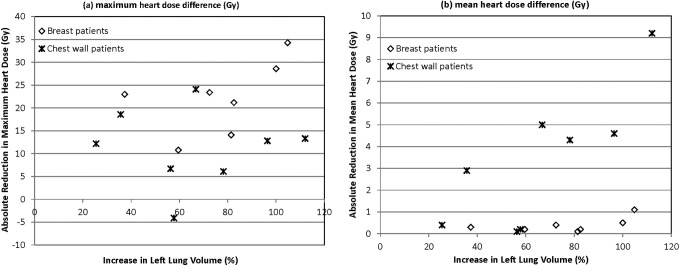
We also analyzed the percentage increase in left lung volume and compared this value with absolute reduction in maximum heart dose and mean heart dose. This method improved our ability to correlate a measurement with reduction in heart dose. The intact breast patients exhibited a moderate positive correlation between percentage left lung increase and maximum heart dose (r = .54) as well as mean heart dose (r = .57; Figure 6). Percentage left lung volume increase displayed a high positive correlation with absolute reduction in the mean heart dose (r = .80) in CW patients but did not correlate well with the maximum heart dose (r = −.0009; Figure 6).
Figure 6.
The correlation between percentage increase in left lung volume and maximum heart dose (A) and mean heart dose (B) for intact breast patients.
Discussion
Numerous studies have confirmed an improvement in locoregional disease recurrence, disease-specific survival, and OS with the addition of RT following breast conservation therapy or mastectomy in properly selected patients.1,3,4 However, this benefit originally established with several older trials has been associated with cardiac and lung toxicity.5–9,26 It is difficult to determine the absolute risk of long-term toxicity for several heart and lung end points as they do not mature until about the second decade following treatment.5,7,8,10,27,28 Therefore, these risk assessments are established from outdated techniques and are not necessarily applicable to current practice.5,7,8,10,27,28 Not only are these older techniques obsolete, but in most cases, the doses delivered to normal tissues are retrospectively estimated without the ability to evaluate 3D CT scans.5,29 Furthermore, the risk of cardiac mortality is multifactorial, and having a diagnosis of cardiac ischemia prior to receiving radiation for left-sided breast cancer results in a significantly higher risk than for those without a prior diagnosis.5,8 Awareness of this long-term and potentially lethal toxicity has spurred the development of several techniques to maximize the therapeutic ratio.11–15,30,31 Here, we report our strategy treating left-sided breast and CW using DIBH treatment monitored with RPM and patient coaching as well as experimentally using AlignRT.
It is clear that RT using outdated RT techniques contributes a nontrivial risk to the development of cardiac disease.5,29 The less established aspects include the absolute risk when using current RT planning and delivery techniques, whether a threshold for safe cardiac dose exists, which dosimetric factors most closely correlate with cardiac toxicity, and what functional metrics best measure cardiac toxicity. One study examining radiation treatment of over 2000 patients from 1958 to 2001 in Sweden and Denmark evaluated an association with major coronary events (myocardial infarction, coronary revascularization, or death from ischemic heart disease).5 The authors calculated the mean heart dose at 4.9 Gy, and they suggested that there was actually no safe dose threshold that did not increase future cardiac events.5 Not surprisingly, they also detected an association with preexisting cardiac risk factors and greater absolute increases in risk from RT. It should be noted that this study is hampered by the retrospective design and difficulty measuring cardiac dose accurately from outdated techniques.5 A prospective study evaluated percentage of the left ventricle included in the radiation field and correlated perfusion defects as well as corresponding wall motion abnormalities as a surrogate for RT-induced cardiac toxicity.32 Although this group did conclude significant differences if >5% of the left ventricle was included in the radiation field as opposed to <5%, suggesting a volume relationship, new defects were still detected in 10% to 20% of patients receiving <5%, and therefore, no safe volume threshold was detected.32 Conversely, in a prospective study evaluating cardiac perfusion changes with single-photon emission computed tomography (SPECT)–CT in 32 patients, there were no changes in cardiac perfusion noted when average cardiac dose was below 5 Gy, suggesting that a safe threshold from mean cardiac dose may exist.31 However, one consideration with these results is that the end point was cardiac perfusion or function changes at 1 year, which may be premature for the development of RT-induced perfusion defects. A similar study was conducted that randomized patients to DIBH with an ABC device versus FB and showed that although the use of ABC significantly reduced the mean heart dose, there were no differences in perfusion on SPECT at 6 months following RT.30 The authors concluded that even very small doses of radiation can potentially induce cardiac toxicity, suggesting no safe dose. Admittedly, this study has a short end point time interval, low patient numbers, and also imperfect sensitivity of SPECT imaging.30 Although current guidelines provide some recommendations on appropriate cardiac dose, such as mean heart dose less than 5 Gy, reduction in heart dose as low as reasonably achievable may result in the lowest risk of cardiac toxicity.
The results from our cohort suggest that the CW excursion from DIBH in both intact breast and postmastectomy patients resulted in heart dose reductions. Several groups have evaluated heart dose to particular regions including the left anterior descending artery, but this has not been widely accepted and is not integrated in current practice. However, the mean heart dose has been proven to be an important surrogate for late heart complications in many studies and therefore was selected for a heart dose surrogate in our study.5,29,31 Since the CW is not in the target volume for intact breast, the prescription isodose line is further from the heart than in the postmastectomy setting, which is likely the underlying reason for the less significant decrease in mean heart dose for our breast cohort. However, this group did exhibit a decrease in maximum heart dose of 22.2 Gy, since a small fraction of the heart can actually be within the treatment fields during FB, therefore receiving direct high dose. The significance of this remains unknown, but theoretically, if critical vascular structures are exposed to this high dose, it could result in vascular damage and stenosis. As discussed previously, a study by Marks et al supports this concept by showing that even when <5% of the left ventricle was included in the radiation field, there was a 10% to 20% incidence of new perfusion defects and associated cardiac wall motion abnormalities within the first 2 years.32 The differences in mean heart dose and maximum heart dose in the CW group were each significant in the CW group likely because the target is in closer proximity to the heart, and therefore, the increase in distance with DIBH provides a more significant reduction. In summary, there is a significant reduction in mean heart dose and maximum heart dose for the CW patients and a significant decrease in maximum heart dose for the intact breast patients with DIBH.
The connection with breast or CW radiation and lung toxicity has been established with outdated techniques, but these data are less robust than that for cardiac toxicity. Our findings suggest that there are insignificant differences in total or ipsilateral lung mean dose or V20. The rates of pneumonitis are low, and generally, all dose constraint recommendations are easily met with or without DIBH. In one study including 540 patients, no radiation pneumonitis was observed for 64 patients who received a mean lung dose up to 8 Gy, whereas 8 (10%) of 81 patients developed radiation pneumonitis those exposed to a mean lung dose of 8 to 12 Gy.27 We observed a mean dose for the total lung of 2.9 Gy (FB) and 3.0 Gy (DIBH) for the breast patients and 7 Gy (FB) and 6.7 Gy (DIBH) for the CW patients, with insignificant differences between the groups. Graham et al showed that total lung V20 was the only significant factor to predict ≥grade II pneumonitis and a V20 < 22% resulted in no pneumonitis.33 Again, this was easily met in our patients with an average total lung V20 of 4.9% (FB) and 5.1% (DIBH) for the breast patients and 12.7% (FB) and 13.2% (DIBH) for the CW patients. Our data indicate that DIBH does not significantly reduce V20 or mean dose for the ipsilateral or total lung volume. This finding was expected, and a likely explanation is that as the lung volume expands, a similar relative volume is included in the radiation dose, and therefore, the percentage volume of lung receiving a particular dose remains largely unchanged. It is plausible that the modest benefits observed in the CW group would have been statistically significant with more patient numbers, but it remains questionable whether a 1 to 2 Gy decrease in V20 or mean lung dose is clinically significant. As the true purpose for DIBH treatment is to avoid heart dose, it was important to show that this technique does not result in a detriment to lung dose.
The most desirable CW excursion for the minimization of normal tissue toxicity remains unknown. We did not find any reliable correlation with mean heart dose, maximum heart dose, or lung V20 with difference in CW excursion. Furthermore, our data support the notion that more CW expansion does not necessarily mean more normal tissue sparing and in some cases can paradoxically increase normal tissue dose. It should be noted that an increase in heart dose only occurred in one CW treatment and that all other patients either had similar or decreased heart dose. Our patient population had a minimum and maximum CW excursion of 1 and 2.5 cm, respectively. This would suggest that a 1 cm expansion of the CW using our measurement definition could be enough to significantly reduce cardiac dose. Since reliable correlation between increased CW expansion and dosimetric advantage was not determined, we would recommend evaluating each treatment plan on all patients prior to determining which is most beneficial. It should also be noted that our definition of CW excursion is a surrogate for increased lung inflation selected for ease of measurement and comparison among plans, but a different definition such as CW expansion as measured from the heart to the point perpendicular to the tangential radiation beams could show a correlation with normal tissue dose. There was a much better correlation with percentage increase in left lung volume and reduction in mean and maximum heart dose in breast patients and in mean heart dose in CW patients. This value is likely more accurate because it essentially evaluates a change that is integrated across all CT slices and better encapsulates the increased distance of the target from the heart than a measurement on 1 slice. Although it appears that further increase in lung volume leads to increased heart avoidance, this also must be reproducible and tolerable. Enhanced patient comfort likely results in more reliable positioning and reproducibility of treatment leading to decreased chances of marginal misses, which are possible as determined by our previous work and the verification CT in our current study.25 The large variation in dose coverage in the verification plans in Table 3 demonstrates this reduced dose coverage and emphasizes the benefit of using AlignRT for target setup and monitoring during DIBH treatments. The most apparent limitation of our work is that we had reduced power to detect any reliable correlation between CW excursion and dosimetric advantage, and even very few outliers could affect our results. Furthermore, a more complete understanding of what heart dose reductions are clinically meaningful would be helpful, but until then, a prudent approach is to limit heart dose to as low as reasonably achievable, which for many patients would include utilization of DIBH. Future studies are warranted to establish possible correlations with more patient data points.
In conclusion, we showed that using DIBH in left-sided intact breast radiation, the maximum heart dose is significantly reduced and the mean heart dose is reduced to a lesser degree. Furthermore, this technique for postmastectomy left-sided CW radiation resulted in significantly reduced mean heart dose and maximum heart dose with a paradoxical increase in maximum heart dose for 1 patient. This was achieved while resulting in the same total lung V20 and left lung V20. Percentage left lung volume increase allowed for better correlation with reduced mean and maximum heart dose than CW excursion as defined in our study, however, it is possible that other metrics will allow better correlation of heart avoidance.
Acknowledgments
The authors would like to thank Nilendu Gupta, Patricia Glass, and Monica Fullenkamp for their support in the development and application of this technique.
Abbreviations
- ABC
active breathing control
- BCS
breast conservation surgery
- CT
computed tomography
- CTV
clinical target volume
- CW
chest wall
- DIBH
deep inspiration breath-hold
- 2D
2-dimensional
- 3D
3-dimensional
- FB
free breathing
- IM
internal mammary
- OS
overall survival
- RPM
Real-Time Position Management
- RSM
reference surface model
- RT
radiation therapy
- RTD
real-time delta
- RTOG
Radiation Therapy Oncology Group
- SPECT
single-photon emission computed tomography
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jagsi R, Pierce L. Postmastectomy radiation therapy for patients with locally advanced breast cancer. Seminars Radiat Oncol. 2009;19(4):236–243. [DOI] [PubMed] [Google Scholar]
- 3. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. [DOI] [PubMed] [Google Scholar]
- 4. McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New Eng J Med. 2013;368(11):987–998. [DOI] [PubMed] [Google Scholar]
- 6. Hurkmans CW, Borger JH, Bos LJ, et al. Cardiac and lung complication probabilities after breast cancer irradiation. Radiother Oncol. 2000;55(2):145–151. [DOI] [PubMed] [Google Scholar]
- 7. Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557–565. [DOI] [PubMed] [Google Scholar]
- 8. McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100(2):167–175. [DOI] [PubMed] [Google Scholar]
- 9. Bouillon K, Haddy N, Delaloge S, et al. Long-term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol. 2011;57(4):445–452. [DOI] [PubMed] [Google Scholar]
- 10. Prochazka M, Hall P, Gagliardi G, et al. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: case-only design. J Clin Oncol. 2005;23(30):7467–7474. [DOI] [PubMed] [Google Scholar]
- 11. Lu HM, Cash E, Chen MH, et al. Reduction of cardiac volume in left-breast treatment fields by respiratory maneuvers: A CT study. Int J Radiat Oncol Biol Phys. 2000;47(4):895–904. [DOI] [PubMed] [Google Scholar]
- 12. Sixel KE, Aznar MC, Ung YC. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int J Radiat Oncol Biol Phys. 2001;49(1):199–204. [DOI] [PubMed] [Google Scholar]
- 13. Remouchamps VM, Letts N, Vicini FA, et al. Initial clinical experience with moderate deep-inspiration breath hold using an active breathing control device in the treatment of patients with left-sided breast cancer using external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56(3):704–715. [DOI] [PubMed] [Google Scholar]
- 14. Mageras GS, Yorke E. Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semi Radiat Oncol. 2004;14(1):65–75. [DOI] [PubMed] [Google Scholar]
- 15. Vikstrom J, Hjelstuen MH, Mjaaland I, Dybvik KI. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol. 2011;50(1):42–50. [DOI] [PubMed] [Google Scholar]
- 16. Korreman SS, Pedersen AN, Nottrup TJ, Specht L, Nystrom H. Breathing adapted radiotherapy for breast cancer: comparison of free breathing gating with the breath-hold technique. Radiother Oncol. 2005;76(3):311–318. [DOI] [PubMed] [Google Scholar]
- 17. Stranzl H, Zurl B, Langsenlehner T, Kapp KS. Wide tangential fields including the internal mammary lymph nodes in patients with left-sided breast cancer. Influence of respiratory-controlled radiotherapy (4D-CT) on cardiac exposure. Strahlenther Onkol. 2009;185(3):155–160. [DOI] [PubMed] [Google Scholar]
- 18. Pepin EW, Wu H, Shirato H. Dynamic gating window for compensation of baseline shift in respiratory-gated radiation therapy. Med Phys. 2011;38(4):1912–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55(2):392–406. [DOI] [PubMed] [Google Scholar]
- 20. Bert C, Metheany KG, Doppke KP, Taghian AG, Powell SN, Chen GT. Clinical experience with a 3D surface patient setup system for alignment of partial-breast irradiation patients. Int J Radiat Oncol Biol Phys. 2006;64(4):1265–1274. [DOI] [PubMed] [Google Scholar]
- 21. Cervino LI, Gupta S, Rose MA, Yashar C, Jiang SB. Using surface imaging and visual coaching to improve the reproducibility and stability of deep-inspiration breath hold for left-breast-cancer radiotherapy. Phys Med Biol. 2009;54(22):6853–6865. [DOI] [PubMed] [Google Scholar]
- 22. Cervino LI, Pawlicki T, Lawson JD, Jiang SB. Frame-less and mask-less cranial stereotactic radiosurgery: a feasibility study. Phys Med Biol. 2010;55(7):1863–1873. [DOI] [PubMed] [Google Scholar]
- 23. Schoffel PJ, Harms W, Sroka-Perez G, Schlegel W, Karger CP. Accuracy of a commercial optical 3D surface imaging system for realignment of patients for radiotherapy of the thorax. Phys Med Biol. 2007;52(13):3949–3963. [DOI] [PubMed] [Google Scholar]
- 24. Shah AP, Dvorak T, Curry MS, Buchholz DJ, Meeks SL. Clinical evaluation of interfractional variations for whole breast radiotherapy using 3-dimensional surface imaging. Pract Radiat Oncol. 2013;3(1):16–25. [DOI] [PubMed] [Google Scholar]
- 25. Rong Y, Walston S, Welliver MX, Chakravarti A, Quick AM. Improving intra-fractional target position accuracy using a 3D surface surrogate for left breast irradiation using the respiratory-gated deep-inspiration breath-hold technique. PLoS One. 2014;9(5):e97933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24(25):4100–4106. [DOI] [PubMed] [Google Scholar]
- 27. Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42(1):1–9. [DOI] [PubMed] [Google Scholar]
- 28. Ford MB, Sigurdson AJ, Petrulis ES, et al. Effects of smoking and radiotherapy on lung carcinoma in breast carcinoma survivors. Cancer. 2003;98(7):1457–1464. [DOI] [PubMed] [Google Scholar]
- 29. Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiat Oncol Biol Phys. 2007;69(5):1484–1495. [DOI] [PubMed] [Google Scholar]
- 30. Zellars R, Bravo PE, Tryggestad E, et al. SPECT analysis of cardiac perfusion changes after whole-breast/chest wall radiation therapy with or without active breathing coordinator: results of a randomized phase 3 trial. Int J Radiat Oncol Biol Phys. 2014;88(4):778–785. [DOI] [PubMed] [Google Scholar]
- 31. Chung E, Corbett JR, Moran JM, et al. Is there a dose-response relationship for heart disease with low-dose radiation therapy? Int J Radiat Oncol Biol Phys. 2013;85(4):959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63(1):214–223. [DOI] [PubMed] [Google Scholar]
- 33. Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999;45(2):323–329. [DOI] [PubMed] [Google Scholar]